Abstract
Background/Objectives: Stroke remains a leading cause of disability in Japan, and early mobilization is an important strategy to prevent muscle atrophy and promote independence. However, the optimal intensity and duration of early rehabilitation remain unclear. This study aims to examine the association between rehabilitation dose during the acute phase of stroke and functional outcomes at 90 days post-onset. Methods: This multicenter prospective cohort study will enroll patients from twelve acute care hospitals across Japan, beginning in June 2026. Eligible patients are aged ≥ 18 years, expected to be hospitalized for ≥7 days, and initiated rehabilitation by day 2 after stroke onset. Rehabilitation dose will be quantified using the Mobilization Quantification Score (MQS). The primary outcome is functional status measured by the modified Rankin Scale (mRS) at 90 days. Secondary outcomes include muscle atrophy assessed by ultrasound, the Barthel Index, and physical performance measures. Subgroup analyses will evaluate how stroke severity modifies the dose–response relationship. Results: As this is a study protocol, results are not yet available. The study is designed to clarify the relationship between early rehabilitation dose and functional recovery after stroke. Conclusions: This is the first large-scale Japanese study to assess early stroke rehabilitation dosage using a standardized tool. Findings are expected to provide evidence for individualized, evidence-based mobilization strategies to optimize functional outcomes in stroke patients.
1. Introduction
Stroke is a leading cause of disability worldwide [] and ranks fourth among the top ten causes of death in Japan. Although advances in medical science have improved survival rates for patients with severe stroke in recent years, the number of individuals living with long-term physical and cognitive sequelae has increased [,]. Among these, muscle atrophy in the paralyzed limbs of patients with hemiplegia, referred to as central atrophy, significantly affects both functional independence and quality of life []. In addition to stroke-related disability, critically ill patients may experience other complications, such as intensive care unit (ICU) acquired weakness, characterized by generalized weakness of the limbs and respiratory muscles, further impeding recovery [,]. Skeletal muscle atrophy in severely ill patients progresses rapidly by approximately 2% per day, amounting to a 12% reduction in muscle mass per week, and is directly associated with loss of functional independence and reduced quality of life. Therefore, early detection and prevention of muscle atrophy are crucial [].
Rehabilitation is a primary strategy to promote functional recovery and enhance independence in stroke patients []. Once the patient’s neurological symptoms and vital signs stabilize during the acute phase, those with functional impairments transition to the post-acute phase and begin rehabilitation. Traditionally, active rehabilitation has been implemented during the post-acute phase, when patients are medically stable, with the goal of minimizing long-term disability []. More recently, increasing attention has been paid to early mobilization in critically ill patients. While several studies have reported the safety of early mobilization, its effectiveness remains a subject of debate []. Several systematic reviews and guideline papers have highlighted both the potential benefits and uncertainties of very early mobilization after stroke, noting variability in outcomes depending on patient severity, timing, and dose of interventions [,,]. For example, one study reported that very early mobilization was associated with poorer functional outcomes and higher rates of adverse events at 3 months post-stroke compared to standard care []. However, a post hoc analysis of the same cohort revealed that patients who received shorter and more frequent mobilization sessions had significantly improved functional outcomes [].
Previous research investigating the impact of early mobilization has examined patient outcomes in relation to age, stroke severity, and specific rehabilitation parameters, including intensity, duration, frequency, and timing of interventions [,,]. It has also been suggested that optimal rehabilitation parameters should be tailored to the individual’s disease status and clinical characteristics []. Furthermore, considering both intensity and duration as components of physical activity may enable a more precise quantification of rehabilitation interventions, potentially leading to optimized clinical outcomes []. The acute phase of stroke is characterized by hemodynamic instability, rapid neurological deterioration, and accelerated skeletal muscle wasting, which can begin within hours of onset and progress by approximately 2% per day in severely ill patients []. During this period, patients are also at high risk for complications such as pneumonia, deep vein thrombosis, and pressure ulcers, all of which are exacerbated by immobility []. Therefore, timely initiation of rehabilitation is considered crucial not only to prevent secondary complications and muscle atrophy but also to maximize the potential for neurological recovery. This critical window highlights the need to carefully evaluate the appropriate dose and timing of early mobilization interventions. However, there is currently no consensus on the ideal dose, intensity, or timing of early mobilization for stroke patients in the acute phase. Each healthcare facility is thus exploring these parameters independently in pursuit of best practices []. Notably, there have been no large-scale studies in Japan evaluating the optimal daily dose of rehabilitation for stroke patients, nor have there been investigations focusing on the dosage of early-stage rehabilitation interventions.
Despite growing interest in early mobilization after stroke, key uncertainties remain. First, the optimal dose and timing in the acute phase are unresolved, with heterogeneous and sometimes conflicting results [,,]. Second, most studies lack a standardized, quantitative dose metric, limiting cross-study comparability; the Mobilization Quantification Score (MQS) addresses this by integrating mobilization level and duration [,]. Finally, early-phase studies rarely incorporate objective muscle-atrophy assessments (e.g., rectus femoris ultrasound) despite prognostic relevance [,]. We hypothesize that the optimal rehabilitation dose during the acute phase of stroke varies by stroke severity: higher-intensity mobilization may promote greater independence in activities of daily living at 90 days post-stroke in patients with mild strokes, whereas lower-intensity mobilization may be more beneficial in those with severe strokes. We hypothesize that a higher early rehabilitation dose will be associated with better functional outcomes at 90 days. We further hypothesize that baseline stroke severity modifies this association: patients with mild-to-moderate stroke will benefit from higher MQS [], whereas patients with severe stroke may benefit from moderate doses. Secondary hypotheses are that higher MQS will be associated with less rectus femoris muscle atrophy on ultrasound and better performance on physical assessment. To test this hypothesis, we will use the MQS to assess rehabilitation dose. In addition, we will analyze patient background characteristics, nutritional status, muscle atrophy, and differences in rehabilitation protocols across participating facilities. This study aims to establish standardized and optimized early mobilization practices and to evaluate the relationship between MQS-based rehabilitation dosage and functional independence in stroke patients.
2. Methods
This study protocol is reported in accordance with the Standard Protocol Items: Recommendations for Interventional Trials checklist (see Supplementary Materials Table S1).
2.1. Study Design
This is a multicenter, prospective, observational cohort study with a 90-day follow-up. The study will begin with the enrollment of the first patient and continue until the 90-day follow-up of the last enrolled participant. Twelve acute care hospitals across Japan will participate. Enrollment is scheduled to begin in June 2026 and continue for 18 months.
2.2. Ethical Approval
The study protocol has been approved by the Ethics Committee of Gifu Health Science University (approval number: 2025-012). All participating institutions will obtain approval from their respective ethics committees before patient recruitment. The study will be conducted in accordance with the Declaration of Helsinki and relevant Japanese ethical guidelines. Written informed consent will be obtained from all patients or their legally authorized representatives (e.g., next of kin) within 24 h of admission, if not already obtained during hospitalization. The study has been registered with the University Hospital Medical Information Network (UMIN000057876).
2.3. Study Setting
Twelve regional acute care hospitals in the Chubu region will participate (Supplementary materials Figure S1). Institutional characteristics (including the presence of unique clinical protocols, nurse-to-patient ratios, and availability of neurologists or other specialists) will be documented prior to enrollment and remain fixed throughout the study period. Rehabilitation practices vary among institutions but are based on standard references, including the Stroke Treatment Guidelines [], Japanese Guidelines for Rehabilitation of Critically Ill Patients [], and the Japanese Guidelines for Nutrition in Critically Ill Patients [].
All hospitalized stroke patients will be screened within 24 h of admission by the research staff or departmental rehabilitation teams on working days. Eligible patients (if awake and cooperative) or their family members will be approached for consent within the first 24 h of admission, after which data collection will begin (Table 1).

Table 1.
Schedule of enrolment, interventions, and assessments.
2.4. Timeline
After enrollment in the BRIDGE cohort, patients will remain in the study until loss to follow-up or completion of the 90-day assessment post-onset. Data will be collected at four time points: (1) enrollment, (2) day 7 of hospitalization, (3) hospital discharge, and (4) 90 days after stroke onset (Figure 1).
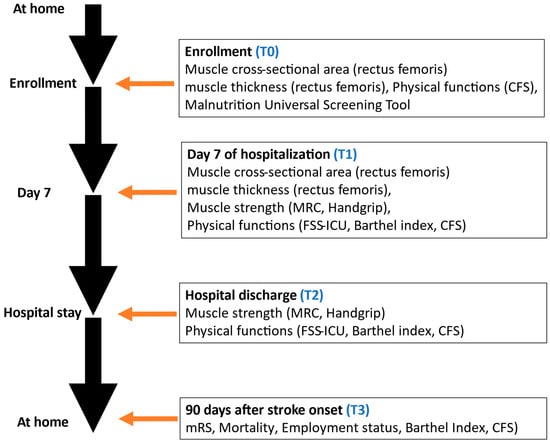
Figure 1.
Flowchart of performed questionnaires during the study. Clinical Frailty Scale, CFS; Medical Research Council, MRC; Functional Status Score for the Intensive Care Unit, FSS-ICU.
2.5. Participants
a. Inclusion criteria:
- Patients diagnosed with acute stroke, specifically including ischemic stroke (cerebral infarction) and hemorrhagic stroke (intracerebral hemorrhage), who are expected to be hospitalized for ≥7 days. Patients with subarachnoid hemorrhage or transient ischemic attack (TIA) will be excluded because their rehabilitation course differs substantially from that of ischemic and hemorrhagic stroke;
- Age ≥ 18 years;
- Provision of informed consent;
- Initiation of rehabilitation by day 2 of admission.
b. Exclusion criteria:
- Pre-hospitalization mRS ≥ 3 (unable to walk even with aids);
- Terminal care patients or those with non-curative intent;
- Patients with anticipated prolonged immobility due to trauma (e.g., multiple unstable fractures, burns, amputations);
- Inability to communicate in Japanese;
- Explicit refusal to allow use of clinical data for research.
c. Anticipated cohort characteristics and planned reporting.
Based on stroke case-mix in the participating hospitals, we anticipate a sex distribution of approximately 55–60% male and 40–45% female, with a mean age around 70 years. For transparency and comparability, we predefine age strata as <65, 65–79, and ≥80 years. Baseline characteristics will be summarized overall and, where appropriate, by sex and age group.
2.6. Primary Outcome
The primary outcome is the modified Rankin Scale (mRS) score at 90 days post-stroke. The mRS ranges from 0 (no symptoms) to 6 (death) and is the most widely used functional outcome measure in stroke trials []. An mRS ≤ 2 at 90 days is defined as a favorable outcome (Table 2).

Table 2.
Details of outcome measures at follow-up.
2.7. Secondary Outcomes
As secondary outcomes, we first selected the item of change in muscle atrophy using ultrasound examination [,]. This will allow us to determine the optimal MQS dose for muscle atrophy results. Next, we selected the items of the Barthel Index [], Medical Research Council score [], functional status score in ICU [], grip strength [], Clinical Frailty Scale [], return to work, and complications. We aim to investigate these items and quantify ICU-acquired weakness from multiple perspectives (Table 2). We will also record adverse events during the rehabilitation intervention (falls, cardiac arrest, tachycardia > 130 bpm, systolic blood pressure < 80 or >200, ventricular tachycardia, other dangerous arrhythmias, arterial oxygen saturation of pulse oximetry < 80% for >3 min, and other device removal).
2.8. Baseline Characteristics and Treatment
The baseline characteristics of enrolled patients will be prospectively collected, including baseline factors such as age, height, weight, handedness, employment status, existing comorbidities, pre-admission mRS, and frailty; disease factors such as culprit vessel, lesion, and national institutes of health stroke scale []; biochemical data such as C-reactive protein, lymphocyte count, and serum albumin; and nutrition factors such as the Malnutrition Universal Screening Tool [] at admission. The National Institutes of Health Stroke Scale will be recorded at admission, day 7, and at discharge. Details of treatments that may affect outcomes, such as surgery, use of Tissue Plasminogen Activator, noninvasive ventilation, mechanical ventilation, and tracheostomy, will also be prospectively collected.
2.9. Data Source/Measurements
At each of the above time points, ultrasound will be used to evaluate the cross-sectional area and muscle thickness of the rectus femoris. All collected data will be prepared according to standard protocols published in the field and analyzed by a team experienced in muscle ultrasound (physiotherapists and/or neurologists and rehabilitation physicians currently working in the hospital).
2.10. Ultrasonography Assessment
Ultrasound will be used to measure the rectus femoris muscle cross-sectional area and thickness in supine patients with extended knees. Measurements will be taken at the anterior superior iliac spine and the distal third of the thigh using B-mode with a linear probe. For consistency, anatomical landmarks will be marked for repeated measures. If one image cannot capture the entire muscle, it will be reconstructed from multiple sections. Each measurement is taken three times, and the median value is used. Pre-study training ensures that intra- and inter-rater variation remains below 3%.
2.11. Rehabilitation Protocol
Participants in the BRIDGE cohort will receive their usual rehabilitation at their respective institutions. We aim to exercise all participants equally every day, based on a five-stage protocol (Level 1: passive range of motion and respiratory physiotherapy, Level 2: active range of motion, Level 3: sitting exercise, Level 4: standing exercise, Level 5: walking exercise) tailored to each participating hospital. In addition, adverse events during implementation will also be indicated with appropriate values in the categories of medical, cardiovascular, respiratory, and neurological problems. In case of deviation from these values, the patient will be immediately placed on bed rest, and the event will be considered an adverse event. After level 5 is achieved, a physiotherapist or occupational therapist will provide each patient with rehabilitation, including muscle strengthening, balance, walking, and stair climbing, for at least 20 min on weekdays, according to the rehabilitation policy of each hospital.
2.12. Data Management and Follow-Up
Patient data will be managed in compliance with Japanese data protection guidelines. A unique study number will be assigned upon enrollment. Follow-up assessments will be conducted via telephone at 90 days post-onset, collecting data on survival, employment, and mRS. If unreachable after multiple attempts, patients will be considered lost to follow-up. Informed consent includes potential secondary use of anonymized data, contingent on new ethical approval.
2.13. Rehabilitation Dose
Rehabilitation dose is defined using the MQS over days 1–7 post-admission. MQS combines mobilization level (assessed using the ICU mobility scale []) and duration (measured with a stopwatch). Only active mobilization time is included; preparation or rest is excluded. The MQS is averaged across the first 7 days to reflect the daily rehabilitation dose (Japanese version MQS []. It should be noted that rehabilitation itself continues beyond the first 7 days until hospital discharge, according to each institution’s standard practice. After discharge, patients typically continue rehabilitation in post-acute care facilities or outpatient/community settings, depending on their medical condition and regional healthcare resources. Functional outcomes at 90 days will therefore reflect the combined effect of early inpatient rehabilitation and subsequent standard care.
2.14. Statistical Methods
There is no maximum sample size for this study; however, the outcome may be subject to targets or maximum sample sizes, which will be specified in the relevant sub-protocol. The expected sample size is ≥200 patients (minimum 10 patients/facilities, ≥20 facilities) to provide a sufficient number of different rehabilitation doses to predict functional outcomes and build robust models for protocol use. To avoid over-representation of some centers, data collection is limited to 50 patients per facility. Patient enrollment will therefore not be strictly equal across hospitals; each facility is expected to enroll at least 10 patients, with a maximum of 50 patients, depending on their clinical volume. This strategy ensures diversity in practice patterns while preventing over-representation of any single center. The number of enrolled patients and facilities is a sufficient sample size to capture the various variations in practice and treatment. It is considered feasible to collect a sample of this calculated size because each participating facility should be able to enroll at least one patient per month based on the number of stroke patients hospitalized at each facility in the past.
Baseline characteristics and outcomes will be summarized using descriptive statistics. Continuous variables will be presented as means with standard deviations (SDs) or medians with interquartile ranges (IQRs), while categorical variables will be presented as frequencies and percentages (n, %).
The primary analysis will examine the association between the mean rehabilitation dose (MQS score) during the first 7 days of hospitalization and the primary outcome of a favorable functional outcome (mRS ≤ 2) at 90 days. This association will be evaluated using a multivariable logistic regression model. The model will be adjusted for a pre-specified set of covariates selected for their established prognostic significance, including age, sex, pre-admission mRS, baseline National Institutes of Health Stroke Scale (NIHSS) score, stroke subtype (ischemic or hemorrhagic), and participating facility (as a random effect).
For secondary outcomes, multivariable linear regression models will be used for continuous variables, such as the Barthel Index. A pre-specified subgroup analysis will be conducted to explore whether the effect of rehabilitation dose is modified by initial stroke severity. An interaction term between the MQS score and the severity category (defined by baseline NIHSS) will be introduced into the primary model.
Time-to-event outcomes will be analyzed using Kaplan–Meier curves and Cox proportional hazards regression models, adjusted for baseline characteristics. For survival analysis, patients will be stratified into groups based on quartiles of rehabilitation volume and pre-defined stroke severity categories. Missing data for covariates will be handled using multiple imputation under the missing-at-random assumption. The primary analysis will be based on the pooled results from the imputed datasets, with a complete-case analysis performed as a sensitivity analysis.
All statistical analyses will be performed using JMP (version 13.0; SAS Institute, Cary, NC, USA) and IBM SPSS software (version 23.0; IBM, Armonk, NY, USA). A two-sided p-value of <0.05 will be considered statistically significant.
3. Discussion
This multicenter prospective cohort study aims to evaluate the association between rehabilitation dose quantified using MQS and functional outcomes in patients with acute stroke. The study is designed to address the current lack of evidence regarding optimal early mobilization parameters, particularly in the acute phase of stroke care. While early rehabilitation has been widely promoted in recent years, clinical practices remain variable, with no established consensus on ideal dose, timing, or intensity []. Our study is the first large-scale investigation in Japan to quantitatively assess daily rehabilitation dose using a validated measure and its relationship with long-term independence in activities of daily living.
Prior studies on early mobilization in stroke patients have yielded inconsistent findings. For instance, while very early mobilization was associated with poorer outcomes in some randomized controlled trials [], further post hoc analyses have suggested that mobilization delivered in shorter, more frequent sessions may improve recovery []. These conflicting findings may be attributed to differences in rehabilitation intensity, timing, patient severity, and methodological inconsistencies across studies []. By incorporating MQS, which integrates both mobilization level and duration, our study provides a more comprehensive and standardized approach to measuring rehabilitation exposure []. Furthermore, by stratifying patients based on stroke severity, our study will allow exploration of differential responses to rehabilitation dose, which may help define individualized, severity-specific strategies.
Another strength of this study is the integration of multiple functional, physical, and clinical outcomes, including mRS, Barthel Index, Medical Research Council score, muscle ultrasound, and frailty assessments, which enables a multifaceted evaluation of recovery. The inclusion of ultrasound to objectively assess atrophy of the rectus femoris provides novel insight into the impact of early mobilization on muscle preservation, which has been relatively underexplored in stroke rehabilitation research [,]. Additionally, our protocol controls for key patient-level and institutional factors, including comorbidities, nutritional status, rehabilitation policy, and institutional staffing, enhancing the robustness of our multivariate analyses.
By quantifying early rehabilitation dose with MQS over days 1–7 and linking it to 90-day outcomes, this protocol is designed to yield directly actionable outputs for practice. First, clinicians can translate MQS into severity-specific dose targets during daily rounds, facilitating individualized prescriptions rather than one-size-fits-all approaches. Second, routine rectus femoris ultrasound enables early detection of muscle atrophy and dose adjustment when deterioration is observed. Third, aggregating MQS and outcomes at the facility level provides benchmarking metrics that support quality-improvement cycles and alignment across centers. Finally, these data can inform guideline refinement and the design of future randomized trials by identifying dose bands and patient subgroups most likely to benefit.
Nevertheless, some limitations must be acknowledged. First, as an observational study, causal relationships cannot be definitively established. Second, rehabilitation practices are not standardized across facilities, which may introduce variability. However, this also enhances external validity by reflecting real-world conditions. Finally, the use of telephone interviews for follow-up assessments may be susceptible to recall bias, although structured questionnaires and clear documentation (e.g., who assisted in responses) aim to minimize this limitation [].
4. Conclusions
This study will be the first multicenter prospective cohort study in Japan to investigate the association between rehabilitation dose and functional outcomes in patients with acute stroke using a standardized measure, the MQS. By exploring dose–response relationships stratified by stroke severity and incorporating multiple physical and functional indicators, the study seeks to identify optimal rehabilitation strategies that are individualized, evidence-based, and implementable in real-world clinical settings. The findings of this study are expected to contribute to the standardization of early rehabilitation protocols and to improve long-term outcomes for stroke patients across diverse healthcare environments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm14196786/s1, Table S1: SPIRIT Checklist for Trials; Figure S1: Participating hospitals; Table S2: List of Collaborators.
Author Contributions
Conceptualization, S.W., W.Y., K.S., A.K., S.S., K.K. (Kousuke Kanamori), K.K. (Kanari Kiritani), T.F., Y.N., N.T., and K.U.; methodology, S.W., W.Y., K.S., A.K., S.S., K.K. (Kousuke Kanamori), K.K. (Kanari Kiritani), H.T., and Y.M. (Yasunari Morita); software, W.Y., K.S., A.K., S.S., K.K. (Kousuke Kanamori), K.K. (Kanari Kiritani), T.F., Y.N., N.T., K.U., N.H., and K.T.; validation, S.W., W.Y., K.S., A.K., S.S., H.T., and Y.M. (Yasunari Morita); formal analysis, S.W., S.S., N.H., and H.T.; investigation, W.Y., K.S., A.K., S.S., K.K. (Kousuke Kanamori)., K.K. (Kanari Kiritani), T.F., Y.N., N.T., K.U., N.H., H.T., Y.M. (Yushi Mitani), and D.H.; resources, Y.N., N.T., K.U., N.H., H.T., Y.M. (Yushi Mitani), and D.H.; data curation, K.S., A.K., S.S., K.K. (Kousuke Kanamori), K.K. (Kanari Kiritani), T.F., Y.N., N.T., and K.U.; writing—original draft preparation, S.W. and S.S.; writing—review and editing, all authors; visualization, W.Y., K.S., A.K., S.S., K.K. (Kousuke Kanamori), K.K. (Kanari Kiritani), T.F., Y.N., K.U., and N.H.; supervision, S.W., H.T., N.H., and K.U.; project administration, S.W., W.Y., K.S., A.K., S.S., and Y.M. (Yasunari Morita); funding acquisition, S.W., H.T., and K.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Gifu University of Health Science (approval number: 2025-012, approved on 20 August 2025) as well as the ethics committees of all participating institutions. This study is registered as a clinical trial in the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR), registration number UMIN000057876.
Informed Consent Statement
Informed consent will be obtained in writing from all subjects involved in the study.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
We would like to thank the study coordinators. We also thank the members of the Bridge Study Committee Office. We thank all collaborators from the participating sites listed in Table S2.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ICU | Intensive Care Unit |
| mRS | Modified Rankin Scale |
| MQS | Mobilization Quantification Score |
| CFS | Clinical Frailty Scale |
| FSS-ICU | Functional Status Score for the Intensive Care Unit |
| MRC | Medical Research Council (score) |
| NIHSS | National Institutes of Health Stroke Scale |
| UMIN | University Hospital Medical Information Network |
| MUST | Malnutrition Universal Screening Tool |
| SD | Standard Deviation |
| IQR | Interquartile Range |
| SPIRIT | Standard Protocol Items: Recommendations for Interventional Trials |
References
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Dhamoon, M.S.; Moon, Y.P.; Paik, M.C.; Boden-Albala, B.; Rundek, T.; Sacco, R.L.; Elkind, M.S. Quality of life declines after first ischemic stroke: The Northern Manhattan Study. Neurology 2010, 75, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Avan, A.; Digaleh, H.; Di Napoli, M.; Stranges, S.; Behrouz, R.; Shojaeianbabaei, G.; Amiri, A.; Tabrizi, R.; Mokhber, N.; Spence, J.D.; et al. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: An ecological analysis from the Global Burden of Disease Study 2017. BMC Med. 2019, 17, 191. [Google Scholar] [CrossRef] [PubMed]
- van Crevel, H. A note on muscle atrophy of “central” origin. Psychiatr. Neurol. Neurochir. 1969, 72, 29–35. [Google Scholar] [PubMed]
- Stevens, R.D.; Marshall, S.A.; Cornblath, D.R.; Hoke, A.; Needham, D.M.; de Jonghe, B.; Ali, N.A.; Sharshar, T. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit. Care Med. 2009, 37 (Suppl. 10), S299–S308. [Google Scholar] [CrossRef]
- Vincent, J.L.; Norrenberg, M. Intensive care unit-acquired weakness: Framing the topic. Crit. Care Med. 2009, 37 (Suppl. S10), S296–S298. [Google Scholar] [CrossRef]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Padhke, R.; Dew, T.; Sidhu, P.S.; et al. Acute skeletal muscle wasting in critical illness. JAMA 2013, 310, 1591–1600. [Google Scholar] [CrossRef]
- Lee, K.E.; Choi, M.; Jeoung, B. Effectiveness of rehabilitation exercise in improving physical function of stroke patients: A systematic review. Int. J. Environ. Res. Public Health 2022, 19, 12739. [Google Scholar] [CrossRef]
- Sundseth, A.; Thommessen, B.; Rønning, O.M. Outcome after mobilization within 24 hours of acute stroke: A randomized controlled trial. Stroke 2012, 43, 2389–2394. [Google Scholar] [CrossRef]
- Warner, J.J.; Harrington, R.A.; Sacco, R.L.; Elkind, M.S.V. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines. Stroke 2019, 50, 3331–3332. [Google Scholar] [CrossRef]
- Xu, T.; Yu, X.; Ou, S.; Liu, X.; Yuan, J.; Chen, Y.; Huang, H.; He, W.; Hu, X. Efficacy and safety of very early mobilization in patients with acute stroke: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 6550. [Google Scholar] [CrossRef]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for adult stroke rehabilitation and recovery: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef]
- Mazwi, N.; Lissak, I.; Wongtangman, K.; Bakar, A.; Badreldin, A.; Azabou, E.; Kang, M.; Ngo, L.; Ely, E.W.; Parker, A.M.; et al. Effects of mobility dose on discharge disposition in critically ill stroke patients. PM&R 2023, 15, 1547–1556. [Google Scholar] [CrossRef]
- AVERT Trial Collaboration Group. Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): A randomised controlled trial. Lancet 2015, 386, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, J.; Churilov, L.; Ellery, F.; Collier, J.; Chamberlain, J.; Langhorne, P.; Lindley, R.I.; Moodie, M.; Dewey, H.; Thrift, A.G.; et al. Prespecified dose-response analysis for A Very Early Rehabilitation Trial (AVERT). Neurology 2016, 86, 2138–2145. [Google Scholar] [CrossRef]
- Watanabe, S.; Liu, K.; Hirota, Y.; Naito, Y.; Sato, N.; Ishii, S.; Yano, H.; Ogata, R.; Koyanagi, Y.; Yasumura, D.; et al. Investigating dose level and duration of rehabilitation of mechanically ventilated patients in the ICU (IPAM). Respir. Care 2025, 70, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Liu, K.; Kozu, R.; Yasumura, D.; Yamauchi, K.; Katsukawa, H.; Suzuki, K.; Koike, T.; Morita, Y. Association between mobilization level and activity of daily living independence in critically ill patients. Ann. Rehabil. Med. 2023, 47, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Liu, K.; Nakamura, K.; Kozu, R.; Horibe, T.; Ishii, K.; Yasumura, D.; Takahashi, Y.; Nanba, T.; Morita, Y.; et al. Association between early mobilization in the ICU and psychiatric symptoms after surviving a critical illness: A multi-center prospective cohort study. J. Clin. Med. 2022, 11, 2587. [Google Scholar] [CrossRef]
- Fuest, K.E.; Ulm, B.; Daum, N.; Lindholz, M.; Lorenz, M.; Blobner, K.; Langer, N.; Hodgson, C.; Herridge, M.; Blobner, M.; et al. Clustering of critically ill patients using an individualized learning approach enables dose optimization of mobilization in the ICU. Crit. Care 2023, 27, 1. [Google Scholar] [CrossRef]
- Miyamoto, S.; Ogasawara, K.; Kuroda, S.; Itabashi, R.; Toyoda, K.; Itoh, Y.; Iguchi, Y.; Shiokawa, Y.; Takagi, Y.; Ohtsuki, T.; et al. Japan Stroke Society Guideline 2021 for the treatment of stroke. Int. J. Stroke 2022, 17, 1039–1049. [Google Scholar] [CrossRef]
- Watanabe, S.; Yamauchi, K.; Yasumura, D.; Suzuki, K.; Koike, T.; Katsukawa, H.; Morita, Y.; Scheffenbichler, F.T.; Schaller, S.J.; Eikermann, M. Reliability and effectiveness of the Japanese version of the Mobilization Quantification Score. Cureus 2023, 15, e43440. [Google Scholar] [CrossRef]
- Mueller, N.; Murthy, S.; Tainter, C.R.; Lee, J.; Riddell, K.; Fintelmann, F.J.; Grabitz, S.D.; Timm, F.P.; Levi, B.; Kurth, T.M.; et al. Can sarcopenia quantified by ultrasound of the rectus femoris muscle predict adverse outcome of surgical intensive care unit patients as well as frailty? A prospective, observational cohort study. Ann. Surg. 2016, 264, 1116–1124. [Google Scholar] [CrossRef]
- Unoki, T.; Hayashida, K.; Kawai, Y.; Taito, S.; Ando, M.; Iida, Y.; Kasai, F.; Kawasaki, T.; Kozu, R.; Kondo, Y.; et al. Japanese Clinical Practice Guidelines for Rehabilitation in Critically Ill Patients 2023 (J-ReCIP 2023). J. Intensive Care 2023, 11, 47. [Google Scholar] [CrossRef]
- Nakamura, K.; Yamamoto, R.; Higashibeppu, N.; Yoshida, M.; Tatsumi, H.; Shimizu, Y.; Izumino, H.; Oshima, T.; Hatakeyama, J.; Ouchi, A.; et al. The Japanese Critical Care Nutrition Guideline 2024. J. Intensive Care 2025, 13, 18. [Google Scholar] [CrossRef]
- Zou, G.; Zou, L.; Choi, Y.H. Distribution-free approach to the design and analysis of randomized stroke trials with the modified Rankin Scale. Stroke 2022, 53, 3025–3031. [Google Scholar] [CrossRef]
- Needham, D.M.; Sepulveda, K.A.; Dinglas, V.D.; Chessare, C.M.; Friedman, L.A.; Bingham, C.O.; Turnbull, A.E. Core outcome measures for clinical research in acute respiratory failure survivors: An international modified Delphi consensus study. Am. J. Respir. Crit. Care Med. 2017, 196, 1122–1130. [Google Scholar] [CrossRef]
- Inoue, S.; Hatakeyama, J.; Kondo, Y.; Hifumi, T.; Sakuramoto, H.; Kawasaki, T.; Taito, S.; Nakamura, K.; Unoki, T.; Kawai, Y.; et al. Post-intensive care syndrome: Its pathophysiology, prevention, and future directions. Acute Med. Surg. 2019, 6, 233–246. [Google Scholar] [CrossRef]
- Huang, M.; Chan, K.S.; Zanni, J.M.; Parry, S.M.; Neto, S.-C.G.B.; Neto, J.A.A.M.; da Silva, V.Z.M.M.; Kho, M.E.; Needham, D.M.F. Functional Status Score for the ICU: An international clinimetric analysis of validity, responsiveness, and minimal important difference. Crit. Care Med. 2016, 44, e1155–e1164. [Google Scholar] [CrossRef] [PubMed]
- Bragança, R.D.; Ravetti, C.G.; Barreto, L.; Ataíde, T.B.L.S.; Carneiro, R.M.; Teixeira, A.L.; Nobre, V. Use of handgrip dynamometry for diagnosis and prognosis assessment of intensive care unit acquired weakness: A prospective study. Heart Lung 2019, 48, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Church, S.; Rogers, E.; Rockwood, K.; Theou, O. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020, 20, 393. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, P.; Melamed, I.; Collins, M.; Lieberman, N.; Sharma, V.; Goren, O.; Zand, R.; Schirmer, C.M.; Griessenauer, C.J. NIHSS 24 h after mechanical thrombectomy predicts 90-day functional outcome. Clin. Neuroradiol. 2022, 32, 401–406. [Google Scholar] [CrossRef]
- Elia, M. The “MUST” Report: Nutritional Screening of Adults. Malnutrition Advisory Group (MAG); BAPEN: Letchworth Garden, UK, 2003. [Google Scholar]
- Hodgson, C.; Needham, D.; Haines, K.; Bailey, M.; Ward, A.; Harrold, M.; Young, P.; Zanni, J.; Buhr, H.; Higgins, A.; et al. Feasibility and inter-rater reliability of the ICU Mobility Scale. Heart Lung 2014, 43, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Japanese Stroke Society. Japanese Guidelines for the Management of Stroke 2021; Kyowa Kikaku: Tokyo, Japan, 2021. (In Japanese) [Google Scholar]
- Tillquist, M.; Kutsogiannis, D.J.; Wischmeyer, P.E.; Kummerlen, C.; Leung, R.; Stollery, D.; Karvellas, C.J.; Preiser, J.; Bird, N.; Kozar, R.; et al. Bedside ultrasound is a practical and reliable measurement tool for assessing quadriceps muscle layer thickness. J. Parenter. Enteral Nutr. 2014, 38, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Nozoe, M.; Kanai, M.; Kubo, H.; Takeuchi, Y.; Kobayashi, M.; Yamamoto, M.; Furuichi, A.; Yamazaki, M.; Shimada, S.; Mase, K. Efficacy of neuromuscular electrical stimulation for preventing quadriceps muscle wasting in patients with moderate or severe acute stroke: A pilot study. NeuroRehabilitation 2017, 41, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.M.; Visser, N.A.; Dorhout Mees, S.M.; Klijn, C.J.; Algra, A.; Rinkel, G.J. Comparison of telephone and face-to-face assessment of the modified Rankin Scale. Cerebrovasc. Dis. 2010, 29, 137–139. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).