The Platelet-to-Hemoglobin Ratio as a Prognostic Marker in Patients with Diabetes Mellitus and Acute Coronary Syndrome
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Study Endpoint and Follow-Up Procedures
2.3. Statistical Analysis
3. Results
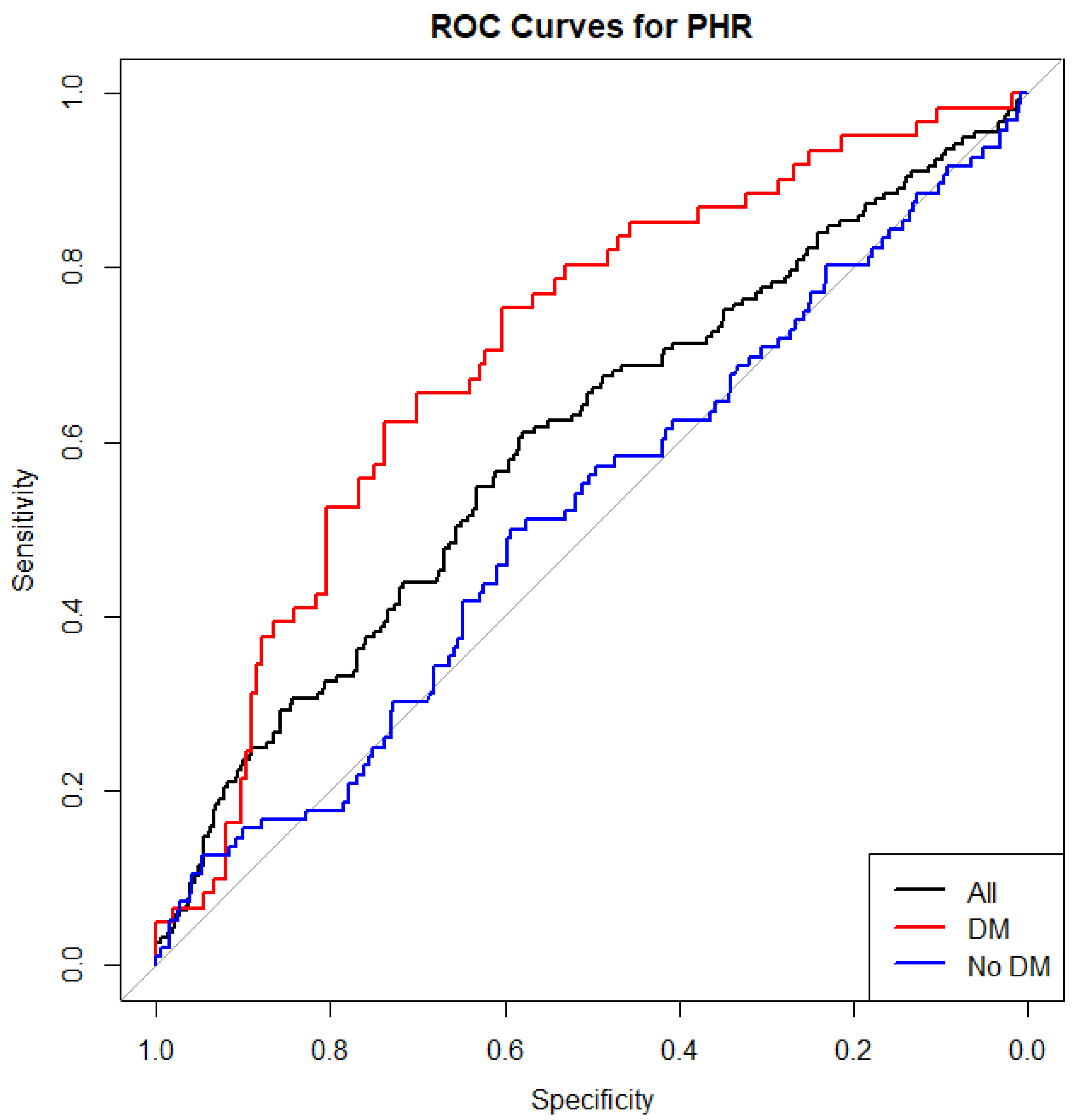
3.1. ROC Curve Analysis
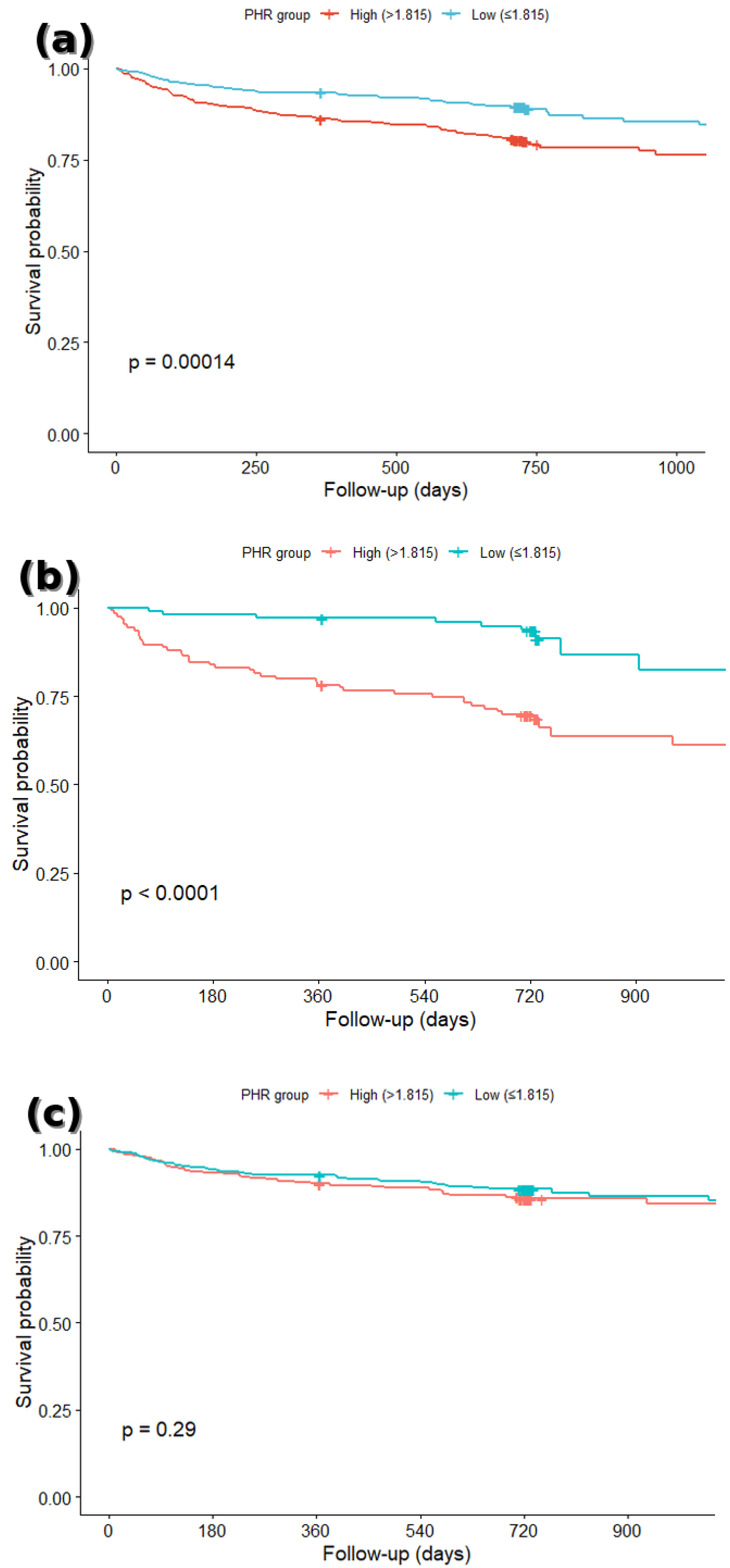
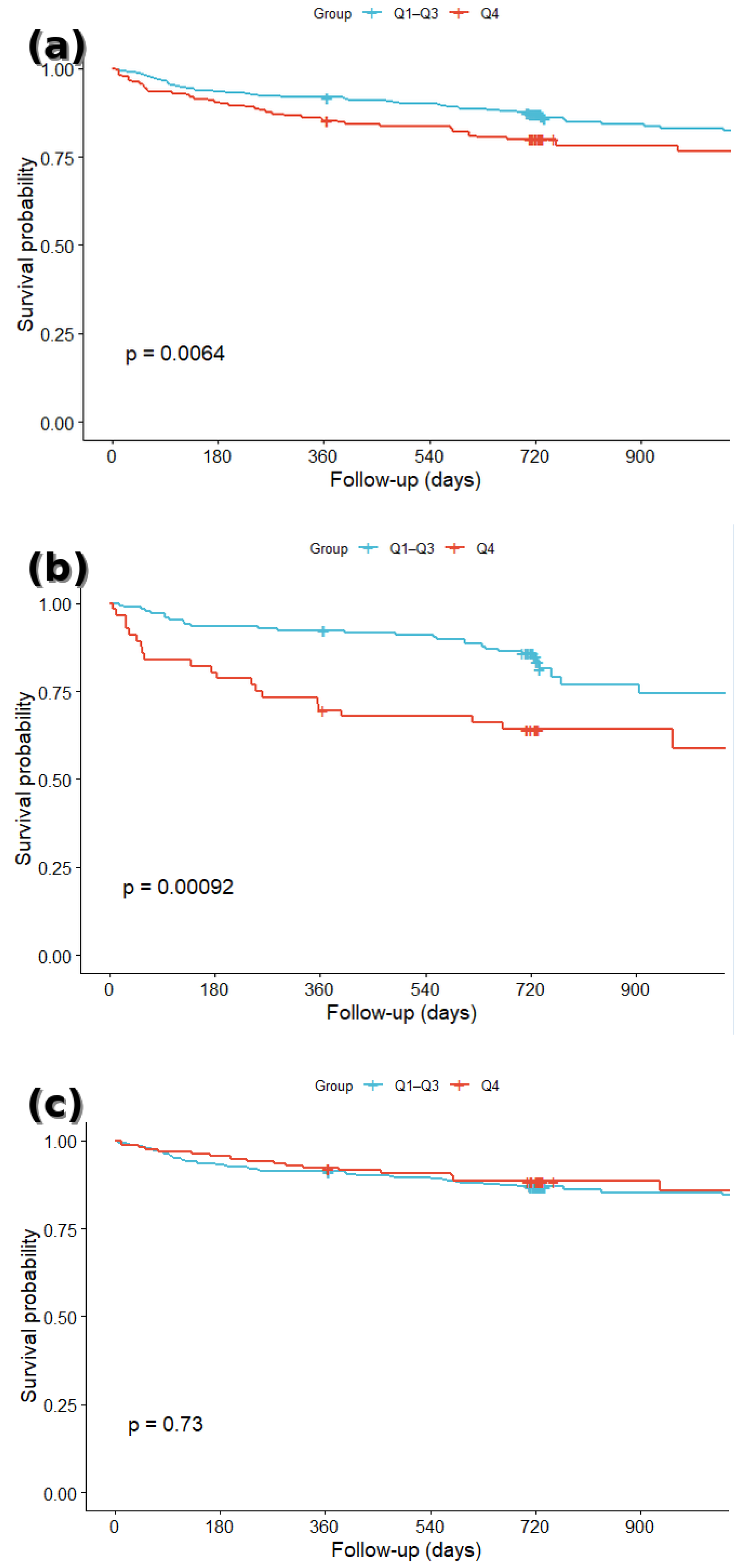
3.2. Survival Analysis
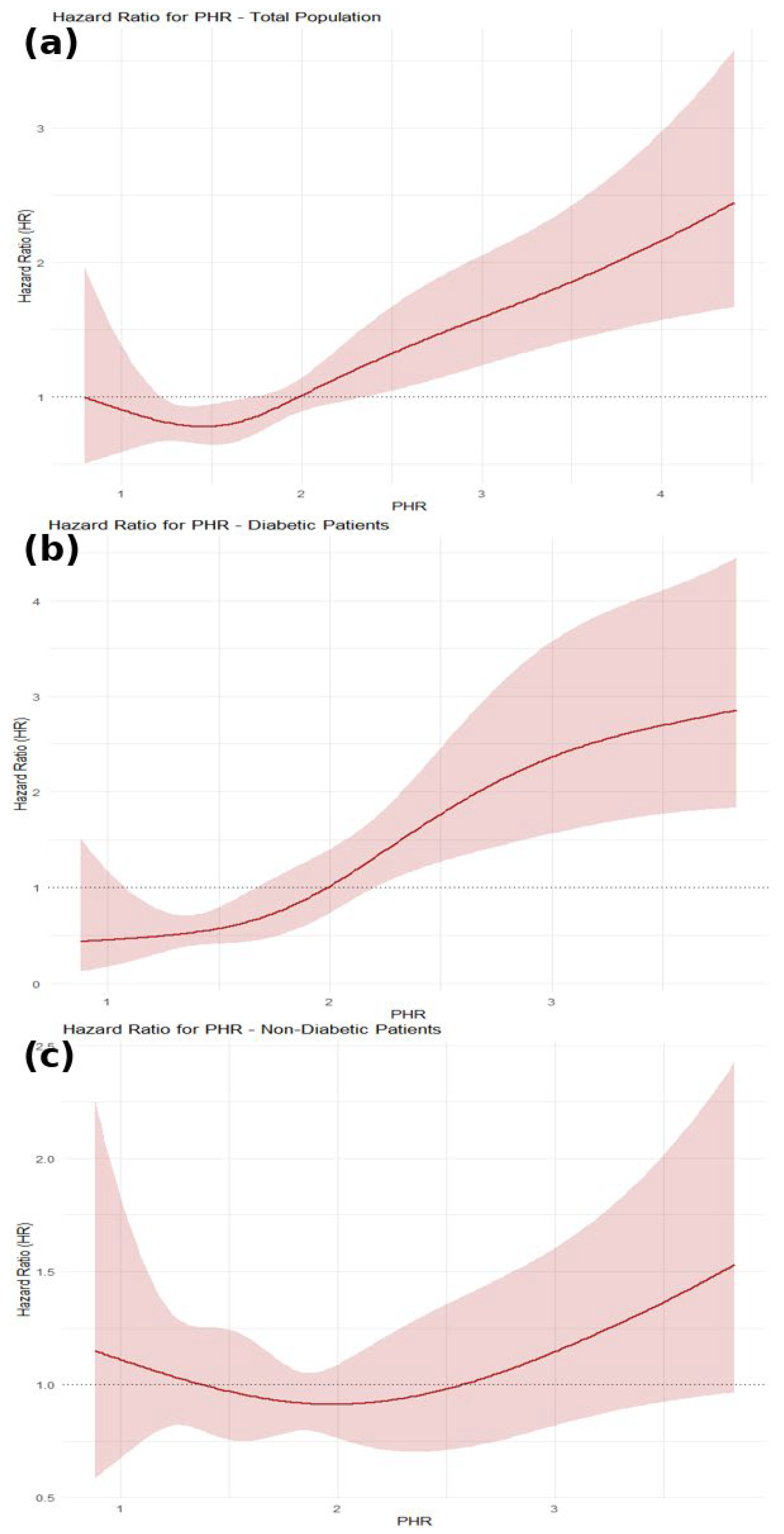
3.3. Restricted Cubic Spline (RCS) Analysis
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bergmark, B.A.; Mathenge, N.; Merlini, P.A.; Lawrence-Wright, M.B.; Giugliano, R.P. Acute Coronary Syndromes. Lancet 2022, 399, 1347–1358. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Eggers, K.M.; Lindahl, B. Prognostic Biomarkers in Acute Coronary Syndromes: Risk Stratification Beyond Cardiac Troponins. Curr. Cardiol. Rep. Curr. Med. Group LLC 1. 2017, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- de Moura Monteiro Júnior, J.G.; de Oliveira Cipriano Torres, D.; da Silva, M.C.F.C.; de Holanda Martins, C.M.; da Silva, I.K.; Nascimento, M.E.M.D.; Santos, A.C.O.D.; Montarroyos, U.R.; Filho, D.C.S. Prognostic value of hematological parameters in patients with acute myocardial infarction: Intrahospital outcomes. PLoS ONE 2018, 13, e0194897. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Dai, C.; Xu, K.; Wu, M. Predictive value of neutrophil to lymphocyte ratio and red cell distribution width on death for ST segment elevation myocardial infarction. Sci. Rep. 2021, 11, 11506. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Morrow, D.A.; Giugliano, R.P.; Burton, P.B.; Murphy, S.A.; McCabe, C.H.; Gibson, C.M.; Braunwald, E. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation 2005, 111, 2042–2049. [Google Scholar] [CrossRef]
- Bao, K.; Huang, H.; Huang, G.; Wang, J.; Liao, Y.; Pan, Y.; Chen, W.; Lu, J.; Yang, Y.; Huang, Z.; et al. Platelet-to-hemoglobin ratio as a valuable predictor of long-term all-cause mortality in coronary artery disease patients with congestive heart failure. BMC Cardiovasc. Disord. 2021, 21, 618. [Google Scholar] [CrossRef]
- Gamal, N.M.; Badawy, E.R.; Gheita, T.A. Emerging Value of Platelet-to-Hemoglobin Ratio and Monocyte-to-Hemoglobin Ratio in Assessment of Rheumatoid Arthritis Patients. Med. J. Cairo Univ. 2021, 89, 1991–1999. [Google Scholar]
- Gawaz, M.; Langer, H.; May, A.E. Platelets in inflammation and atherogenesis. J. Clin. Investig. 2005, 115, 3378–3384. [Google Scholar] [CrossRef]
- Wester, A.; Attar, R.; Mohammad, M.A.; Andell, P.; Hofmann, R.; Jensen, J.; Szummer, K.; Erlinge, D.; Koul, S. Impact of Baseline Anemia in Patients with Acute Coronary Syndromes Undergoing Percutaneous Coronary Intervention: A Prespecified Analysis From the VALIDATE-SWEDEHEART Trial. J. Am. Heart Assoc. 2019, 8, e012741. [Google Scholar] [CrossRef]
- Beckman, J.A.; Creager, M.A.; Libby, P. Diabetes and Atherosclerosis Epidemiology, Pathophysiology, and Management. Available online: http://jama.jamanetwork.com/ (accessed on 27 April 2025).
- Stampouloglou, P.K.; Anastasiou, A.; Bletsa, E.; Lygkoni, S.; Chouzouri, F.; Xenou, M.; Katsarou, O.; Theofilis, P.; Zisimos, K.; Tousoulis, D.; et al. Diabetes Mellitus in Acute Coronary Syndrome. Life Multidiscip. Digit. Publ. Inst. 2023, 13, 2226. [Google Scholar] [CrossRef]
- Khafaji, H.A.H. Atypical presentation of acute and chronic coronary artery disease in diabetics. World J. Cardiol. 2014, 6, 802. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.J. Factors contributing to increased platelet reactivity in people with diabetes. Diabetes Care. 2009, 32, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Antoniadou, C.; Gavriilidis, E.; Ritis, K.; Tsilingiris, D. Anemia in diabetes mellitus: Pathogenetic aspects and the value of early erythropoietin therapy. Metabol. Open. 2025, 25, 100344. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef]
- World Medical Association. WMA Declaration of Helsinki–Ethical Principles for Medical Research Involving Human Subjects. Jama 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Croxford, R. Restricted Cubic Spline Regression: A Brief Introduction. Available online: http://pages.cs.wisc.edu/~deboor/draftspline.html (accessed on 27 April 2025).
- Işık, F.; Soner, S. Platelet-to-Hemoglobin Ratio Is an Important Predictor of In-Hospital Mortality in Patients With ST-Segment Elevation Myocardial Infarction. Cureus 2022, 14, e26833. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Wu, T.T.; Chen, Y.; Hou, X.G.; Yang, Y.; Zhang, J.Y.; Ma, Y.T.; Xie, X. Platelet-to-hemoglobin ratio as a novel predictor of long-term adverse outcomes in patients after percutaneous coronary intervention: A retrospective cohort study. Eur. J. Prev. Cardiol. 2020, 27, 2216–2219. [Google Scholar] [CrossRef]
- Seitun, S.; Mantini, C.; Clemente, A.; Sambuceti, V.; Francese, G.; Carpaneto, S.; Porto, I. Role of CT and CMR in the Management of Chronic Coronary Syndrome. Echocardiogr. John Wiley Sons Inc. 2025, 42, e70117. [Google Scholar] [CrossRef]
- Lossnitzer, D.; Klenantz, S.; Andre, F.; Goerich, J.; Schoepf, U.J.; Pazzo, K.L.; Sommer, A.; Brado, M.; Gückel, F.; Sokiranski, R.; et al. Stable patients with suspected myocardial ischemia: Comparison of machine-learning computed tomography-based fractional flow reserve and stress perfusion cardiovascular magnetic resonance imaging to detect myocardial ischemia. BMC Cardiovasc. Disord. 2022, 22, 34. [Google Scholar] [CrossRef]
- Stark, K.; Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. Nat. Res. 2021, 18, 666–682. [Google Scholar] [CrossRef]
- Mozos, I. Mechanisms linking red blood cell disorders and cardiovascular diseases. BioMed Res. Int. Hindawi Publ. Corp. 2015, 2015, 682054. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.J.; Mix, D.S.; Ture, S.K.; Schmidt, R.A.; Mohan, A.; Pariser, D.; Stoner, M.C.; Shah, P.; Chen, L.; Zhang, H.; et al. Hypoxia and Ischemia Promote a Maladaptive Platelet Phenotype. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1594–1606. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Goodnough, L.T. Anemia of Chronic Disease. Available online: https://www.nejm.org (accessed on 27 April 2025).
- De Bosscher, R.; Dausin, C.; Claus, P. Lifelong endurance exercise and its relation with coronary atherosclerosis. Eur. Heart J. 2023, 44, 2388–2399. [Google Scholar] [CrossRef] [PubMed]
- Jeon, K.H.; Jeong, Y.H.; Chae, I.H.; Kim, B.K.; Joo, H.J.; Chang, K.; Park, Y.; Song, Y.; Ahn, S.G.; Lee, S.Y.; et al. Implication of diabetic status on platelet reactivity and clinical outcomes after drug-eluting stent implantation: Results from the PTRG-DES consortium. Cardiovasc. Diabetol. 2023, 22, 245. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, U.; Toto, R.D. Anemia, diabetes, and chronic kidney disease. Diabetes Care 2009, 32, 1320–1326. [Google Scholar] [CrossRef]
- Danese, E.; Montagnana, M. An historical approach to the diagnostic biomarkers of acute coronary syndrome. Ann. Transl. Med. 2016, 4, 194. [Google Scholar] [CrossRef]
- Sawant, A.C.; Adhikari, P.; Narra, S.R.; Srivatsa, S.V.; Mills, P.K.; Srivatsa, S.S. Neutrophil to lymphocyte ratio predicts short- and long-term mortality following revascularization therapy for ST elevation myocardial infarction. Cardiol. J. 2014, 21, 500–508. [Google Scholar] [CrossRef]
- Li, W.; Liu, Q.; Tang, Y. Platelet to lymphocyte ratio in the prediction of adverse outcomes after acute coronary syndrome: A meta-analysis. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Ranka, S.; Lahan, S.; Dalia, T.; Tripathi, A.; Goyal, A.; Sreenivasan, J.; Taduru, S.; Muhammed, M.; Moriarty, P.M. Association between Red Cell Distribution Width and Cardiovascular Outcomes-Systematic Review and Meta-Analysis. Med. Res. Arch. 2021, 9, 1–9. [Google Scholar] [CrossRef]
| Characteristic | Total (n = 843) | Non-DM (n = 618) | DM (n = 225) | p-Value 1 |
|---|---|---|---|---|
| Age, years | 64.4 ± 13.0 | 62.7 ± 13.1 | 69.0 ± 11.6 | <0.001 |
| PHR | 1.90 ± 0.78 | 1.84 ± 0.69 | 2.08 ± 0.95 | <0.001 |
| Serum creatinine, mg/dL | 1.19 ± 1.00 | 1.12 ± 0.82 | 1.40 ± 1.37 | <0.001 |
| High-sensitivity troponin I | 1440.8 ± 3194.4 | 1546.9 ± 3404.0 | 1155.0 ± 2531.3 | 0.123 |
| Male sex, n (%) | 614 (72.8) | 460 (74.4) | 154 (68.4) | 0.101 |
| Heart failure, n (%) | 24 (2.8) | 11 (2.0) | 13 (6.2) | 0.005 |
| Hypertension, n (%) | 413 (49.0) | 245 (39.7) | 168 (74.7) | <0.001 |
| Dyslipidemia, n (%) | 217 (25.8) | 128 (20.8) | 89 (39.6) | <0.001 |
| Chronic kidney disease, n (%) | 55 (7.2) | 23 (4.1) | 32 (15.3) | <0.001 |
| Atrial fibrillation, n (%) | 72 (9.4) | 42 (7.5) | 30 (14.4) | 0.006 |
| Family history of CVD, n (%) | 124 (14.8) | 102 (16.6) | 22 (9.9) | 0.021 |
| Smoking, n (%) | 379 (45.1) | 297 (48.2) | 82 (36.6) | 0.004 |
| Anticoagulant use, n (%) | 160 (19.1) | 102 (16.6) | 58 (25.8) | 0.004 |
| Statin use, n (%) | 404 (48.4) | 272 (44.6) | 132 (58.9) | <0.001 |
| Beta-blocker use, n (%) | 394 (47.3) | 271 (44.5) | 123 (54.9) | 0.010 |
| Variable | Univariate OR (95% CI) | p-Value (Univariate) | Multivariate aOR (95% CI) | p-Value (Multivariate) |
|---|---|---|---|---|
| PHR | 1.607 (1.304–1.981) | <0.001 | 1.672 (1.289–2.169) | <0.001 |
| Heart Failure | 4.038 (1.806–9.029) | <0.001 | 3.077 (1.286–9.207) | 0.014 |
| Gender | 0.793 (0.552–1.140) | 0.210 | 1.367 (0.852–2.195) | 0.195 |
| Hypertension | 0.945 (0.676–1.320) | 0.739 | 0.692 (0.444–1.078) | 0.103 |
| Diabetes Mellitus | 2.119 (1.492–3.011) | <0.001 | 1.441 (0.904–2.298) | 0.125 |
| Dyslipidemia | 0.613 (0.402–0.936) | 0.024 | 0.567 (0.341–0.944) | 0.029 |
| Smoking | 0.477 (0.333–0.685) | <0.001 | 0.731 (0.454–1.175) | 0.195 |
| Age | 1.048 (1.033–1.063) | <0.001 | 1.029 (1.010–1.049) | 0.003 |
| Chronic Kidney Disease | 3.868 (2.249–6.651) | <0.001 | 0.987 (0.432–2.256) | 0.976 |
| Atrial Fibrillation | 2.083 (1.251–3.468) | 0.005 | 0.889 (0.451–1.750) | 0.733 |
| Serum creatinine | 1.506 (1.274–1.779) | <0.001 | 1.277 (1.044–1.563) | 0.017 |
| Anticoagulant Use | 2.149 (1.473–3.135) | <0.001 | 1.822 (1.109–2.996) | 0.018 |
| Beta-blocker Use | 1.492 (1.064–2.092) | 0.020 | 1.078 (0.705–1.648) | 0.729 |
| High-sensitive Troponin I | 1.002 (1.001–1.003) | 0.047 | 1.002 (1.001–1.003) | 0.354 |
| Variable | Univariate HR (95% CI) | p-Value | Multivariate HR (95% CI) | p-Value |
|---|---|---|---|---|
| PHR | 1.414 (1.224–1.642) | <0.001 | 1.406 (1.210–1.634) | <0.001 |
| Heart Failure | 3.82 (2.114–6.921) | <0.001 | 2.864 (1.469–5.582) | 0.002 |
| Gender | 0.93 (0.672–1.283) | 0.649 | 1.307 (0.875–1.951) | 0.191 |
| Hypertension | 0.97 (0.721–1.314) | 0.838 | 0.833 (0.573–1.213) | 0.341 |
| Diabetes Mellitus | 1.629 (1.192–2.227) | 0.002 | 1.298 (0.884–1.907) | 0.183 |
| Dyslipidemia | 0.856 (0.573–1.269) | 0.408 | 0.930 (0.588–1.472) | 0.758 |
| Smoking | 0.734 (0.526–1.033) | 0.077 | 0.881 (0.574–1.352) | 0.562 |
| Age | 1.035 (1.025–1.042) | <0.001 | 1.019 (1.003–1.036) | 0.020 |
| Chronic Kidney Disease | 3.086 (2.043–4.701) | <0.001 | 1.284 (0.686–2.403) | 0.435 |
| Atrial Fibrillation | 1.724 (1.123–2.646) | 0.014 | 0.949 (0.549–1.639) | 0.851 |
| Serum creatinine | 1.203 (1.087–1.312) | <0.001 | 1.164 (1.022–1.324) | 0.022 |
| Anticoagulant Use | 1.575 (1.127–2.182) | 0.008 | 1.307 (0.663–1.982) | 0.207 |
| Statin Use | 0.725 (0.531–0.987) | 0.035 | 0.636 (0.444–0.912) | 0.014 |
| High-sensitive Troponin I | 1.002 (1.001–1.003) | 0.004 | 1.002 (1.001–1.003) | 0.098 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kofos, C.; Stachteas, P.; Fyntanidou, B.; Papazoglou, A.S.; Samaras, A.; Nasoufidou, A.; Apostolopoulou, A.; Karakasis, P.; Arvanitaki, A.; Bantidos, M.G.; et al. The Platelet-to-Hemoglobin Ratio as a Prognostic Marker in Patients with Diabetes Mellitus and Acute Coronary Syndrome. J. Clin. Med. 2025, 14, 6780. https://doi.org/10.3390/jcm14196780
Kofos C, Stachteas P, Fyntanidou B, Papazoglou AS, Samaras A, Nasoufidou A, Apostolopoulou A, Karakasis P, Arvanitaki A, Bantidos MG, et al. The Platelet-to-Hemoglobin Ratio as a Prognostic Marker in Patients with Diabetes Mellitus and Acute Coronary Syndrome. Journal of Clinical Medicine. 2025; 14(19):6780. https://doi.org/10.3390/jcm14196780
Chicago/Turabian StyleKofos, Christos, Panagiotis Stachteas, Barbara Fyntanidou, Andreas S. Papazoglou, Athanasios Samaras, Athina Nasoufidou, Aikaterini Apostolopoulou, Paschalis Karakasis, Alexandra Arvanitaki, Marios G. Bantidos, and et al. 2025. "The Platelet-to-Hemoglobin Ratio as a Prognostic Marker in Patients with Diabetes Mellitus and Acute Coronary Syndrome" Journal of Clinical Medicine 14, no. 19: 6780. https://doi.org/10.3390/jcm14196780
APA StyleKofos, C., Stachteas, P., Fyntanidou, B., Papazoglou, A. S., Samaras, A., Nasoufidou, A., Apostolopoulou, A., Karakasis, P., Arvanitaki, A., Bantidos, M. G., Moysidis, D. V., Stalikas, N., Patoulias, D., Sagris, M., Tzikas, A., Kassimis, G., Fragakis, N., & Karagiannidis, E. (2025). The Platelet-to-Hemoglobin Ratio as a Prognostic Marker in Patients with Diabetes Mellitus and Acute Coronary Syndrome. Journal of Clinical Medicine, 14(19), 6780. https://doi.org/10.3390/jcm14196780









