Abstract
Background: Smoking in patients with hepatitis C (HCV) amplifies the risk of cirrhosis and hepatocellular carcinoma. We aimed to estimate the prevalence of active smoking over the last decade at the population level among adults living with HCV in the U.S., estimate temporal trends in active smoking, and identify factors associated with active smoking. Methods: We analyzed repeated cross-sectional NHANES data (2007–2018) of adults ≥20 years old with serologic evidence of HCV and complete smoking data (unweighted [n = 621] and weighted [n = 3,620,603] sample size). Temporal trends were evaluated using linear regression and joinpoint regression. Survey-weighted multivariable logistic regression was used to identify factors associated with active smoking. Results: The cumulative prevalence of active smoking was 56.4% (95% CI, 49.2–63.4). Linear trend testing was not significant (p = 0.93). Joinpoint regression suggested a slope change near 2013–2014, but neither segment-specific annual percent changes nor the slope change reached significance. Factors associated with higher odds of active smoking included female sex (aOR = 2.23; 95% CI, 1.17–4.24), low poverty income ratio (aOR = 3.33; 1.41–7.84), lifetime substance use (aOR = 10.63; 3.08–36.70), and depression (aOR = 2.65; 1.29–5.45). Lower odds were observed with >high-school education (aOR = 0.50; 0.26–0.94), obesity (aOR = 0.32; 0.18–0.58), and ≥2 yearly healthcare visits (aOR = 0.27; 0.10–0.68). Conclusions: Smoking appears to be endemic within the HCV population, and rates have remained alarmingly high and stagnant (i.e., unchanged or have not decreased) over the last decade, which consequently can lead to heightened incident cases of HCV-related cirrhosis and hepatocellular carcinoma in the near future.
1. Introduction
Worldwide, it is estimated that 50 million people are living with chronic hepatitis C virus (HCV), with about 1 million new cases each year and roughly 242,000 deaths due to HCV-associated cirrhosis or HCV-associated hepatocellular carcinoma [1]. In the United States, an estimated ~2.2 million adults are living with active HCV infection [2] HCV often leads to advanced liver disease, including cirrhosis, decompensated cirrhosis, and HCC.
Smoking, a behavior that alone increases the risk for cirrhosis and hepatocellular carcinoma, ref. [3] is disproportionately higher among people living with HCV compared to the general population. Smoking worsens HCV-related liver injury by increasing oxidative stress, inducing inflammatory cytokines, and activating hepatic stellate cells, which accelerates fibrosis and raises the risk for cirrhosis and hepatocellular carcinoma [4,5]. Although direct-acting antivirals have transformed HCV management, clinical guidelines recommend addressing modifiable behaviors (e.g., alcohol and tobacco) within HCV care [6].
Several studies have delineated smoking patterns among individuals infected with HCV. Utilizing the National Health and Nutrition Examination Survey (NHANES) data spanning 1999 to 2014, a study identified a substantial smoking prevalence among HCV-infected adults and elucidated socioeconomic and clinical factors associated with this behavior [7]. Complementary qualitative and mixed-methods research explored HCV-specific smoking motivations and obstacles, including stress management beliefs and inadequate utilization of cessation support mechanisms [8,9]. Another study conducted among HCV patients who inject drugs who were in opioid agonist treatment revealed exceptionally high smoking rates, which were linked to specific substance use contexts and treatment engagement patterns [10,11]. These findings collectively suggest the pervasive nature of tobacco use within HCV-affected populations, implicating socioeconomic disadvantage, mental health comorbidity, and substance use as pivotal drivers of this phenomenon. For comparative context, the prevalence of cigarette smoking among U.S. adults in 2018 was approximately 14%, while earlier NHANES analyses indicated a prevalence of around 62% among adults with HCV [12].
However, data on long-term smoking trends at the population level among people living with HCV remain limited. Many studies only provide cross-sectional snapshots of smoking prevalence or use small samples from clinical settings, and it is unknown if smoking rates have declined among adults living with HCV in the U.S. To help fill this gap at the population-level, we aimed to estimate the prevalence of active smoking over the last decade among adults living with HCV in the U.S., estimate temporal trends in active smoking, and identify demographic, clinical, and behavioral factors that predict active smoking.
2. Materials and Methods
2.1. Study Design
By combing annual data from the National Health and Nutrition Examination Survey (NHANES) cycles from 2007 to 2018, we performed a repeated cross-sectional, retrospective analysis of data. NHANES is a publicly available, de-identified database that uses a multistage, stratified probability sampling strategy to recruit a nationally representative sample of non-institutionalized adults living in the U.S. We pooled (“stacked”) six two-year waves and applied survey design variables (weights, strata, primary sampling units) per NCHS guidance to generate nationally representative estimates for adults living with HCV.
Sample Selection
Inclusion criteria were age ≥ 20 years, serologic evidence of HCV (anti-HCV antibody and/or HCV RNA positive per NHANES laboratory files), and non-missing smoking status. Across six waves of data (i.e., 2007–2018), 622 unweighted participants met HCV serologic criteria; one lacked smoking status and was excluded, yielding a final unweighted analytic sample of n = 621 (weighted sample size n = 3,620,603). The mean unweighted number of HCV-seropositive participants per two-year wave was 103.5 (range, 93–116). Exclusion criteria was missing data on smoking status (n = 1).
2.2. Primary Outcomes
The primary outcomes were the cumulative prevalence of active smoking over the last decade, trend changes in the prevalence of active smoking over the last decade, and factors that predict active smoking. Smoking status was ascertained using standardized NHANES questionnaires that assessed whether participants had smoked ≥100 cigarettes in their lifetime and whether they currently smoke cigarettes (every day or some days). Participants who answered “yes” to both questions were classified as active smokers, and all others were considered non-smokers.
2.3. Independent Variables
2.3.1. Demographic Characteristics
Demographic variables included age, sex, ethnicity (non-Hispanic White, non-Hispanic Black, Other), relationship status (married/partnered vs. other), insurance status (insured vs. uninsured), education level (<High School [HS], High School/GED, >High School), and poverty income ratio (low vs. middle/high).
2.3.2. Clinical and Laboratory Characteristics
Clinical variables encompassed obesity (body mass index ≥ 30 kg/m2), hypertension, hyperlipidemia, hepatitis B virus (HBV) co-infection, and advanced liver fibrosis—determined by calculating the Fibrosis-4 (FIB-4) score [age, aminotransferase (ALT), aspartate aminotransferase (AST), and platelet count were used to calculate the FIB-4 score]—serum cotinine, gamma glutamyl transferase (GGT), creatinine, and total bilirubin. HCV serology components (anti-HCV and HCV RNA) were obtained from NHANES laboratory datasets and used solely to define HCV status.
2.3.3. Behavioral and Psychosocial Characteristics
Behavioral and psychosocial characteristics included current marijuana use (self-reported past 30-day use) and lifetime substance use. The Patient Health Questionnaire-9 (PHQ-9) was used to assess levels of depression, and those with a score ≥ 10 were classified as having depression.
2.4. Statistical Analysis
Survey design variables (weights, strata, primary sampling units) were applied to account for complex design and to obtain nationally representative estimates. Descriptive statistics included weighted means/frequencies with 95% CIs. Between-group comparisons used design-based t-tests (continuous) and Rao–Scott χ2 tests (categorical).
To assess temporal trends in smoking prevalence (2007–2018), we first computed wave-specific weighted prevalences and then fit (i) a linear regression of prevalence on survey wave (treated as continuous) and (ii) a join-point (piecewise log-linear) regression allowing at most one join-point to avoid overfitting. Wave-level estimates were analyzed with inverse-variance weights derived from each wave’s CI on the log scale. We compared 0- vs. 1-joinpoint models using an F-test and reported annual percent change (APC) for each segment and average annual percent change (AAPC) for 2007–2018 with 95% CIs; a Davies test assessed slope change.
For determinants of active smoking, we fitted a survey-weighted binary logistic regression to the pooled sample across all waves, reporting adjusted odds ratios (aORs) with 95% CIs. Two-sided α = 0.05. Analyses used R (v4.4.1) with the survey package; join-point modeling used the segmented package. We limited trend analyses to 2007–2018 because NHANES field operations and public release structures changed during 2019–2020 (COVID-19), and the combined “2017–March 2020 pre-pandemic” files are not an independent wave for these HCV laboratory components; including them would duplicate 2017–2018 estimates and compromise trend comparability.
2.5. Ethical Considerations
NHANES data are publicly available and de-identified. In accordance with Cleveland Clinic IRB policy, analyses of these public-use files do not constitute human subjects research and did not require IRB review or informed consent beyond that obtained by the National Center for Health Statistics.
3. Results
3.1. Active Smoking: Prevalence and Trends
Wave-specific prevalences with 95% CIs and unweighted counts are shown in (Table 1). The cumulative prevalence of active smoking among adults with HCV in the U.S. from 2007 to 2018 was 56.4% (95% CI, 49.2–63.4). Neither a simple linear model (p = 0.93) nor a join-point model (Davies p = 0.44; F-test p = 0.43) identified a statistically significant temporal trend; estimates suggested a decline through ~2013–2014 followed by a modest uptick, but segment-specific APCs were not different from zero (Figure 1).

Table 1.
Prevalence of Active Smoking Among Adults ≥20 Years Old Living with HCV by NHANES Wave and Regression Summary for Trend Analysis.
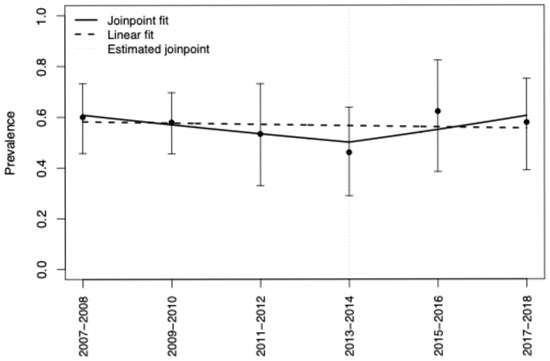
Figure 1.
Overall Smoking Prevalence [2007–2018] and Annual Trends in the Prevalence of Active Smoking Among Adults Living with HCV in the U.S.: Joinpoint and Linear Regression Modeling. Notes: Points show survey-weighted prevalence (95% CI) for each two-year wave. The dashed line is a single-slope linear fit. The solid line is a joinpoint (piecewise log-linear) fit allowing one joinpoint; the estimated joinpoint is near 2013–2014. Neither the overall linear trend nor the slope change reached statistical significance (see Table 1 for model summaries).
3.2. Active Smoking: Prevalence and Trends by Demographic Characteristics
The overall cumulative prevalence of active smoking (2007–2018) was higher among adults 20–64 vs. ≥65 years old (59.4% vs. 26.9%), non-Hispanic Whites vs. non-Hispanic Blacks and those of other ethnicities (59.4% vs. 49.8% vs. 49.8%, respectively), those who were not in a relationship vs. those who were married/partnered (57.7% vs. 55.5%), those with a low vs. middle/high poverty income ratio (67.7% vs. 43.8%), those with <HS or HS/GED vs. >HS education (62.4–65.3% vs. 44.9%), and those with 0–1 vs. ≥2 yearly health care visits (73.5% vs. 49.7%) (Table 2).

Table 2.
Trends in the Prevalence of Active Smoking Among Adults ≥20 Years Old Living with HCV—Stratified by Demographics: NHANES 2007–2008 to 2017–2018.
Though trends in active smoking did not decrease or increase significantly across these demographic groups, certain groups had noteworthy increases in the prevalence of active smoking from 2007 to 2018. For instance, the prevalence of active smoking increased from 2007 to 2018 among those 20–64 years old (61.7% to 67.1%), non-Hispanic Blacks (41.8% to 52.9%), married/partnered (51.1% to 69.1%), with a low poverty income ratio (72.1% to 76.2%), with >HS education (41.5% vs. 49.5%), and 0–1 yearly healthcare visits (62.6% to 89.0%).
3.3. Active Smoking: Prevalence and Trends by Medical Conditions, Alcohol Use, Marijuana Use, and Depression
Trends in active smoking were not statistically significantly (i.e., did not increase or decrease) across any medical group, but certain groups exhibited notable upward changes from 2007 to 2018 (Table 3). The prevalence of active smoking among those with HBV co-infection increased from 52.8% to 68.5%, increased from 48.1% to 62.7% among those with advanced fibrosis, and rose from 46.0% to 72.5% among those with alcohol use and from 72.3% to 89.1% among those with current marijuana use.

Table 3.
Trends in the Prevalence of Active Smoking Among Adults ≥20 Years Old Living with HCV—Stratified by Medical Conditions, and Alcohol Use, Marijuana Use, and Depression: NHANES 2007–2008 to 2017–2018.
3.4. Bivariate Analysis
Compared with non-smokers, active smokers had a higher proportion with a low poverty-income ratio (61.90% vs. 37.65%, p = 0.0004), a high school/GED education (37.23% vs. 25.61%, p = 0.0257), and depression (23.89% vs. 11.67%, p = 0.0084), as well as a higher mean platelet count (231 ± 7.07 vs. 208 ± 7.74, p = 0.0352) and serum cotinine (260.41 vs. 51.08 ng/mL, p < 0.001) (Table 4). Non-smokers were older on average, more likely to be married/partnered, had higher income and >high-school education, greater obesity prevalence, more diabetes, more frequent healthcare visits, and higher mean creatinine and total bilirubin.

Table 4.
Demographic and Clinical Characteristics of Adults Living with HCV With and Without Active Smoking: NHANES 2007–2008 to 2017–2018.
3.5. Multivariate Binary Logistic Regression
In survey-weighted multivariate binary logistic regression, female sex (adjusted odds ratio [aOR] = 2.23, 95% CI 1.174.24, p = 0.0156), low poverty income ratio (aOR = 3.33, 95% CI 1.41–7.84, p = 0.0070), lifetime substance use (aOR = 10.63, 95% CI 3.08–36.70, p = 0.0004), and depression (aOR = 2.65, 95% CI 1.29–5.45, p = 0.0089) were associated with increased odds of active smoking (Table 5). Conversely, having >high school education (aOR = 0.50, 95% CI 0.26–0.94, p = 0.0329), obesity (aOR = 0.32, 95% CI 0.18–0.58, p = 0.0004), and ≥2 yearly health care visits (aOR = 0.27, 95% CI 0.10–0.68, p = 0.0065) were associated with lower odds of active smoking.

Table 5.
Weighted Multivariate Binary Logistic Regression: Active Smoking.
4. Discussion
In this large, population-level analysis of NHANES data over the past decade (an 11-year period) in the U.S., the prevalence of active smoking among adults living with HCV was 56.4%, and there were no significant declines in smoking over the 11-year period. Female sex, low socioeconomic status, lifetime substance use, and depression were factors associated with increased odds of active smoking, whereas greater educational attainment, obesity, and regular healthcare utilization were associated with lower odds of active smoking.
Smoking appears to be endemic within the HCV population [9,10], which further complicates management of cirrhosis and hepatocellular carcinoma. Although cigarette smoking in the general U.S. population declined from 19.8% in 2007 to 13.8% in 2018 [12], smoking rates among people living with HCV have remained alarmingly stagnant. Undoubtedly, this phenomenon will likely accelerate incident cases of HCV-related advanced liver disease, especially if high-risk subgroups (e.g., those with low income or psychiatric comorbidities) remain uninformed of the negative effects of smoking on HCV, disregard cessation advisement, or do not receive effective cessation interventions. Evidence-based pharmacotherapies—including varenicline, bupropion, and nicotine replacement therapy—have demonstrated efficacy for smoking cessation across diverse populations, including those with psychiatric comorbidities [13]. Although no large, randomized trials have specifically examined integrated HCV care in combination with smoking cessation, these medications, along with behavioral support, may hold promise for people living with HCV. Digital or text-based interventions have also shown cost-effective, scalable benefits for hard-to-reach groups and could be adapted for HCV patients. Future studies are warranted to fully assess integrated cessation interventions within HCV treatment programs.
The link between depression and active smoking may reflect a bidirectional cycle in which nicotine transiently alleviates mood disturbances (e.g., stress or anxiety) but ultimately exacerbates depressive symptoms via neurochemical or inflammatory pathways [7]. Integrating depression screening and treatment within smoking-cessation efforts could help break this cycle. Likewise, lifetime substance use often co-occurs with smoking due to shared neurobiological circuits and environmental reinforcement [10], underscoring the importance of integrated interventions that address both HCV management and substance use disorders. Another notable finding was the heightened odds of smoking among women with HCV, whereas other studies indicate men are more likely to smoke than women with HCV [14]. This divergence could be due to evolving demographic patterns, shifts in substance use trends, or previously under-recognized psychosocial factors influencing women—particularly as many older studies were cross-sectional and had smaller samples.
Smoking prevalence among adults with HCV is likely much higher than the general U.S. population due to differences in socioeconomic disadvantages, co-occurring substance and alcohol use, and psychiatric disorders. All of these respective factors are over-represented among adults with HCV and are strongly linked with tobacco use. Similarly, this is consistent with our multivariable findings.
We did notice an uptick in the 2015–2016 active smoking prevalence estimate. We re-checked wave-level sample sizes, missingness, and survey question wording and found no methodological artifacts. This interval coincided with a marked escalation of the U.S. opioid epidemic [15], tightly intertwined with HCV epidemiology via injection drug use. Smoking prevalence is exceptionally high among individuals with opioid use disorder, often exceeding 80% [16] and remains elevated among individuals with opioid use disorder (~73%), which could reinforce tobacco use within this comorbid population [17]. The observed pattern may therefore reflect broader syndemic dynamics rather than a sustained secular change. Nevertheless, our joinpoint regression modeling did not indicate a statistically significant slope change.
Given the high prevalence of undiagnosed cases of HCV in the U.S., several medical institutions have implemented universal HCV testing for all patients who present in their respective emergency departments [18]. This point of care, universal HCV testing model in the emergency department uses the SBIRT (Screening, Brief Intervention, and Referral to Treatment), which identifies undiagnosed cases of HCV, provides brief education on HCV and harmful effects of alcohol use, and refers and links patients to HCV direct-acting antiviral treatment with primary care, infectious disease, or hepatology providers. Integration of the harmful effects of smoking during the brief intervention of SBIRT and provision of tobacco cessation counseling or resources may be an optimal time to engender thoughts of behavioral change among patients at the time of diagnosis. Future studies are needed to evaluate the feasibility and impact of patient psychoeducation on the harmful, toxic hepatic effects of smoking on HCV at the time of testing and diagnosis in emergency department settings.
This study has several limitations and strengths. First, self-reported smoking—without biochemical verification may introduce recall or social desirability bias. Second, data on the number of cigarettes smoked per day were not included, limiting our ability to model changes in smoking intensity over the 11-year period. Furthermore, the absence of data on alternative tobacco products (e.g., e-cigarettes, cigars, smokeless tobacco) for this HCV-defined analytic subset precludes product-specific analyses. However, the repeated cross-sectional design across multiple NHANES cycles is significantly more robust than single cross-sectional designs. The large, nationally representative sample, use of survey weighting, and incorporation of joinpoint regression modeling enhanced both the internal and external validity of our findings.
Active smoking is still shockingly prevalent among people living with HCV, suggesting a great need for focused cessation programs addressing sex-specific risk factors, socioeconomic-driven inequalities, co-occurring substance use, and mental health issues. Future prospective studies should evaluate integrated treatment approaches that concurrently treat HCV, help smokers quit, and address psychiatric or substance use comorbidities. Such initiatives could significantly reduce morbidity and mortality, thereby improving population health and clinical care.
5. Conclusions
Among U.S. adults living with HCV, active smoking remains common and shows no statistically significant decline over the last decade. Integrating targeted tobacco cessation—with attention to socioeconomic disadvantage, co-occurring substance use, and depression—into HCV care pathways is an immediate actionable strategy to mitigate progression to advanced liver disease.
Author Contributions
Conceptualization, O.T.S.; methodology, J.S., Y.G. and O.T.S.; software, J.S. and Y.G.; validation, J.S., Y.G. and O.T.S.; formal analysis, J.S., Y.G. and O.T.S.; investigation, M.A., J.S., Y.G. and O.T.S.; resources, O.T.S.; data curation, J.S., Y.G. and O.T.S.; writing—original draft preparation, M.A., J.S., Y.G., H.W., I.O., M.C., W.M., S.S. and O.T.S.; writing—review and editing, M.A., J.S., Y.G., H.W., I.O., M.C., W.M., S.S. and O.T.S.; visualization, M.A.; supervision, O.T.S.; project administration, O.T.S.; funding acquisition, O.T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA), grant number L30AA023401-04 (to O.T.S.).
Institutional Review Board Statement
The study analyzed publicly available, de-identified NHANES data and therefore did not require ethical approval according to the Cleveland Clinic Institutional Review Board policy.
Informed Consent Statement
The analysis used secondary, de-identified public-use NHANES files for which informed consent had already been obtained by the National Center for Health Statistics.
Data Availability Statement
All data underpinning this study are openly available from the National Health and Nutrition Examination Survey (NHANES) repository at https://www.cdc.gov/nchs/nhanes/ (accessed on 17 October 2024).
Acknowledgments
The authors thank the participants and staff of NHANES for making these data publicly accessible. Administrative support was provided by the Department of Gastroenterology, Hepatology, and Nutrition, Cleveland Clinic.
Conflicts of Interest
O.T.S. reports grant support from NIAAA (L30AA023401-04) related to this work. All other authors declare no conflicts of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| NHANES | National Health and Nutrition Examination Survey |
| HCV | Hepatitis C Virus |
| CI | Confidence Interval |
| OR/aOR | (Adjusted) Odds Ratio |
| NIAAA | National Institute on Alcohol Abuse and Alcoholism |
| APC | Article Processing Charge |
References
- Hepatitis C. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 18 April 2025).
- Lewis, K.C.; Barker, L.K.; Jiles, R.B.; Gupta, N. Estimated Prevalence and Awareness of Hepatitis C Virus Infection Among US Adults: National Health and Nutrition Examination Survey, January 2017–March 2020. Clin. Infect. Dis. 2023, 77, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-J.; Park, M.Y.; Cho, E.J.; Yu, S.J.; Kim, S.G.; Kim, Y.J.; Kim, Y.S.; Yoon, J.-H. Smoking Increases the Risk of Hepatocellular Carcinoma and Cardiovascular Disease in Patients with Metabolic-Associated Fatty Liver Disease. J. Clin. Med. 2023, 12, 3336. [Google Scholar] [CrossRef] [PubMed]
- Marti-Aguado, D.; Clemente-Sanchez, A.; Bataller, R. Cigarette Smoking and Liver Diseases. J. Hepatol. 2022, 77, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Taye, B.W. Hepatitis C Virus Infection and Tobacco Smoking—Joint Health Effects and Implications for Treatment of Both: A Systematic Review. medRxiv 2021. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the Screening, Care and Treatment of Persons with Chronic Hepatitis C Infection; Updated Version, April 2016; World Health Organization: Geneva, Switzerland, 2016; ISBN 978-92-4-154961-5.
- Kim, R.S.; Weinberger, A.H.; Chander, G.; Sulkowski, M.S.; Norton, B.; Shuter, J. Cigarette Smoking in Persons Living with Hepatitis C: The National Health and Nutrition Examination Survey (NHANES), 1999–2014. Am. J. Med. 2018, 131, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Shuter, J.; Litwin, A.H.; Sulkowski, M.S.; Feinstein, A.; Bursky-Tammam, A.; Maslak, S.; Weinberger, A.H.; Esan, H.; Segal, K.S.; Norton, B. Cigarette Smoking Behaviors and Beliefs in Persons Living with Hepatitis C. Nicotine Tob. Res. 2016, 19, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.F.; Ward, K.M.; Gittleman, J.; Perez, E.; Pia, T.; Shuter, J.; Weinberger, A.H.; Sulkowski, M. Hepatitis C and Cigarette Smoking Behavior: Themes from Focus Groups. Nicotine Tob. Res. 2024, 26, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Pericot-Valverde, I.; Heo, M.; Akiyama, M.J.; Norton, B.L.; Agyemang, L.; Niu, J.; Litwin, A.H. Factors and HCV Treatment Outcomes Associated with Smoking among People Who Inject Drugs on Opioid Agonist Treatment: Secondary Analysis of the PREVAIL Randomized Clinical Trial. BMC Infect. Dis. 2020, 20, 928. [Google Scholar] [CrossRef] [PubMed]
- Bjørnestad, E.D.; Vederhus, J.-K.; Clausen, T. High Smoking and Low Cessation Rates among Patients in Treatment for Opioid and Other Substance Use Disorders. BMC Psychiatry 2022, 22, 649. [Google Scholar] [CrossRef] [PubMed]
- Creamer, M.R.; Wang, T.W.; Babb, S.; Cullen, K.A.; Day, H.; Willis, G.; Jamal, A.; Neff, L. Tobacco Product Use and Cessation Indicators Among Adults—United States, 2018. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Evins, A.E.; Benowitz, N.L.; West, R.; Russ, C.; McRae, T.; Lawrence, D.; Krishen, A.; St Aubin, L.; Maravic, M.C.; Anthenelli, R.M. Neuropsychiatric Safety and Efficacy of Varenicline, Bupropion, and Nicotine Patch in Smokers with Psychotic, Anxiety, and Mood Disorders in the EAGLES Trial. J. Clin. Psychopharmacol. 2019, 39, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lin, J.-L.; Liu, J.; Jiang, X.-W.; Zhang, H.; Peng, L. Estimates of Prevalence, Time-Trend, and Association of Smoking in Adults Living with HIV, HBV, and HCV (NHANES 1999–2018). Sci. Rep. 2022, 12, 19925. [Google Scholar] [CrossRef] [PubMed]
- Hedegaard, H. Drug Overdose Deaths in the United States, 1999–2018; National Center for Health Statistics: Hyattsville, MD, USA, 2020.
- Guydish, J.; Passalacqua, E.; Pagano, A.; Martínez, C.; Le, T.; Chun, J.; Tajima, B.; Docto, L.; Garina, D.; Delucchi, K. An International Systematic Review of Smoking Prevalence in Addiction Treatment: Smoking Prevalence in Addiction Treatment. Addiction 2016, 111, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.A.; Weinberger, A.H.; Villanti, A.C. Quit Ratios for Cigarette Smoking among Individuals with Opioid Misuse and Opioid Use Disorder in the United States. Drug Alcohol Depend. 2020, 214, 108164. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.-H.; Rothman, R.E.; Laeyendecker, O.B.; Kelen, G.D.; Avornu, A.; Patel, E.U.; Kim, J.; Irvin, R.; Thomas, D.L.; Quinn, T.C. Evaluation of the Centers for Disease Control and Prevention Recommendations for Hepatitis C Virus Testing in an Urban Emergency Department. Clin. Infect. Dis. 2016, 62, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).