Comparison of the Effect of CFTR Modulators elexacaftor/tezacaftor/ivacaftor and lumacaftor/ivacaftor via Serum Human Epididymis Protein 4 Concentration in p.Phe508del-CFTR Homozygous Cystic Fibrosis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Study
2.2. Study Population and Design
2.3. Serum HE4 Measurement
2.4. Clinical Correlation Analysis
2.5. In Vitro Experiments
2.6. Cell Culture and Treatment with CFTR Modulators
2.7. HE4 Protein Analysis in Cell Supernatants
2.8. CFTR Functional Assessment (Whole-Cell Patch Clamp)
2.9. Statistical Analyses
3. Results
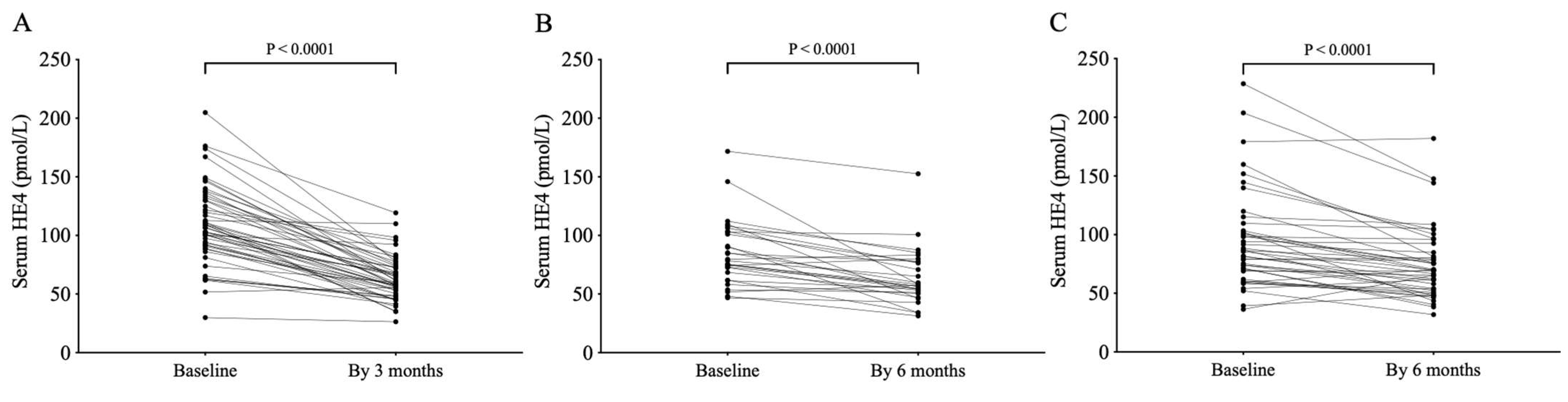
3.1. Treatment with ETI or LUM/IVA Lowers Serum HE4 Levels in CF Subjects Homozygous for p.Phe508del-CFTR Variant
3.2. HE4 Serum Levels Strongly Correlate with ppFEV1
3.3. Change in Serum HE4 Levels Predicts the Improvement of CF Lung Disease Under Kaftrio® Therapy
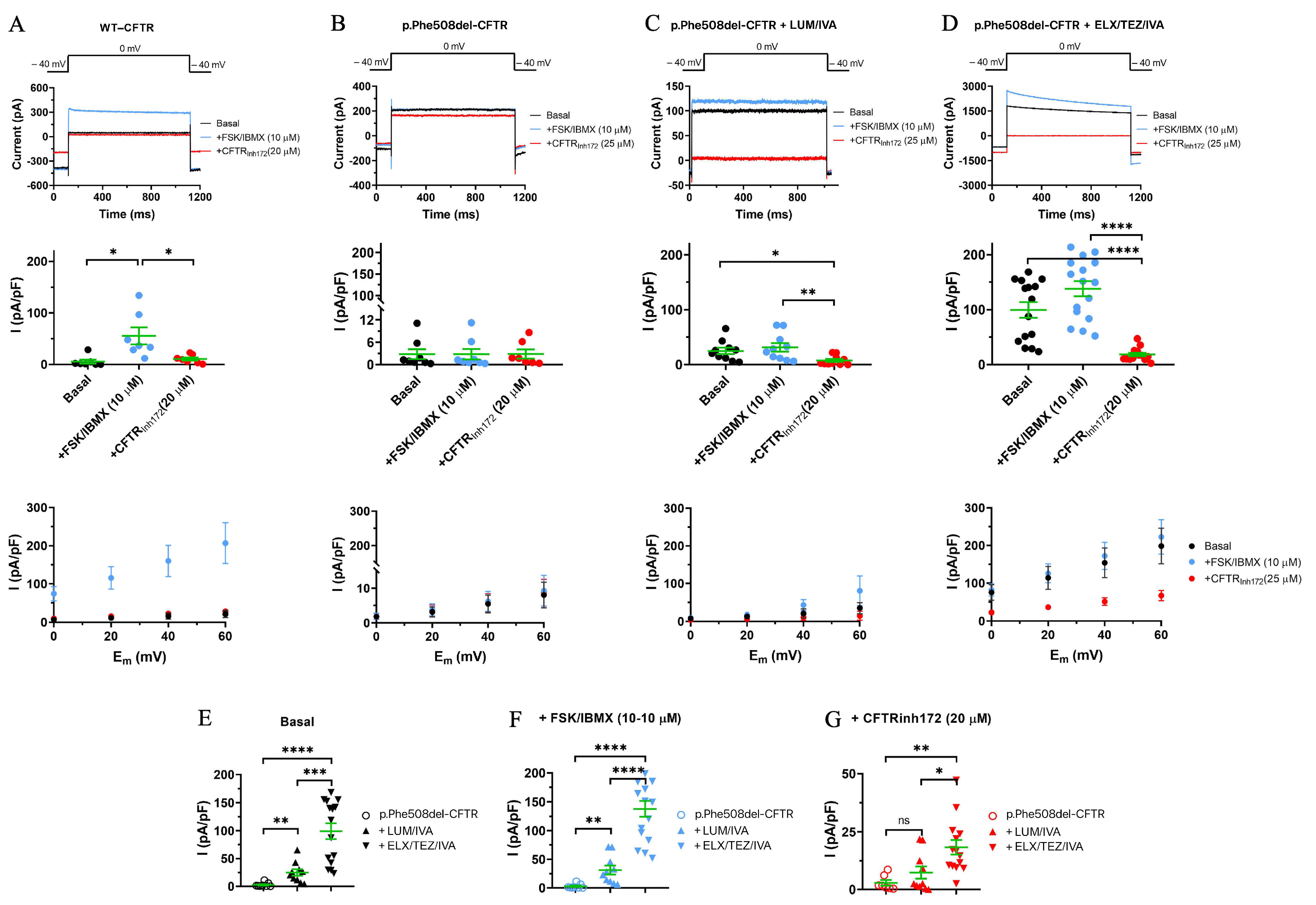
3.4. Comparison of the Effects of the Two Different CFTRm on p.Phe508del-CFTR Cl- Currents in CFBE Cells
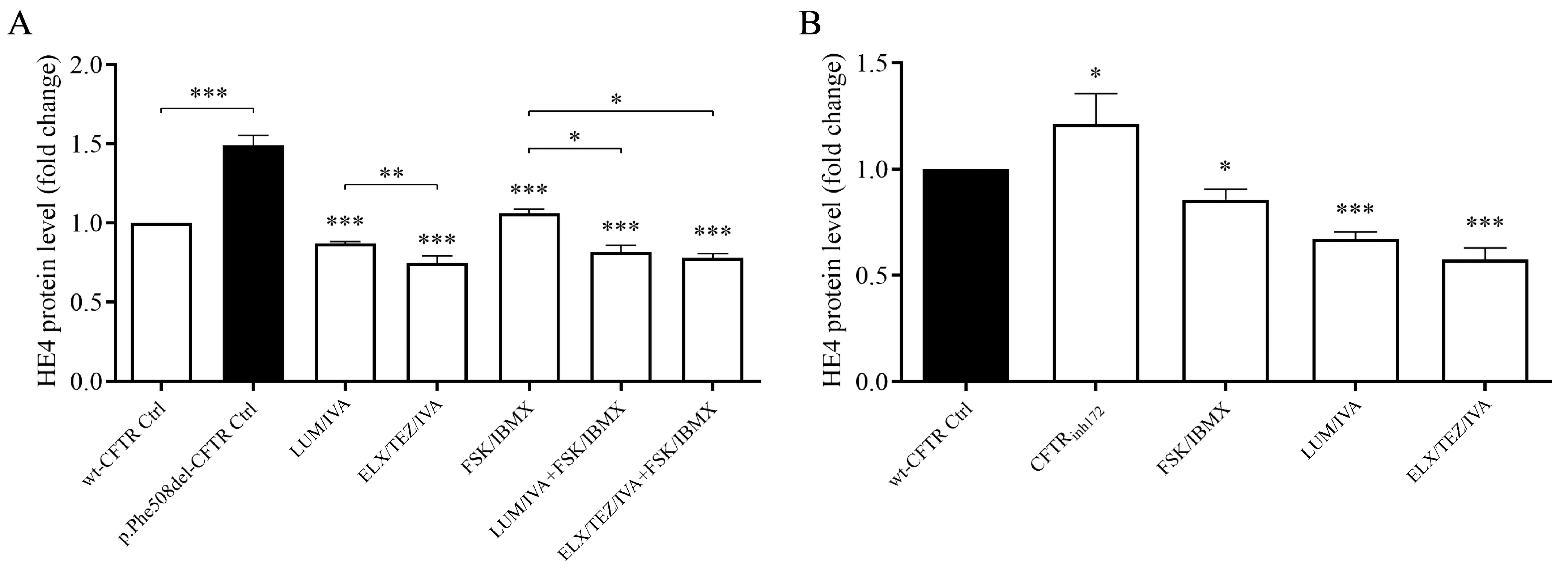
3.5. ELX/TEZ/IVA Treatment Reduced HE4 Concentrations in the Supernatant of CFBE 41o- Cell Cultures In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HE4 | human epididymis protein 4 |
| pwCF | patients with CF |
| CFTRm | CFTR modulator |
| CFTR | CF transmembrane conductance regulator |
| CFBE | cystic fibrosis bronchial epithelial cell |
| LUM/IVA | lumacaftor/ivacaftor |
| ETI | elexacaftor/tezacaftor/ivacaftor |
| ppFEV1 | FEV1% predicted value |
| CFTRm | CFTR modulators |
| CRP | C-reactive protein |
| FSK | Forskolin |
| IBMX | 3-isobutyl-1-methylxanthine |
| DMSO | dimethyl sulfoxide |
| ROC | the receiver operating characteristic curve |
| AUC | area under the curve |
| IL-18 | interleukin-18 |
| TNF-α | tumor necrosis factor-α |
References
- Rowe, S.M.; Miller, S.; Sorscher, E.J. Cystic fibrosis. N. Engl. J. Med. 2005, 352, 1992–2001. [Google Scholar] [CrossRef] [PubMed]
- ECFS Patient Registry Annual Report. 2023. Available online: https://www.ecfs.eu/projects/ecfs-patient-registry/annual-reports (accessed on 8 July 2025).
- De Boeck, K.; Amaral, M.D. Progress in Therapies for Cystic Fibrosis. Lancet Respir. Med. 2016, 4, 662–674. [Google Scholar] [CrossRef]
- Van Goor, F.; Hadida, S.; Grootenhuis, P.D.; Burton, B.; Cao, D.; Neuberger, T.; Turnbull, A.; Singh, A.; Joubran, J.; Hazlewood, A.; et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc. Natl. Acad. Sci. USA 2009, 106, 18825–18830. [Google Scholar] [CrossRef]
- Van Goor, F.; Hadida, S.; Grootenhuis, P.D.; Burton, B.; Stack, J.H.; Straley, K.S.; Decker, C.J.; Miller, M.; McCartney, J.; Olson, E.R.; et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc. Natl. Acad. Sci. USA 2011, 108, 18843–18848. [Google Scholar] [CrossRef]
- Pranke, I.M.; Hatton, A.; Simonin, J.; Jais, J.P.; Le Pimpec-Barthes, F.; Carsin, A.; Bonnette, P.; Fayon, M.; Stremler-Le Bel, N.; Grenet, D.; et al. Correction of CFTR function in nasal epithelial cells from cystic fibrosis patients predicts improvement of respiratory function by CFTR modulators. Sci. Rep. 2017, 7, 7375. [Google Scholar] [CrossRef]
- Ridley, K.; Condren, M. Elexacaftor-Tezacaftor-Ivacaftor: The First Triple-Combination Cystic Fibrosis Transmembrane Conductance Regulator Modulating Therapy. J. Pediatr. Pharmacol. Ther. 2020, 25, 192–197. [Google Scholar] [CrossRef]
- Middleton, P.G.; Mall, M.A.; Dřevínek, P.; Lands, L.C.; McKone, E.F.; Polineni, D.; Ramsey, B.W.; Taylor-Cousar, J.L.; Tullis, E.; Vermeulen, F.; et al. Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019, 381, 1809–1819. [Google Scholar] [CrossRef]
- Heijerman, H.G.M.; McKone, E.F.; Downey, D.G.; Van Braeckel, E.; Rowe, S.M.; Tullis, E.; Mall, M.A.; Welter, J.J.; Ramsey, B.W.; McKee, C.M.; et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: A double-blind, randomised, phase 3 trial. Lancet 2019, 394, 1940–1948. [Google Scholar] [CrossRef]
- Goralski, J.L.; Hoppe, J.E.; Mall, M.A.; McColley, S.A.; McKone, E.; Ramsey, B.; Rayment, J.H.; Robinson, P.; Stehling, F.; Taylor-Cousar, J.L.; et al. Phase 3 Open-Label Clinical Trial of Elexacaftor/Tezacaftor/Ivacaftor in Children Aged 2–5 Years with Cystic Fibrosis and at Least One F508del Allele. Am. J. Respir. Crit. Care Med. 2023, 208, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Sutharsan, S.; McKone, E.F.; Downey, D.G.; Duckers, J.; MacGregor, G.; Tullis, E.; Van Braeckel, E.; Wainwright, C.E.; Watson, D.; Ahluwalia, N.; et al. Efficacy and safety of elexacaftor plus tezacaftor plus ivacaftor versus tezacaftor plus ivacaftor in people with cystic fibrosis homozygous for F508del-CFTR: A 24-week, multicentre, randomised, double-blind, active-controlled, phase 3b trial. Lancet Respir. Med. 2022, 10, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Mall, M.A.; Brugha, R.; Gartner, S.; Legg, J.; Moeller, A.; Mondejar-Lopez, P.; Prais, D.; Pressler, T.; Ratjen, F.; Reix, P.; et al. Efficacy and Safety of Elexacaftor/Tezacaftor/Ivacaftor in Children 6 Through 11 Years of Age with Cystic Fibrosis Heterozygous for F508del and a Minimal Function Mutation: A Phase 3b, Randomized, Placebo-controlled Study. Am. J. Respir. Crit. Care Med. 2022, 206, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Milla, C.E.; Ratjen, F.; Marigowda, G.; Liu, F.; Waltz, D.; Rosenfeld, M.; VX13-809-011 Part B Investigator Group. Lumacaftor/Ivacaftor in Patients Aged 6-11 Years with Cystic Fibrosis and Homozygous for F508del-CFTR. Am. J. Respir. Crit. Care Med. 2017, 195, 912–920. [Google Scholar] [CrossRef]
- Burgel, P.R.; Munck, A.; Durieu, I.; Chiron, R.; Mely, L.; Prevotat, A.; Murris-Espin, M.; Porzio, M.; Abely, M.; Reix, P.; et al. Real-Life Safety and Effectiveness of Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2020, 201, 188–197. [Google Scholar] [CrossRef]
- Nagy, B., Jr.; Nagy, B.; Fila, L.; Clarke, L.A.; Gönczy, F.; Bede, O.; Nagy, D.; Újhelyi, R.; Szabó, Á.; Anghelyi, A.; et al. Human epididymis protein 4: A novel serum inflammatory biomarker in cystic fibrosis. Chest 2016, 150, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B., Jr.; Bene, Z.; Fejes, Z.; Heltshe, S.L.; Reid, D.; Ronan, N.J.; McCarthy, Y.; Smith, D.; Nagy, A.; Joseloff, E.; et al. Human epididymis protein 4 (HE4) levels inversely correlate with lung function improvement (delta FEV1) in cystic fibrosis patients receiving ivacaftor treatment. J. Cyst. Fibros. 2019, 18, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Pócsi, M.; Fejes, Z.; Bene, Z.; Nagy, A.; Balogh, I.; Amaral, M.D.; Macek, M., Jr.; Nagy, B., Jr. Human epididymis protein 4 (HE4) plasma concentration inversely correlates with the improvement of cystic fibrosis lung disease in p.Phe508del-CFTR homozygous cases treated with the CFTR modulator lumacaftor/ivacaftor combination. J. Cyst. Fibros. 2023, 22, 1085–1092. [Google Scholar]
- Bene, Z.; Fejes, Z.; Szanto, T.G.; Fenyvesi, F.; Váradi, J.; Clarke, L.A.; Panyi, G.; Macek, M., Jr.; Amaral, M.D.; Balogh, I.; et al. Enhanced expression of human epididymis protein 4 (HE4) reflecting pro-inflammatory status is regulated by CFTR in cystic fibrosis bronchial epithelial cells. Front. Pharmacol. 2021, 12, 592184. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.; ERS Global Lung Function Initiative; et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Nagy, B., Jr.; Krasznai, Z.T.; Balla, H.; Csobán, M.; Antal-Szalmás, P.; Hernádi, Z.; Kappelmayer, J. Elevated human epididymis protein 4 concentrations in chronic kidney disease. Ann. Clin. Biochem. 2012, 49 Pt 4, 377–380. [Google Scholar] [CrossRef]
- Hunter, M.J.; Treharne, K.J.; Winter, A.K.; Cassidy, D.M.; Land, S.; Mehta, A. Expression of Wild-type CFTR Suppresses NF-kappaB-Driven Inflammatory Signalling. PLoS One 2010, 5, e11598. [Google Scholar] [CrossRef]
- Kmit, A.; Marson, F.A.L.; Pereira, S.V.; Vinagre, A.M.; Leite, G.S.; Servidoni, M.F.; Ribeiro, J.D.; Ribeiro, A.F.; Bertuzzo, C.S.; Amaral, M.D. Extent of rescue of F508del-CFTR function by VX-809 and VX-770 in human nasal epithelial cells correlates with SNP rs7512462 in SLC26A9 gene in F508del/F508del Cystic Fibrosis patients. Biochim. Biophys. Acta. Mol. Basis. Dis. 2019, 1865, 1323–1331. [Google Scholar] [CrossRef]
- Boinot, C.; Jollivet Souchet, M.; Ferru-Clément, R.; Becq, F. Searching for combinations of small-molecule correctors to restore f508del-cystic fibrosis transmembrane conductance regulator function and processing. J. Pharmacol. Exp. Ther. 2014, 350, 624–634. [Google Scholar] [CrossRef]
- Billet, A.; Froux, L.; Hanrahan, J.W.; Becq, F. Development of Automated Patch Clamp Technique to Investigate CFTR Chloride Channel Function. Front. Pharmacol. 2017, 8, 195. [Google Scholar] [CrossRef]
- Graeber, S.Y.; Vitzthum, C.; Pallenberg, S.T.; Naehrlich, L.; Stahl, M.; Rohrbach, A.; Drescher, M.; Minso, R.; Ringshausen, F.C.; Rueckes-Nilges, C.; et al. Effects of Elexacaftor/Tezacaftor/Ivacaftor Therapy on CFTR Function in Patients with Cystic Fibrosis and One or Two F508del Alleles. Am. J. Respir. Crit. Care Med. 2022, 205, 540–549. [Google Scholar] [CrossRef]
- Szczesniak, R.; Heltshe, S.L.; Stanojevic, S.; Mayer-Hamblett, N. Use of FEV1 in cystic fibrosis epidemiologic studies and clinical trials: A statistical perspective for the clinical researcher. J. Cyst. Fibros. 2017, 16, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Greaves, R.F.; Jolly, L.; Massie, J.; Scott, S.; Wiley, V.C.; Metz, M.P.; Mackay, R.J.; Australasian Association of Clinical Biochemists Sweat Test Working Party in association with the Royal Australasian College of Pathologists Quality Assurance Programs. Laboratory performance of sweat conductivity for the screening of cystic fibrosis. Clin. Chem. Lab. Med. 2018, 28, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Bene, Z.; Fejes, Z.; Macek, M., Jr.; Amaral, M.D.; Balogh, I.; Nagy, B., Jr. Laboratory biomarkers for lung disease severity and progression in cystic fibrosis. Clin. Chim. Acta 2020, 508, 277–286. [Google Scholar] [CrossRef]
- Jarosz-Griffiths, H.H.; Scambler, T.; Wong, C.H.; Lara-Reyna, S.; Holbrook, J.; Martinon, F.; Savic, S.; Whitaker, P.; Etherington, C.; Spoletini, G.; et al. Different CFTR modulator combinations downregulate inflammation differently in cystic fibrosis. Elife 2020, 9, e54556 pii. [Google Scholar] [CrossRef] [PubMed]
- García, M.S.; Madrid-Carbajal, C.J.; Peláez, A.; Moreno, R.M.G.; Alonso, E.F.; García, B.P.; Punter, R.M.G.; Ancochea, J.; Bachiller, J.M.E.; Ruiz, J.D.H.; et al. The Role of Triple CFTR Modulator Therapy in Reducing Systemic Inflammation in Cystic Fibrosis. Lung 2025, 203, 55. [Google Scholar] [CrossRef]
- Maher, R.E.; Cytlak-Chaudhuri, U.M.; Aleem, S.; Barry, P.; Brice, D.P.; Caamaño Gutiérrez, E.; Driver, K.; Emmott, E.; Rothwell, A.; Smith, E.; et al. Effect of elexacaftor/tezacaftor/ivacaftor on systemic inflammation in cystic fibrosis. Thorax 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Carnovale, V.; Scialò, F.; Gelzo, M.; Iacotucci, P.; Amato, F.; Zarrilli, F.; Celardo, A.; Castaldo, G.; Corso, G. Cystic Fibrosis Patients with F508del/Minimal Function Genotype: Laboratory and Nutritional Evaluations after One Year of Elexacaftor/Tezacaftor/Ivacaftor Treatment. J. Clin. Med. 2022, 11, 6900. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, D.; Pepe, A. Cystic Fibrosis Transmembrane Conductance Regulator Modulators in Cystic Fibrosis: A Review of Registry-Based Evidence. J. Clin. Med. 2025, 14, 3978. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Höpfer, L.M.; Wohlgemuth, L.; Knapp, C.L.; Mohamed, A.O.K.; Stukan, L.; Münnich, F.; Hüsken, D.; Koller, A.S.; Stratmann, A.E.P.; et al. Multimodal analysis of granulocytes, monocytes, and platelets in patients with cystic fibrosis before and after Elexacaftor-Tezacaftor-Ivacaftor treatment. Front. Immunol. 2023, 14, 1180282. [Google Scholar] [CrossRef]
- Capraro, M.; Pedrazzi, M.; De Tullio, R.; Manfredi, M.; Cresta, F.; Castellani, C.; Averna, M. Modulation of Plasmatic Matrix Metalloprotease 9: A Promising New Tool for Understanding the Variable Clinical Responses of Patients with Cystic Fibrosis to Cystic Fibrosis Transmembrane Conductance Regulator Modulators. Int. J. Mol. Sci. 2023, 24, 13384. [Google Scholar] [CrossRef]
- Tümmler, B. Post-approval studies with the CFTR modulators Elexacaftor-Tezacaftor-Ivacaftor. Front. Pharmacol. 2023, 14, 1158207. [Google Scholar] [CrossRef]
- Liu, J.; Bihler, H.; Farinha, C.M.; Awatade, N.T.; Romão, A.M.; Mercadante, D.; Cheng, Y.; Musisi, I.; Jantarajit, W.; Wang, Y.; et al. Partial rescue of F508del-cystic fibrosis transmembrane conductance regulator channel gating with modest improvement of protein processing, but not stability, by a dual-acting small molecule. Br. J. Pharmacol. 2018, 175, 1017–1038. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | CF Cohort 1 on Kaftrio® (n = 51) | CF Cohort 2 on Kaftrio® (n = 29) | CF Cohort on Orkambi® (n = 43) |
|---|---|---|---|
| Age (years) (median, min–max) | 27 (20–47) | 12 (6–50) | 14 (11–53) |
| Gender (f/m), n | 28/23 | 17/12 | 23/20 |
| Baseline sweat chloride (mmol/L) (median, min–max) | 103 (61–1 19) | 112 (66–149) | 128 (61–166) |
| Baseline ppFEV1 (%) (median, min–max) | 73 (24–107) | 89 (40–147) | 70 (23–120) |
| Pre-treatment BMI (kg/m2) (median, min–max) | 21.9 (17.0–34.4) | 16.3 (13.2–25.3) | 17.9 (13.2–27.9) |
| Change in ppFEV1 by 3 * or 6 m (%) (mean, 95% CI) | 17.5 (14.5 to 20.5) * | 4.0 (−3.9 to 12.1) | 1.6 (−1.6 to 4.7) |
| Baseline serum C-reactive protein (mg/L) (median, min–max) | 4.5 (0.5–43.4) | 2.4 (0.2–96.4) | 2.3 (0.1–57.4) |
| Baseline serum creatinine (µmol/L) (median, min–max) | - | 71 (32–96) | 68 (32–107) |
| Bacterial colonization (y/n), n | - | 7/22 | 10/33 |
| Responders/non-responders, n | 46/5 | 19/10 | 22/21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pócsi, M.; Fila, L.; Péterfia, C.; Halász, A.; Szanto, T.G.; Mészáros, B.; Major, J.; Laki, I.; Szabó, H.; Panyi, G.; et al. Comparison of the Effect of CFTR Modulators elexacaftor/tezacaftor/ivacaftor and lumacaftor/ivacaftor via Serum Human Epididymis Protein 4 Concentration in p.Phe508del-CFTR Homozygous Cystic Fibrosis Patients. J. Clin. Med. 2025, 14, 6188. https://doi.org/10.3390/jcm14176188
Pócsi M, Fila L, Péterfia C, Halász A, Szanto TG, Mészáros B, Major J, Laki I, Szabó H, Panyi G, et al. Comparison of the Effect of CFTR Modulators elexacaftor/tezacaftor/ivacaftor and lumacaftor/ivacaftor via Serum Human Epididymis Protein 4 Concentration in p.Phe508del-CFTR Homozygous Cystic Fibrosis Patients. Journal of Clinical Medicine. 2025; 14(17):6188. https://doi.org/10.3390/jcm14176188
Chicago/Turabian StylePócsi, Marianna, Libor Fila, Csaba Péterfia, Adrien Halász, Tibor G. Szanto, Beáta Mészáros, Judit Major, István Laki, Hajnalka Szabó, György Panyi, and et al. 2025. "Comparison of the Effect of CFTR Modulators elexacaftor/tezacaftor/ivacaftor and lumacaftor/ivacaftor via Serum Human Epididymis Protein 4 Concentration in p.Phe508del-CFTR Homozygous Cystic Fibrosis Patients" Journal of Clinical Medicine 14, no. 17: 6188. https://doi.org/10.3390/jcm14176188
APA StylePócsi, M., Fila, L., Péterfia, C., Halász, A., Szanto, T. G., Mészáros, B., Major, J., Laki, I., Szabó, H., Panyi, G., Balogh, I., Amaral, M. D., Macek Jr., M., & Nagy Jr., B. (2025). Comparison of the Effect of CFTR Modulators elexacaftor/tezacaftor/ivacaftor and lumacaftor/ivacaftor via Serum Human Epididymis Protein 4 Concentration in p.Phe508del-CFTR Homozygous Cystic Fibrosis Patients. Journal of Clinical Medicine, 14(17), 6188. https://doi.org/10.3390/jcm14176188








