The Positive Effect of Negative Stimuli: Exposure to Negative Emotional Stimuli Improves Mood in Individuals with Major Depressive Disorder
Abstract
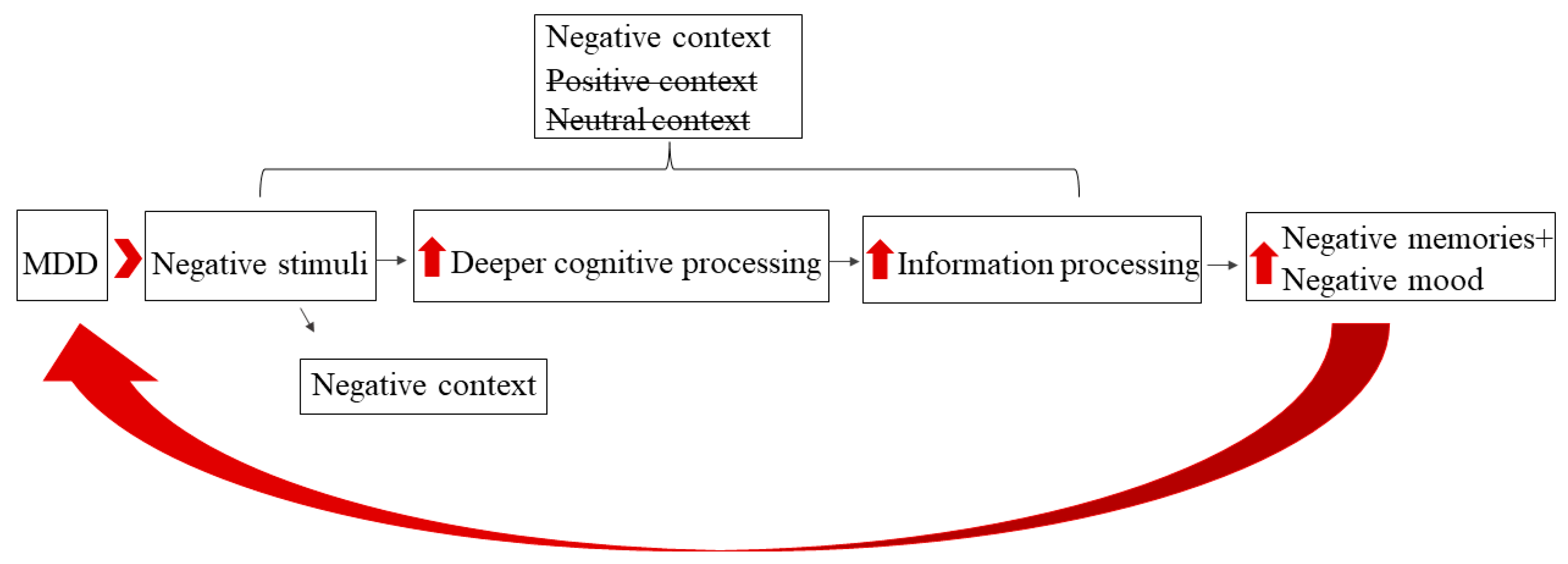
1. Introduction
2. Methods
2.1. Participants
2.2. Procedure
2.3. The Emotional Recall Task (Figure 2)
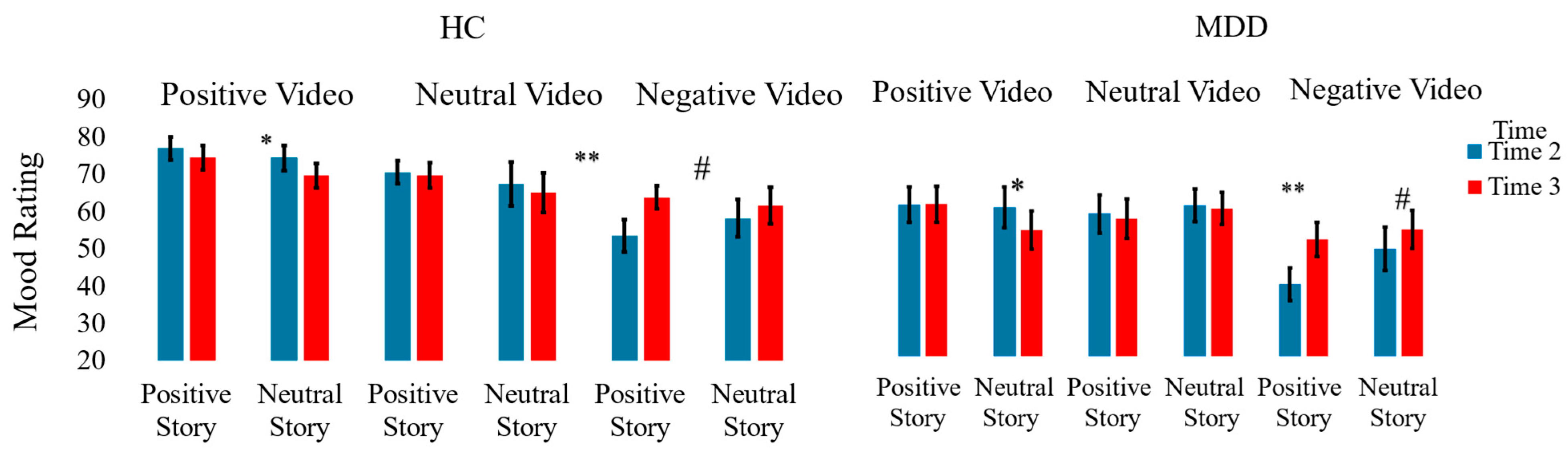
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Boland, R.J.; Keller, M.B. Course and outcome of depression. In Handbook of Depression; Gotlib, I.A., Hammen, C.L., Eds.; The Guilford Press: New York, NY, USA, 2009; Volume 2, pp. 23–43. [Google Scholar]
- Kessler, R.C.; Bromet, E.J. The epidemiology of depression across cultures. Annu. Rev. Public Health 2013, 34, 119–138. [Google Scholar] [CrossRef]
- Slavich, G.M.; Irwin, M.R. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol. Bull. 2014, 140, 774–815. [Google Scholar] [CrossRef]
- Cuijpers, P.; Sijbrandij, M.; Koole, S.L.; Andersson, G.; Beekman, A.T.; Reynolds, C.F. Adding psychotherapy to antidepressant medication in depression and anxiety disorders: A meta-analysis. World Psychiatry 2014, 13, 56–67. [Google Scholar] [CrossRef]
- Munder, T.; Flückiger, C.; Leichsenring, F.; Abbass, A.A.; Hilsenroth, M.J.; Luyten, P.; Rbung s Steinert, C.; Wampold, B.E. Is psychotherapy effective? A re-analysis of treatments for depression. Epidemiol. Psychiatr. Sci. 2019, 28, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Vittengl, J.R.; Clark, L.A.; Dunn, T.W.; Jarrett, R.B. Reducing relapse and recurrence in unipolar depression: A comparative metaanalysis of cognitive-behavioral therapy’s effects. J. Consult. Clin. Psychol. 2007, 75, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Hollon, S.D.; Thase, M.E.; Markowitz, J.C. Treatment and prevention of depression. Psychol. Sci. Public Interest 2002, 3, 39–77. [Google Scholar] [CrossRef]
- Beck, A.T.; Haigh, E.A.P. Advances in cognitive theory and therapy: The generic cognitive model. Annu. Rev. Clin. Psychol. 2014, 10, 1–24. [Google Scholar] [CrossRef]
- Clark, D.A.; Beck, A.T.; Alford, B.A. Scientific Foundations of Cognitive Theory and Therapy of Depression; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Ingram, R.E. Toward an information-processing analysis of depression. Cogn. Ther. Res. 1984, 8, 443–477. [Google Scholar] [CrossRef]
- Mathews, A.; MacLeod, C. Cognitive vulnerability to emotional disorders. Annu. Rev. Clin. Psychol. 2005, 1, 167–195. [Google Scholar] [CrossRef]
- Duque, A.; Vázquez, C. Double attention bias for positive and negative emotional faces in clinical depression: Evidence from an eye-tracking study. J. Behav. Ther. Exp. Psychiatry 2015, 46, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Gotlib, I.H.; Joormann, J. Cognition and depression: Current status and future directions. Annu. Rev. Clin. Psychol. 2010, 6, 285–312. [Google Scholar] [CrossRef]
- Lazarov, A.; Ben-Zion, Z.; Shamai, D.; Pine, D.S.; Bar-Haim, Y. Free viewing of sad and happy faces in depression: A potential target for attention bias modification. J. Affect. Disord. 2018, 238, 94–100. [Google Scholar] [CrossRef]
- Peckham, A.D.; McHugh, R.K.; Otto, M.W. A meta-analysis of the magnitude of biased attention in depression. Depress. Anxiety 2010, 27, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Winer, E.S.; Salem, T. Reward devaluation: Dot-probe meta-analytic evidence of avoidance of positive information in depressed persons. Psychol. Bull. 2016, 142, 18–78. [Google Scholar] [CrossRef] [PubMed]
- Everaert, J.; Koster, E.H. The interplay among attention, interpretation, and memory biases in depression: Revisiting the combined cognitive bias hypothesis. In Cognitive Biases in Health and Psychiatric Disorders; Academic Press: Cambridge, MA, USA, 2020; pp. 193–213. [Google Scholar] [CrossRef]
- Bar-Haim, Y. Research review: Attention bias modification (ABM): A novel treatment for anxiety disorders. J. Child Psychol. Psychiatry 2010, 51, 859–870. [Google Scholar] [CrossRef]
- Yang, W.; Ding, Z.; Dai, T.; Peng, F.; Zhang, J.X. Attention bias modification training in individuals with depressive symptoms: A randomized controlled trial. J. Behav. Ther. Exp. Psychiatry 2015, 49, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Wells, T.T.; Beevers, C.G. Biased attention and dysphoria: Manipulating selective attention reduces subsequent depressive symptoms. Cogn. Emot. 2010, 24, 719–728. [Google Scholar] [CrossRef]
- Woolridge, S.M.; Harrison, G.W.; Best, M.W.; Bowie, C.R. Attention bias modification in depression: A randomized trial using a novel, reward-based, eye-tracking approach. J. Behav. Ther. Exp. Psychiatry 2021, 71, 101621. [Google Scholar] [CrossRef]
- Joormann, J.; Waugh, C.E.; Gotlib, I.H. Cognitive bias modification for interpretation in major depression: Effects on memory and stress reactivity. Clin. Psychol. Sci. 2015, 3, 126–139. [Google Scholar] [CrossRef]
- Arditte Hall, K.A.; De Raedt, R.; Timpano, K.R.; Joormann, J. Positive memory enhancement training for individuals with major depressive disorder. Cogn. Behav. Ther. 2018, 47, 155–168. [Google Scholar] [CrossRef]
- Miron, S.; Kalanthroff, E. Negative emotional cues improve free recall of positive and neutral words in unmedicated patients with major depressive disorder. Cogn. Behav. Ther. 2024, 53, 409–422. [Google Scholar] [CrossRef]
- Miron, S.; Naftalovich, H.; Kalanthroff, E. The effect of emotional primes on attentional focus in high versus low depression. Atten. Percept. Psychophys. 2020, 82, 377–382. [Google Scholar] [CrossRef]
- Craik, F.I.M.; Lockhart, R.S. Levels of processing: A framework for memory research. J. Verbal Learn. Verbal Behav. 1972, 11, 671–684. [Google Scholar] [CrossRef]
- Miron, S.; Kalanthroff, E. Remembering the blues: Negative emotion during encoding improves memory recall in major depressive Disorder. Cogn. Emot. 2024, 39, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Foland-Ross, L.C.; Gotlib, I.H. Cognitive and neural aspects of information processing in major depressive disorder: An integrative perspective. Front. Psychol. 2012, 3, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Beck Depression Inventory Manual, 2nd ed.; Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Tolin, D.F.; Bowe, W.; Davis, E.; Hannan, S.; Springer, K.; Worden, B.; Steinman, S.A. Diagnostic Interview for Anxiety, Mood, and OCD and Related Neuropsychiatric Disorders (DIAMOND); Institute of Living/Hartford HealthCare Corporation: Hartford, CT, USA, 2016. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Van Bergen, P.; Wall, J.; Salmon, K. The good, the bad, and the neutral: The influence of emotional valence on young children’s recall. J. Appl. Res. Mem. Cogn. 2015, 4, 29–35. [Google Scholar] [CrossRef]
- Gross, J.J.; Levenson, R.W. Emotion elicitation using films. Cogn. Emot. 1995, 9, 87–108. [Google Scholar] [CrossRef]
- Jenkins, L.M.; Andrewes, D.G. A new set of standardised verbal and non-verbal contemporary film stimuli for the elicitation of emotions. Brain Impair. 2012, 13, 212–227. [Google Scholar] [CrossRef]
- Sommers, P.; Birkin, J. Mr Bean 10th Anniversary Collection: Mr Bean Goes to Town; [Television Series]; Universal Studios: Bedford, UK, 1990. [Google Scholar]
- Disney, W.; Hand, D. Bambi [Film]; Walt Disney Home Video: Burbank, CA, USA, 1942. [Google Scholar]
- Rottenberg, J.; Ray, R.D.; Gross, J.J. Emotion elicitation using films. In Handbook of Emotion Elicitation and Assessment; Coan, J.A., Allen, J.J.B., Eds.; Oxford University Press: New York, NY, USA, 2007; pp. 9–28. [Google Scholar]
- Bishop, S.; Dalgleish, T.; Yule, W. Memory for emotional stories in high and low depressed children. Memory 2004, 12, 214–230. [Google Scholar] [CrossRef]
- Lenth, R. Emmeans: Estimated Marginal Means, aka Least-Squares Means, R package Version 1.1. 2018. Available online: https://cran.r-project.org/package=emmeans (accessed on 26 August 2025).
- Williams, J.M.G.; Watts, F.N.; MacLeod, C.; Mathews, A. Cognitive Psychology and Emotional Disorders; Wiley: Chichester, UK, 1997; Volume 2. [Google Scholar]
- Armstrong, T.; Olatunji, B.O. Eye tracking of attention in the affective disorders: A meta-analytic review and synthesis. Clin. Psychol. Rev. 2012, 32, 704–723. [Google Scholar] [CrossRef]
- Gotlib, I.H.; Krasnoperova, E.; Yue, D.N.; Joormann, J. Attentional biases for negative interpersonal stimuli in clinical depression. J. Abnorm. Psychol. 2004, 113, 121–135. [Google Scholar] [CrossRef]
- Shane, M.S.; Peterson, J.B. An evaluation of early and late stage attentional processing of positive and negative information in dysphoria. Cogn. Emot. 2007, 21, 789–815. [Google Scholar] [CrossRef]
- Matt, G.E.; Vázquez, C.; Campbell, W.K. Mood-congruent recall of affectively toned stimuli: A meta-analytic review. Clin. Psychol. Rev. 1992, 12, 227–255. [Google Scholar] [CrossRef]
- Werner-Seidler, A.; Moulds, M.L. Autobiographical memory characteristics in depression vulnerability: Formerly depressed individuals recall less vivid positive memories. Cogn. Emot. 2011, 25, 1087–1103. [Google Scholar] [CrossRef] [PubMed]
- Werner-Seidler, A.; Moulds, M.L. Characteristics of self-defining memory in depression vulnerability. Memory 2012, 20, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Begovic, E.; Panaite, V.; Bylsma, L.M.; George, C.; Kovacs, M.; Yaroslavsky, I.; Baji, I.; Benák, I.; Dochnal, R.; Kiss, E.; et al. Positive autobiographical memory deficits in youth with depression histories and their never-depressed siblings. Br. J. Clin. Psychol. 2017, 56, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Joormann, J.; Siemer, M. Memory accessibility, mood regulation, and dysphoria: Difficulties in repairing sad mood with happy memories? J. Abnorm. Psychol. 2004, 113, 179–188. [Google Scholar] [CrossRef]
- Rusting, C.L.; DeHart, T. Retrieving positive memories to regulate negative mood: Consequences for mood-congruent memory. J. Personal. Soc. Psychol. 2000, 78, 737–752. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miron, S.; Keha, E.; Kalanthroff, E. The Positive Effect of Negative Stimuli: Exposure to Negative Emotional Stimuli Improves Mood in Individuals with Major Depressive Disorder. J. Clin. Med. 2025, 14, 6189. https://doi.org/10.3390/jcm14176189
Miron S, Keha E, Kalanthroff E. The Positive Effect of Negative Stimuli: Exposure to Negative Emotional Stimuli Improves Mood in Individuals with Major Depressive Disorder. Journal of Clinical Medicine. 2025; 14(17):6189. https://doi.org/10.3390/jcm14176189
Chicago/Turabian StyleMiron, Sapir, Eldad Keha, and Eyal Kalanthroff. 2025. "The Positive Effect of Negative Stimuli: Exposure to Negative Emotional Stimuli Improves Mood in Individuals with Major Depressive Disorder" Journal of Clinical Medicine 14, no. 17: 6189. https://doi.org/10.3390/jcm14176189
APA StyleMiron, S., Keha, E., & Kalanthroff, E. (2025). The Positive Effect of Negative Stimuli: Exposure to Negative Emotional Stimuli Improves Mood in Individuals with Major Depressive Disorder. Journal of Clinical Medicine, 14(17), 6189. https://doi.org/10.3390/jcm14176189






