The Role of Imaging Modalities in Estimating Myocardial Viability: A Narrative Review
Abstract
1. Introduction
1.1. Viable Myocardium: Myocardial Stunning and Myocardial Hibernation
1.2. Imaging Techniques for Myocardial Viability
2. Echocardiography
2.1. Dobutamine Stress Echocardiography
2.2. Contrast Enhanced Echocardiography (CEE)
2.3. Myocardial Strain Imaging with Speckle Tracking Echocardiography
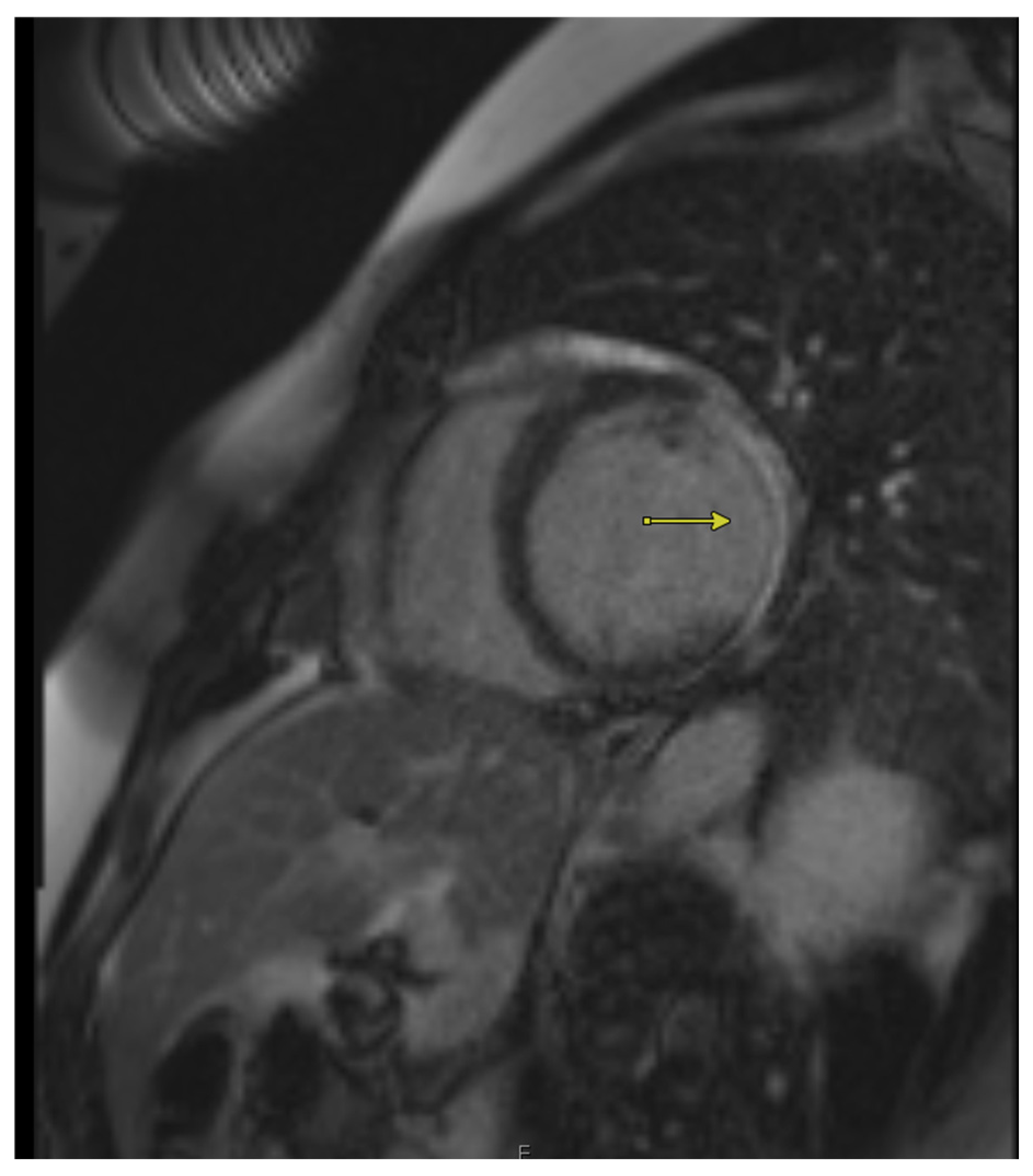
3. Cardiac Magnetic Resonance Imaging (CMR)
3.1. Quantification of Transmural Infarction to Determine Viability
3.2. Role of CMR in Quantifying Wall Thickness to Assess Viability
3.3. Advancements in AI-Enhanced MRI for Myocardial Viability Assessment
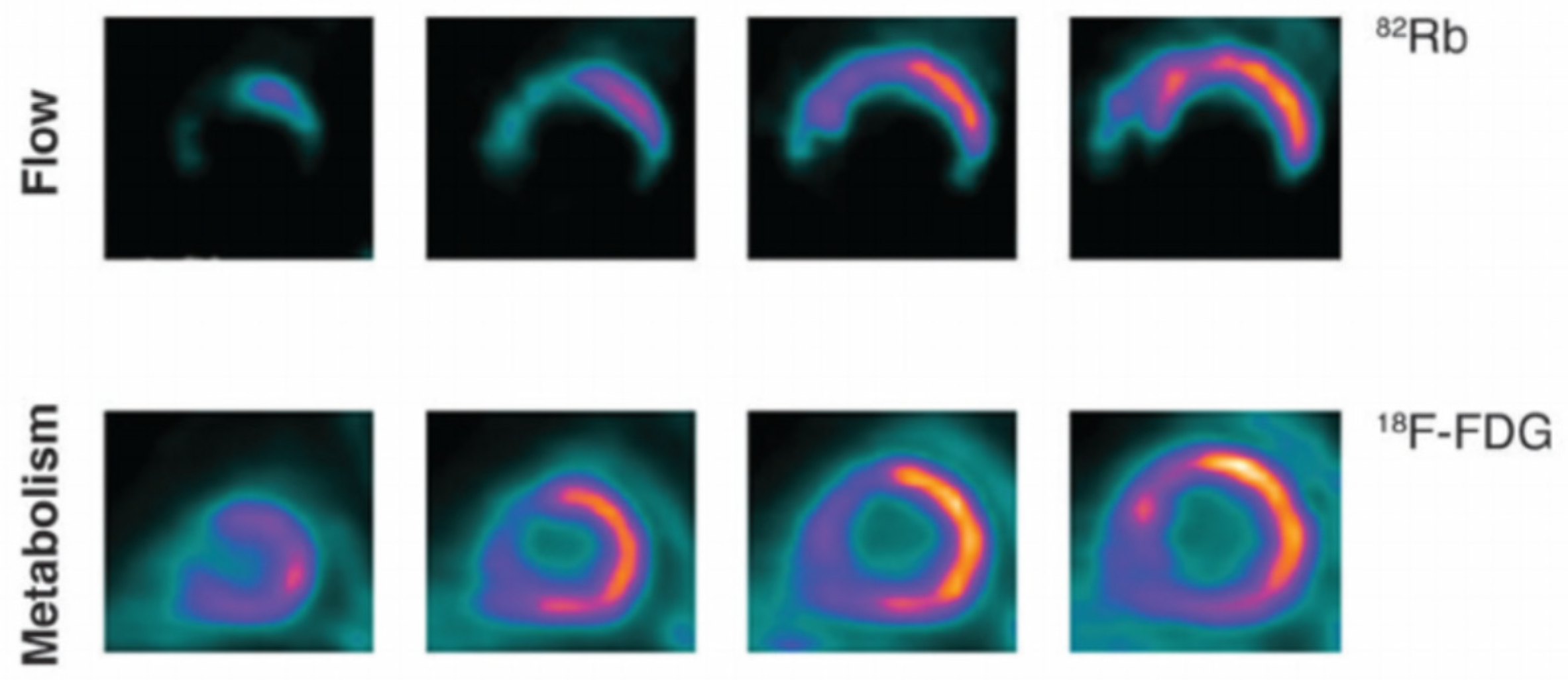
4. Positron Emission Tomography (PET Scan)
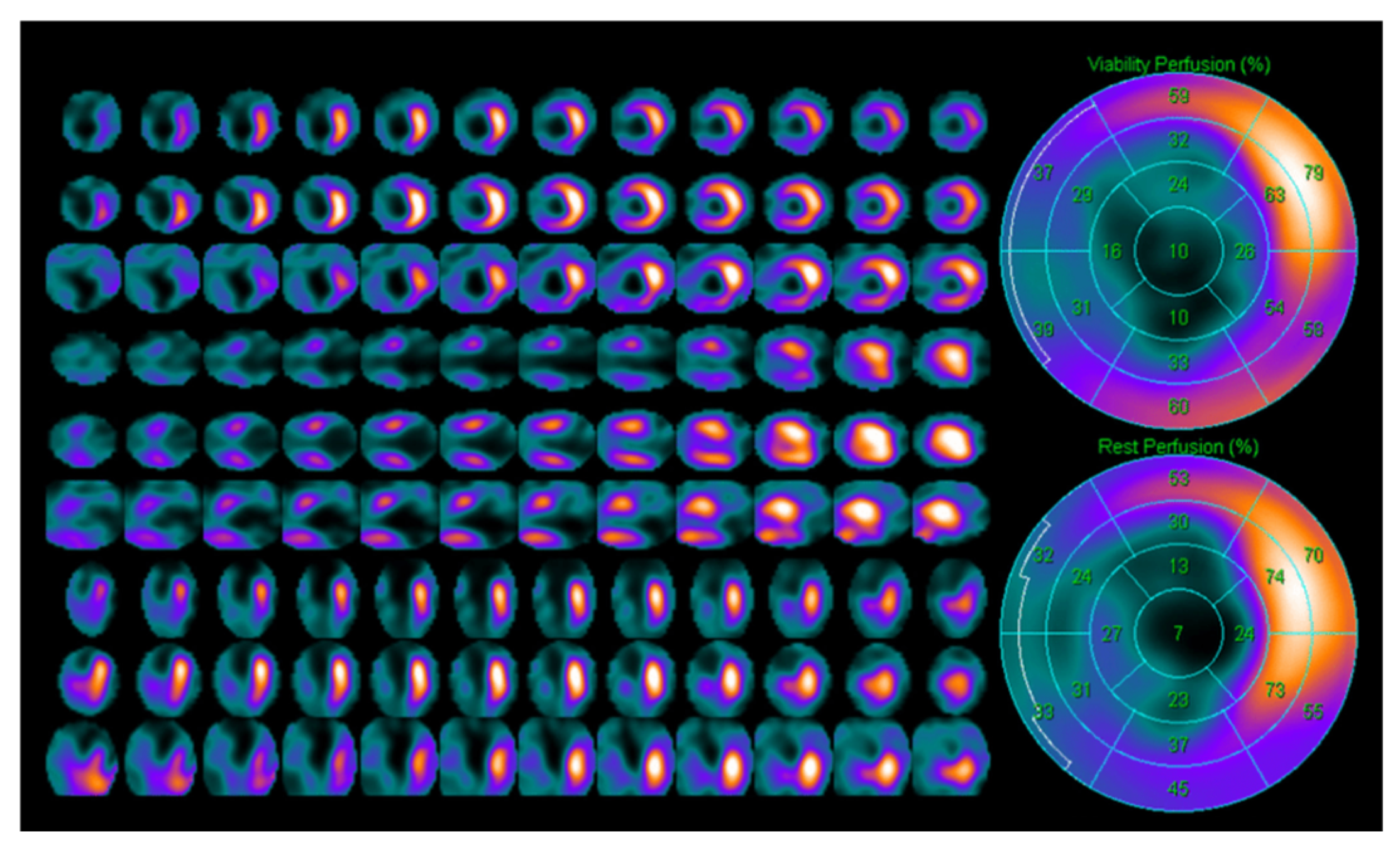
5. Single Photon Emission Computed Tomography (SPECT)
6. Computer Tomography Scan (CT SCAN)
7. Discussion
8. Future Directions
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Safiri, S.; Karamzad, N.; Singh, K.; Carson-Chahhoud, K.; Adams, C.; Nejadghaderi, S.A.; Almasi-Hashiani, A.; Sullman, M.J.M.; Mansournia, M.A.; Bragazzi, N.L.; et al. Burden of ischemic heart disease and its attributable risk factors in 204 countries and territories, 1990–2019. Eur. J. Prev. Cardiol. 2022, 29, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E.; Kloner, R.A. The stunned myocardium: Prolonged, postischemic ventricular dysfunction. Circulation 1982, 66, 1146–1149. [Google Scholar] [CrossRef]
- Kloner, R.A.; DeBoer, L.W.; Darsee, J.R.; Ingwall, J.S.; Braunwald, E. Recovery from prolonged abnormalities of canine myocardium salvaged from ischemic necrosis by coronary reperfusion. Proc. Natl. Acad. Sci. USA 1981, 78, 7152–7156. [Google Scholar] [CrossRef]
- Guaricci, A.I.; Bulzis, G.; Pontone, G.; Scicchitanoet, P.; Carbonara, R.; Rabbat, M.; De Santis, D.; Ciccone, M.M. Current interpretation of myocardial stunning. Trends Cardiovasc. Med. 2018, 28, 263–271. [Google Scholar] [CrossRef]
- Leung, J.M. Clinical Evidence of Myocardial Stunning in Patients Undergoing CABG Surgery. J. Card. Surg. 1993, 8, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Rahimtoola, S.H. The hibernating myocardium. Am. Heart J. 1989, 117, 211–221. [Google Scholar] [CrossRef]
- Ryan, M.J.; Perera, D. Identifying and Managing Hibernating Myocardium: What’s New and What Remains Unknown? Curr. Heart Fail. Rep. 2018, 15, 214. [Google Scholar] [CrossRef]
- Tarakji, K.G.; Brunken, R.; McCarthy, P.M.; Al-Chekakie, M.O.; Abdel-Latif, A.; Pothier, C.E.; Blackstone, E.H.; Lauer, M.S. Myocardial viability testing and the effect of early intervention in patients with advanced left ventricular systolic dysfunction. Circulation 2006, 113, 230–237. [Google Scholar] [CrossRef]
- Hernandez-Pampaloni, M.; Peral, V.; Carreras, J.L.; Sanchez-Harguindey, L.; Vilacosta, I. Biphasic response to dobutamine predicts improvement of left ventricular dysfunction after revascularization: Correlation with positron emission and rest-redistribution 201Tl tomographies. Int. J. Cardiovasc. Imaging 2003, 19, 519–528. [Google Scholar] [CrossRef]
- Cornel, J.H.; Bax, J.J.; Maat, A.P.; Kimman, G.J.; Geleijnse, M.L.; Rambaldi, R.; Boersma, E.; Fioretti, P.M. Biphasic Response to Dobutamine Predicts Improvement of Global Left Ventricular Function After Surgical Revascularization in Patients With Stable Coronary Artery Disease: Implications of Time Course of Recovery on Diagnostic Accuracy. J. Am. Coll. Cardiol. 1998, 31, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Gilstrap, L.G.; Bhatia, R.S.; Weiner, R.B.; Dudzinski, D.M. Dobutamine stress echocardiography: A review and update. Res. Rep. Clin. Cardiol. 2014, 5, 69–81. [Google Scholar] [CrossRef][Green Version]
- Bigi, R. Complications of pharmacologic stress echocardiography in coronary artery disease. Clin. Cardiol. 1996, 19, 776–780. [Google Scholar] [CrossRef]
- Porter, T.R.; Mulvagh, S.L.; Abdelmoneim, S.S.; Becher, H.; Belcik, J.T.; Bierig, M.; Choy, J.; Gaibazzi, N.; Gillam, L.D.; Janardhanan, R.; et al. Clinical Applications of Ultrasonic Enhancing Agents in Echocardiography: 2018 American Society of Echocardiography Guidelines Update. J. Am. Soc. Echocardiogr. 2018, 31, 241–274. [Google Scholar] [CrossRef]
- Ohmori, K.; Cotter, B.; Leistad, E.; Bhargava, V.; Wolf, P.L.; Mizushige, K.; DeMaria, A.N. Assessment of myocardial postreperfusion viability by intravenous myocardial contrast echocardiography: Analysis of the intensity and texture of opacification. Circulation 2001, 103, 2021–2027. [Google Scholar] [CrossRef]
- Funaro, S.; La Torre, G.; Madonna, M.; Galiuto, L.; Scarà, A.; Labbadia, A.; Canali, E.; Mattatelli, A.; Fedele, F.; Alessandrini, F.; et al. Incidence, determinants, and prognostic value of reverse left ventricular remodelling after primary percutaneous coronary intervention: Results of the Acute Myocardial Infarction Contrast Imaging (AMICI) multicenter study. Eur. Heart J. 2009, 30, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Mollema, S.A.; Delgado, V.; Bertini, M.; Antoni, M.L.; Boersma, E.; Holman, E.R.; Stokkel, M.P.; van der Wall, E.E.; Schalij, M.J.; Bax, J.J. Viability assessment with global left ventricular longitudinal strain predicts recovery of left ventricular function after acute myocardial infarction. Circ. Cardiovasc. Imaging 2010, 3, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Hanekom, L.; Jenkins, C.; Jeffries, L.; Case, C.; Mundy, J.; Hawley, C.; Marwick, T.H. Incremental value of strain rate analysis as an adjunct to wall-motion scoring for assessment of myocardial viability by dobutamine echocardiography: A follow-up study after revascularization. Circulation 2005, 112, 3892–3900. [Google Scholar] [CrossRef] [PubMed]
- Weinsaft, J.W.; Klem, I.; Judd, R.M. MRI for the Assessment of Myocardial Viability. Cardiol. Clin. 2007, 25, 35–56. [Google Scholar] [CrossRef]
- Choi, I.Y.; Kim, H.W.; Gim, D.H.; Ki, Y.J.; Kim, H.K.; Kim, H.K.; Park, K.H.; Song, H.; Choi, D.H. Long-Term Prognostic Value of Infarct Transmurality Determined by Contrast-Enhanced Cardiac Magnetic Resonance after ST-Segment Elevation Myocardial Infarction. Chonnam Med. J. 2024, 60, 120–128. [Google Scholar] [CrossRef]
- Shah, A.H.; Olivero, J.J. Gadolinium-Induced Nephrogenic Systemic Fibrosis. Methodist Debakey Cardiovasc. J. 2017, 13, 172. [Google Scholar] [CrossRef]
- GhaffariJolfayi, A.; Salmanipour, A.; Heshmat-Ghahdarijani, K.; MozafaryBazargany, M.; Azimi, A.; Pirouzi, P.; Mohammadzadeh, A. Machine learning-based interpretation of non-contrast feature tracking strain analysis and T1/T2 mapping for assessing myocardial viability. Sci. Rep. 2025, 15, 753. [Google Scholar] [CrossRef]
- Dilsizian, V.; Bacharach, S.L.; Beanlands, R.S.; Bergmann, S.R.; Delbeke, D.; Dorbala, S.; Gropler, R.J.; Knuuti, J.; Schelbert, H.R.; Travin, M.I. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J. Nucl. Cardiol. 2016, 23, 1187–1226. [Google Scholar] [CrossRef]
- Tillisch, J.; Brunken, R.; Marshall, R.; Schwaiger, M.; Mandelkern, M.; Phelps, M.; Schelbert, H. Reversibility of cardiac wall-motion abnormalities predicted by positron tomography. N. Engl. J. Med. 1986, 314, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Mhlanga, J.; Derenoncourt, P.; Haq, A.; Bhandiwad, A.; Laforest, R.; Siegel, B.A.; Dehdashti, F.; Gropler, R.J.; Schindler, T.H. 18F-FDG PET in Myocardial Viability Assessment: A Practical and Time-Efficient Protocol. J. Nucl. Med. 2021, 63, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Osterholt, M.; Sen, S.; Dilsizian, V.; Taegtmeyer, H. Targeted Metabolic Imaging to Improve the Management of Heart Disease. JACC Cardiovasc. Imaging 2012, 5, 214. [Google Scholar] [CrossRef]
- Kauffman, G.J.; Boyne, T.S.; Watson, D.D.; Smith, W.H.; Beller, G.A. Comparison of rest thallium-201 imaging and rest technetium-99m sestamibi imaging for assessment of myocardial viability in patients with coronary artery disease and severe left ventricular dysfunction. J. Am. Coll. Cardiol. 1996, 27, 1592–1597. [Google Scholar] [CrossRef]
- Perrone-Filardi, P.; Pace, L.; Prastaro, M.; Squame, F.; Betocchi, S.; Soricelli, A.; Piscione, F.; Indolfi, C.; Crisci, T.; Salvatore, M.; et al. Assessment of myocardial viability in patients with chronic coronary artery disease: Rest-4-hour-24-hour 201Tl tomography versus dobutamine echocardiography. Circulation 1996, 94, 2712–2719. [Google Scholar] [CrossRef]
- Bisi, G.; Sciagrà, R.; Santoro, G.M.; Fazzini, P.F. Rest technetium-99m sestamibi tomography in combination with short-term administration of nitrates: Feasibility and reliability for prediction of postrevascularization outcome of asynergic territories. J. Am. Coll. Cardiol. 1994, 24, 1282–1289. [Google Scholar] [CrossRef]
- Santoyo-Saavedra, A.H.; Espinola-Zavaleta, N.; Bermudez-Gonzalez, J.L.; Gonzalez-Hernandez, M.Á.; García-Cárdenas, M.; Canseco-León, N.; Luna-Álvarez-Amezquita, J.A.; Straface, J.I.; Perez-Partida, A.M.; Alexanderson-Rosas, E. The importance of time for the evaluation of viability by SPECT. J. Nucl. Cardiol. 2023, 30, 1272–1276. [Google Scholar] [CrossRef] [PubMed]
- Brodoefel, H.; Reimann, A.; Klumpp, B.; Fenchel, M.; Ohmer, M.; Miller, S.; Schroeder, S.; Claussen, C.; Scheule, A.; Kopp, A.F. Assessment of myocardial viability in a reperfused porcine model: Evaluation of different MSCT contrast protocols in acute and subacute infarct stages in comparison with MRI. J. Comput. Assist. Tomogr. 2007, 31, 290–298. [Google Scholar] [CrossRef]
- Jacquier, A.; Boussel, L.; Amabile, N.; Bartoli, J.M.; Douek, P.; Moulin, G.; Paganelli, F.; Saeed, M.; Revel, D.; Croisille, P. Multidetector computed tomography in reperfused acute myocardial infarction. Assessment of infarct size and no-reflow in comparison with cardiac magnetic resonance imaging. Investig. Radiol. 2008, 43, 773–781. [Google Scholar] [CrossRef]
- Baks, T.; Cademartiri, F.; Moelker, A.D.; Weustink, A.C.; van Geuns, R.J.; Mollet, N.R.; Krestin, G.P.; Duncker, D.J.; de Feyter, P.J. Multislice computed tomography and magnetic resonance imaging for the assessment of reperfused acute myocardial infarction. J. Am. Coll. Cardiol. 2006, 48, 144–152. [Google Scholar] [CrossRef]
- Mahnken, A.H.; Koos, R.; Katoh, M.; Wildberger, J.E.; Spuentrup, E.; Buecker, A.; Günther, R.W.; Kühl, H.P. Assessment of myocardial viability in reperfused acute myocardial infarction using 16-slice computed tomography in comparison to magnetic resonance imaging. J. Am. Coll. Cardiol. 2005, 45, 2042–2047. [Google Scholar] [CrossRef]
- Zdanowicz, A.; Guzinski, M.; Pula, M.; Witkowska, A.; Reczuch, K. Dynamic CT Myocardial Perfusion: The Role of Functional Evaluation in the Diagnosis of Coronary Artery Disease. J. Clin. Med. 2023, 12, 7062. [Google Scholar] [CrossRef] [PubMed]
- Nieman, K.; Balla, S. Dynamic CT myocardial perfusion imaging. J. Cardiovasc. Comput. Tomogr. 2020, 14, 303–306. [Google Scholar] [CrossRef]
- Allman, K.C.; Shaw, L.J.; Hachamovitch, R.; Udelson, J.E. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: A meta-analysis. J. Am. Coll. Cardiol. 2002, 39, 1151–1158. [Google Scholar] [CrossRef]
- Velazquez, E.J.; Lee, K.L.; Jones, R.H.; Al-Khalidi, H.R.; Hill, J.A.; Panza, J.A.; Michler, R.E.; Bonow, R.O.; Doenst, T.; Petrie, M.C.; et al. Coronary-Artery Bypass Surgery in Patients with Ischemic Cardiomyopathy. N. Engl. J. Med. 2016, 374, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Panza, J.A.; Ellis, A.M.; Al-Khalidi, H.R.; Holly, T.A.; Berman, D.S.; Oh, J.K.; Pohost, G.M.; Sopko, G.; Chrzanowski, L.; Mark, D.B.; et al. Myocardial Viability and Long-Term Outcomes in Ischemic Cardiomyopathy. N. Engl. J. Med. 2019, 381, 739–748. [Google Scholar] [CrossRef]
- Perera, D.; Clayton, T.; O'Kane, P.D.; Greenwood, J.P.; Weerackody, R.; Ryan, M.; Morgan, H.P.; Dodd, M.; Evans, R.; Canter, R.; et al. Percutaneous Revascularization for Ischemic Left Ventricular Dysfunction. N. Engl. J. Med. 2022, 387, 1351–1360. [Google Scholar] [CrossRef]
- Cleland, J.G.F.; Freemantle, N.; Ball, S.G.; Bonser, R.S.; Camici, P.; Chattopadhyay, S.; Dutka, D.; Eastaugh, J.; Hampton, J.; Large, S.; et al. The heart failure revascularisation trial (HEART): Rationale, design and methodology. Eur. J. Heart Fail. 2003, 5, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Beanlands, R.S.B.; Nichol, G.; Huszti, E.; Humen, D.; Racine, N.; Freeman, M.; Gulenchyn, K.Y.; Garrard, L.; deKemp, R.; Guo, A.; et al. F-18-fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: A randomized, controlled trial (PARR-2). J. Am. Coll. Cardiol. 2007, 50, 2002–2012. [Google Scholar] [CrossRef]
- D’Egidio, G.; Nichol, G.; Williams, K.A.; Guo, A.; Garrard, L.; deKemp, R.; Ruddy, T.D.; DaSilva, J.; Humen, D.; Gulenchyn, K.Y.; et al. Increasing Benefit From Revascularization Is Associated With Increasing Amounts of Myocardial Hibernation: A Substudy of the PARR-2 Trial. JACC Cardiovasc. Imaging 2009, 2, 1060–1068. [Google Scholar] [CrossRef]
- van Loon, R.B.; Veen, G.; Baur, L.H.; Kamp, O.; Bronzwaer, J.G.; Twisk, J.W.; Verheugt, F.W.; van Rossum, A.C. Improved clinical outcome after invasive management of patients with recent myocardial infarction and proven myocardial viability: Primary results of a randomized controlled trial (VIAMI-trial). Trials 2012, 13, 1. [Google Scholar] [CrossRef]
- Pica, S.; Di Giovine, G.; Bollati, M.; Testa, L.; Bedogni, F.; Camporeale, A.; Pontone, G.; Andreini, D.; Monti, L.; Gasparini, G.; et al. Cardiac magnetic resonance for ischaemia and viability detection. Guiding patient selection to revascularization in coronary chronic total occlusions: The CARISMA_CTO study design. Int. J. Cardiol. 2018, 272, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Canton, L.; Suma, N.; Amicone, S.; Impellizzeri, A.; Bodega, F.; Marinelli, V.; Ciarlantini, M.; Casuso, M.; Bavuso, L.; Belà, R.; et al. Clinical impact of multimodality assessment of myocardial viability. Echocardiography 2024, 41, e15854. [Google Scholar] [CrossRef] [PubMed]
- Pagano, D.; Townend, J.N.; Parums, D.V.; Bonser, R.S.; Camici, P.G. Hibernating myocardium: Morphological correlates of inotropic stimulation and glucose uptake. Heart 2000, 83, 456. [Google Scholar] [CrossRef][Green Version]
- Kim, R.J.; Wu, E.; Rafael, A.; Chen, E.L.; Parker, M.A.; Simonetti, O.; Klocke, F.J.; Bonow, R.O.; Judd, R.M. The Use of Contrast-Enhanced Magnetic Resonance Imaging to Identify Reversible Myocardial Dysfunction. N. Engl. J. Med. 2000, 343, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Galli, A.; Lombardi, F. Postinfarct Left Ventricular Remodelling: A Prevailing Cause of Heart Failure. Cardiol. Res. Pract. 2016, 2016, 2579832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Burrage, M.K.; Shanmuganathan, M.; Gonzales, R.A.; Lukaschuk, E.; Thomas, K.E.; Mills, R.; Leal Pelado, J.; Nikolaidou, C.; Popescu, I.A.; et al. Artificial Intelligence for Contrast-Free MRI: Scar Assessment in Myocardial Infarction Using Deep Learning-Based Virtual Native Enhancement. Circulation 2022, 146, 1492–1503. [Google Scholar] [CrossRef]
| Myocardium Type | Low-Dose Dobutamine Response | High-Dose Dobutamine Response | Clinical Interpretation |
|---|---|---|---|
| Normal Myocardium | Increased contractility | No contractility or further increase in contractility | Healthy tissue with adequate contractile reserve |
| Viable Myocardium | Improved contractility | No contractility | Hibernating/stunned myocardium with contractile reserve, potential for recovery post revascularization |
| Non-viable Myocardium | No contractility | No contractility | Necrotic tissue (no contractile reserve) |
| Feature | CMR | PET | SPECT | DSE |
|---|---|---|---|---|
| Mechanism | Scar/fibrosis (LGE), contractile reserve | Metabolism (FDG), perfusion | Perfusion | Contractile reserve |
| Sensitivity | High | Very high | Moderate | Moderate |
| Specificity | High | High | Moderate | Moderate to high |
| Spatial Resolution | Excellent (1–2 mm) | Moderate (4–5 mm) | Low (10–15 mm) | Moderate |
| Radiation | None | Yes | Yes | None |
| Availability | Moderate | Limited | Widely available | Widely available |
| Cost | High | High | Moderate | Low |
| Time | Moderate (~45–60 min) | Long (60–90 min) | Long (~60–120 min for Tc-99m; longer for Thallium-201) | Short (~30 min) |
| Prognostic Value | Strong | Strong | Moderate | Moderate |
| Limitations | Contraindicated in devices, breath-hold needed | Limited access, expensive | Poor resolution, attenuation artifacts | Operator-dependent, suboptimal in poor windows |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modak, V.; Satish, V.; Maliha, M.; Kumar, S.S.; Christia, P. The Role of Imaging Modalities in Estimating Myocardial Viability: A Narrative Review. J. Clin. Med. 2025, 14, 5529. https://doi.org/10.3390/jcm14155529
Modak V, Satish V, Maliha M, Kumar SS, Christia P. The Role of Imaging Modalities in Estimating Myocardial Viability: A Narrative Review. Journal of Clinical Medicine. 2025; 14(15):5529. https://doi.org/10.3390/jcm14155529
Chicago/Turabian StyleModak, Vishakha, Vikyath Satish, Maisha Maliha, Sriram S. Kumar, and Panagiota Christia. 2025. "The Role of Imaging Modalities in Estimating Myocardial Viability: A Narrative Review" Journal of Clinical Medicine 14, no. 15: 5529. https://doi.org/10.3390/jcm14155529
APA StyleModak, V., Satish, V., Maliha, M., Kumar, S. S., & Christia, P. (2025). The Role of Imaging Modalities in Estimating Myocardial Viability: A Narrative Review. Journal of Clinical Medicine, 14(15), 5529. https://doi.org/10.3390/jcm14155529






