Combination Therapy with Trehalose and Hyaluronic Acid Restores Tear Lipid Layer Functionality by Ameliorating Inflammatory Response Protein Markers on the Ocular Surface of Dry Eye Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Clinical Data Analysis
2.4. Tear Sample Collection and Preparation for Proteomics Analysis
2.5. Mass Spectrometry (MS)-Based Clinical Proteomics Analysis
2.6. Label-Free Quantitative Proteomics Analysis
2.7. Functional Annotation and Pathway Analyses
3. Results
3.1. Clinical Investigation on the Effects of Thealoz® Duo Eye Drops on the Ocular Surface
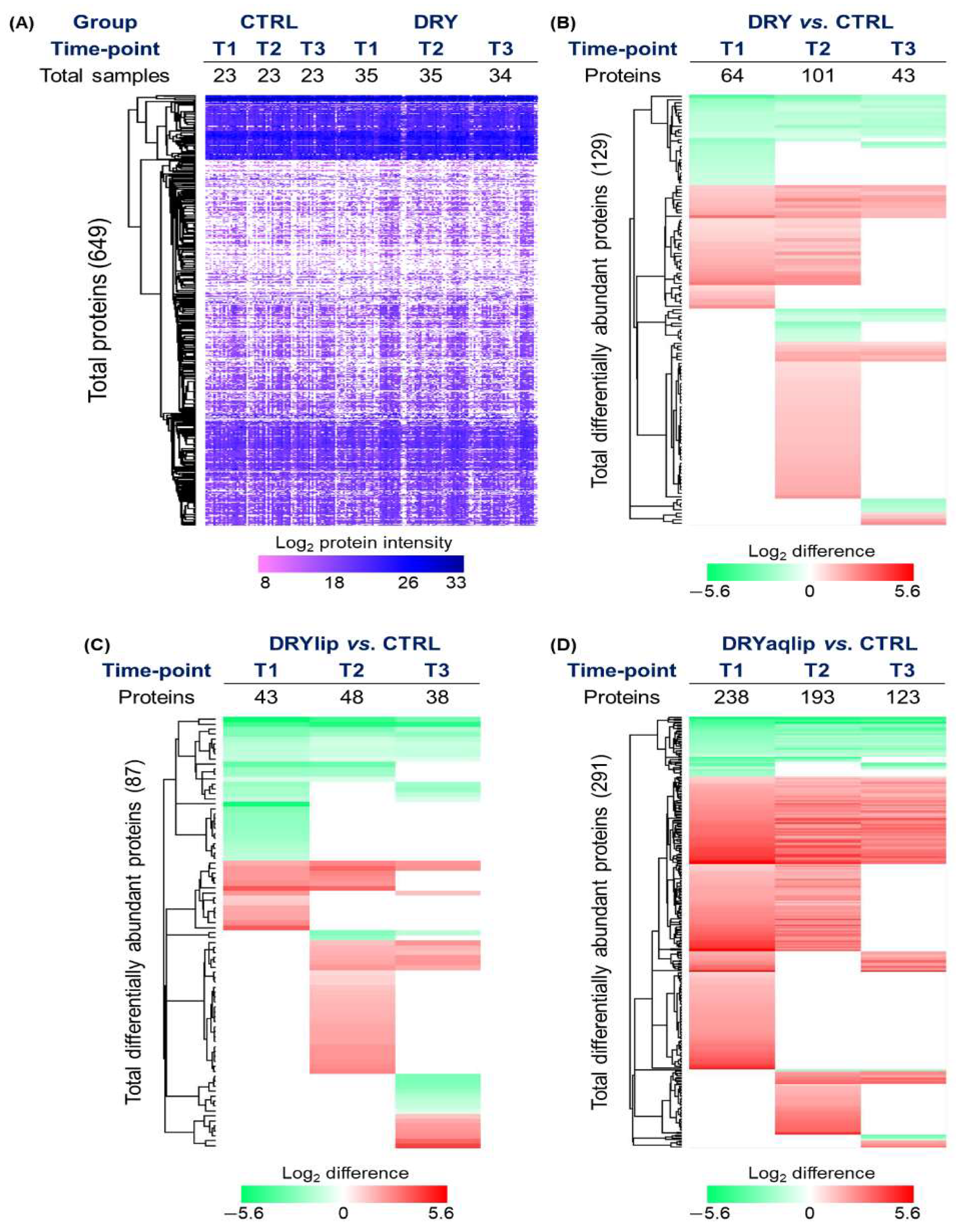
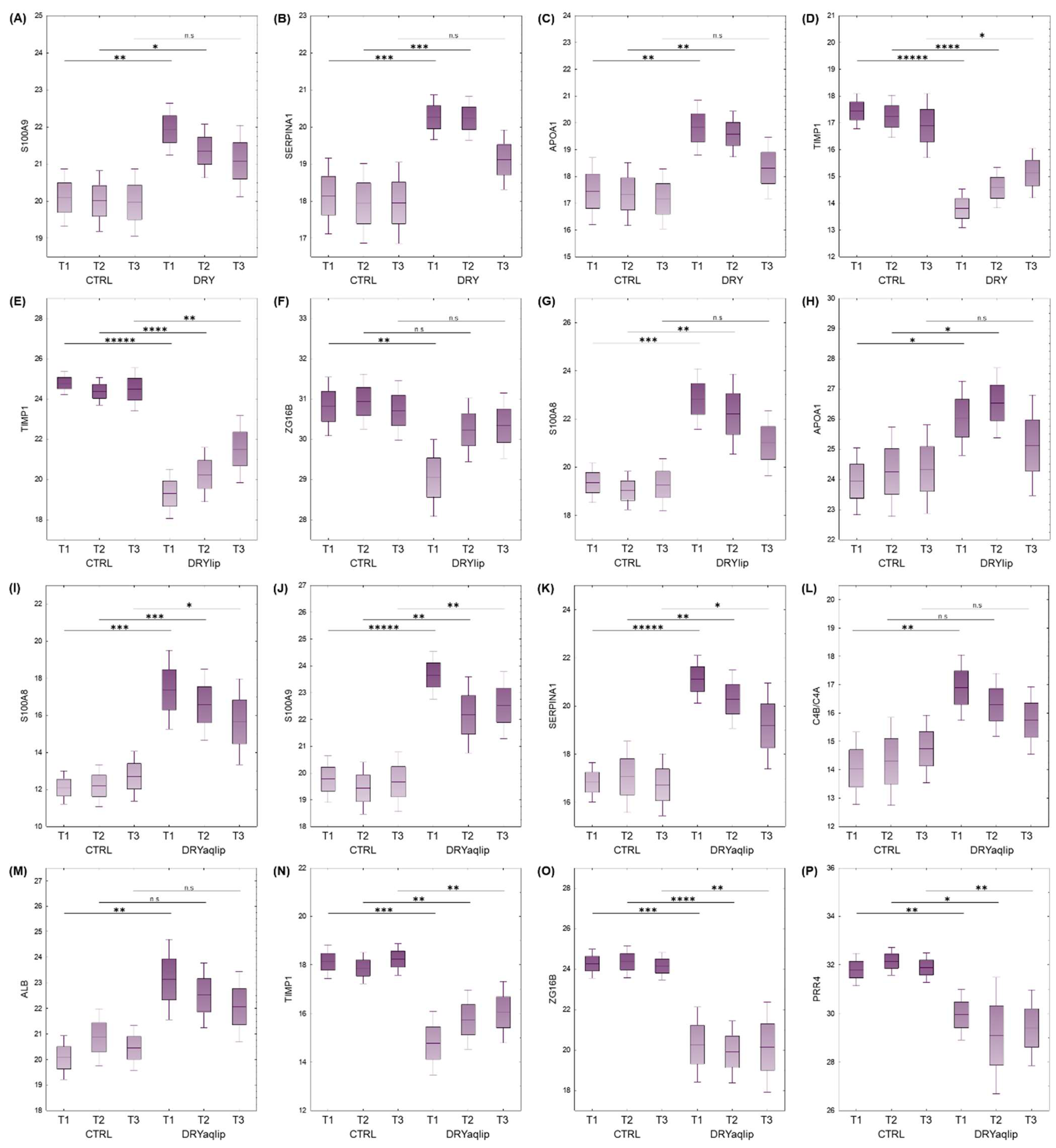
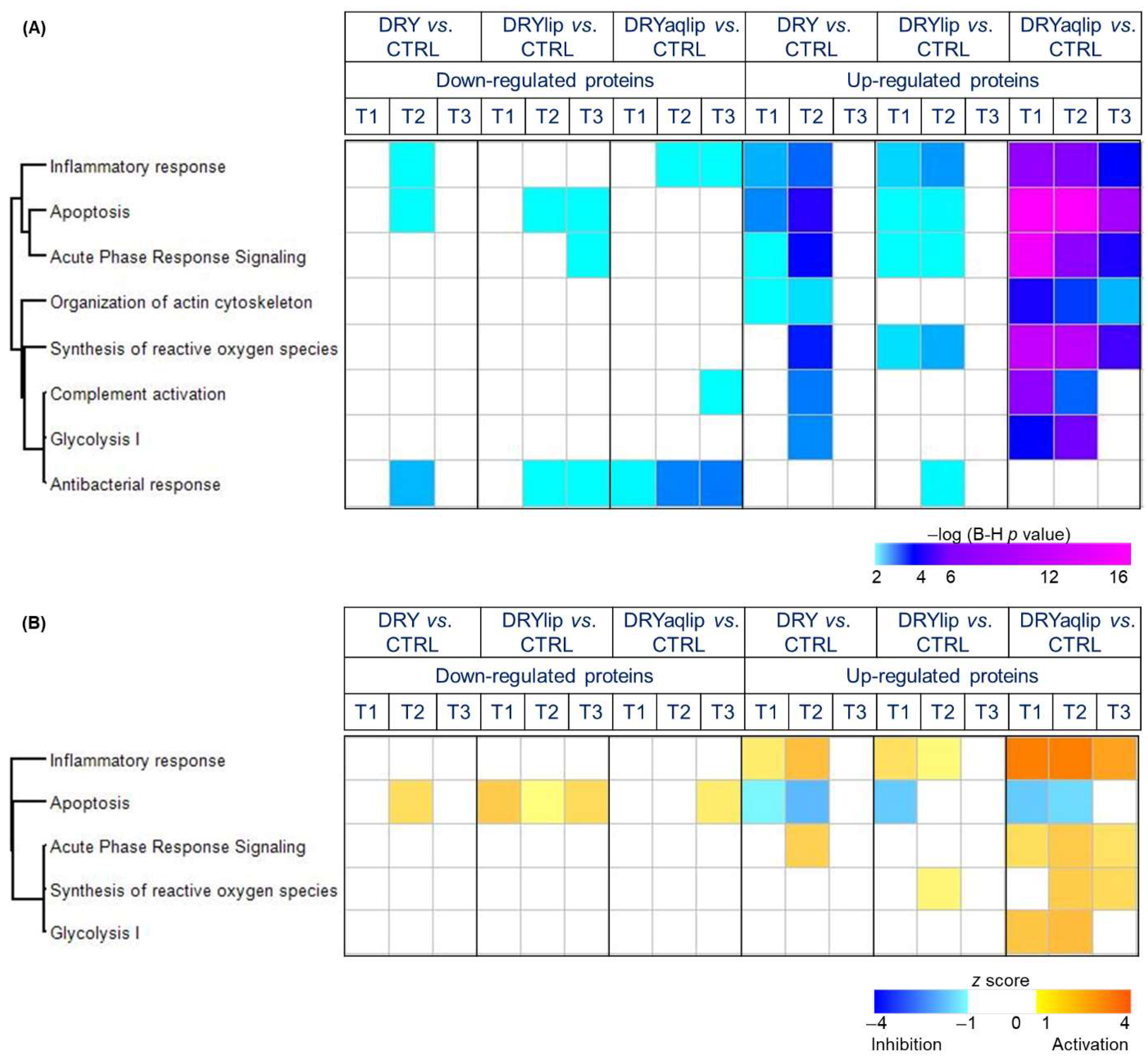
3.2. Proteomics Investigation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Yokoi, N.; Uchino, Y.; Dogru, M.; Kawashima, M.; Komuro, A.; Sonomura, Y.; Kato, H.; Kinoshita, S.; Schaumberg, D.A.; et al. Prevalence of dry eye disease and its risk factors in visual display terminal users: The Osaka study. Am. J. Ophthalmol. 2013, 156, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Kathuria, A.; Shamloo, K.; Jhanji, V.; Sharma, A. Categorization of marketed artificial tear formulations based on their ingredients: A rational approach for their use. J. Clin. Med. 2021, 10, 1289. [Google Scholar] [CrossRef]
- Tong, L.; Petznick, A.; Lee, S.; Tan, J. Choice of artificial tear formulation for patients with dry eye: Where do we start? Cornea 2012, 31, S32–S36. [Google Scholar] [CrossRef]
- Dogru, M.; Tsubota, K. Pharmacotherapy of dry eye. Expert. Opin. Pharmacother. 2011, 12, 325–334. [Google Scholar] [CrossRef]
- Ozek, D.; Kemer, O.E. Effect of the bioprotectant agent trehalose on corneal epithelial healing after corneal cross-linking for keratoconus. Arq. Bras. Oftalmol. 2018, 81, 505–509. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Liu, W.; Xie, L.; Wei, B.; Xu, C.; Xu, Y.; Zheng, M.; Wang, H. Improvement of collagen self-assembly and thermal stability in the presence of trehalose. New J. Chem. 2022, 46, 9264–9271. [Google Scholar] [CrossRef]
- Laihia, J.; Kaarniranta, K. Trehalose for Ocular Surface Health. Biomolecules 2020, 10, 809. [Google Scholar] [CrossRef]
- Hill-Bator, A.; Misiuk-Hojlo, M.; Marycz, K.; Grzesiak, J. Trehalose-based eye drops preserve viability and functionality of cultured human corneal epithelial cells during desiccation. BioMed Res. Int. 2014, 2014, 292139. [Google Scholar] [CrossRef]
- Cejkova, J.; Ardan, T.; Cejka, C.; Luyckx, J. Favorable effects of trehalose on the development of UVB-mediated antioxidant/pro-oxidant imbalance in the corneal epithelium, proinflammatory cytokine and matrix metalloproteinase induction, and heat shock protein 70 expression. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 1185–1194. [Google Scholar] [CrossRef]
- Ohtake, S.; Wang, Y.J. Trehalose: Current use and future applications. J. Pharm. Sci. 2011, 100, 2020–2053. [Google Scholar] [CrossRef]
- Doan, S.; Bremond-Gignac, D.; Chiambaretta, F. Comparison of the effect of a hyaluronate-trehalose solution to hyaluronate alone on Ocular Surface Disease Index in patients with moderate to severe dry eye disease. Curr. Med. Res. Opin. 2018, 34, 1373–1376. [Google Scholar] [CrossRef]
- Mateo-Orobia, A.J.; Martínez, A.B.; Sanz, E.D.P.; Julvez, L.P.; Llorens, V.P.; Ojeda, N.L.; Pina, D.S.; Corta, M.I. Efficacy of Artificial Tears Containing Trehalose and Hyaluronic Acid (Thealoz Duo®) for Dry Eye Disease (DED in Peri-and Postmenopausal Women. Investig. Ophthalmol. Vis. Sci. 2021, 62, 1229. [Google Scholar]
- Schmidl, D.; Schmetterer, L.; Witkowska, K.J.; Unterhuber, A.; dos Santos, V.A.; Kaya, S.; Nepp, J.; Baar, C.; Rosner, P.; Werkmeister, R.M.; et al. Tear film thickness after treatment with artificial tears in patients with moderate dry eye disease. Cornea 2015, 34, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Fariselli, C.; Giannaccare, G.; Fresina, M.; Versura, P. Trehalose/hyaluronate eyedrop effects on ocular surface inflammatory markers and mucin expression in dry eye patients. Clin. Ophthalmol. 2018, 12, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Troisi, M.; Del Prete, S.; Troisi, S.; Marasco, D.; Rinaldi, M.; Costagliola, C. Scanning Electron Microscopy (SEM) Evaluation of the Ultrastructural Effects on Conjunctival Epithelial Cells of a New Multiple-Action Artificial Tear Containing Cross-Linked Hyaluronic Acid, Cationic Liposomes and Trehalose. Biomedicines 2024, 12, 1945. [Google Scholar] [CrossRef] [PubMed]
- Perumal, N.; Funke, S.; Pfeiffer, N.; Grus, F.H. Proteomics analysis of human tears from aqueous-deficient and evaporative dry eye patients. Sci. Rep. 2016, 6, 29629. [Google Scholar] [CrossRef]
- Manicam, C.; Perumal, N.; Wasielica-Poslednik, J.; Ngongkole, Y.C.; Tschäbunin, A.; Sievers, M.; Lisch, W.; Pfeiffer, N.; Grus, F.H.; Gericke, A. Proteomics unravels the regulatory mechanisms in human tears following acute renouncement of contact lens use: A comparison between hard and soft lenses. Sci. Rep. 2018, 8, 11526. [Google Scholar] [CrossRef]
- Pham, T.N.M.; Perumal, N.; Manicam, C.; Basoglu, M.; Eimer, S.; Fuhrmann, D.C.; Pietrzik, C.U.; Clement, A.M.; Körschgen, H.; Schepers, J.; et al. Adaptive responses of neuronal cells to chronic endoplasmic reticulum (ER) stress. Redox Biol. 2023, 67, 102943. [Google Scholar] [CrossRef]
- Perumal, N.; Herfurth, A.; Pfeiffer, N.; Manicam, C. Short-Term Omega-3 Supplementation Modulates Novel Neurovascular and Fatty Acid Metabolic Proteome Changes in the Retina and Ophthalmic Artery of Mice with Targeted Cyp2c44 Gene Deletion. Cells 2022, 11, 3494. [Google Scholar] [CrossRef]
- Perumal, N.; Yurugi, H.; Dahm, K.; Rajalingam, K.; Grus, F.H.; Pfeiffer, N.; Manicam, C. Proteome landscape and interactome of voltage-gated potassium channel 1.6 (Kv1.6) of the murine ophthalmic artery and neuroretina. Int. J. Biol. Macromol. 2024, 257, 128464. [Google Scholar] [CrossRef]
- Grandjean, T.; Perumal, N.; Manicam, C.; Matthey, B.; Wu, T.; Thiem, D.G.; Stein, S.; Henrich, D.; Kämmerer, P.W.; Al-Nawas, B. Towards optimized tissue regeneration: A new 3D printable bioink of alginate/cellulose hydrogel loaded with thrombocyte concentrate. Front. Bioeng. Biotechnol. 2024, 12, 1363380. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Luber, C.A.; Cox, J.; Lauterbach, H.; Fancke, B.; Selbach, M.; Tschopp, J.; Akira, S.; Wiegand, M.; Hochrein, H.; O’Keeffe, M.; et al. Quantitative proteomics reveals subset-specific viral recognition in dendritic cells. Immunity 2010, 32, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Hein, M.Y.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteom. 2014, 13, 2513–2526. [Google Scholar] [CrossRef]
- Cox, J.; Neuhauser, N.; Michalski, A.; Scheltema, R.A.; Olsen, J.V.; Mann, M. Andromeda: A peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 2011, 10, 1794–1805. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef]
- Perumal, N.; Funke, S.; Pfeiffer, N.; Grus, F.H. Characterization of lacrimal proline-rich protein 4 (PRR4) in human tear proteome. Proteomics 2014, 14, 1698–1709. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote) omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 2013, 30, 523–530. [Google Scholar] [CrossRef]
- Pinto-Bonilla, J.C.; del Olmo-Jimeno, A.; Llovet-Osuna, F.; Hernández-Galilea, E. A randomized crossover study comparing trehalose/hyaluronate eyedrops and standard treatment: Patient satisfaction in the treatment of dry eye syndrome. Ther. Clin. Risk Manag. 2015, 11, 595–603. [Google Scholar] [CrossRef]
- Chiambaretta, F.; Doan, S.; Labetoulle, M.; Rocher, N.; Fekih, L.E.; Messaoud, R.; Khairallah, M.; Baudouin, C.; HA-trehalose Study Group. A randomized, controlled study of the efficacy and safety of a new eyedrop formulation for moderate to severe dry eye syndrome. Eur. J. Ophthalmol. 2017, 27, 1–9. [Google Scholar] [CrossRef]
- Tsubota, K. Short tear film breakup time–type dry eye. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES64–DES70. [Google Scholar] [CrossRef] [PubMed]
- Rolando, M.; Merayo-Lloves, J. Management Strategies for Evaporative Dry Eye Disease and Future Perspective. Curr. Eye Res. 2022, 47, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef] [PubMed]
- Willcox, M.D.P.; Argueso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef]
- Grus, F.H.; Podust, V.N.; Bruns, K.; Lackner, K.; Fu, S.; Dalmasso, E.A.; Wirthlin, A.; Pfeiffer, N. SELDI-TOF-MS ProteinChip array profiling of tears from patients with dry eye. Investig. Ophthalmol. Vis. Sci. 2005, 46, 863–876. [Google Scholar] [CrossRef]
- Zhou, L.; Beuerman, R.W.; Chan, C.M.; Zhao, S.Z.; Li, X.R.; Yang, H.; Tong, L.; Liu, S.; Stern, M.E.; Tan, D. Identification of tear fluid biomarkers in dry eye syndrome using iTRAQ quantitative proteomics. J. Proteome Res. 2009, 8, 4889–4905. [Google Scholar] [CrossRef]
- Boehm, N.; Funke, S.; Wiegand, M.; Wehrwein, N.; Pfeiffer, N.; Grus, F.H. Alterations in the tear proteome of dry-eye patients—A matter of the clinical phenotype. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2385–2392. [Google Scholar] [CrossRef]
- Soria, J.; Duran, J.A.; Etxebarria, J.; Merayo, J.; Gonzalez, N.; Reigada, R.; Garcia, I.; Acera, A.; Suarez, T. Tear proteome and protein network analyses reveal a novel pentamarker panel for tear film characterization in dry eye and meibomian gland dysfunction. J. Proteom. 2013, 78, 94–112. [Google Scholar] [CrossRef]
- Li, B.; Sheng, M.; Li, J.; Yan, G.; Lin, A.; Li, M.; Wang, W.; Chen, Y. Tear proteomic analysis of Sjögren syndrome patients with dry eye syndrome by two-dimensional-nano-liquid chromatography coupled with tandem mass spectrometry. Sci. Rep. 2014, 4, 5772. [Google Scholar] [CrossRef]
- Kushner, I. Regulation of the acute phase response by cytokines. Perspect. Biol. Med. 1993, 36, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Markiewski, M.M.; Lambris, J.D. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am. J. Pathol. 2007, 171, 715–727. [Google Scholar] [CrossRef]
- Ivanov, A.I.; Parkos, C.A.; Nusrat, A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am. J. Pathol. 2010, 177, 512–524. [Google Scholar] [CrossRef]
- Werner, T.; Haller, D. Intestinal epithelial cell signalling and chronic inflammation: From the proteome to specific molecular mechanisms. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2007, 622, 42–57. [Google Scholar] [CrossRef]
- Chen, D.-B.; Zhao, Y.-J.; Wang, X.-Y.; Liao, W.-J.; Chen, P.; Deng, K.-J.; Cong, X.; Fei, R.; Wu, X.; Shao, Q.-X. Regulatory factor X5 promotes hepatocellular carcinoma progression by transactivating tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein theta and suppressing apoptosis. Chin. Med. J. 2019, 132, 1572–1581. [Google Scholar] [CrossRef]
- Kim, J.W.; Dang, C.V. Multifaceted roles of glycolytic enzymes. Trends Biochem. Sci. 2005, 30, 142–150. [Google Scholar] [CrossRef]
- Nichols, J.J.; Green-Church, K.B. Mass spectrometry-based proteomic analyses in contact lens-related dry eye. Cornea 2009, 28, 1109–1117. [Google Scholar] [CrossRef]
- Srinivasan, S.; Thangavelu, M.; Zhang, L.; Green, K.B.; Nichols, K.K. iTRAQ quantitative proteomics in the analysis of tears in dry eye patients. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5052–5059. [Google Scholar] [CrossRef]
- Aluru, S.V.; Agarwal, S.; Srinivasan, B.; Iyer, G.K.; Rajappa, S.M.; Tatu, U.; Padmanabhan, P.; Subramanian, N.; Narayanasamy, A. Lacrimal Proline Rich 4 (LPRR4) Protein in the Tear Fluid Is a Potential Biomarker of Dry Eye Syndrome. PLoS ONE 2012, 7, e51979. [Google Scholar] [CrossRef] [PubMed]
- Saijyothi, A.V.; Angayarkanni, N.; Syama, C.; Utpal, T.; Shweta, A.; Bhaskar, S.; Geetha, I.K.; Vinay, P.S.; Thennarasu, M.; Sivakumar, R.M.; et al. Two dimensional electrophoretic analysis of human tears: Collection method in dry eye syndrome. Electrophoresis 2010, 31, 3420–3427. [Google Scholar] [CrossRef]
- Karnati, R.; Laurie, D.E.; Laurie, G.W. Lacritin and the tear proteome as natural replacement therapy for dry eye. Exp. Eye Res. 2013, 117, 39–52. [Google Scholar] [CrossRef] [PubMed]
- McKown, R.L.; Wang, N.; Raab, R.W.; Karnati, R.; Zhang, Y.; Williams, P.B.; Laurie, G.W. Lacritin and other new proteins of the lacrimal functional unit. Exp. Eye Res. 2009, 88, 848–858. [Google Scholar] [CrossRef][Green Version]
- Soto-Heredero, G.; Gomez de las Heras, M.M.; Gabandé-Rodríguez, E.; Oller, J.; Mittelbrunn, M. Glycolysis–A key player in the inflammatory response. FEBS J. 2020, 287, 3350–3369. [Google Scholar] [CrossRef]
- Brandes, R.P.; Rezende, F. Glycolysis and Inflammation: Partners in Crime! Circ. Res. 2021, 129, 30–32. [Google Scholar] [CrossRef]
- Veras, F.P.; Peres, R.S.; Saraiva, A.L.; Pinto, L.G.; Louzada-Junior, P.; Cunha, T.M.; Paschoal, J.A.; Cunha, F.Q.; Alves-Filho, J.C. Fructose 1, 6-bisphosphate, a high-energy intermediate of glycolysis, attenuates experimental arthritis by activating anti-inflammatory adenosinergic pathway. Sci. Rep. 2015, 5, 15171. [Google Scholar] [CrossRef]
- Gomez, D.; Alonso, D.; Yoshiji, H.; Thorgeirsson, U. Tissue inhibitors of metalloproteinases: Structure, regulation and biological functions. Eur. J. Cell Biol. 1997, 74, 111–122. [Google Scholar]
- Waszczykowska, A.; Podgórski, M.; Waszczykowski, M.; Gerlicz-Kowalczuk, Z.; Jurowski, P. Matrix metalloproteinases MMP-2 and MMP-9, their inhibitors TIMP-1 and TIMP-2, vascular endothelial growth factor and sVEGFR-2 as predictive markers of ischemic retinopathy in patients with systemic sclerosis—Case series report. Int. J. Mol. Sci. 2020, 21, 8703. [Google Scholar] [CrossRef]
- Khamar, P.; Nair, A.P.; Shetty, R.; Vaidya, T.; Subramani, M.; Ponnalagu, M.; Dhamodaran, K.; D’souza, S.; Ghosh, A.; Pahuja, N. Dysregulated tear fluid nociception-associated factors, corneal dendritic cell density, and vitamin D levels in evaporative dry eye. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2532–2542. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, B.G.; Kim, J.S.; Hwang, J.H. Matrix metalloproteinase 9 point-of-care immunoassay result predicts response to topical cyclosporine treatment in dry eye disease. Transl. Vis. Sci. Technol. 2018, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Li, D.-Q.; Doshi, A.; Farley, W.; Corrales, R.M.; Pflugfelder, S.C. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4293–4301. [Google Scholar] [CrossRef]
- Aragona, P.; Aguennouz, M.H.; Rania, L.; Postorino, E.; Sommario, M.S.; Roszkowska, A.M.; De Pasquale, M.G.; Pisani, A.; Puzzolo, D. Matrix metalloproteinase 9 and transglutaminase 2 expression at the ocular surface in patients with different forms of dry eye disease. Ophthalmology 2015, 122, 62–71. [Google Scholar] [CrossRef]
- Corrales, R.M.; Stern, M.E.; De Paiva, C.S.; Welch, J.; Li, D.-Q.; Pflugfelder, S.C. Desiccating stress stimulates expression of matrix metalloproteinases by the corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3293–3302. [Google Scholar] [CrossRef]
- Dor, M.; Eperon, S.; Lalive, P.H.; Guex-Crosier, Y.; Hamedani, M.; Salvisberg, C.; Turck, N. Investigation of the global protein content from healthy human tears. Exp. Eye Res. 2019, 179, 64–74. [Google Scholar] [CrossRef]
- Perumal, N.; Jeong, E.; Runde, S.; Manicam, C.; Pfeiffer, N.; Grus, F. Clinical Proteomics Unravel Novel Protective Mechanisms of Thealoz® Duo Eye Drops in Evaporative Dry Eye Patients. In Proceedings of the ARVO (Association of Research in Vision and Ophthalmology) Annual Meeting Abstract June 2023, Investigative Ophthalmology & Visual Science, New Orleans, LA, USA, 23–27 April 2023; Volume 64, p. 688. [Google Scholar]
- Perumal, N.; Jeong, E.; Runde, S.; Wolter, D.; Manicam, C.; Pfeiffer, N.; Grus, F. Thealoz ® Duo eye drops protect the ocular surface of dry eye patients by breaking the inflammatory vicious cycle. In Proceedings of the ARVO Annual Meeting: Abstract June 2022, Investigative Ophthalmology & Visual Science, Denver, CO, USA, 1–4, 11–12 May 2022; Volume 63, p. 2007-A0337. [Google Scholar]
- Perumal, N.; Jeong, E.; Runde, S.; Manicam, C.; Pfeiffer, N.; Grus, F. Application of Thealoz (R) duo eye drops confers novel protective effects on the ocular surface of dry eye patients. In Proceedings of the Acta Ophthalmologica, Presented at EVER (European Association for Vision and Eye Research), Valencia, Spain, 13–15 October 2022; Volume 100. [Google Scholar] [CrossRef]
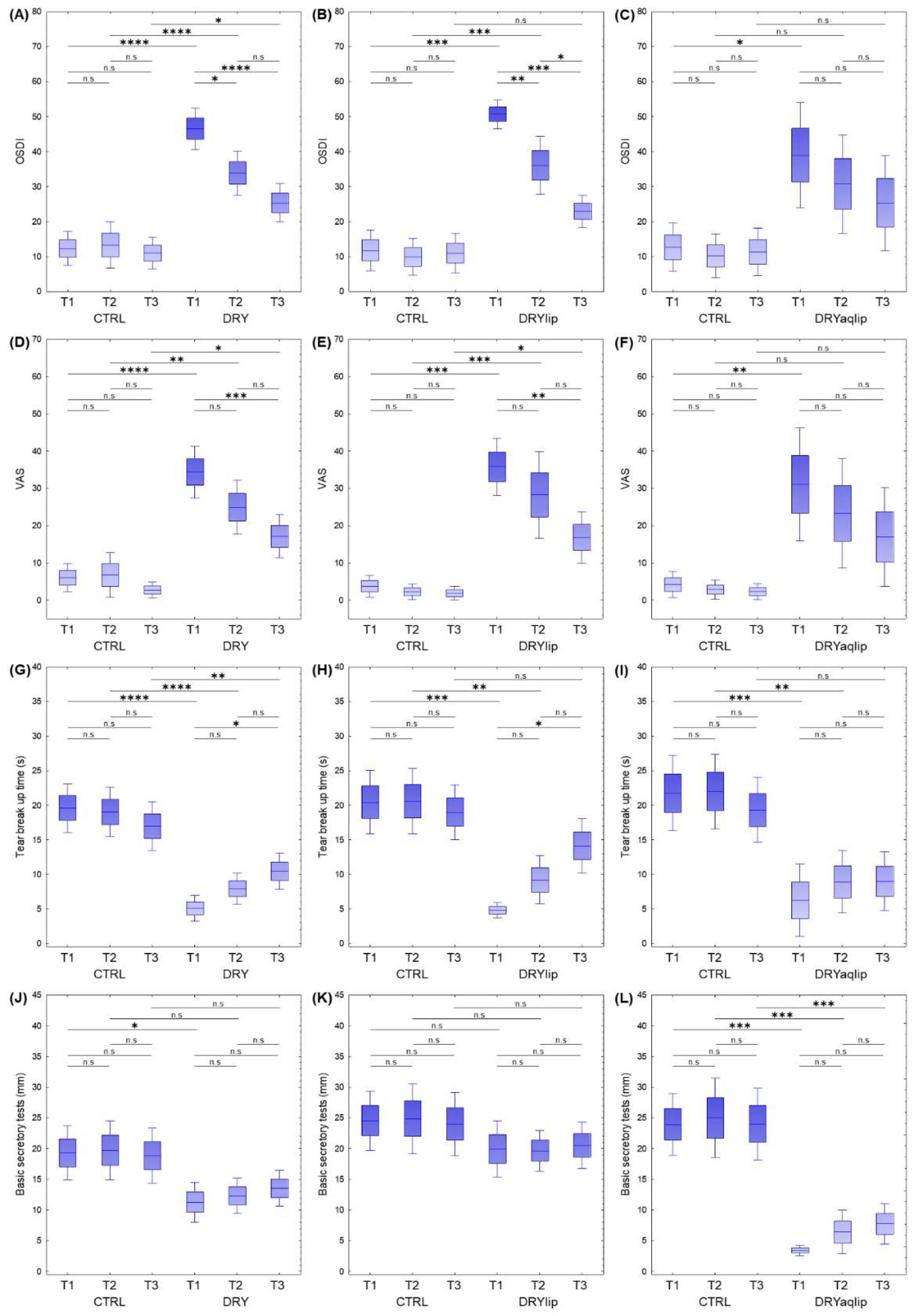

| Groups | Samples (n) | Gender | Age | BST (mm) | TBUT (s) | OSDI | VAS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | ||
| DRY vs. CTRL | |||||||||||||
| CTRL_T1 | 23 | 13 | 10 | 46.52 | 3.06 | 19.30 | 2.25 | 19.61 | 1.79 | 12.32 | 2.46 | 6.00 | 1.92 |
| CTRL_T2 | 23 | 13 | 10 | 46.52 | 3.06 | 19.78 | 2.49 | 18.57 | 1.86 | 13.34 | 3.38 | 6.80 | 3.06 |
| CTRL_T3 | 23 | 13 | 10 | 46.61 | 3.04 | 18.74 | 2.27 | 17.48 | 1.75 | 11.02 | 2.31 | 2.68 | 1.09 |
| DRY_T1 | 35 | 22 | 13 | 57.60 | 2.48 | 11.29 | 1.65 | 5.09 | 0.95 | 46.53 | 3.01 | 34.40 | 3.55 |
| DRY_T2 | 35 | 22 | 13 | 57.66 | 2.48 | 12.31 | 1.47 | 7.91 | 1.15 | 33.93 | 3.22 | 24.97 | 3.68 |
| DRY_T3 | 34 | 21 | 13 | 57.88 | 2.54 | 13.53 | 1.50 | 10.44 | 1.32 | 25.37 | 2.80 | 17.16 | 2.94 |
| DRYlip vs. CTRL | |||||||||||||
| CTRL_T1 | 15 | 9 | 6 | 42.67 | 4.17 | 24.53 | 2.46 | 20.47 | 2.35 | 11.82 | 2.97 | 3.72 | 1.48 |
| CTRL_T2 | 15 | 9 | 6 | 42.67 | 4.17 | 25.00 | 2.92 | 19.87 | 2.54 | 9.92 | 2.68 | 2.29 | 1.06 |
| CTRL_T3 | 15 | 9 | 6 | 42.80 | 4.15 | 23.87 | 2.58 | 19.73 | 1.91 | 10.96 | 2.87 | 1.86 | 0.93 |
| DRYlip_T1 | 15 | 8 | 7 | 59.27 | 4.25 | 19.93 | 2.34 | 4.80 | 0.57 | 50.68 | 2.06 | 35.80 | 3.92 |
| DRYlip_T2 | 15 | 8 | 7 | 59.27 | 4.25 | 19.67 | 1.69 | 9.20 | 1.77 | 36.08 | 4.20 | 28.27 | 5.95 |
| DRYlip_T3 | 15 | 8 | 7 | 59.27 | 4.25 | 20.53 | 1.91 | 14.13 | 2.03 | 22.94 | 2.37 | 16.83 | 3.51 |
| DRYaqlip vs. CTRL | |||||||||||||
| CTRL_T1 | 12 | 7 | 5 | 43.58 | 4.72 | 23.92 | 2.56 | 21.75 | 2.76 | 12.69 | 3.56 | 4.23 | 1.80 |
| CTRL_T2 | 12 | 7 | 5 | 43.58 | 4.72 | 25.00 | 3.31 | 22.00 | 2.77 | 10.19 | 3.18 | 2.86 | 1.27 |
| CTRL_T3 | 12 | 7 | 5 | 43.75 | 4.68 | 24.00 | 2.98 | 19.33 | 2.38 | 11.33 | 3.46 | 2.32 | 1.12 |
| DRYaqlip_T1 | 12 | 8 | 4 | 57.92 | 2.93 | 3.42 | 0.42 | 6.25 | 2.67 | 38.98 | 7.67 | 31.07 | 7.75 |
| DRYaqlip_T2 | 12 | 8 | 4 | 58.00 | 2.93 | 6.42 | 1.82 | 8.92 | 2.32 | 30.73 | 7.18 | 23.30 | 7.45 |
| DRYaqlip_T3 | 12 | 8 | 4 | 58.00 | 2.93 | 7.75 | 1.68 | 9.00 | 2.17 | 25.37 | 6.92 | 16.95 | 6.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perumal, N.; Manicam, C.; Jeong, E.; Runde, S.; Pfeiffer, N.; Grus, F.H. Combination Therapy with Trehalose and Hyaluronic Acid Restores Tear Lipid Layer Functionality by Ameliorating Inflammatory Response Protein Markers on the Ocular Surface of Dry Eye Patients. J. Clin. Med. 2025, 14, 5525. https://doi.org/10.3390/jcm14155525
Perumal N, Manicam C, Jeong E, Runde S, Pfeiffer N, Grus FH. Combination Therapy with Trehalose and Hyaluronic Acid Restores Tear Lipid Layer Functionality by Ameliorating Inflammatory Response Protein Markers on the Ocular Surface of Dry Eye Patients. Journal of Clinical Medicine. 2025; 14(15):5525. https://doi.org/10.3390/jcm14155525
Chicago/Turabian StylePerumal, Natarajan, Caroline Manicam, Eunjin Jeong, Sarah Runde, Norbert Pfeiffer, and Franz H. Grus. 2025. "Combination Therapy with Trehalose and Hyaluronic Acid Restores Tear Lipid Layer Functionality by Ameliorating Inflammatory Response Protein Markers on the Ocular Surface of Dry Eye Patients" Journal of Clinical Medicine 14, no. 15: 5525. https://doi.org/10.3390/jcm14155525
APA StylePerumal, N., Manicam, C., Jeong, E., Runde, S., Pfeiffer, N., & Grus, F. H. (2025). Combination Therapy with Trehalose and Hyaluronic Acid Restores Tear Lipid Layer Functionality by Ameliorating Inflammatory Response Protein Markers on the Ocular Surface of Dry Eye Patients. Journal of Clinical Medicine, 14(15), 5525. https://doi.org/10.3390/jcm14155525





