Abstract
Background: High-intensity gait training (HIT) is an evidence-based intervention recommended for stroke rehabilitation; however, its implementation in routine practice is inconsistent. This study examined the real-world implementation of HIT in an inpatient rehabilitation setting in Norway, focusing on fidelity, barriers, and knowledge translation (KT) strategies. Methods: Using the Knowledge-to-Action (KTA) framework, HIT was implemented in three phases: pre-implementation, implementation, and competency. Fidelity metrics and coverage were assessed in 99 participants post-stroke. Barriers and facilitators were documented and categorized using the Consolidated Framework for Implementation Research. Results: HIT was delivered with improved fidelity during the implementation and competency phases, reflected by increased stepping and heart rate metrics. A coverage rate of 52% was achieved. Barriers evolved over time, beginning with logistical and knowledge challenges and shifting toward decision-making complexity. The KT interventions, developed collaboratively by clinicians and external facilitators, supported implementation. Conclusions: Structured pre-implementation planning, clinician engagement, and external facilitation enabled high-fidelity HIT implementation in a real-world setting. Pragmatic, context-sensitive strategies were critical to overcoming evolving barriers. Future research should examine scalable, adaptive KT strategies that balance theoretical guidance with clinical feasibility to sustain evidence-based practice in rehabilitation.
1. Introduction
The field of knowledge translation (KT) bridges the gap between research and clinical practice by providing methods for the systematic implementation of evidence-based practices into patient care [1]. When planning a KT project, it is critical to target a practice with robust evidence supporting its effectiveness and safety [1]. Such practices must be backed by a comprehensive body of high-quality research, typically including well-conducted randomized controlled trials and systematic reviews, which demonstrate consistent positive outcomes across various settings and populations.
Stroke rehabilitation has an extensive amount of literature, including national and international clinical practice guidelines, designed to standardize care and improve patient outcomes [2,3,4,5,6]. These guidelines recommend interventions that are supported by strong evidence, such as high-intensity gait training (HIT), which has demonstrated efficacy in improving gait outcomes for individuals undergoing stroke rehabilitation [3]. HIT involves task-specific walking while targeting high heart rates (≥70% of predicted maximum heart rate) and is delivered on the treadmill, overground, and on stairs [7]. While studies demonstrate that HIT is effective in improving gait and balance outcomes when compared to usual care in controlled laboratory settings [8], successful implementation in real-world rehabilitation settings depends on fidelity, or applying the intervention as designed or intended. The fidelity of HIT is commonly measured using metrics such as stepping activity, heart rate targets, and frequency. Inpatient rehabilitation implementation studies have also demonstrated improvements in HIT result in substantial improvements in patient outcomes, although these studies did not achieve the levels of fidelity demonstrated in the research [9,10,11]. Achieving fidelity in routine clinical practice remains a challenge. More research is needed to examine implementation strategies, barriers, and facilitators to ensure that HIT is delivered with high fidelity when implemented into stroke rehabilitation practice.
This study observed HIT implementation in an inpatient rehabilitation facility that provided care to individuals in the subacute and chronic phases post-stroke. The specific aims of this study were to (1) describe barriers encountered during the implementation of HIT; (2) identify strategies to implement HIT with fidelity; and (3) monitor fidelity metrics to identify changes in clinical practice.
2. Methods
2.1. Study Design and Setting
This observational study was conducted at Skogli Helse- og Rehabiliteringssenter AS (Skogli, Lillehammer, Norway), a private interdisciplinary rehabilitation center located in Lillehammer, Norway, in collaboration with the Regional Rehabilitation Knowledge Center (RKR). RKR is an integral part of the health system in Norway, focusing on promoting best practices in rehabilitation across the region. This center collaborates with rehabilitation centers to enhance the quality of care using evidence-based implementation strategies. Skogli participated as a site in a larger Norwegian multi-site implementation project, Focused Intensive Repetitive Step Training (FIRST), that aimed to implement HIT in nine facilities. While HIT was implemented at these facilities, RKR guided the implementation and ensured that the interdisciplinary teams had the necessary skills and knowledge to implement HIT effectively. In addition, RKR provided education and training for the clinicians on standardized assessments and HIT. While each site conducted their projects separately, the teams at each facility came together for in-person and online training, mentoring, and project meetings. They also shared experiences related to strategies to overcome local barriers.
Skogli maintains an average occupancy of 82 patients per day under a contract with the Helse Sør-Øst RHF (i.e., government funding). The Skogli interdisciplinary care team consists of physiotherapists (PTs), occupational therapists, nurses, medical doctors, social workers, psychologists, a vision specialist, and a speech therapistwho work collaboratively to provide comprehensive rehabilitation. The facility employs ~12 full-time PT positions that are shared between 13 clinicians. Rehabilitation services are provided to individuals who have experienced a stroke, with approximately 120 patients with stroke treated per year. Historically, the typical length of stay at the facility is 21 days for patients with chronic stroke and 28 days for patients with subacute stroke. The stroke team includes three physical therapists, equivalent to 1.7 full-time employees, dedicated to providing stroke rehabilitation.
Traditional stroke rehabilitation at Skogli, prior to the implementation of HIT, included functional tests that were conducted at admission and discharge, with clinicians individually selecting tests based on each patient’s functional level and clinician preferences. Standard testing protocols were not used and varied among the clinicians. Treatment interventions focused on a variety of therapeutic techniques tailored to individual patient needs. Key components included functional training and mobility techniques, strength training, range of motion exercises, and gait and balance training. For patients with motor deficits in the upper extremities, task-specific training was provided in collaboration with occupational therapists. The treatment interventions were selected by the treating therapist and varied among clinicians.
2.2. Participating Clinicians and Patients
Both clinicians treating patients with stroke and individuals undergoing stroke rehabilitation were included in the study. Full or part-time clinicians who treated patients receiving care for stroke rehabilitation were invited to participate. The participants undergoing post-stroke recovery were over 18 years of age and received inpatient rehabilitation for post-stroke functional deficits at the participating facility. They also had a physiotherapy goal of improving walking function. If patients had previously received HIT, either at Skogli or another FIRST project site, they were also excluded from data collection. Additionally, all participants were required to have the ability to provide informed consent, with the option for designated caregivers to provide consent on their behalf if necessary. Participants were excluded if they used instrumentation, such as a ventilator, that restricted walking activities. Patients with significant co-morbidities, including uncontrolled cardiovascular, metabolic, respiratory, infectious, or psychiatric disorders, or malignancies, were also excluded. Furthermore, individuals with a previous history of orthopedic or neurologic disorders that prevented them from walking more than 45 m before the stroke, such as an amputation or lower extremity fracture, were not eligible for the study.
Coverage, an important fidelity metric that assesses whether all patients who might benefit from HIT were offered the intervention, was monitored and documented by the leader of the stroke unit. While the FIRST project inclusion and exclusion criteria are listed above, some patients were not offered HIT for reasons such as prioritizing other rehabilitation goals (e.g., vision training, speech, fatigue, and pain management), referral to Skogli specifically for the constraint-induced movement therapy program, or medical instability as determined by the patient’s physician. If a patient had a short length of stay (typically <3 days) due to re-hospitalization, new-onset COVID diagnosis, or violating local rules, they were not offered HIT. Similarly, some patients who did not meet the FIRST project data collection criteria were offered HIT because they could benefit from the intervention.
2.3. Implementation Framework and Phases
The implementation plan was developed collaboratively by a KT expert from RKR and the interdisciplinary team at Skogli. The planning was guided by the Knowledge-to-Action (KTA) framework, which facilitated a systematic process for translating knowledge into practice [1]. The KTA includes two components, visually represented as the knowledge creation funnel (the generation of evidence and tailoring this evidence for implementation), and the action cycle, which includes seven phases to guide implementation processes. The seven phases are as follows: the selection of the practice to be implemented and assessing the know–do gap; adapting knowledge to the local context; assessing barriers and facilitators to knowledge use; selecting and using KT interventions (i.e., implementation strategies); monitoring knowledge use (i.e., fidelity); evaluating outcomes; and sustaining the implemented practice [1]. The project efforts were categorized into three phases: pre-implementation, implementation, and competency [12]. While a general timeline guided these activities, this was adjusted as needed to address the evolving demands of the project. The timeline and KTA plan are shown in Figure 1 and Table 1.
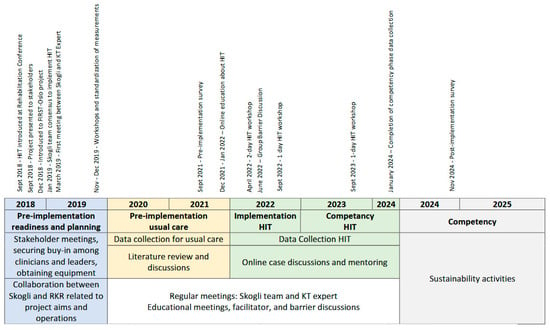
Figure 1.
Timeline of pre-implementation, implementation, and competency activities.

Table 1.
Knowledge translation plan and results.
The pre-implementation phase followed the first four phases of the Knowledge-to-Action (KTA) framework, encompassing the identification of the know–do gap, the adaptation of evidence-based knowledge to the local context, the assessment of barriers and facilitators, and selection of targeted knowledge translation strategies. Discussions about implementing HIT were initiated by the leader of the stroke team, who was a PT who learned about HIT at a rehabilitation conference. Recognizing the need for a structured and evidence-based approach to gait training, the stroke team leader engaged the leader of the PT department, emphasizing HIT’s clinical benefits, alignment with evidence-based practice guidelines, and potential to enhance patient care and facility reputation. Together, they introduced the concept to the broader clinical team, facilitating discussions on feasibility, implementation challenges, and the potential impact on patient outcomes. These discussions highlighted HIT’s advantages, including standardized training parameters, greater consistency in care delivery, and opportunities for improved referrals and contractual relationships.
Following a consensus among clinicians and leadership, the leaders approached the Chief Executive Officer and positioned HIT as a strategic investment to modernize rehabilitation services. With leadership approval, Skogli joined the RKR-led FIRST project, gaining access to external facilitation, structured implementation support, clinician training, mentoring, and an implementation toolkit.
2.4. Pre-Implementation Phase: Monitoring Usual Care, Implementation of Standardized Assessments, and Fidelity Metrics
During the pre-implementation phase, we assessed the characteristics of usual care and implemented assessments to measure the patients’ functional level and rehabilitation outcomes. Heart rate was monitored using the OH1 (Polar Electro, Kempele, Finland) and a smartphone for recording. Fidelity metrics were gathered, including peak heart rate during PT sessions, not including sessions with >50% of the time spent on outcome measures, and the time patients spent at ≥70% of their maximum heart rate, calculated using Equation 211 − (0.64 × age) [13]. Borg’s Rating of Perceived Exertion (RPE) was also collected throughout PT sessions [14,15]. Stepping metrics were monitored, including steps per day, steps per PT session, stepping rate, and the number of minutes spent stepping using the StepWatch (Modus, Inc., Washington, DC, USA) [15]. These data were described as both absolute and relative values, where the absolute values described the number of minutes and the relative values referred to the percentage of the session. The fidelity metrics are defined in Table 2.

Table 2.
Definitions of terms used in fidelity calculations.
Measures implemented included gait speed, which was assessed using self-selected velocity (SSV) and fast velocity (FV) [2]. Walking distance was measured using the 6-Minute Walk Test (6MWT) using the instructions to “cover as much ground as possible over 6 min” [2]. Balance was assessed using the Berg Balance Scale (BBS), and for patients scoring above 50 on the admission BBS, the Mini-Balance Evaluation Systems Test (Mini-BESTest) was used [2]. These measures have excellent psychometric properties and are strongly recommended to be used in patients with stroke [2]. Clinicians were trained on standardized assessment protocols to ensure consistent data collection methods.
2.5. Implementation of HIT
After observing usual care practices to understand baseline rehabilitation characteristics and outcomes, the focus shifted to implementing HIT. The process emphasized selecting and applying tailored KT interventions to overcome barriers. A primary goal of the implementation plan was to deliver HIT with fidelity, meaning that it was delivered similarly to how it was studied [16]. The KT plan followed an iterative cycle of assessing barriers, selecting and using KT interventions, and monitoring knowledge use. If fidelity goals were not met, the cycle was repeated, allowing for continuous adjustments to optimize the integration of HIT into clinical practice.
HIT was recommended to be used in practice at a frequency of four times per week for one-hour sessions [8,15]. Recommendations also included delivering HIT at an intensity of 70% to 85% of maximum HR or 14–18 on the RPE scale (hard or very hard), with approximately 40% of each one-hour session spent in the target heart rate zone (70–85% of maximum heart rate). Fidelity metrics were monitored throughout the implementation phase to understand adherence to HIT recommendations. These are outlined above (see the pre-implementation phase). Annual feedback sessions provided clinicians with synthesized fidelity metrics, which facilitated ongoing efforts to improve fidelity. In addition, coverage was monitored to determine if all patients who may benefit from the intervention were offered it [16]. Each patient was screened for the potential to benefit from HIT, and information about why HIT was not offered was documented.
2.6. Barrier and Facilitator Identification
To understand barriers and facilitators, clinicians participated in informal discussions about barriers and facilitators to implementing standardized assessments and HIT in clinical practice. In addition, a designated PT systematically documented barriers encountered during the implementation phase, recording detailed information on the timing and nature of the barrier and strategies employed to overcome it. After the completion of the project, the team also reflected on and documented the facilitators of the implementation of HIT.
The barriers and facilitators identified during the project were systematically identified and categorized using the Consolidated Framework for Implementation Research (CFIR) [17]. Using its updated version, CFIR organizes these factors into five domains that describe the innovation (i.e., practice being implemented), outer setting (e.g., setting in which the rehabilitation center exists, such as the health system), inner setting (i.e., setting in which the practice is being implemented), individuals (i.e., roles and characteristics of individuals), and implementation process (activities and strategies used to implement the intervention). In addition, there are several constructs that are associated with each domain. Operational definitions of the domains and constructs are located on the CFIR website [18]. While the CFIR did not guide the selection of KT interventions, it is presented here as a method to describe the barriers and facilitators.
2.7. Selection of KT Interventions
Barriers encountered during the project were systematically identified and addressed through an iterative and collaborative process of developing KT interventions. Practical barriers, such as logistical challenges, were often resolved directly by clinicians, leveraging their detailed understanding of culture, daily operations, and team dynamics. However, more complex barriers required guidance from RKR’s KT expert and HIT content experts. The clinical team met routinely to discuss these challenges, allowing clinicians to share insights and propose potential solutions. This ongoing, adaptive process ensured that the strategies developed were practical and effective, enabling the team to respond dynamically to evolving needs. The KT interventions developed during the project are detailed in Table 3.

Table 3.
Barriers and facilitators observed throughout the project.
In addition to these tailored KT interventions, the team was provided with an implementation toolkit from RKR. This toolkit included resources such as online courses that teach clinicians and students about the practices, evidence-based protocols for measurement administration and HIT delivery, evidence-based resources that summarize measurement and HIT information, target heart rate calculators, clinical prediction rule calculators, gym and lanyard signs (e.g., rating of perceived exertion, heart rate zones, decision-support tools), and short videos on strategies to overcome barriers (e.g., ankle taping). In addition, the toolkit included implementation resources such as standardization instructions, data collection sheets, and screening forms. The team adapted these resources to meet their local needs.
2.8. Ethics Approval
The project was approved by the Southeastern Norway Ethics Committee (approval 2016/873), and all participants provided written informed consent.
2.9. Data Collection and Statistical Analysis
Data were grouped and analyzed by implementation phase. Normality was assessed using Shapiro–Wilk tests, and homogeneity of variances was evaluated with Levene’s test. Based on these assessments, appropriate parametric or non-parametric tests were applied. An independent samples Kruskal–Wallis test and the Kruskal–Wallis H statistic were used to compare the demographics and baseline function of patient groups. For stepping metrics, an Analysis of Covariance (ANCOVA) or Quade’s Non-parametric ANCOVA was used, adjusting for 6MWT distance as a covariate. Session times, number of sessions, HR metrics, and RPEs were analyzed without a covariate using one-way ANOVA or Kruskal–Wallis tests, depending on normality and variance assumptions. When significant group differences were identified, post hoc comparisons were conducted using Tukey’s HSD for parametric tests and Bonferroni-adjusted pairwise comparisons for non-parametric tests. Statistical analyses were conducted using IBM SPSS Statistics (Version 28, IBM Corp., Armonk, NY, USA) with significance set at α = 0.05.
To mitigate potential bias and ensure objectivity, data processing and analysis were conducted collaboratively by the lead author, a researcher is employed at Skogli, and the broader project team. Team members had access to the data and contributed to the interpretation of results through joint discussions. This collaborative approach ensured transparency, challenged assumptions, and reduced the risk of confirmation bias related to the lead author’s dual role as project facilitator and researcher.
3. Results
The project was divided into distinct phases over a seven-year period, where the timeline was adjusted to respond to the needs of the project and to facilitate successful implementation. The timeline for the project is shown in Figure 1.
Three clinicians contributed to the collection of data on 99 patients (n = 38 pre-implementation; n = 30 implementation; n = 31 competency). Participants with subacute (n = 40) and chronic stroke (n = 59) were recruited across the three phases. Table 4 provides an overview of clinician demographics, and Supplementary Material Table S1 describes patient demographics and baseline function. Participants with subacute stroke were similar across phases in terms of age, the time since stroke, and length of stay. The median age ranged from 71 to 73 years, and time since stroke at admission was consistent, with medians of approximately 40–56 days. Average gait speeds (SSV and FV) were not significantly different across phases. Among individuals with chronic stroke, baseline characteristics were also generally comparable across phases. The median age ranged from 65.5 to 71 years, and time since stroke varied more widely from ~450 to >1000 days. There were no statistically significant differences in self-selected or fast gait speed or FAC scores across the phases. While the baseline 6MWT was not statistically different between groups, the differences in 6 MWTs were near the threshold of the minimally important clinical difference of 65–70 m [19] and 50 m [20] for subacute and chronic stroke groups, respectively. Therefore, we utilized the 6MWT as a covariate for the stepping fidelity metric analysis for both groups.

Table 4.
Characteristics of the clinicians (n = 3).
3.1. Fidelity Metrics
A total of 271 patients were screened at admission. Of these, 91 were not offered HIT due to referral for other goals, such as the constraint-induced movement therapy program, 7 were not offered HIT due to medical instability, and 2 declined treatment. Thus, 167 patients could have benefited from HIT, and 87 received the intervention, resulting in a 52% coverage rate. While 87 patients received the intervention, only 61 are reported here from the implementation and competency phases due to data collection exclusions (see participants section). Detailed data related to coverage are presented in Table 5.

Table 5.
Coverage for HIT.
Patients with subacute stroke completed 14 to 17 sessions, and individuals with chronic stroke completed 12 to 14 sessions as part of their plan of care. For both groups, there were no significant differences in the total number of sessions across phases. After adjusting for admission 6MWT, average daily stepping did not significantly differ across implementation phases. However, steps per session, stepping duration, and stepping rate were significantly higher in the implementation and competency phases compared to the pre-implementation phase. No significant differences were observed between the implementation and competency phases for these stepping metrics. Similarly, heart rate and RPE metrics increased from pre-implementation to implementation and remained elevated in the competency phase.
In the subacute stroke group, fidelity metrics improved substantially across phases. Steps per session increased from a mean of 748 (435–1044) in the pre-implementation phase to 1853 (774–2195) during the implementation and 2306 (1492–2608) in the competency phase (p < 0.001). Minutes stepping per session also increased from 19 (6) minutes to 31 (11) and 37 (8) minutes, respectively (p < 0.001), and the stepping rate increased from 35 (9) to 49 (17) and 54 (12) steps per minute (p < 0.001). Time spent in the target heart rate (HR) zone increased from a median of 1 min (1% of session) in the pre-implementation phase to 18 min (40%) during implementation phase, and 20 min (45%) in the competency phase (p < 0.01). Similarly, time spent in the RPE zone increased from 2 min (5%) to 22 min (48%) and 21 min (34%) (p < 0.001). These fidelity gains occurred without significant increases in the total session time or number of sessions, suggesting improved quality of HIT delivery over time. For full fidelity metrics, please refer to Supplementary Material Table S2a.
For individuals with chronic stroke, fidelity also improved significantly. Steps per session increased from 584 (280 to 809) in the pre-implementation phase to 1803 (1130–2155) in the implementation phase and 2001 (1467–2552) in the competency phase (p < 0.001). Stepping rate improved from 32 (11) to 48 (12) and 52 (15) steps per minute (p < 0.001), and the number of minutes stepping increased from 16 (12 to 19) to 33 (26–37) and 38 (29–43) minutes (p < 0.001). Time in the target HR zone increased from 2 min (5% of session) to 17 min (31%) and 13 min (21%) across phases (p < 0.001). The RPE zone time also increased significantly from 3 min (6%) to 19 min (37%) and 20 min (36%) (p < 0.001). These improvements occurred despite similar session lengths and highlight enhanced adherence to HIT intensity targets, reflecting successful implementation over time. For full fidelity metrics, please refer to Supplementary Material Table S2b.
3.2. Barriers and Facilitators
The barriers and facilitators are shown in Table 3. Barriers were identified across the phases of the project. Early-stage barriers occurred during the pre-implementation phase and were related to the implementation of assessments and planning for HIT implementation. These barriers were spread across several CFIR domains and included inner setting, innovation, and individual characteristics. The barriers were related to learning testing procedures and interpretation, project-related processes, and securing equipment and space. As the clinicians began to learn about how to use HIT in clinical practice, the barriers mainly focused on individual characteristics. Specifically, they focused on the knowledge and skills related to delivering HIT in clinical practice. During this phase, barriers evolved to include the domain of the inner setting as the team recognized different equipment and space needs. In addition, the need to further adapt HIT for their practice was identified, which was a strategy that targeted a barrier categorized as innovation adaptability. Later in the implementation phase of HIT, the barriers continued to evolve. While they mainly focused on individuals, the barriers were described as a need to refine various aspects of clinical decision-making related to HIT.
The facilitators were spread across the inner setting, individuals, outer setting, innovation, and implementation process domains. Many facilitators focused on the inner setting and described a learning-centered culture, the alignment of the mission and priorities, and the connections among the clinicians and leaders. The individual facilitators were described as clinicians’ motivation and experience, and supportive leaders. The implementation process facilitators were related to the team initiating the project and their collaboration during implementation. The outer setting facilitators included partnerships and connections with professional associations, RKR, and other participating sites, and the belief that providing high-quality care could result in increased patient referrals. Lastly, the packaging of the HIT implementation program facilitated the project.
4. Discussion
This study demonstrates the real-world feasibility of HIT implementation in inpatient stroke rehabilitation, highlighting fidelity improvements, barriers, facilitators, and implementation strategies. Notably, improvements in HIT fidelity metrics (i.e., stepping and heart rate metrics) were observed, indicating that the intervention was delivered with increased fidelity. While these metrics are not as high as observed in research laboratories, they are aligned with fidelity metrics achieved in other inpatient rehabilitation implementation studies of patients who had a lower functional level at admission [8,9,10]. Although a structured implementation plan facilitated implementation fidelity improvements between 2022 and 2023, achieving high coverage levels in clinical practice remains challenging. Future implementation efforts should explore strategies to enhance coverage while maintaining clinical feasibility, including workflow adaptations, clinician training on patient selection, and system-level support to optimize HIT integration into routine rehabilitation.
The overall coverage rate was 52%; thus, approximately half of the patients who may have benefited from HIT received the intervention. The lower coverage observed in 2024 (29%) reflects a brief data collection window, as the project concluded in January and only 17 patients were admitted during that month. This contrasts with the 125+ patients admitted during 2022 and 2023 and likely underrepresents typical coverage trends due to normal fluctuations among patient admission rates. While the overall coverage rate is higher than that of another published Norwegian HIT implementation study [9], it is much lower than the ~96% coverage rate observed in a US study [10]. We did not systematically collect the goals of patients not enrolled in this project; however, notes from patient screening forms indicated that many patients were treated for other rehabilitation goals, such as fatigue management, cognitive rehabilitation, upper extremity function, or vision training. In addition, many of these patients had relatively high baseline functions and reported a perception of no difficulty with walking on a subjective assessment. This suggests that clinicians may have prioritized other areas of need based on patient goals and functional status. Recognizing and tracking these factors in future projects could support the development of a targeted implementation plan to improve coverage if needed.
The evolving nature of barriers encountered during HIT implementation in inpatient rehabilitation is demonstrated in this study. Early challenges centered on logistical issues and shifted toward optimizing HIT delivery as implementation progressed. Later, barriers focused on refining treatment, decision-making, and sustaining fidelity. Previous studies have described many barriers that arise during HIT implementation, such as resource constraints, clinician knowledge, and fidelity challenges [11,21]. While previous research identified the presence of barriers, this study uniquely documents their evolution over time. By illustrating their progression, this study highlights the need for adaptive and dynamic implementation strategies. Recognizing how barriers change over time allows teams to apply targeted strategies at different stages. Further research should investigate this progression across different healthcare settings and determine effective and efficient implementation strategies.
The implementation science literature emphasizes the importance of the pre-implementation phase in establishing a strong foundation for implementation with fidelity [22]. The pre-implementation phase in this project required 14 months and was marked by activities such as securing support from the leadership team and clinicians, understanding the HIT-related barriers and facilitators, and implementation planning. In a comparable HIT implementation project, clinicians spent approximately two years completing this phase, focusing on building consensus for HIT implementation and facilitating readiness for change [21]. Both studies included time for these activities to occur, which may have impacted the success of the project. Studies have demonstrated that comprehensive planning and completion of activities during the pre-implementation phase significantly predict implementation with fidelity and sustainment [22,23]. Furthermore, studies indicate that incomplete pre-implementation activities can undermine the effectiveness of later implementation efforts, as even a well-executed implementation phase cannot compensate for early gaps [22]. While implementation science has recognized the importance of pre-implementation, there is limited research on how to optimize pre-implementation activities. Future research should focus on identifying the most effective strategies for engaging stakeholders, addressing context-specific barriers, and designing a structured pre-implementation plan.
This project demonstrates a pragmatic, real-world implementation of evidence-based practices, illustrating how a practical and context-sensitive approach to KT has the potential to address the inherent complexities of clinical practice. While we used the KTA framework to plan and guide the overall implementation process and the CFIR to categorize barriers and facilitators, we did not use a theory to guide implementation strategy selection [24]. Instead, we took a pragmatic approach, leveraging the team’s understanding of the local clinical context and barriers to iteratively select and refine KT interventions. This approach allowed for flexibility in addressing emerging challenges in real time and ensured that strategies were directly relevant to clinicians’ workflow, resource availability, and patient needs. Although the literature often recommends frameworks such as the Theoretical Domains Framework [25] or the CFIR [24] to guide the selection of KT interventions, studies suggest that these approaches can sometimes be complex and difficult for clinicians without formal KT training to apply in real-world settings [24,26]. Notably, Graham and colleagues emphasize that both theory-driven and common-sense approaches have value in implementation and should be studied, particularly when tailored to the needs and capacities of frontline clinicians [1]. A pragmatic approach, such as the one used in this study, might be more intuitive and feasible for clinicians who are embedded in the clinical environment but have limited experience with implementation science. However, a potential trade-off is the risk of missing theoretically informed strategies that could enhance implementation success, particularly when barriers are complex and require multi-level interventions. Future research should explore how pragmatic approaches can be balanced with theory-driven methods to optimize implementation planning while maintaining feasibility and adaptability in practice.
External facilitation and participation in the larger FIRST project may have contributed to the implementation of HIT by providing structured guidance, external expertise, resources, and a sense of collective learning [27]. The RKR team played a key role in facilitating the implementation process by offering targeted training, structured feedback, and ongoing mentorship, which may enhance clinician engagement and sustain motivation [27]. External facilitation is a well-established implementation strategy that helps bridge knowledge gaps, sustain motivation, and support problem-solving by integrating both internal and external expertise [28,29]. It is particularly effective in complex interventions by fostering organizational learning and adapting strategies to fit the local context [30]. Additionally, external facilitators may play an important role in guiding teams through the implementation process and helping them navigate barriers and reinforcing accountability [31]. Skogli’s involvement in the larger FIRST project also allowed clinicians to connect with peers from other facilities, exchange knowledge, and collaboratively address common barriers. Research suggests that being part of a larger initiative strengthens professional identity, encourages shared problem-solving, and enhances accountability, all of which may have contributed to implementation success [32,33]. Furthermore, the availability of a structured implementation toolkit that was available to FIRST project sites included online courses, decision-support tools, and evidence-based support for clinicians in overcoming barriers related to resources. The local adaptation of these resources may have improved clinician engagement and self-efficacy, aligning with findings that highlight the importance of packaged resources and structured training in enhancing adherence to implemented practices [33,34,35]. These combined factors likely reinforced the successful integration of HIT into clinical practice, demonstrating the value of external facilitation, structured resources, and engagement in a broader implementation effort. Future research should explore how these factors interact and influence implementation over time to better understand their role in implementing evidence-based practices with fidelity.
Limitations
While this study provides valuable insights into the real-world implementation of HIT in inpatient stroke rehabilitation, several limitations should be acknowledged. First, this was a single-site study within a broader multi-site implementation effort, which may limit the generalizability of the findings to other settings with different organizational structures, healthcare policies, or patient populations. While the larger FIRST project provided opportunities for cross-site learning, this study focused on implementation at one site, making it difficult to determine whether similar implementation strategies would be effective in other rehabilitation facilities with different resources and clinical workflows. Second, the pragmatic approach to selecting KT interventions, while contextually relevant and feasible for frontline clinicians, may have omitted theoretically driven strategies that could have further optimized implementation success. The absence of a structured framework to systematically map barriers to specific KT interventions presents a potential limitation, as it may have resulted in missed opportunities to apply more targeted, evidence-based strategies. Third, fidelity metrics were calculated using the entire duration of each therapy session, rather than isolating only the time actively spent delivering HIT. While this reflects how sessions are structured and delivered in practice, it may underestimate the fidelity achieved during the actual HIT portions. Although sessions with more than 50% of time spent on outcome measures were excluded, the inclusion of sessions with some testing may have influenced fidelity metrics by reducing the total time available for HIT. It is likely that the total fidelity to HIT principles, if calculated solely based on the active HIT time, could be higher. However, we did not have valid or consistent methods to isolate HIT time alone. Patient characteristics may have also influenced their ability to engage in the intervention, which could impact fidelity metrics. Although inclusion criteria and baseline functional measures were consistent across groups, variability in time since stroke occurrence in the chronic stroke group may have influenced participation and fidelity. Additionally, we did not directly measure patient motivation or readiness, which may have contributed to differences in implementation fidelity across phases.
Another limitation is the lack of comprehensive data collection on the reasons HIT was not offered to eligible patients. While many of those who were not offered HIT were noted as pursuing alternative rehabilitation goals, which were often aligned with high baseline functional levels, this information was not consistently documented. Similarly, systemic constraints such as therapist availability or vacation schedules were not tracked, limiting our ability to assess their influence on HIT coverage. Future research should incorporate the structured documentation of these factors to better understand and address factors contributing to the underutilization of evidence-based interventions.
5. Conclusions
This study demonstrates the feasibility of implementing HIT in inpatient stroke rehabilitation, highlighting the importance of structured pre-implementation planning, clinician engagement, and external facilitation. These findings underscore the evolving nature of implementation barriers and the need for adaptive, pragmatic knowledge translation strategies to sustain fidelity in clinical practice. Research is needed to identify the most impactful implementation strategies for optimizing fidelity and to evaluate scalable, pragmatic approaches that balance theory-driven and clinician-led implementation methods across diverse rehabilitation settings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm14155409/s1, Table S1: Patient demographics and baseline measures; Table S2. (a) Fidelity Metrics in Patients with Subacute Stroke; (b) Fidelity Metrics in Patients with Chronic Stroke.
Author Contributions
Conceptualization, J.L.M., C.E.H., T.G.H. and S.A.R.; Methodology, J.L.M., P.K., C.E.H., T.G.H., I.L. and A.O.; Formal analysis, J.L.M., C.E.H. and A.O.; Investigation, A.O., P.K., H.H.B., M.B.S. and R.A.; Data curation, J.L.M., P.K., C.E.H. and A.O.; Writing—original draft, J.L.M.; Writing—review & editing, J.L.M., P.K., H.H.B., M.B.S., R.A., C.E.H., T.G.H., S.A.R. and I.L.; Supervision, J.L.M. and I.L.; Project administration, J.L.M., P.K., S.A.R. and A.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Southeastern Norway Ethics Committee (approval 2016/873).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
Jennifer Moore is an advisor for the Southeastern Norway Regional Center for Knowledge Translation in Rehabilitation and the Founder of the Institute for Knowledge Translation.
References
- Straus, S.; Tetroe, J.; Graham, I. Knowledge Translation in Health Care: Moving from Evidence to Practice, 2nd ed.; BMJ Books: West Sussex, UK, 2013. [Google Scholar]
- Moore, J.L.; Potter, K.; Blankshain, K.; Kaplan, S.L.; O’Dwyer, L.C.; Sullivan, J.E. A Core Set of Outcome Measures for Adults with Neurologic Conditions Undergoing Rehabilitation: A Clinical Practice Guideline. J. Neurol. Phys. Ther. JNPT 2018, 42, 174–220. [Google Scholar] [CrossRef]
- Hornby, T.G.; Reisman, D.S.; Ward, I.G.; Scheets, P.L.; Miller, A.; Haddad, D.; Fox, E.J.; Fritz, N.E.; Hawkins, K.; Henderson, C.E.; et al. Clinical Practice Guideline to Improve Locomotor Function Following Chronic Stroke, Incomplete Spinal Cord Injury, and Brain Injury. J. Neurol. Phys. Ther. JNPT 2020, 44, 49–100. [Google Scholar] [CrossRef]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef]
- Tang, E.; Moran, N.; Cadman, M.; Hill, S.; Sloan, C.; Warburton, E. Stroke rehabilitation in adults: Summary of updated NICE guidance. Bmj 2024, 384, q498. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.D.; Dominguez-Vargas, A.U.; Rosso, C.; Branscheidt, M.; Sheehy, L.; Quandt, F.; Zamora, S.A.; Fleming, M.K.; Azzollini, V.; Mooney, R.A.; et al. A translational roadmap for transcranial magnetic and direct current stimulation in stroke rehabilitation: Consensus-based core recommendations from the third stroke recovery and rehabilitation roundtable. Int. J. Stroke 2024, 19, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Holleran, C.L.; Straube, D.D.; Kinnaird, C.R.; Leddy, A.L.; Hornby, T.G. Feasibility and potential efficacy of high-intensity stepping training in variable contexts in subacute and chronic stroke. Neurorehabilit. Neural Repair 2014, 28, 643–651. [Google Scholar] [CrossRef]
- Hornby, T.G.; Holleran, C.L.; Hennessy, P.W.; Leddy, A.L.; Connolly, M.; Camardo, J.; Woodward, J.; Mahtani, G.; Lovell, L.; Roth, E.J. Variable Intensive Early Walking Poststroke (VIEWS): A Randomized Controlled Trial. Neurorehabilit. Neural Repair 2015, 30, 440–445. [Google Scholar] [CrossRef]
- Moore, J.L.; Nordvik, J.E.; Erichsen, A.; Rosseland, I.; Bø, E.; Hornby, T.G.; Barkenæs, T.; Bratlie, H.; Byhring, M.; Grimstad, I.; et al. Implementation of High-Intensity Stepping Training During Inpatient Stroke Rehabilitation Improves Functional Outcomes. Stroke 2020, 51, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Henderson, C.E.; Plawecki, A.; Lucas, E.; Lotter, J.K.; Scofield, M.; Carbone, A.; Jang, J.H.; Hornby, T.G. Increasing the Amount and Intensity of Stepping Training During Inpatient Stroke Rehabilitation Improves Locomotor and Non-Locomotor Outcomes. Neurorehabilit. Neural Repair 2022, 36, 621–632. [Google Scholar] [CrossRef]
- Hornby, T.G.; Holleran, C.L.; Leddy, A.L.; Hennessy, P.; Leech, K.A.; Connolly, M.; Moore, J.L.; Straube, D.; Lovell, L.; Roth, E. Feasibility of Focused Stepping Practice During Inpatient Rehabilitation Poststroke and Potential Contributions to Mobility Outcomes. Neurorehabilit. Neural Repair 2015, 29, 923–932. [Google Scholar] [CrossRef]
- Chamberlain, P.; Brown, C.H.; Saldana, L. Observational measure of implementation progress in community based settings: The stages of implementation completion (SIC). Implement. Sci. IS 2011, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Nes, B.M.; Janszky, I.; Wisloff, U.; Stoylen, A.; Karlsen, T. Age-predicted maximal heart rate in healthy subjects: The HUNT fitness study. Scand. J. Med. Sci. Sports 2013, 23, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Ratings of perceived exertion and heart rates during short-term cycle exercise and their use in a new cycling strength test. Int. J. Sports Med. 1982, 3, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Carroll, C.; Patterson, M.; Wood, S.; Booth, A.; Rick, J.; Balain, S. A conceptual framework for implementation fidelity. Implement. Sci. IS 2007, 2, 40. [Google Scholar] [CrossRef]
- Damschroder, L.J.; Reardon, C.M.; Widerquist, M.A.O.; Lowery, J. The updated Consolidated Framework for Implementation Research based on user feedback. Implement. Sci. 2022, 17, 75. [Google Scholar] [CrossRef]
- Consolidated Framework for Implementation Resaearch. Available online: https://cfirguide.org/constructs-old/ (accessed on 7 January 2025).
- Fulk, G.; He, Y. Minimal Clinically Important Difference of the 6-Minute Walk Test in People with Stroke. J. Neurol. Phys. Ther. 2018, 42, 235–240. [Google Scholar] [CrossRef]
- Perera, S.; Mody, S.H.; Woodman, R.C.; Studenski, S.A. Meaningful change and responsiveness in common physical performance measures in older adults. J. Am. Geriatr. Soc. 2006, 54, 743–749. [Google Scholar] [CrossRef]
- Moore, J.L.; Bø, E.; Erichsen, A.; Rosseland, I.; Halvorsen, J.; Bratlie, H.; Hornby, T.G.; Nordvik, J.E. Development and Results of an Implementation Plan for High-Intensity Gait Training. J. Neurol. Phys. Ther. JNPT 2021, 45, 282–291. [Google Scholar] [CrossRef]
- Alley, Z.M.; Chapman, J.E.; Schaper, H.; Saldana, L. The relative value of Pre-Implementation stages for successful implementation of evidence-informed programs. Implement. Sci. 2023, 18, 30. [Google Scholar] [CrossRef]
- Saldana, L.; Chamberlain, P.; Wang, W.; Hendricks Brown, C. Predicting Program Start-Up Using the Stages of Implementation Measure. Adm. Policy Ment. Health Ment. Health Serv. Res. 2012, 39, 419–425. [Google Scholar] [CrossRef]
- Powell, B.J.; Beidas, R.S.; Lewis, C.C.; Aarons, G.A.; McMillen, J.C.; Proctor, E.K.; Mandell, D.S. Methods to improve the selection and tailoring of implementation strategies. J. Behav. Health Serv. Res. 2017, 44, 177–194. [Google Scholar] [CrossRef] [PubMed]
- French, S.D.; Green, S.E.; O’Connor, D.A.; McKenzie, J.E.; Francis, J.J.; Michie, S.; Buchbinder, R.; Schattner, P.; Spike, N.; Grimshaw, J.M. Developing theory-informed behaviour change interventions to implement evidence into practice: A systematic approach using the Theoretical Domains Framework. Implement. Sci. IS 2012, 7, 38. [Google Scholar] [CrossRef]
- Rapport, F.; Smith, J.; Hutchinson, K.; Clay-Williams, R.; Churruca, K.; Bierbaum, M.; Braithwaite, J. Too much theory and not enough practice? The challenge of implementation science application in healthcare practice. J. Eval. Clin. Pract. 2022, 28, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Mbalilaki, J.A.; Lilleheie, I.; Rimehaug, S.A.; Tveitan, S.N.; Linnestad, A.M.; Krøll, P.; Lundberg, S.; Molle, M.; Moore, J.L. Facilitators and Barriers to Implementing High-Intensity Gait Training in Inpatient Stroke Rehabilitation: A Mixed-Methods Study. J. Clin. Med. 2024, 13, 3708. [Google Scholar] [CrossRef]
- Stetler, C.B.; Legro, M.W.; Rycroft-Malone, J.; Bowman, C.; Curran, G.; Guihan, M.; Hagedorn, H.; Pineros, S.; Wallace, C.M. Role of “external facilitation” in implementation of research findings: A qualitative evaluation of facilitation experiences in the Veterans Health Administration. Implement. Sci. 2006, 1, 23. [Google Scholar] [CrossRef]
- Berta, W.; Cranley, L.; Dearing, J.W.; Dogherty, E.J.; Squires, J.E.; Estabrooks, C.A. Why (we think) facilitation works: Insights from organizational learning theory. Implement. Sci. IS 2015, 10, 141. [Google Scholar] [CrossRef]
- Girard, A.; Doucet, A.; Lambert, M.; Ouadfel, S.; Caron, G.; Hudon, C. What is known about the role of external facilitators during the implementation of complex interventions in healthcare settings? A scoping review. BMJ Open 2024, 14, e084883. [Google Scholar] [CrossRef]
- Ritchie, M.J.; Parker, L.E.; Kirchner, J.E. From novice to expert: A qualitative study of implementation facilitation skills. Implement. Sci. Commun. 2020, 1, 25. [Google Scholar] [CrossRef]
- Moore, J.L.; Bjørkli, C.; Havdahl, R.T.; Lømo, L.L.; Midthaug, M.; Skjuve, M.; Klokkerud, M.; Nordvik, J.E. A qualitative study exploring contributors to the success of a community of practice in rehabilitation. BMC Med. Educ. 2021, 21, 282. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.H.; Majnemer, A.; Pietrangelo, F.; Dickson, L.; Shikako, K.; Dahan-Oliel, N.; Steven, E.; Iliopoulos, G.; Ogourtsova, T. Evidence-based early rehabilitation for children with cerebral palsy: Co-development of a multifaceted knowledge translation strategy for rehabilitation professionals. Front. Rehabil. Sci. 2024, 5, 1413240. [Google Scholar] [CrossRef] [PubMed]
- Yamada, J.; Shorkey, A.; Barwick, M.; Widger, K.; Stevens, B.J. The effectiveness of toolkits as knowledge translation strategies for integrating evidence into clinical care: A systematic review. BMJ Open 2015, 5, e006808. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Amatya, B.; Elmalik, A.; Song, K.; Diaz, D.; Dickinson, M. Embedding rehabilitation into cancer care continuum: An implementation study. J. Rehabil. Med. 2024, 56, jrm40855. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).