Medium-Frequency Neuromuscular Electrical Stimulation in Critically Ill Patients Promoted Larger Functional Capacity Improvement During Recovery than Low-Frequency Neuromuscular Electrical Stimulation: Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Intervention
2.3.1. Standard Physical Therapy
2.3.2. Low- and Medium-Frequency Electrical Stimulation
3. Outcome Measures
3.1. Clinical Assessment of Muscle Strength
3.2. Handgrip Strength
3.3. Functional Status
3.4. Timed Up and Go Test
3.5. Barthel Index
3.6. Quality of Life
3.7. Patient Clinical Registry
4. Statistical Analysis
4.1. Power Calculation and Sample Size
4.2. Data Analysis
5. Results
5.1. Participants Characteristics
5.2. Admission Diagnosis, Comorbidities and Pharmacological Treatment
5.3. Muscle Strength and Handgrip Strength
5.4. Functional Status
5.5. Functional Mobility and Dynamic Balance
5.6. Independence for Activities of Daily Living
5.7. Quality of Life
5.8. Physical Therapy Sessions and Days in MV, ICU and in Hospital
6. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lad, H.; Saumur, T.M.; Herridge, M.S.; dos Santos, C.C.; Mathur, S.; Batt, J.; Gilbert, P.M. Intensive Care Unit-Acquired Weakness: Not Just Another Muscle Atrophying Condition. Int. J. Mol. Sci. 2020, 21, 7840. [Google Scholar] [CrossRef]
- Pravda, J. Metabolic theory of septic shock. World J. Crit. Care Med. 2014, 3, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G. Metabolic Response to Injury and Sepsis: Changes in Protein Metabolism. Nutrition 1997, 13 (Suppl. 9), 52S–57S. [Google Scholar] [CrossRef] [PubMed]
- Preiser, J.-C.; Ichai, C.; Orban, J.-C.; Groeneveld, A.B.J. Metabolic response to the stress of critical illness. Br. J. Anaesth. 2014, 113, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Reid, C.L.; Campbell, I.T.; Little, R.A. Muscle wasting and energy balance in critical illness. Clin. Nutr. 2004, 23, 273–280. [Google Scholar] [CrossRef]
- Rocheteau, P.; Chatre, L.; Briand, D.; Mebarki, M.; Jouvion, G.; Bardon, J.; Crochemore, C.; Serrani, P.; Lecci, P.P.; Latil, M.; et al. Sepsis induces long-term metabolic and mitochondrial muscle stem cell dysfunction amenable by mesenchymal stem cell therapy. Nat. Commun. 2015, 6, 10145. [Google Scholar] [CrossRef]
- De Jonghe, B.; Bastuji-Garin, S.; Durand, M.-C.; Malissin, I.; Rodrigues, P.; Cerf, C.; Outin, H.; Sharshar, T. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness*. Crit. Care Med. 2007, 35, 2007–2015. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- de Jonghe, B.; Lacherade, J.-C.; Sharshar, T.; Outin, H. Intensive care unit-acquired weakness: Risk factors and prevention. Crit. Care Med. 2009, 37, S309–S315. [Google Scholar] [CrossRef]
- Appleton, R.T.; Kinsella, J.; Quasim, T. The incidence of intensive care unit-acquired weakness syndromes: A systematic review. J. Intensiv. Care Soc. 2014, 16, 126–136. [Google Scholar] [CrossRef]
- Schreiber, A.; Bertoni, M.; Goligher, E.C. Avoiding Respiratory and Peripheral Muscle Injury During Mechanical Ventilation. Crit. Care Clin. 2018, 34, 357–381. [Google Scholar] [CrossRef]
- Apostolakis, E.; Papakonstantinou, N.A.; Baikoussis, N.G.; Papadopoulos, G. Intensive care unit-related generalized neuromuscular weakness due to critical illness polyneuropathy/myopathy in critically ill patients. J. Anesthesia 2014, 29, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Batt, J.; Herridge, M.S.; dos Santos, C.C. From skeletal muscle weakness to functional outcomes following critical illness: A translational biology perspective. Thorax 2019, 74, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Edbrooke, D.L.; Minelli, C.; Mills, G.H.; Iapichino, G.; Pezzi, A.; Corbella, D.; Jacobs, P.; Lippert, A.; Wiis, J.; Pesenti, A.; et al. Implications of ICU triage decisions on patient mortality: A cost-effectiveness analysis. Crit. Care 2011, 15, R56. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, M. Intensive care unit-acquired weakness: Recent insights. J. Intensiv. Med. 2023, 4, 73–80. [Google Scholar] [CrossRef]
- Zorowitz, R.D. ICU–Acquired Weakness. Chest 2016, 150, 966–971. [Google Scholar] [CrossRef]
- Fontela, P.C.; Abdala, F.A.N.B.; Forgiarini, S.G.I.; Júnior, L.A.F. Quality of life in survivors after a period of hospitalization in the intensive care unit: A systematic review. Rev. Bras. Ter. Intensiv. 2018, 30, 496–507. [Google Scholar] [CrossRef]
- Gardner, A.K.; Ghita, G.L.; Wang, Z.; Ozrazgat-Baslanti, T.; Raymond, S.L.; Mankowski, R.T.; Brumback, B.A.; Efron, P.A.; Bihorac, A.; Moore, F.A.; et al. The Development of Chronic Critical Illness Determines Physical Function, Quality of Life, and Long-Term Survival Among Early Survivors of Sepsis in Surgical ICUs*. Crit. Care Med. 2019, 47, 566–573. [Google Scholar] [CrossRef]
- Herridge, M.S.; Tansey, C.M.; Matté, A.; Tomlinson, G.; Diaz-Granados, N.; Cooper, A.; Guest, C.B.; Mazer, C.D.; Mehta, S.; Stewart, T.E.; et al. Functional Disability 5 Years after Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2011, 364, 1293–1304. [Google Scholar] [CrossRef]
- Potcovaru, C.G.; Salmen, T.; Potcovaru, A.M.; Săndulescu, I.M.; Chiriac, O.; Balasa, A.C.; Diaconu, L.S.; Poenaru, D.; Stoian, A.P.; Cinteza, D.; et al. The Long-Term Impact of COVID-19 on Disability after Post-Acute Rehabilitation: A Pilot Study. J. Clin. Med. 2024, 13, 4694. [Google Scholar] [CrossRef]
- Bolton, C.E.; Bevan-Smith, E.F.; Blakey, J.D.; Crowe, P.; Elkin, S.L.; Garrod, R.; Greening, N.J.; Heslop, K.; Hull, J.H.; Man, W.D.-C.; et al. British Thoracic Society guideline on pulmonary rehabilitation in adults: Accredited by NICE. Thorax 2013, 68, ii1–ii30. [Google Scholar] [CrossRef]
- Quittan, M.; Wiesinger, G.F.; Sturm, B.; Puig, S.; Mayr, W.; Sochor, A.; Paternostro, T.; Resch, K.L.; Pacher, R.; Fialka-Moser, V. Improvement of Thigh Muscles by Neuromuscular Electrical Stimulation in Patients with Refractory Heart Failure. Am. J. Phys. Med. Rehabilit. 2001, 80, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Gruther, W.; Zorn, C.; Paternostro-Sluga, T.; Quittan, M.; Spiss, C.; Kainberger, F.; Crevenna, R.; Fialka-Moser, V. Effects of neuromuscular electrical stimulation on muscle layer thickness of knee extensor muscles in intensive care unit patients: A pilot study. J. Rehabilit. Med. 2010, 42, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Vega, P.; Cuyul-Vásquez, I.; Artigas-Arias, M.; Muñoz-Cofre, R.; Curi, R.; Marzuca-Nassr, G.N. Physical Therapy Protocols to Attenuate Skeletal Muscle Atrophy in Critically ill Patients: Narrative Review. Int. J. Morphol. 2022, 40, 640–649. [Google Scholar] [CrossRef]
- Zaidman, C.M.; Malkus, E.C.; Siener, C.; Florence, J.; Pestronk, A.; Al-Lozi, M. Qualitative and quantitative skeletal muscle ultrasound in late-onset acid maltase deficiency. Muscle Nerve 2011, 44, 418–423. [Google Scholar] [CrossRef]
- Watanabe, K.; Kawade, S.; Moritani, T. Effect of electrode position of low intensity neuromuscular electrical stimulation on the evoked force in the quadriceps femoris muscle. BMC Res. Notes 2017, 10, 300. [Google Scholar] [CrossRef][Green Version]
- Eggmann, S.; Verra, M.L.; Luder, G.; Takala, J.; Jakob, S.M. Effects of early, combined endurance and resistance training in mechanically ventilated, critically ill patients: A study protocol for a randomised controlled trial. Trials 2016, 17, 403. [Google Scholar] [CrossRef]
- Mettler, J.A.; Magee, D.M.; Doucet, B.M. High-Frequency Neuromuscular Electrical Stimulation Increases Anabolic Signaling. Med. Sci. Sports Exerc. 2018, 50, 1540–1548. [Google Scholar] [CrossRef]
- A Maffiuletti, N.; Roig, M.; Karatzanos, E.; Nanas, S. Neuromuscular electrical stimulation for preventing skeletal-muscle weakness and wasting in critically ill patients: A systematic review. BMC Med. 2013, 11, 137. [Google Scholar] [CrossRef]
- Pinfildi, C.E.; Andraus, R.A.C.; Iida, L.M.; Prado, R.P. Neuromuscular electrical stimulation of medium and low frequency on the quadriceps femoris. Acta Ortop. Bras. 2018, 26, 346–349. [Google Scholar] [CrossRef]
- Ward, A.R.; Shkuratova, N. Russian electrical stimulation: The early experiments. Phys. Ther. 2002, 82, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Maffiuletti, N.A. Physiological and methodological considerations for the use of neuromuscular electrical stimulation. Eur. J. Appl. Physiol. 2010, 110, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Dirks, M.L.; Hansen, D.; Van Assche, A.; Dendale, P.; Van Loon, L.J.C. Neuromuscular electrical stimulation prevents muscle wasting in critically ill comatose patients. Clin. Sci. 2015, 128, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Gerovasili, V.; Stefanidis, K.; Vitzilaios, K.; Karatzanos, E.; Politis, P.; Koroneos, A.; Chatzimichail, A.; Routsi, C.; Roussos, C.; Nanas, S. Electrical muscle stimulation preserves the muscle mass of critically ill patients: A randomized study. Crit. Care 2009, 13, R161. [Google Scholar] [CrossRef] [PubMed]
- Truong, A.D.; Fan, E.; Brower, R.G.; Needham, D.M. Bench-to-bedside review: Mobilizing patients in the intensive care unit—From pathophysiology to clinical trials. Crit. Care 2009, 13, 216. [Google Scholar] [CrossRef]
- Laghi, F.; Jubran, A. Treating the septic muscle with electrical stimulations*. Crit. Care Med. 2011, 39, 585–586. [Google Scholar] [CrossRef]
- Jones, S.; Man, W.D.; Gao, W.; Higginson, I.J.; Wilcock, A.; Maddocks, M. Neuromuscular electrical stimulation for muscle weakness in adults with advanced disease. Cochrane Database Syst. Rev. 2016, 10, CD009419. [Google Scholar] [CrossRef]
- Parry, S.M.; Puthucheary, Z.A. The impact of extended bed rest on the musculoskeletal system in the critical care environment. Extreme Physiol. Med. 2015, 4, 16. [Google Scholar] [CrossRef]
- Patsaki, I.; Gerovasili, V.; Sidiras, G.; Karatzanos, E.; Mitsiou, G.; Papadopoulos, E.; Christakou, A.; Routsi, C.; Kotanidou, A.; Nanas, S. Effect of neuromuscular stimulation and individualized rehabilitation on muscle strength in Intensive Care Unit survivors: A randomized trial. J. Crit. Care 2017, 40, 76–82. [Google Scholar] [CrossRef]
- García-Peña, C.; García-Fabela, L.C.; Gutiérrez-Robledo, L.M.; García-González, J.J.; Arango-Lopera, V.E.; Pérez-Zepeda, M.U.; Bayer, A. Handgrip Strength Predicts Functional Decline at Discharge in Hospitalized Male Elderly: A Hospital Cohort Study. PLoS ONE 2013, 8, e69849. [Google Scholar] [CrossRef]
- Mohamed-Hussein, A.A.R.; Makhlouf, H.A.; Selim, Z.I.; Saleh, W.G. Association between hand grip strength with weaning and intensive care outcomes in COPD patients: A pilot study. Clin. Respir. J. 2018, 12, 2475–2479. [Google Scholar] [CrossRef] [PubMed]
- González-Seguel, F.; Camus-Molina, A.; Leppe, J.; Hidalgo-Cabalín, V.; Gutiérrez-Panchana, T.; Needham, D.M.; Guimarães, F.S. Chilean version of the Functional Status Score for the Intensive Care Unit: A translation and cross-cultural adaptation. Medwave 2019, 19, e7439. [Google Scholar] [CrossRef]
- Knobe, M.; Giesen, M.; Plate, S.; Gradl-Dietsch, G.; Buecking, B.; Eschbach, D.; van Laack, W.; Pape, H.-C. The Aachen Mobility and Balance Index to measure physiological falls risk: A comparison with the Tinetti POMA Scale. Eur. J. Trauma Emerg. Surg. 2016, 42, 537–545. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Chrispin, P.S.; Scotton, H.; Rogers, J.; Lloyd, D.; Ridley, S.A. Short Form 36 in the intensive care unit: Assessment of accepta-bility, reliability and validity of the questionnaire. Anaesthesia 1997, 52, 15–23. [Google Scholar] [CrossRef]
- Karatzanos, E.; Gerovasili, V.; Zervakis, D.; Tripodaki, E.-S.; Apostolou, K.; Vasileiadis, I.; Papadopoulos, E.; Mitsiou, G.; Tsimpouki, D.; Routsi, C.; et al. Electrical Muscle Stimulation: An Effective Form of Exercise and Early Mobilization to Preserve Muscle Strength in Critically Ill Patients. Crit. Care Res. Pr. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Phadke, R.; Dew, T.; Sidhu, P.S.; et al. Acute skeletal muscle wasting in critical illness. JAMA 2013, 310, 1591–1600. [Google Scholar] [CrossRef]
- Parry, S.M.; El-Ansary, D.; Cartwright, M.S.; Sarwal, A.; Berney, S.; Koopman, R.; Annoni, R.; Puthucheary, Z.; Gordon, I.R.; Morris, P.E.; et al. Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J. Crit. Care 2015, 30, 1151.e9–1151.e14. [Google Scholar] [CrossRef]
- Ferrante, L.E.; Pisani, M.A.; Murphy, T.E.; Gahbauer, E.A.; Leo-Summers, L.S.; Gill, T.M. Factors associated with functional recovery among older intensive care unit survivors. Am. J. Respir. Crit. Care Med. 2016, 194, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda-Jofré, P.; Guerra-Vega, P.; Fu, C.; Marzuca-Nassr, G.N. Skeletal muscle atrophy in critical ill patients and the use of electrical stimulation as a treatment strategy: Recommendations for clinical practice. Trends Anaesth. Crit. Care 2021, 40, 14–22. [Google Scholar] [CrossRef]
- Gerovasili, V.; Routsi, C.; Vasileiadis, I.; Karatzanos, E.; Pitsolis, T.; Tripodaki, E.S.; Markaki, V.; Zervakis, D.; Nanas, S. Electrical muscle stimulation prevents critical illness polyneuromyopathy: A randomized parallel intervention trial. Crit. Care 2010, 14, R74. [Google Scholar] [CrossRef]
- Xu, C.; Yang, F.; Wang, Q.; Gao, W. Effect of neuromuscular electrical stimulation in critically ill adults with mechanical ventilation: A systematic review and network meta-analysis. BMC Pulm. Med. 2024, 24, 56. [Google Scholar] [CrossRef]
- Kho, M.E.; Truong, A.D.; Zanni, J.M.; Ciesla, N.D.; Brower, R.G.; Palmer, J.B.; Needham, D.M. Neuromuscular electrical stimulation in mechanically ventilated patients: A randomized, sham-controlled pilot trial with blinded outcome assessment. J. Crit. Care 2015, 30, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Wollersheim, T.; Grunow, J.J.; Carbon, N.M.; Haas, K.; Malleike, J.; Ramme, S.F.; Schneider, J.; Spies, C.D.; Märdian, S.; Mai, K.; et al. Muscle wasting and function after muscle activation and early protocol-based physiotherapy: An explorative trial. J. Cachex-Sarcopenia Muscle 2019, 10, 734–747. [Google Scholar] [CrossRef] [PubMed]
- Nonoyama, T.; Shigemi, H.; Kubota, M.; Matsumine, A.; Shigemi, K.; Ishizuka, T. Neuromuscular electrical stimulation in the intensive care unit prevents muscle atrophy in critically ill older patients: A retrospective cohort study. Medicine 2022, 101, e29451. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2004, 19, 1–22. [Google Scholar] [CrossRef]
- Symons, T.B.; Sheffield-Moore, M.; Wolfe, R.R.; Paddon-Jones, D. A Moderate Serving of High-Quality Protein Maximally Stimulates Skeletal Muscle Protein Synthesis in Young and Elderly Subjects. J. Am. Diet. Assoc. 2009, 109, 1582–1586. [Google Scholar] [CrossRef]
- Nakanishi, N.; Yoshihiro, S.M.; Kawamura, Y.; Aikawa, G.R.; Shida, H.; Shimizu, M.; Fujinami, Y.; Matsuoka, A.; Watanabe, S.; Taito, S.; et al. Effect of Neuromuscular Electrical Stimulation in Patients With Critical Illness: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Care Med. 2023, 51, 1386–1396. [Google Scholar] [CrossRef]
- Campos, D.R.; Bueno, T.B.C.; Anjos, J.S.G.G.; Zoppi, D.; Dantas, B.G.; Gosselink, R.P.; Guirro, R.R.J.; Borges, M.C. Early Neuromuscular Electrical Stimulation in Addition to Early Mobilization Improves Functional Status and Decreases Hospitalization Days of Critically Ill Patients. Crit. Care Med. 2022, 50, 1116–1126. [Google Scholar] [CrossRef]
- Tsuchikawa, Y.; Tanaka, S.; Kasugai, D.; Nakagawa, R.; Shimizu, M.; Inoue, T.; Nagaya, M.; Nasu, T.; Omote, N.; Higashi, M.; et al. Effects of acute phase intensive electrical muscle stimulation in COVID-19 patients requiring invasive mechanical ventilation: An observational case-control study. Sci. Rep. 2024, 14, 5254. [Google Scholar] [CrossRef]
- Eggmann, S.; Luder, G.; Verra, M.L.; Irincheeva, I.; Bastiaenen, C.H.G.; Jakob, S.M.; Scherag, A. Functional ability and quality of life in critical illness survivors with intensive care unit acquired weakness: A secondary analysis of a randomised controlled trial. PLoS ONE 2020, 15, e0229725. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.-L.; Wang, L.-Y.; Wu, C.-P.; Wu, H.-D.; Wu, Y.-T. Effects of Physical Training on Functional Status in Patients With Prolonged Mechanical Ventilation. Phys. Ther. 2006, 86, 1271–1281. [Google Scholar] [CrossRef]
- A Doiron, K.; Hoffmann, T.C.; Beller, E.M. Cochrane Emergency and Critical Care Group Early intervention (mobilization or active exercise) for critically ill adults in the intensive care unit. Cochrane Database Syst. Rev. 2018, 2018, CD010754. [Google Scholar]
- Bohannon, R.W. Hand-Grip Dynamometry Predicts Future Outcomes in Aging Adults. J. Geriatr. Phys. Ther. 2008, 31, 3–10. [Google Scholar] [CrossRef]
- Vanpee, G.; Hermans, G.; Segers, J.; Gosselink, R. Assessment of Limb Muscle Strength in Critically Ill Patients. Crit. Care Med. 2014, 42, 701–711. [Google Scholar] [CrossRef]
- Lee, J.J.; Waak, K.; Grosse-Sundrup, M.; Xue, F.; Lee, J.; Chipman, D.; Ryan, C.; Bittner, E.A.; Schmidt, U.; Eikermann, M. Global Muscle Strength But Not Grip Strength Predicts Mortality and Length of Stay in a General Population in a Surgical Intensive Care Unit. Phys. Ther. 2012, 92, 1546–1555. [Google Scholar] [CrossRef]
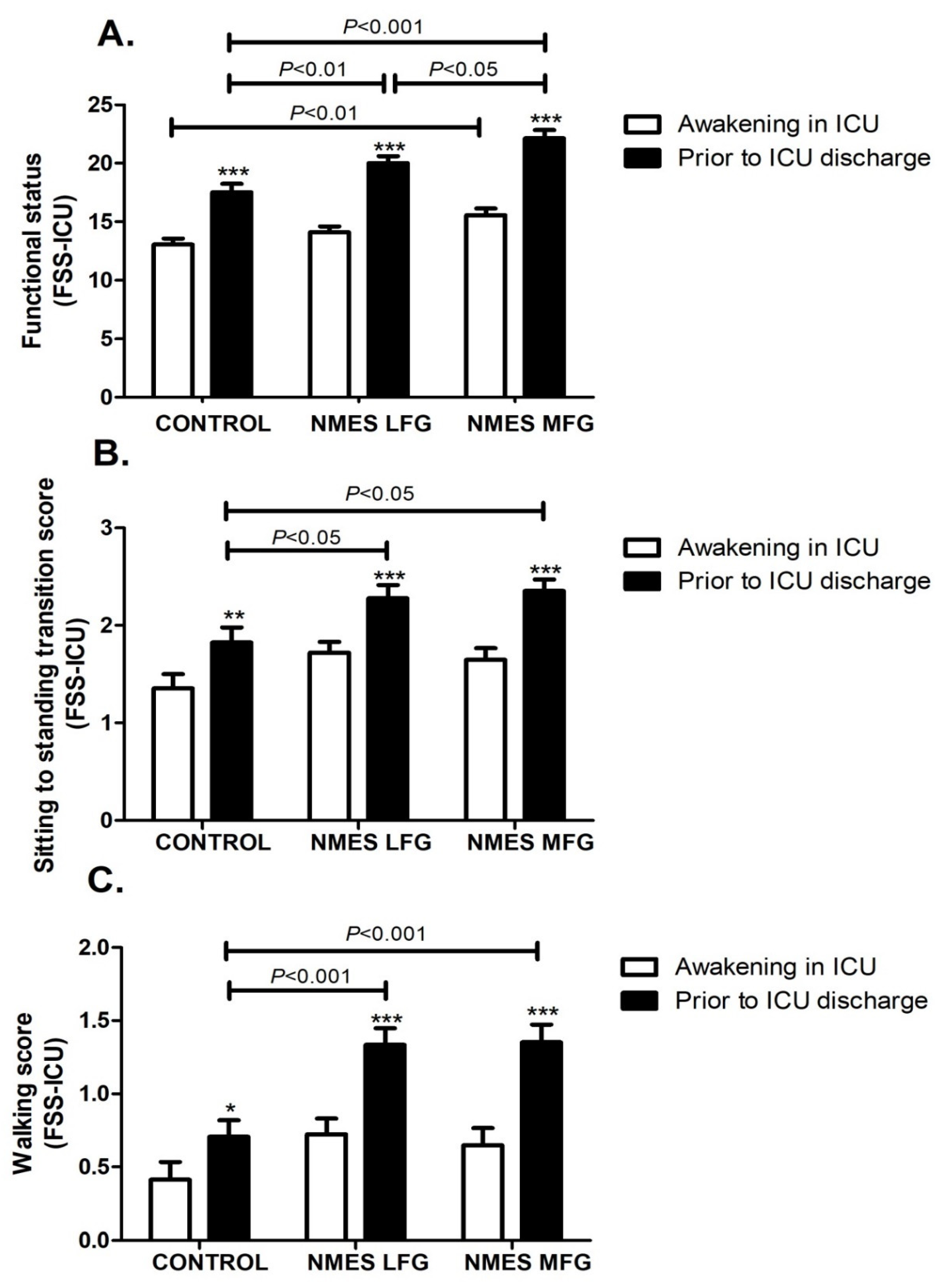


| CONTROL (n = 17) | NMES LFG (n = 18) | NMES MFG (n = 17) | p Value | |
|---|---|---|---|---|
| Women | 9 | 6 | 8 | |
| Men | 8 | 12 | 9 | |
| Age (years) | 53 ± 18 | 62 ± 11 | 59 ± 16 | 0.205 |
| Height (cm) | 164 ± 6.1 | 165 ± 8.3 | 166 ± 6.6 | 0.654 |
| Weight (kg) | 70 ± 8.2 | 76 ± 10.4 | 75 ± 8.1 | 0.099 |
| BMI | 25.8 ± 2.6 | 27.9 ± 2.8 | 27.1 ± 1.7 | 0.054 |
| APACHE II | 31 ± 2 | 33 ± 3 | 31 ± 3 | 0.182 |
| SOFA | 14 ± 1 | 14 ± 1 | 15 ± 2 | 0.117 |
| Awakening in ICU | Prior to ICU Discharge | Prior to IMCU Discharge | Prior to Hospital Discharge | |
|---|---|---|---|---|
| Control group | ||||
| MRC-SS | 33.3 ± 1.8 | 36.9 ± 1.7 | 41.4 ± 2.1 | 46.0 ± 2.5 |
| ICUAW, n (%) | 17 (100%) | 17 (100%) | 17 (100%) | 9 (53%) |
| Knee extension (MRC) | 5.5 + 0.7 | 6.5 ± 0.7 | 6.8 ±0.8 | 7.5 ± 0.7 |
| Handgrip strength (kg) | 9.0 ± 0.9 | 9.9 ± 0.9 | 11.6 ± 1.0 | 13.7 ± 0.9 |
| NMES LFG | ||||
| MRC-SS | 35.3 ± 2.7 | 38.7 ± 3.0 | 42.7 ± 3.2 | 48.2 ± 3.2 |
| ICUAW, n (%) | 18 (100%) | 18 (100%) | 16 (89%) | 6 (33%) |
| Knee extension (MRC) | 6.4 ± 0.5 *** | 7.4 ± 0.5 *** | 7.8 ± 0.7 *** | 8.4 ± 0.5 *** |
| Handgrip strength (kg) | 9.3 ± 1.2 | 10.7 ± 1.5 | 12.6 ± 1.6 | 14.9 ± 1.9 * |
| NMES MFG | ||||
| MRC-SS | 35.6 ± 2.9 * | 39.6 ± 2.8 * | 44.5 ± 2.9 ** | 49.9 ± 3.0 *** |
| ICUAW, n (%) | 17 (100%) | 17 (100%) | 15 (88%) | 1 (6%) |
| Knee extension (MRC) | 6.6 ± 0.5 *** | 7.6 ± 0.5 *** | 8.3 ± 0.7 ***,# | 8.9 ± 0.5 *** |
| Handgrip strength (kg) | 9.8 ± 1.2 | 11.2 ± 1.2 * | 13.3 ± 1.5 ** | 15.9 ± 2.1 *** |
| Prior to IMCU Discharge | Prior Hospital Discharge | |
|---|---|---|
| Control group | ||
| TUG test (s) | 26.5 ± 2.4 | 22.5 ± 2.5 |
| NMES LFG | ||
| TUG test (s) | 23.8 ± 2.5 * | 20.0 ± 2.4 * |
| NMES MFG | ||
| TUG test (s) | 21.1 ± 1.8 **,# | 16.8 ± 1.8 **,## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra-Vega, P.; Guzmán, R.; Betancourt, C.; Grage, M.; Vera, C.; Artigas-Arias, M.; Muñoz-Cofré, R.; Vitzel, K.F.; Marzuca-Nassr, G.N. Medium-Frequency Neuromuscular Electrical Stimulation in Critically Ill Patients Promoted Larger Functional Capacity Improvement During Recovery than Low-Frequency Neuromuscular Electrical Stimulation: Randomized Clinical Trial. J. Clin. Med. 2025, 14, 5407. https://doi.org/10.3390/jcm14155407
Guerra-Vega P, Guzmán R, Betancourt C, Grage M, Vera C, Artigas-Arias M, Muñoz-Cofré R, Vitzel KF, Marzuca-Nassr GN. Medium-Frequency Neuromuscular Electrical Stimulation in Critically Ill Patients Promoted Larger Functional Capacity Improvement During Recovery than Low-Frequency Neuromuscular Electrical Stimulation: Randomized Clinical Trial. Journal of Clinical Medicine. 2025; 14(15):5407. https://doi.org/10.3390/jcm14155407
Chicago/Turabian StyleGuerra-Vega, Pablo, Rodrigo Guzmán, Claudio Betancourt, Mario Grage, Cristian Vera, Macarena Artigas-Arias, Rodrigo Muñoz-Cofré, Kaio F. Vitzel, and Gabriel Nasri Marzuca-Nassr. 2025. "Medium-Frequency Neuromuscular Electrical Stimulation in Critically Ill Patients Promoted Larger Functional Capacity Improvement During Recovery than Low-Frequency Neuromuscular Electrical Stimulation: Randomized Clinical Trial" Journal of Clinical Medicine 14, no. 15: 5407. https://doi.org/10.3390/jcm14155407
APA StyleGuerra-Vega, P., Guzmán, R., Betancourt, C., Grage, M., Vera, C., Artigas-Arias, M., Muñoz-Cofré, R., Vitzel, K. F., & Marzuca-Nassr, G. N. (2025). Medium-Frequency Neuromuscular Electrical Stimulation in Critically Ill Patients Promoted Larger Functional Capacity Improvement During Recovery than Low-Frequency Neuromuscular Electrical Stimulation: Randomized Clinical Trial. Journal of Clinical Medicine, 14(15), 5407. https://doi.org/10.3390/jcm14155407






