Abstract
Pre-oxygenation is the key step prior to endotracheal intubation, particularly in a critically ill patient, to prevent life-threatening peri-procedural hypoxemia. This narrative review explores the emerging interest of Non-Invasive Positive Pressure Ventilation (NIPPV) as a pre-oxygenation modality in the intensive care unit (ICU) context. We reviewed data from randomized controlled trials (RCTs) and observational studies published from 2000 to 2024 that compare NIPPV to conventional oxygen therapy and High Flow Nasal Cannula Oxygen (HFNCO). The pathophysiological mechanisms for the successful use of NIPPV, including alveolar recruitment, the decrease of shunting, and the maintenance of functional residual capacity, were reviewed in depth. Existing studies show that NIPPV significantly prolongs the apnea time, reduces the rate of peri-intubation severe hypoxaemia in selected patients and is especially effective for patients with acute hypoxaemic respiratory failure. Nevertheless, appropriate patient selection is still crucial because some diseases can contraindicate or even be harmful with NIPPV. We further discussed the practical aspects of how to use this ventilatory support (the best ventilator settings, which interface, and when to apply it). We lastly discuss unanswered questions and offer suggestions and opportunities for future exploration in guiding the role of NIPPV use in the pre-oxygenation of the critically ill patient requiring emergent airway management.
1. Introduction
Endotracheal intubation is a high-risk procedure in critically ill patients, with complications occurring in up to 40% of cases [1]. Among these complications, cardiovascular collapse and severe hypoxemia represent the most life-threatening events, associated with cardiac arrest, neurological damage, and increased mortality [2,3]. Pre-oxygenation before intubation aims to extend the safe apnea time by maximizing oxygen reserves, primarily within the functional residual capacity, thus providing a critical safety buffer during the apneic period of airway instrumentation [4]. Conventional pre-oxygenation techniques using a face mask with 100% oxygen via a Mapleson circuit have been the standard of care for decades [5]. However, these approaches often prove inadequate in critically ill patients due to ventilation-perfusion mismatch, shunt, increased oxygen consumption, and reduced functional residual capacity [6,7]. Critically ill patients, now characterized as having a “physiologically difficult airway” [8], often present with baseline hypoxemia that makes them particularly vulnerable during intubation attempts, with reported desaturation rates as high as 50% despite standard pre-oxygenation techniques [9]. Non-invasive positive pressure ventilation (NIPPV) has emerged as a promising alternative for pre-oxygenation in this challenging population. By delivering positive pressure throughout the respiratory cycle, NIPPV can recruit collapsed alveoli, reduce shunting, and maintain functional residual capacity, potentially addressing the limitations of conventional approaches [10,11]. The first significant study exploring this concept was published by Baillard et al. in 2006, demonstrating that NIPPV improved oxygenation parameters either before and after intubation compared to standard face mask oxygenation [12]. Since this landmark study, numerous investigations have assessed the efficacy of NIPPV for pre-oxygenation in various clinical scenarios. Jaber et al. reported in a multicenter randomized controlled trial that non-invasive ventilation combined with post-intubation recruitment maneuvers significantly reduced the incidence of severe hypoxemia during intubation of critically ill patients [13]. These findings were supported by a meta-analysis by Fong et al., which showed a significant reduction in peri-intubation desaturation events with NIPPV compared to conventional methods [14]. The diffusion of high-flow nasal cannula oxygen (HFNCO) therapy has further complicated the pre-oxygenation landscape. Unlike NIPPV, HFNCO may allow continuous oxygenation during laryngoscopy, potentially offering advantages through apneic oxygenation [15,16,17]. Comparisons between NIPPV and HFNCO have yielded conflicting results, with some studies favoring NIPPV for patients with severe baseline hypoxemia [18], while others suggest comparable efficacy with potentially better tolerance for HFNCO [19]. The decisional crossroad is probably the residual amount of available functional residual capacity. Patient selection appears crucial when considering the modality of pre-oxygenation.
Certain patient populations, such as those with hypercapnic respiratory failure or acute respiratory distress syndrome (ARDS), may benefit more substantially from NIPPV [6,20]. Conversely, NIPPV may be less effective or even counterproductive in patients with hemodynamic instability, altered mental status, or upper airway obstruction [21]. The technical aspects of NIPPV delivery—including interface selection, pressure settings, and duration of application—vary considerably across studies, complicating the interpretation of results and establishment of standardized protocols [22]. Furthermore, the potential adverse effects of NIPPV, such as gastric insufflation, aspiration risk, and hemodynamic compromise, must be carefully weighed against its benefits [22,23].
The focus of this narrative review is to critically discuss ill patients who require intubation and address whether pre-oxygenation with NIPPV offers a reliable alternative to the standard approach. We will discuss the physiologic basis, clinical results, practical considerations, pitfalls, and caveats of such a strategy and curate the available evidence in this rapidly emerging field and the future of research.
2. Methods
This narrative review aimed to examine the evidence for non-invasive positive pressure ventilation (NIPPV) as a pre-oxygenation modality in critically ill patients before intubation. We conducted a comprehensive literature search of PubMed, EMBASE, and Cochrane Library databases from January 2000 to March 2024. Search terms included combinations of “non-invasive ventilation,” “non-invasive positive pressure ventilation,” “NIPPV,” “NIV,” “pre-oxygenation,” “preoxygenation,” “intubation,” “critically ill,” “intensive care,” and “emergency.”
We included randomized controlled trials, observational studies, systematic reviews, and meta-analyses that investigated NIPPV for pre-oxygenation in adult critically ill patients requiring intubation. Studies comparing NIPPV to conventional oxygen therapy, high-flow nasal cannula oxygen, or combined approaches were prioritized. We excluded pediatric studies, animal studies, case reports, and non-English publications.
Two authors independently screened titles and abstracts, followed by a full-text review of potentially relevant articles. Disagreements were resolved through discussion with a third author. We extracted data on study design, patient populations, interventions, comparators, outcomes (particularly oxygenation parameters, desaturation events, and complications), and methodological quality. The quality of evidence was assessed using the Oxford Centre for Evidence-Based Medicine levels of evidence framework, with randomized controlled trials and meta-analyses given the highest priority in formulating recommendations. Studies were also evaluated for risk of bias using appropriate tools based on study design.
3. Physiological Basis for NIPPV in Pre-Oxygenation
During apnea following induction of anesthesia, oxygen consumption continues at approximately 250–300 mL/min in critically ill patients, while carbon dioxide production generates about 200 mL/min [24].
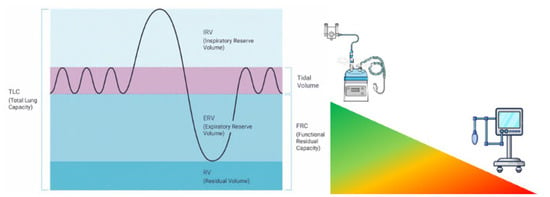
Oxygen reserves are primarily stored in three compartments: the lungs (specifically the functional residual capacity, FRC), blood, and tissues, with the FRC representing the largest readily available oxygen reservoir [25]. In healthy adults breathing room air, the FRC contains approximately 2–2.5 L of air, equal to 450 mL of oxygen, which may increase to about 3000 mL after adequate pre-oxygenation with 100% oxygen [26]. This oxygen reserve typically allows 8–10 min of apnea before critical desaturation occurs in healthy individuals, but this duration is dramatically shortened in critically ill patients [4].
Conventional pre-oxygenation using a face mask with 100% oxygen via a Mapleson circuit faces significant limitations in the critically ill population. These patients often present with shunt fraction as high as 20–30% due to atelectasis, pulmonary edema, or pneumonia, compared to 1–2% in healthy individuals [27]. Additionally, critically ill patients frequently exhibit increased oxygen consumption from systemic inflammation, work of breathing, and catecholamine surge [28]. Their FRC is further compromised by supine positioning, obesity, pulmonary edema, and atelectasis [29]. Together, these factors create a “perfect storm” that significantly reduces the effectiveness of standard pre-oxygenation techniques, resulting in rapid desaturation during intubation attempts [9]. NIPPV addresses these physiological limitations through several mechanisms. First, the application of positive pressure throughout the respiratory cycle—particularly positive end-expiratory pressure (PEEP)—recruits collapsed alveoli and prevents derecruitment, effectively reducing shunt fraction [30]. Studies have demonstrated that NIPPV can increase end-expiratory lung volume by 10–25% in patients with acute respiratory failure [31]. Second, NIPPV maintains and potentially increases FRC, creating a larger oxygen reservoir [32]. Third, the inspiratory pressure support component of NIPPV enhances tidal volume and alveolar ventilation, facilitating more efficient oxygen loading and carbon dioxide elimination [33]. This results in higher arterial oxygen content prior to apnea, extending the safe apnea time. The physiological advantages of NIPPV over conventional oxygen delivery methods are substantial. Unlike standard face mask oxygenation, which relies on the patient’s respiratory efforts and cannot overcome the negative effects of atelectasis, NIPPV actively recruits lung units and supports ventilation [34]. Compared to HFNCO, NIPPV typically generates higher and more consistent positive airway pressures (10–15 cmH2O vs. a flow-dependent 2–5 cmH2O with HFNCO), resulting in superior alveolar recruitment [35]. However, HFNCO has the advantage of continuing oxygenation during laryngoscopy, whereas NIPPV must be discontinued [16]. Physiologically, NIPPV also differs from apneic oxygenation techniques that maintain oxygen flow during laryngoscopy. While apneic techniques rely on the mass flow of oxygen from the pharynx to the alveoli via diffusion, NIPPV actively ventilates the lungs before apnea [36]. The combination of these approaches—NIPPV for pre-oxygenation followed by apneic oxygenation during laryngoscopy—represents a physiologically sound strategy that addresses both the pre-intubation oxygen loading and the ongoing oxygen consumption during the procedure [13].
4. Evidence Base for NIPPV Pre-Oxygenation
The rationale for the use of NIPPV as a technique of pre-oxygenation has been fleshed out well over the last 20 years, moving from physiology to large RCTs. The idea was initially developed by observational studies concluding that PEEP had some beneficial effects on gas exchange in the critically ill. The landmark study by Baillard et al. in 2006 [12] marked the first significant clinical investigation specifically examining NIPPV for pre-oxygenation, probably standing between the first paper highlighting the difference between anatomically and physiologically difficult airways [37]. This prospective randomized study of 53 hypoxemic patients requiring intubation in the ICU compared non-invasive pressure support ventilation (PSV 5–15 cmH2O, PEEP 5 cmH2O) with standard bag-valve-mask pre-oxygenation. The results were striking: the NIPPV group achieved significantly higher mean SpO2 after pre-oxygenation (98 ± 2% vs. 93 ± 6%, p < 0.001) and maintained better oxygenation during intubation (93 ± 8% vs. 81 ± 15%, p < 0.001). Most importantly, the incidence of severe desaturation (SpO2 < 80%) decreased dramatically from 46% to 7% in the NIPPV group. This study established the proof of concept that would drive subsequent research. More interestingly, the research highlighted that arterial oxygen partial pressure remained higher during and 30 min after laryngoscopy, whereas conventional pre-oxygenation resulted in much lower values in both phases. This finding clearly suggests not only efficiency, but sustained efficacy in the peri-intubation period. Building on these findings, Vourc’h et al. [38] conducted a multicenter RCT comparing pre-oxygenation with NIPPV versus HFNCO in 124 patients with acute hypoxemic respiratory failure. While they found no significant difference in the lowest SpO2 during intubation between groups, a post-hoc analysis revealed a potential advantage of NIPPV in the most severely hypoxemic patients (PaO2/FiO2 < 200). This highlighted the importance of patient selection when considering NIPPV for pre-oxygenation, suggesting that HFNCO may be effective if functional residual capacity is available for being filled with oxygen; should it not be so, NIPPV has the physiologic advantage of recruiting the FRC to be later filled with oxygen [39] (Figure 1).
Figure 1.
The efficacy and efficiency of the pre-oxygenation technique depend upon the amount of available functional residual capacity. The larger the reduction, the lesser the effect of non-positive pressure pre-oxygenation techniques.
A methodological and logical advance came with the OPTINIV trial by Jaber et al. [13], which examined a combined approach of NIPPV pre-oxygenation with HFNCO peri-oxygenation before and during laryngoscopy. This single-center RCT of 25 patients demonstrated that the combination significantly improved the lowest SpO2 during intubation compared to NIPPV alone (100% vs. 96%, p = 0.029) and reduced the incidence of severe hypoxemia (SpO2 < 80%) from 21% to 0%. This study highlighted the complementary physiological benefits of combining these techniques. The FLORALI-2 study by Frat et al. [18] represented a significant advancement, directly comparing NIPPV against HFNCO with apneic oxygenation in 313 patients with acute hypoxemic respiratory failure. This multicenter RCT found that NIPPV led to better pre-oxygenation (median SpO2 100% vs. 99%, p < 0.01) and reduced the incidence of severe desaturation below 80% (23% vs. 35%, p = 0.03). Notably, the benefit was most pronounced in patients with severe hypoxemia at baseline (PaO2/FiO2 < 200). Another important contribution came from Frat et al. [19], who conducted an RCT comparing HFNCO to NIPPV for pre-oxygenation in 192 critically ill patients. While they found no significant difference in the median lowest SpO2 during intubation (92% vs. 90%, p = 0.44), they observed better patient comfort with HFNCO, suggesting that factors beyond oxygenation should be considered when selecting a pre-oxygenation strategy. Several meta-analyses have synthesized this growing body of evidence. Zhao et al. [40] analyzed 11 RCTs (1472 patients) comparing NIPPV, HFNCO and conventional oxygen therapy for pre-oxygenation. They found that NIPPV significantly reduced the risk of severe desaturation compared to conventional oxygen therapy (RR 0.43, 95% CI 0.23–0.80) but found no significant difference between NIPPV and HFNCO. A network meta-analysis by Fong et al. [14] examined 25 studies (3232 patients) and concluded that NIPPV was associated with the lowest risk of desaturation events compared to all other pre-oxygenation methods. Beyond oxygenation parameters, some studies have investigated the impact of pre-oxygenation strategies on procedural outcomes. Jaber et al. [41] found that NIPPV pre-oxygenation was associated with improved first-attempt success rates compared to standard pre-oxygenation (67% vs. 54%, p = 0.02) in a cohort of 1000 intubations. This effect was attributed to better operator conditions due to reduced time pressure from desaturation events. Similarly, Bailly et al. [42] reported that effective pre-oxygenation was independently associated with reduced complications during intubation in a prospective observational study of 1400 ICU intubations. The most recent meta-analysis by Higgs et al. [43], including 24 RCTs (3521 patients), demonstrated that NIPPV pre-oxygenation reduced the relative risk of severe desaturation by 30% compared to conventional methods (RR 0.70, 95% CI 0.59–0.85). They also found that NIPPV was associated with reduced ICU length of stay (mean difference −1.8 days, 95% CI −3.1 to −0.5), though no significant mortality benefit was detected. This is indicative that the prevention of severe hypoxemia at intubation might have downstream effects on patient outcomes beyond the initial procedure. Although the evidence overwhelmingly supports the use of NIPPV for pre-oxygenation for most patients (especially those who are very hypoxemic), there are still questions regarding the best way to apply NIPPV, who to use it on, and the feasibility of combining approaches that would maximize the benefits while minimizing the risks.
5. Comparison with Alternative Techniques
Multiple pre-oxygenation strategies have recently been studied to determine optimal approaches for different clinical scenarios, and a summary of key studies is provided in Table 1.
Table 1.
Summary of Key Studies on NIPPV for Pre-oxygenation in Critically Ill Patients. NIPPV = Non-Invasive Positive Pressure Ventilation; HFNCO = High-Flow Nasal Cannula Oxygen; RCT = Randomized Controlled Trial; PSV = Pressure Support Ventilation; PEEP = Positive End-Expiratory Pressure; SpO2 = Peripheral Oxygen Saturation; ICU = Intensive Care Unit; RR = Relative Risk; CI = Confidence Interval.
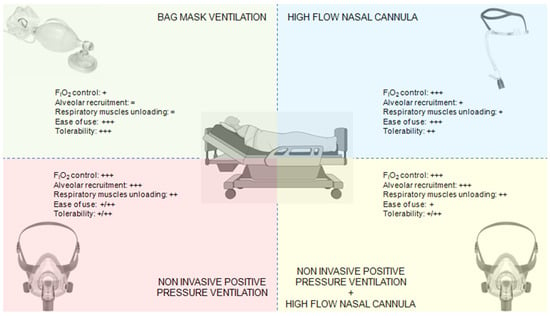
The primary alternatives to NIPPV for pre-oxygenation include HFNCO, standard bag-valve-mask (BVM) ventilation, and various combined approaches (Figure 2).
5.1. NIPPV vs. HFNCO
HFNCO has gained popularity for pre-oxygenation due to its ability to deliver high FiO2, provide modest positive airway pressure, and continue oxygenation during laryngoscopy [15]. Direct comparisons between NIPPV and HFNCO have yielded mixed results. The FLORALI-2 trial [18] demonstrated superiority of NIPPV over HFNCO for preventing severe hypoxemia during intubation in patients with acute hypoxemic respiratory failure. Conversely, Frat et al. [19] found no significant difference in the lowest SpO2 values between techniques but noted better patient comfort with HFNCO. A physiological study by Rittayamai et al. [45] showed that NIPPV achieved higher end-expiratory lung volumes and PaO2 levels compared to HFNCO, particularly in patients with more severe hypoxemia. Notably, the different mechanisms of action suggest specific clinical targets for each modality. Ricard et al. [34] observed that NIPPV was more effective in patients with recruitable lung pathology (e.g., atelectasis, early ARDS), while HFNCO offered advantages in patients with upper airway obstruction or those intolerant of masks. A pragmatic consideration highlighted by Besnier et al. [46] is that HFNCO allows continuous oxygenation during laryngoscopy, while NIPPV requires removal for tube insertion, potentially risking rapid derecruitment.
5.2. NIPPV vs. Bag-Valve-Mask
Traditional BVM pre-oxygenation with 100% oxygen via Mapleson circuit remains widely used but has significant limitations in critically ill patients. Baillard’s landmark study [11] demonstrated clear superiority of NIPPV over BVM, with higher post-pre-oxygenation SpO2 (98% vs. 93%) and reduced severe desaturation events (7% vs. 46%). A subsequent trial by Casey et al. [44] reinforced these findings, showing that BVM often fails to achieve adequate pre-oxygenation in patients with shunt physiology. The primary advantage of NIPPV over BVM lies in its ability to maintain consistent positive pressure, recruit collapsed alveoli, and provide predictable tidal volumes [47].
5.3. Combined Approaches
Recognition that different techniques offer complementary benefits has led to the exploration of combined approaches. The OPTINIV trial [13] demonstrated that combining NIPPV pre-oxygenation with HFNCO apneic oxygenation during laryngoscopy achieved superior results compared to NIPPV alone, effectively addressing both pre-intubation oxygen loading and ongoing oxygen consumption during the procedure. Jaber et al. reported no severe desaturation events with this combination approach. A technique gaining attention is the addition of deliberate nasal insufflation during pre-oxygenation with NIPPV (nasal oxygen during efforts securing a tube—NO DESAT), which Levitan [48] found could extend safe apnea time by up to 100 s and a recent metanalysis showed up to improve all oxygenation parameters with indirect effect on increasing intubation first-pass success [17].
5.4. Special Circumstances
Rapid sequence induction-intubation (RSI-I) presents unique challenges for pre-oxygenation. Traditional teaching discourages positive pressure ventilation due to aspiration concerns, potentially limiting NIPPV use. However, Baillard et al. [49] demonstrated that gentle NIPPV (pressure support ≤ 10 cmH2O) with proper technique minimally increases gastric insufflation while significantly improving pre-oxygenation. For RSI-I in hypoxemic patients, De Jong et al. [50] found that a modified approach using NIPPV pre-oxygenation with immediate sequence induction resulted in fewer desaturation events than conventional RSI with BVM. Nevertheless, the setting of RSII is evolving, with concerns surrounding the available evidence [51] and suggesting a change of practice, for either bag mask ventilation and cricoid force application [44]. Its practice is changing [52], with NIPPV and HFNCO already being part of the modern RSII [8] and further statements coming from the Project for Universal Management of Airways [53]. In obese patients, NIPPV shows particular advantages. Futier et al. [29] demonstrated that NIPPV pre-oxygenation with recruitment maneuvers significantly improved oxygenation and extended safe apnea time in morbidly obese surgical patients compared to conventional pre-oxygenation. This benefit extends to critically ill obese patients, where Baillard et al. [54] found NIPPV reduced atelectasis formation and improved functional residual capacity compared to standard approaches, and a recent sub-analysis of the INTUBE study confirmed these findings [1].
6. Patient Selection and Risk Stratification
Appropriate patient selection is crucial for maximizing the benefits of NIPPV pre-oxygenation while minimizing potential risks. The available evidence suggests that not all critically ill patients may equally benefit from this technique, calling for a careful consideration of individual patient characteristics and underlying pathophysiology.
6.1. Ideal Candidates for NIPPV Pre-Oxygenation
Patients most likely to benefit from NIPPV pre-oxygenation include those with acute hypoxemic respiratory failure, particularly when the PaO2/FiO2 ratio is below 200 mmHg [18]. Also, patients with atelectasis-prone conditions—including obesity, pulmonary edema, and ARDS—represent optimal candidates due to NIPPV’s ability to recruit collapsed lung units [29,55]. Cabrini et al. [56] found that patients already receiving NIPPV for respiratory support had reduced complications when continuing NIPPV during pre-oxygenation compared to transitioning to other methods.
6.2. Contraindications and Cautions
Despite its benefits, NIPPV pre-oxygenation is not appropriate for all patients. Absolute contraindications include inability to protect the airway, recent upper airway or esophageal surgery, undrained pneumothorax, and hemodynamic instability requiring imminent vasopressor support [57]. Relative contraindications include severe agitation, excessive secretions, facial trauma, and high aspiration risk [21]. Mosier et al. [28] cautioned that NIPPV in patients with decreased level of consciousness (GCS < 10) might increase aspiration risk, though Baillard et al. [49] demonstrated that careful application in selected obtunded patients did not increase complications.
6.3. Risk-Benefit Assessment in Different Patient Populations
The risk-benefit profile varies significantly across patient populations. In hypercapnic respiratory failure (e.g., COPD exacerbation), Scala et al. [58] demonstrated that NIPPV pre-oxygenation significantly reduced both desaturation events and post-intubation hypercapnia compared to conventional methods. For cardiogenic pulmonary edema, Baillard et al. [59] found NIPPV particularly effective due to its ability to simultaneously reduce preload and afterload while improving oxygenation. Conversely, in patients with COVID-19-related ARDS, Grieco et al. [33] and a recent expert consensus [60] observed that while NIPPV improved pre-oxygenation parameters, it also increased aerosolization risk, suggesting that benefits must be weighed against infection control considerations. In trauma patients, Bauman et al. [61] reported that NIPPV pre-oxygenation was beneficial only in the absence of significant chest or abdominal injuries, as positive pressure could exacerbate existing pathology. For patients with altered mental status, such as those with encephalopathy, low Glasgow Coma Scale scores, or intoxication, the application of NIPPV requires careful consideration. However, when applied with vigilant monitoring and appropriate positioning, short-duration NIPPV pre-oxygenation could still improve oxygenation parameters without increasing complications in selected patients with mild to moderate altered mental status [58].
6.4. Predictors of Success/Failure
Several factors predict successful NIPPV pre-oxygenation. Nava et al. [21] identified that patients who tolerate the interface well and demonstrate rapid improvement in SpO2 within the first 5 min are likely to achieve optimal pre-oxygenation. Jaber et al. [41] found that patients with a respiratory rate < 30 breaths/min and without accessory muscle use were more likely to benefit from NIPPV pre-oxygenation. Conversely, predictors of failure include agitation requiring sedation, inability to synchronize with the ventilator, and persistent hypoxemia despite optimal settings [62]. De Jong et al. [63] developed a risk score incorporating these factors along with BMI > 35 kg/m2, MACOCHA score > 3, and presence of upper airway secretions to identify patients at high risk for NIPPV pre-oxygenation failure. This score demonstrated good predictive accuracy (AUROC 0.81) and can guide clinician decision-making regarding pre-oxygenation strategy selection. Systematic evaluation of these factors allows for an individualized approach to pre-oxygenation, potentially optimizing outcomes in this high-risk procedure across diverse critically ill populations.
7. Practical Implementation
Successful implementation of NIPPV for pre-oxygenation requires attention to technical details, appropriate ventilator settings, careful interface selection, diligent monitoring, and smooth transition to laryngoscopy. These practical aspects can significantly impact the effectiveness and safety of the procedure.
7.1. Technical Aspects of NIPPV Delivery for Pre-Oxygenation
NIPPV for pre-oxygenation can be delivered through dedicated non-invasive ventilators or ICU ventilators with non-invasive capabilities (flow generators providing adequate flows in response to patients’ peak demands). Ehrmann et al. [64] demonstrated that ICU ventilators with dedicated NIV algorithms provided more stable pressure delivery and better patient-ventilator synchrony compared to basic transport ventilators. The choice of ventilation mode is also important, with pressure support ventilation (PSV) with PEEP being most commonly utilized [12,13]. Bilevel positive airway pressure (BiPAP) offers similar benefits with a potential contribution to aiding tidal volume exchanges, while continuous positive airway pressure (CPAP) alone may be insufficient for patients with high work of breathing [65].
7.2. Optimal Settings
The literature suggests specific ventilator settings to optimize pre-oxygenation while minimizing complications. For pressure levels, most studies recommend an inspiratory positive airway pressure (IPAP) or pressure support of 8–15 cmH2O and PEEP of 5–10 cmH2O [18]. Jaber et al. [13] demonstrated that higher PEEP levels (8–10 cmH2O) were particularly beneficial in severely hypoxemic patients (PaO2/FiO2 < 200). Regarding FiO2, Mort et al. [66] confirmed that 100% oxygen should be used to maximize oxygen storage capacity, though De Jong et al. [67] cautioned that prolonged exposure to high FiO2 might contribute to absorption atelectasis in some patients. The optimal duration of NIPPV pre-oxygenation remains debated. While traditional teaching recommends 3–5 min of pre-oxygenation, Tanoubi et al. [5] demonstrated that critically ill patients often require longer periods (5–8 min) to achieve maximal end-tidal oxygen concentration. A pragmatic approach suggested by Bailly et al. [42] is to continue NIPPV until end-tidal oxygen concentration reaches a plateau (typically > 90%) or for a minimum of 3 min in urgent situations.
7.3. Interface Selection Considerations
Interface selection significantly impacts pre-oxygenation effectiveness. Chaudhuri et al. [68] compared four interfaces (face mask, nasal mask, helmet, and total face mask) for NIPPV pre-oxygenation, finding that oronasal face masks provided the best balance of effectiveness and tolerance. Helmets, while reducing air leakage, introduced dead space that delayed oxygen equilibration. For patients with difficult facial anatomy or a beard, Chiumello et al. [69] demonstrated that total face masks achieved better seal and higher oxygen concentrations. Regardless of interface, proper sizing and fitting are essential to minimize leaks that may compromise pressure delivery and oxygenation and jeopardize patients’ compliance and adherence [70].
7.4. Monitoring During Pre-Oxygenation
Comprehensive monitoring is crucial during NIPPV pre-oxygenation. Beyond standard pulse oximetry and capnography, Mosier et al. [71] and Nimmagadda et Al [4] recommended monitoring expired oxygen concentration as the most reliable indicator of adequate pre-oxygenation, while Jaber et al. [41] highlighted that vigilant hemodynamic monitoring can detect early signs of positive pressure-induced hypotension, especially in volume-depleted patients.
7.5. Transitioning from Pre-Oxygenation to Laryngoscopy
The transition from NIPPV to laryngoscopy represents a critical moment where derecruitment and desaturation may occur. Jaber et al. [13] described a coordinated approach where NIPPV is maintained until immediately before laryngoscopy, with rapid sequence induction medications administered while NIPPV continues. The OPTINIV technique maintains nasal oxygen insufflation during this transition, providing apneic oxygenation through a separate nasal cannula [13]. For patients at high risk of desaturation, Lapinsky [72] recommended a head-up position during both pre-oxygenation and laryngoscopy to optimize functional residual capacity and reduce closing capacity, highlighting the importance of adequate positioning as a fundamental part of an adequate pre-oxygenation strategy, especially in patients with reduced FRC, such as those with obesity [73].
A standard low-flow nasal cannula may be applied during NIPPV, with the double aim of compensating for eventual leaks and being already in position for the apnoic phase of airway instrumentation [74].
8. Limitations and Potential Complications
Despite its benefits, NIPPV for pre-oxygenation is associated with several limitations and potential complications that must be carefully considered when selecting this technique for critically ill patients requiring intubation.
8.1. Potential Adverse Effects of NIPPV for Pre-Oxygenation
NIPPV can induce patient discomfort and anxiety, potentially increasing the need for sedation prior to intubation. Schmidt et al. [75] reported that approximately 30% of patients exhibited poor tolerance to NIPPV pre-oxygenation, necessitating either interface adjustment or sedative administration. Mask-related complications, including pressure ulcers, conjunctival irritation, and claustrophobia, have been documented by Carron et al. [76], though these are less concerning for short-term pre-oxygenation applications than for prolonged NIPPV use. More seriously, Lemiale et al. [77] observed that NIPPV pre-oxygenation in immunocompromised patients occasionally exacerbated respiratory distress through patient-ventilator asynchrony, particularly when inadequate support levels were used.
8.2. Gastric Insufflation and Aspiration Risk
One of the primary concerns with NIPPV pre-oxygenation is the potential for gastric insufflation, which may increase regurgitation and aspiration risk. Frat et al. [78] documented that inspiratory pressures exceeding 20 cmH2O significantly increased gastric air volume measured by ultrasound. In patients with high aspiration risk, Jaber et al. [49] recommended using lower pressure settings (PS ≤ 8 cmH2O, PEEP ≤ 5 cmH2O) and ensuring proper positioning.
8.3. Hemodynamic Effects
The positive intrathoracic pressure generated by NIPPV can have significant hemodynamic consequences. Ricard et al. [79] demonstrated that NIPPV reduced venous return and cardiac preload, potentially precipitating hypotension in volume-depleted patients [80]. This effect is particularly pronounced in patients with right ventricular dysfunction or pulmonary hypertension, where Mosier et al. [28] observed that NIPPV occasionally worsened right heart failure. Using a conventional medication regimen during intubation of critically ill patients, a sub-analysis of the INTUBE study clearly showed the role of propofol in causing cardiovascular collapse; no quality data are available with respect to the assumption that NIPPV may further enhance this phenomenon because of well-known effects on venous return and cardiac performance [81].
8.4. Delays in Securing Definitive Airway
Attempting NIPPV pre-oxygenation in inappropriate candidates may delay definitive airway management. Thille et al. [62] reported that failed NIPPV trials before intubation were associated with longer time to intubation and increased mortality in acute respiratory failure. Additionally, Kang et al. [82] observed that clinicians sometimes persisted with ineffective NIPPV pre-oxygenation despite minimal improvement in oxygenation, potentially missing the optimal window for intubation.
8.5. Resource Considerations
Implementation of NIPPV pre-oxygenation requires appropriate equipment and expertise. Ehrmann et al. [83] noted that dedicated NIPPV ventilators or ICU ventilators with NIV capabilities are not universally available, particularly in resource-limited settings. Furthermore, effective application requires staff familiar with NIPPV setup and troubleshooting, which may be challenging during emergent situations or off-hours when experienced personnel may be limited.
9. Standardized Protocols and Training for Safe NIPPV Implementation
The successful implementation of NIPPV for pre-oxygenation relies not only on equipment availability but also on the proficiency of the clinical team. Despite robust evidence supporting its physiological benefits and clinical efficacy, considerable variability persists in how NIPPV is applied across institutions. This inconsistency underscores the urgent need for standardized protocols that delineate optimal ventilator settings, interface selection, duration of application, and monitoring parameters during the pre-oxygenation phase.
Equally important is the structured training of healthcare providers involved in airway management. Anesthesiologists, intensivists, respiratory therapists, and nursing staff must receive dedicated education not only on the technical aspects of NIPPV use but also on patient selection, troubleshooting, and real-time decision-making. Simulation-based learning and interprofessional training modules can be particularly effective in promoting confidence and competence. Establishing such education as a routine component of airway management curricula may significantly enhance patient safety and procedural outcomes in high-acuity settings.
10. Future Directions: The Role of Artificial Intelligence
The integration of artificial intelligence (AI) into anesthesiology is poised to revolutionize perioperative care by enhancing real-time monitoring and decision-making processes. AI algorithms can analyze complex physiological data streams to predict adverse events, such as hypotension or respiratory distress, guiding airway management and allowing for preemptive interventions [84,85]. Similarly, AI-driven systems are being developed to optimize ventilator settings by continuously assessing patient-ventilator interactions, thereby reducing the incidence of asynchrony and improving overall ventilation strategies [86].
In the context of non-invasive ventilation, AI’s role extends to remote patient monitoring and tele-supervision, which are particularly beneficial in resource-limited settings or during pandemics. Advanced AI models can process data from wearable sensors and home monitoring devices to detect early signs of respiratory compromise, facilitating timely medical responses [87]. Moreover, the development of intelligent intensive care units (ICUs) equipped with AI technologies allows for continuous, autonomous monitoring of patients, enhancing the detection of subtle clinical changes that might precede deterioration. These innovations not only augment the capabilities of anesthesiologists but also contribute to more personalized and responsive patient care.
11. Conclusions
Non-invasive positive pressure ventilation can be a useful modality of pre-oxygenation in suitable patients with critical care-related indications for intubation. There is evidence that NIPPV may correct the physiologic deficiencies of traditional pre-oxygenation methods by opening collapsed alveoli, sustaining FRC, and optimizing ventilation-perfusion relationships. These changes lead to clinically important results that have implications for patient care, such as improved oxygen saturation during pre-oxygenation, a lower rate of high desaturation events, and possibly less intubation-related trauma. Ideally, the use of NIPPV pre-oxygenation should be placed in the management algorithm, and used in those with severe hypoxemia (i.e., PaO2/FiO2 < 200), recruitable lung pathology or with a patient who is already receiving NIPPV for respiratory support. The application should emphasize appropriate patient interface choice, determine optimal pressure values and a continuous reassessment of patient response. The combination of NIPPV pre-oxygenation and apneic oxygenation during laryngoscopy adds to established pre-oxygenation benefits and is likely to mitigate the risk of desaturation. Although not for everyone, NIPPV pre-oxygenation has carved out a place in the arsenal of tools available for airway management in the critically ill. When used appropriately, based on an understanding of the potential advantages and disadvantages, it may be an essential tool to increase the safety of this high-risk procedure and to improve patient outcomes in the intensive care unit.
Author Contributions
Conceptualization, L.L.V., G.C., C.D., I.Z. and M.S.; investigation, L.L.V., G.C., D.S.P. and M.S.; data curation, L.L.V., G.C., G.D.-M., N.N.-L., T.S.T. and A.M.; writing—original draft preparation, L.L.V., G.C. and M.S.; writing—review and editing, I.Z., G.D.-M., N.N.-L., D.S.P., C.D., T.S.T. and A.M.; supervision, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Russotto, V.; Myatra, S.N.; Laffey, J.G.; Tassistro, E.; Antolini, L.; Bauer, P.; Lascarrou, J.B.; Szuldrzynski, K.; Camporota, L.; Pelosi, P.; et al. Intubation Practices and Adverse Peri-intubation Events in Critically Ill Patients from 29 Countries. JAMA 2021, 325, 1164. [Google Scholar] [CrossRef] [PubMed]
- De Jong, A.; Rolle, A.; Molinari, N.; Paugam-Burtz, C.; Constantin, J.-M.; Lefrant, J.-Y.; Asehnoune, K.; Jung, B.; Futier, E.; Chanques, G.; et al. Cardiac Arrest and Mortality Related to Intubation Procedure in Critically Ill Adult Patients: A Multicenter Cohort Study. Crit. Care Med. 2018, 46, 532–539. [Google Scholar] [CrossRef]
- Mort, T.C. The incidence and risk factors for cardiac arrest during emergency tracheal intubation: A justification for incorporating the ASA Guidelines in the remote location. J. Clin. Anesth. 2004, 16, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Nimmagadda, U.; Salem, M.R.; Crystal, G.J. Preoxygenation: Physiologic Basis, Benefits, and Potential Risks. Anesth. Analg. 2017, 124, 507–517. [Google Scholar] [CrossRef]
- Tanoubi, I.; Drolet, P.; Donati, F. Optimizing preoxygenation in adults. Can. J. Anesth. Can. Anesth. 2009, 56, 449–466. [Google Scholar] [CrossRef]
- Mosier, J.M.; Hypes, C.D.; Sakles, J.C. Understanding preoxygenation and apneic oxygenation during intubation in the critically ill. Intensive Care Med. 2017, 43, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Pourmand, A.; Robinson, C.; Dorwart, K.; O’Connell, F. Pre-oxygenation: Implications in emergency airway management. Am. J. Emerg. Med. 2017, 35, 1177–1183. [Google Scholar] [CrossRef]
- Karamchandani, K.; Nasa, P.; Jarzebowski, M.; Brewster, D.J.; De Jong, A.; Bauer, P.R.; Berkow, L.; Brown, C.A.; Cabrini, L.; Casey, J.; et al. Tracheal intubation in critically ill adults with a physiologically difficult airway. An international Delphi study. Intensive Care Med. 2024, 50, 1563–1579. [Google Scholar] [CrossRef]
- Jaber, S.; Amraoui, J.; Lefrant, J.-Y.; Arich, C.; Cohendy, R.; Landreau, L.; Calvet, Y.; Capdevila, X.; Mahamat, A.; Eledjam, J.-J. Clinical practice and risk factors for immediate complications of endotracheal intubation in the intensive care unit: A prospective, multiple-center study. Crit. Care Med. 2006, 34, 2355–2361. [Google Scholar] [CrossRef]
- Goldberg, P.; Reissmann, H.; Maltais, F.; Ranieri, M.; Gottfried, S. Efficacy of noninvasive CPAP in COPD with acute respiratory failure. Eur. Respir. J. 1995, 8, 1894–1900. [Google Scholar] [CrossRef]
- Carteaux, G.; Millán-Guilarte, T.; De Prost, N.; Razazi, K.; Abid, S.; Thille, A.W.; Schortgen, F.; Brochard, L.; Brun-Buisson, C.; Dessap, A.M. Failure of Noninvasive Ventilation for De Novo Acute Hypoxemic Respiratory Failure: Role of Tidal Volume. Crit. Care Med. 2016, 44, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Baillard, C.; Fosse, J.-P.; Sebbane, M.; Chanques, G.; Vincent, F.; Courouble, P.; Cohen, Y.; Eledjam, J.-J.; Adnet, F.; Jaber, S. Noninvasive Ventilation Improves Preoxygenation before Intubation of Hypoxic Patients. Am. J. Respir. Crit. Care Med. 2006, 174, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Jaber, S.; Monnin, M.; Girard, M.; Conseil, M.; Cisse, M.; Carr, J.; Mahul, M.; Delay, J.M.; Belafia, F.; Chanques, G.; et al. Apnoeic oxygenation via high-flow nasal cannula oxygen combined with non-invasive ventilation preoxygenation for intubation in hypoxaemic patients in the intensive care unit: The single-centre, blinded, randomised controlled OPTINIV trial. Intensive Care Med. 2016, 42, 1877–1887. [Google Scholar] [CrossRef]
- Fong, K.M.; Au, S.Y.; Ng, G.W.Y. Preoxygenation before intubation in adult patients with acute hypoxemic respiratory failure: A network meta-analysis of randomized trials. Crit. Care 2019, 23, 319. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Montanes, R.; Hajage, D.; Messika, J.; Bertrand, F.; Gaudry, S.; Rafat, C.; Labbé, V.; Dufour, N.; Jean-Baptiste, S.; Bedet, A.; et al. Use of High-Flow Nasal Cannula Oxygen Therapy to Prevent Desaturation During Tracheal Intubation of Intensive Care Patients With Mild-to-Moderate Hypoxemia. Crit. Care Med. 2015, 43, 574–583. [Google Scholar] [CrossRef]
- Papazian, L.; Corley, A.; Hess, D.; Fraser, J.F.; Frat, J.-P.; Guitton, C.; Jaber, S.; Maggiore, S.M.; Nava, S.; Rello, J.; et al. Use of high-flow nasal cannula oxygenation in ICU adults: A narrative review. Intensive Care Med. 2016, 42, 1336–1349. [Google Scholar] [CrossRef]
- Oliveira, J.E.; Silva, L.; Cabrera, D.; Barrionuevo, P.; Johnson, R.L.; Erwin, P.J.; Murad, M.H.; Bellolio, M.F. Effectiveness of Apneic Oxygenation During Intubation: A Systematic Review and Meta-Analysis. Ann. Emerg. Med. 2017, 70, 483–494.e11. [Google Scholar] [CrossRef]
- Frat, J.P.; Ricard, J.D.; Quenot, J.P.; Pichon, N.; Demoule, A.; Forel, J.M.; Mira, J.-P.; Coudroy, R.; Berquier, G.; Voisin, B.; et al. Non-invasive ventilation versus high-flow nasal cannula oxygen therapy with apnoeic oxygenation for preoxygenation before intubation of patients with acute hypoxaemic respiratory failure: A randomised, multicentre, open-label trial. Lancet Respir. Med. 2019, 7, 303–312. [Google Scholar] [CrossRef]
- Frat, J.P.; Coudroy, R.; Thille, A.W. Non-invasive ventilation or high-flow oxygen therapy: When to choose one over the other? Respirology 2019, 24, 724–731. [Google Scholar] [CrossRef]
- Brindley, P.G.; Beed, M.; Law, J.A.; Hung, O.; Levitan, R.; Murphy, M.F.; Duggan, L.V. Airway management outside the operating room: How to better prepare. Can. J. Anesth. Can. Anesth. 2017, 64, 530–539. [Google Scholar] [CrossRef]
- Misseri, G.; Frassanito, L.; Simonte, R.; Rosà, T.; Grieco, D.L.; Piersanti, A.; De Robertis, E.; Gregoretti, C. Personalized Noninvasive Respiratory Support in the Perioperative Setting: State of the Art and Future Perspectives. J. Pers. Med. 2023, 14, 56. [Google Scholar] [CrossRef]
- Masip, J.; Mas, A. Noninvasive ventilation in acute respiratory failure. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 837–852. [Google Scholar] [CrossRef]
- Keenan, S.P.; Sinuff, T.; Burns, K.E.A.; Muscedere, J.; Kutsogiannis, J.; Mehta, S.; Cook, D.J.; Ayas, N.; Adhikari, N.K.; Hand, L.; et al. Clinical practice guidelines for the use of noninvasive positive-pressure ventilation and noninvasive continuous positive airway pressure in the acute care setting. Can. Med. Assoc. J. 2011, 183, E195–E214. [Google Scholar] [CrossRef] [PubMed]
- Weingart, S.D.; Levitan, R.M. Preoxygenation and Prevention of Desaturation During Emergency Airway Management. Ann. Emerg. Med. 2012, 59, 165–175.e1. [Google Scholar] [CrossRef] [PubMed]
- Benumof, J.L.; Dagg, R.; Benumof, R. Critical Hemoglobin Desaturation Will Occur before Return to an Unparalyzed State following 1 mg/kg Intravenous Succinylcholine. Anesthesiology 1997, 87, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Baraka, A.S.; Taha, S.K.; Aouad, M.T.; El-Khatib, M.F.; Kawkabani, N.I. Preoxygenation: Comparison of Maximal Breathing and Tidal Volume Breathing Techniques. Anesthesiology 1999, 91, 612. [Google Scholar] [CrossRef]
- Hedenstierna, G.; Edmark, L. Effects of anesthesia on the respiratory system. Best Pract. Res. Clin. Anaesthesiol. 2015, 29, 273–284. [Google Scholar] [CrossRef]
- Mosier, J.M.; Sakles, J.C.; Whitmore, S.P.; Hypes, C.D.; Hallett, D.K.; Hawbaker, K.E.; Snyder, L.S.; Bloom, J.W. Failed noninvasive positive-pressure ventilation is associated with an increased risk of intubation-related complications. Ann. Intensive Care 2015, 5, 4. [Google Scholar] [CrossRef]
- Futier, E.; Constantin, J.-M.; Pelosi, P.; Chanques, G.; Massone, A.; Petit, A.; Kwiatkowski, F.; Bazin, J.-E.; Jaber, S. Noninvasive Ventilation and Alveolar Recruitment Maneuver Improve Respiratory Function during and after Intubation of Morbidly Obese Patients: A Randomized Controlled Study. Anesthesiology 2011, 114, 1354–1363. [Google Scholar] [CrossRef]
- Constantin, J.-M.; Futier, E.; Cherprenet, A.-L.; Chanques, G.; Guerin, R.; Cayot-Constantin, S.; Jabaudon, M.; Perbet, S.; Chartier, C.; Jung, B.; et al. A recruitment maneuver increases oxygenation after intubation of hypoxemic intensive care unit patients: A randomized controlled study. Crit. Care 2010, 14, R76. [Google Scholar] [CrossRef]
- Gattinoni, L.; Caironi, P.; Cressoni, M.; Chiumello, D.; Ranieri, V.M.; Quintel, M.; Russo, S.; Patroniti, N.; Cornejo, R.; Bugedo, G. Lung recruitment in patients with the acute respiratory distress syndrome. N. Engl. J. Med. 2006, 354, 1775–1786. [Google Scholar] [CrossRef]
- Maniaci, A.; La Via, L.; Lechien, J.R.; Sangiorgio, G.; Iannella, G.; Magliulo, G.; Pace, A.; Mat, Q.; Lavalle, S.; Lentini, M. Hearing Loss and Oxidative Stress: A Comprehensive Review. Antioxidants 2024, 13, 842. [Google Scholar] [CrossRef]
- Grieco, D.L.; Menga, L.S.; Cesarano, M.; Rosà, T.; Spadaro, S.; Bitondo, M.M.; Montomoli, J.; Falò, G.; Tonetti, T.; Cutuli, S.L.; et al. Effect of Helmet Noninvasive Ventilation vs. High-Flow Nasal Oxygen on Days Free of Respiratory Support in Patients With COVID-19 and Moderate to Severe Hypoxemic Respiratory Failure: The HENIVOT Randomized Clinical Trial. JAMA 2021, 325, 1731. [Google Scholar] [CrossRef] [PubMed]
- Ricard, J.-D.; Roca, O.; Lemiale, V.; Corley, A.; Braunlich, J.; Jones, P.; Kang, B.J.; Lellouche, F.; Nava, S.; Rittayamai, N.; et al. Use of nasal high flow oxygen during acute respiratory failure. Intensive Care Med. 2020, 46, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Parke, R.L.; McGuinness, S.P. Pressures Delivered By Nasal High Flow Oxygen During All Phases of the Respiratory Cycle. Respir. Care 2013, 58, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Lyons, C.; Callaghan, M. Apnoeic oxygenation with high-flow nasal oxygen for laryngeal surgery: A case series. Anaesthesia 2017, 72, 1379–1387. [Google Scholar] [CrossRef]
- Sorbello, M.; Antonelli, M.; Guarino, A.; Merli, G.; Petrini, F.; Frova, G.; Società Italiana di Anestesia, Analgesia, Rianimazione, Terapia Intensiva e Iperbarica (SIAARTI) Difficult Airways Study Group. Noninvasive ventilation and intubation of hypoxic patients: ICU versus operating room. Am. J. Respir. Crit. Care Med. 2008, 177, 357–358, author reply 358. [Google Scholar] [CrossRef]
- Vourc’h, M.; Asfar, P.; Volteau, C.; Bachoumas, K.; Clavieras, N.; Egreteau, P.-Y.; Asehnoune, K.; Mercat, A.; Reignier, J.; Jaber, S.; et al. High-flow nasal cannula oxygen during endotracheal intubation in hypoxemic patients: A randomized controlled clinical trial. Intensive Care Med. 2015, 41, 1538–1548. [Google Scholar] [CrossRef]
- Leone, M.; Einav, S.; Chiumello, D.; Constantin, J.-M.; De Robertis, E.; De Abreu, M.G.; Gregoretti, C.; Jaber, S.; Maggiore, S.M.; Pelosi, P.; et al. Noninvasive respiratory support in the hypoxaemic peri-operative/periprocedural patient: A joint ESA/ESICM guideline. Intensive Care Med 2020, 46, 697–713. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, H.; Sun, F.; Lyu, S.; An, Y. High-flow nasal cannula oxygen therapy is superior to conventional oxygen therapy but not to noninvasive mechanical ventilation on intubation rate: A systematic review and meta-analysis. Crit. Care 2017, 21, 184. [Google Scholar] [CrossRef]
- Jaber, S.; Jung, B.; Corne, P.; Sebbane, M.; Muller, L.; Chanques, G.; Verzilli, D.; Jonquet, O.; Eledjam, J.-J.; Lefrant, J.-Y. An intervention to decrease complications related to endotracheal intubation in the intensive care unit: A prospective, multiple-center study. Intensive Care Med. 2010, 36, 248–255. [Google Scholar] [CrossRef]
- Bailly, A.; Ricard, J.-D.; Le Thuaut, A.; Helms, J.; Kamel, T.; Mercier, E.; Lemiale, V.; Colin, G.; Mira, J.-P.; Clere-Jehl, R.; et al. Compared Efficacy of Four Preoxygenation Methods for Intubation in the ICU: Retrospective Analysis of McGrath Mac Videolaryngoscope Versus Macintosh Laryngoscope (MACMAN) Trial Data. Crit. Care Med. 2019, 47, e340–e348. [Google Scholar] [CrossRef] [PubMed]
- Higgs, A.; McGrath, B.A.; Goddard, C.; Rangasami, J.; Suntharalingam, G.; Gale, R.; Cook, T. Guidelines for the management of tracheal intubation in critically ill adults. Br. J. Anaesth. 2018, 120, 323–352. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.D.; Janz, D.R.; Russell, D.W.; Vonderhaar, D.J.; Joffe, A.M.; Dischert, K.M.; Brown, R.M.; Zouk, A.N.; Gulati, S.; Heideman, B.E.; et al. Bag-Mask Ventilation during Tracheal Intubation of Critically Ill Adults. N. Engl. J. Med. 2019, 380, 811–821. [Google Scholar] [CrossRef]
- Rittayamai, N.; Tscheikuna, J.; Rujiwit, P. High-Flow Nasal Cannula Versus Conventional Oxygen Therapy After Endotracheal Extubation: A Randomized Crossover Physiologic Study. Respir. Care 2014, 59, 485–490. [Google Scholar] [CrossRef]
- Besnier, E.; Guernon, K.; Bubenheim, M.; Gouin, P.; Carpentier, D.; Béduneau, G.; Grangé, S.; Declercq, P.-L.; Marchalot, A.; Tamion, F.; et al. Pre-oxygenation with high-flow nasal cannula oxygen therapy and non-invasive ventilation for intubation in the intensive care unit. Intensive Care Med. 2016, 42, 1291–1292. [Google Scholar] [CrossRef]
- Hirsch, J.; Führer, I.; Kuhly, P.; Schaffartzik, W. Preoxygenation: A comparison of three different breathing systems. Br. J. Anaesth. 2001, 87, 928–931. [Google Scholar] [CrossRef]
- Bhagwan, S.D. Levitan’s no desat with nasal cannula for Infants with pyloric stenosis requiring intubation. Pediatr. Anesth. 2013, 23, 297–298. [Google Scholar] [CrossRef]
- Baillard, C.; Prat, G.; Jung, B.; Futier, E.; Lefrant, J.Y.; Vincent, F.; Hamdi, A.; Vicaut, E.; Jaber, S. Effect of preoxygenation using non-invasive ventilation before intubation on subsequent organ failures in hypoxaemic patients: A randomised clinical trial. Br. J. Anaesth. 2018, 120, 361–367. [Google Scholar] [CrossRef]
- De Jong, A.; Jung, B.; Jaber, S. Intubation in the ICU: We could improve our practice. Crit. Care Lond. Engl. 2014, 18, 209. [Google Scholar] [CrossRef]
- Lyons, C.; Wiles, M.D. Rapid sequence induction: A modern-day example of Theseus’ Paradox? Anaesthesia 2025. early view. [Google Scholar] [CrossRef]
- Sorbello, M.; Paternò, D.S.; Zdravkovic, I.; La Via, L. Pharmacological approach to rapid sequence induction/intubation: A contemporary perspective. Curr. Opin. Anaesthesiol. 2025, 38, 369–374. [Google Scholar] [CrossRef]
- Chrimes, N.; Higgs, A.; Law, J.A.; Baker, P.A.; Cooper, R.M.; Greif, R.; Kovacs, G.; Myatra, S.N.; O’SUllivan, E.P.; Rosenblatt, W.H.; et al. Project for Universal Management of Airways—Part 1: Concept and methods. Anaesthesia 2020, 75, 1671–1682. [Google Scholar] [CrossRef]
- Baillard, C.; Depret, F.; Levy, V.; Boubaya, M.; Beloucif, S. Incidence and prediction of inadequate preoxygenation before induction of anaesthesia. Ann. Fr. D'anesthesie Reanim. 2014, 33, e55–e58. [Google Scholar] [CrossRef]
- Chiumello, D.; Brochard, L.; Marini, J.J.; Slutsky, A.S.; Mancebo, J.; Ranieri, V.M.; Thompson, B.T.; Papazian, L.; Schultz, M.J.; Amato, M.; et al. Respiratory support in patients with acute respiratory distress syndrome: An expert opinion. Crit. Care 2017, 21, 240. [Google Scholar] [CrossRef]
- Cabrini, L.; Landoni, G.; Oriani, A.; Plumari, V.P.; Nobile, L.; Greco, M.; Pasin, L.; Beretta, L.; Zangrillo, A. Noninvasive Ventilation and Survival in Acute Care Settings: A Comprehensive Systematic Review and Metaanalysis of Randomized Controlled Trials. Crit. Care Med. 2015, 43, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Rochwerg, B.; Brochard, L.; Elliott, M.W.; Hess, D.; Hill, N.S.; Nava, S.; Navalesi, P.; Antonelli, M.; Brozek, J.; Conti, G.; et al. Official ERS/ATS clinical practice guidelines: Noninvasive ventilation for acute respiratory failure. Eur. Respir. J. 2017, 50, 1602426. [Google Scholar] [CrossRef] [PubMed]
- Scala, R.; Naldi, M.; Archinucci, I.; Coniglio, G.; Nava, S. Noninvasive Positive Pressure Ventilation in Patients With Acute Exacerbations of COPD and Varying Levels of Consciousness. Chest 2005, 128, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Baillard, C.; Boussarsar, M.; Fosse, J.-P.; Girou, E.; Le Toumelin, P.; Cracco, C.; Jaber, S.; Cohen, Y.; Brochard, L. Cardiac troponin I in patients with severe exacerbation of chronic obstructive pulmonary disease. Intensive Care Med. 2003, 29, 584–589. [Google Scholar] [CrossRef]
- Nasa, P.; Azoulay, E.; Khanna, A.K.; Jain, R.; Gupta, S.; Javeri, Y.; Juneja, D.; Rangappa, P.; Sundararajan, K.; Alhazzani, W.; et al. Expert consensus statements for the management of COVID-19-related acute respiratory failure using a Delphi method. Crit. Care 2021, 25, 106. [Google Scholar] [CrossRef]
- Bauman, Z.M.; Gassner, M.Y.; Coughlin, M.A.; Mahan, M.; Watras, J. Lung Injury Prediction Score Is Useful in Predicting Acute Respiratory Distress Syndrome and Mortality in Surgical Critical Care Patients. Crit. Care Res. Pract. 2015, 2015, 157408. [Google Scholar] [CrossRef][Green Version]
- Thille, A.W.; Contou, D.; Fragnoli, C.; Córdoba-Izquierdo, A.; Boissier, F.; Brun-Buisson, C. Non-invasive ventilation for acute hypoxemic respiratory failure: Intubation rate and risk factors. Crit. Care 2013, 17, R269. [Google Scholar] [CrossRef] [PubMed]
- De Jong, A.; Rolle, A.; Pensier, J.; Capdevila, M.; Jaber, S. First-attempt success is associated with fewer complications related to intubation in the intensive care unit. Intensive Care Med. 2020, 46, 1278–1280. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, S.; Roche-Campo, F.; Sferrazza Papa, G.F.; Isabey, D.; Brochard, L.; Apiou-Sbirlea, G.; REVA Research Network. Aerosol therapy during mechanical ventilation: An international survey. Intensive Care Med. 2013, 39, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Brochard, L.; Mancebo, J.; Wysocki, M.; Lofaso, F.; Conti, G.; Rauss, A.; Simonneau, G.; Benito, S.; Gasparetto, A.; Lemaire, F.; et al. Noninvasive Ventilation for Acute Exacerbations of Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 1995, 333, 817–822. [Google Scholar] [CrossRef]
- Mort, T.C.; Waberski, B.H.; Clive, J. Extending the preoxygenation period from 4 to 8 min in critically ill patients undergoing emergency intubation. Crit. Care Med. 2009, 37, 68–71. [Google Scholar] [CrossRef]
- De Jong, A.; Chanques, G.; Jaber, S. Mechanical ventilation in obese ICU patients: From intubation to extubation. Crit. Care 2017, 21, 63. [Google Scholar] [CrossRef]
- Chaudhuri, D.; Jinah, R.; Burns, K.E.A.; Angriman, F.; Ferreyro, B.L.; Munshi, L.; Goligher, E.; Scales, D.; Cook, D.J.; Mauri, T.; et al. Helmet noninvasive ventilation compared to facemask noninvasive ventilation and high-flow nasal cannula in acute respiratory failure: A systematic review and meta-analysis. Eur. Respir. J. 2022, 59, 2101269. [Google Scholar] [CrossRef]
- Chiumello, D.; Pelosi, P.; Carlesso, E.; Severgnini, P.; Aspesi, M.; Gamberoni, C.; Antonelli, M.; Conti, G.; Chiaranda, M.; Gattinoni, L. Noninvasive positive pressure ventilation delivered by helmet vs. standard face mask. Intensive Care Med. 2003, 29, 1671–1679. [Google Scholar] [CrossRef]
- L’Her, E.; Deye, N.; Lellouche, F.; Taille, S.; Demoule, A.; Fraticelli, A.; Mancebo, J.; Brochard, L. Physiologic Effects of Noninvasive Ventilation during Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2005, 172, 1112–1118. [Google Scholar] [CrossRef]
- Mosier, J.M.; Sakles, J.C.; Stolz, U.; Hypes, C.D.; Chopra, H.; Malo, J.; Bloom, J.W. Neuromuscular Blockade Improves First-Attempt Success for Intubation in the Intensive Care Unit. A Propensity Matched Analysis. Ann. Am. Thorac. Soc. 2015, 12, 734–741. [Google Scholar] [CrossRef]
- Lapinsky, S.E. Endotracheal intubation in the ICU. Crit. Care 2015, 19, 258. [Google Scholar] [CrossRef]
- Boyce, J.R.; Ness, T.; Castroman, P.; Gleysteen, J.J. A Preliminary Study of the Optimal Anesthesia Positioning for the Morbidly Obese Patient. Obes. Surg. 2003, 13, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Hayes-Bradley, C.; Lewis, A.; Burns, B.; Miller, M. Efficacy of Nasal Cannula Oxygen as a Preoxygenation Adjunct in Emergency Airway Management. Ann. Emerg. Med. 2016, 68, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, G.A.; Girard, T.D.; Kress, J.P.; Morris, P.E.; Ouellette, D.R.; Alhazzani, W.; Burns, S.M.; Epstein, S.K.; Esteban, A.; Fan, E.; et al. Official Executive Summary of an American Thoracic Society/American College of Chest Physicians Clinical Practice Guideline: Liberation from Mechanical Ventilation in Critically Ill Adults. Am. J. Respir. Crit. Care Med. 2017, 195, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Carron, M.; Freo, U.; BaHammam, A.S.; Dellweg, D.; Guarracino, F.; Cosentini, R.; Feltracco, P.; Vianello, A.; Ori, C.; Esquinas, A. Complications of non-invasive ventilation techniques: A comprehensive qualitative review of randomized trials. Br. J. Anaesth. 2013, 110, 896–914. [Google Scholar] [CrossRef]
- Lemiale, V.; Mokart, D.; Resche-Rigon, M.; Pène, F.; Mayaux, J.; Faucher, E.; Nyunga, M.; Girault, C.; Perez, P.; Guitton, C.; et al. Effect of Noninvasive Ventilation vs. Oxygen Therapy on Mortality Among Immunocompromised Patients With Acute Respiratory Failure: A Randomized Clinical Trial. JAMA 2015, 314, 1711. [Google Scholar] [CrossRef]
- Frat, J.-P.; Thille, A.W.; Mercat, A.; Girault, C.; Ragot, S.; Perbet, S.; Prat, G.; Boulain, T.; Morawiec, E.; Cottereau, A.; et al. High-Flow Oxygen through Nasal Cannula in Acute Hypoxemic Respiratory Failure. N. Engl. J. Med. 2015, 372, 2185–2196. [Google Scholar] [CrossRef]
- Ricard, J.-D.; Messika, J.; Sztrymf, B.; Gaudry, S. Impact on outcome of delayed intubation with high-flow nasal cannula oxygen: Is the device solely responsible? Intensive Care Med. 2015, 41, 1157–1158. [Google Scholar] [CrossRef]
- Sanfilippo, F.; La Via, L.; Dezio, V.; Santonocito, C.; Amelio, P.; Genoese, G.; Astuto, M.; Noto, A. Assessment of the inferior vena cava collapsibility from subcostal and trans-hepatic imaging using both M-mode or artificial intelligence: A prospective study on healthy volunteers. Intensive Care Med. Exp. 2023, 11, 15. [Google Scholar] [CrossRef]
- Russotto, V.; Tassistro, E.; Myatra, S.N.; Parotto, M.; Antolini, L.; Bauer, P.; Lascarrou, J.B.; Szułdrzyński, K.; Camporota, L.; Putensen, C.; et al. Peri-intubation Cardiovascular Collapse in Patients Who Are Critically Ill: Insights from the INTUBE Study. Am. J. Respir. Crit. Care Med. 2022, 206, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.J.; Koh, Y.; Lim, C.-M.; Huh, J.W.; Baek, S.; Han, M.; Seo, H.-S.; Suh, H.J.; Seo, G.J.; Kim, E.Y.; et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015, 41, 623–632. [Google Scholar] [CrossRef]
- Ehrmann, S.; Li, J.; Ibarra-Estrada, M.; Perez, Y.; Pavlov, I.; McNicholas, B.; Roca, O.; Mirza, S.; Vines, D.; Garcia-Salcido, R.; et al. Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: A randomised, controlled, multinational, open-label meta-trial. Lancet Respir. Med. 2021, 9, 1387–1395. [Google Scholar] [CrossRef]
- Maniaci, A.; Lavalle, S.; Gagliano, C.; Lentini, M.; Masiello, E.; Parisi, F.; Iannella, G.; Cilia, N.D.; Salerno, V.; Cusumano, G.; et al. The Integration of Radiomics and Artificial Intelligence in Modern Medicine. Life 2024, 14, 1248. [Google Scholar] [CrossRef]
- La Via, L.; Maniaci, A.; Gage, D.; Cuttone, G.; Misseri, G.; Lentini, M.; Paternò, D.S.; Pappalardo, F.; Sorbello, M. Exploring the potential of artificial intelligence in airway management. Trends Anaesth. Crit. Care 2024, 59, 101512. [Google Scholar] [CrossRef]
- Rubulotta, F.; Blanch Torra, L.; Naidoo, K.D.; Aboumarie, H.S.; Mathivha, L.R.; Asiri, A.Y.; Uranga, L.S.; Soussi, S. Mechanical Ventilation, Past, Present, and Future. Anesth. Analg. 2024, 138, 308–325. [Google Scholar] [CrossRef]
- Luckscheiter, A.; Thiel, M.; Zink, W.; Eisenberger, J.; Viergutz, T.; Schneider-Lindner, V. Utilization of non-invasive ventilation before prehospital emergency anesthesia in trauma—A cohort analysis with machine learning. Scand. J. Trauma Resusc. Emerg. Med. 2025, 33, 35. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).