Abstract
Bcakground: The deepithelialized free gingival graft (DGG) technique provides high-quality connective tissue grafts (CTGs) with predictable outcomes for recession coverage. This study evaluates a novel method of free gingival graft (FGG) deepithelialization using an Er,Cr:YSGG laser (LDEE) for treating multiple gingival recessions. Methods: A split-mouth study was conducted on 46 (n = 46) recessions in nine patients (23 per test and control group). Sites were randomized. Full-thickness palatal grafts were harvested with a scalpel. In the test group (LDEE), deepithelialization was performed extraorally using an Er,Cr:YSGG laser (2780 nm; 2.5 W, 83.3 mJ, 30 Hz, 600 µm tip). In the control group (DEE), a 15c scalpel was used. All CTGs were applied using the modified coronally advanced tunnel (TUN) technique. Clinical parameters—recession depth (RD), keratinized tissue width (KT), gingival thickness (GT), pocket depth (PD), clinical attachment loss (CAL), pink esthetic score (PES), approximal plaque index (API), mean root coverage (MRC), and complete root coverage (CRC)—were assessed at baseline (T0), 3 months (T1), and 6 months (T2). Results: Both LDEE and DEE groups showed significant improvements in RD, KT, GT, PD, and CAL over time (p < 0.001). At T1 and T2, KT was significantly higher in the LDEE group (T1: 3.73 ± 0.72 mm; T2: 3.98 ± 0.76 mm) compared to the DEE group (T1: 3.21 ± 0.61 mm; T2: 3.44 ± 0.74 mm; p < 0.05). Other parameters (RD, GT, PD, CAL) showed no statistically significant intergroup differences at any time point (p > 0.05). After 6 months, MRC was 95% and CRC 82.6% for LDEE, compared to 94.8% and 82.6% for DEE (p > 0.05). PES scores were similar between groups at all time points (p > 0.05). Conclusions: Both laser- and scalpel-deepithelialized grafts effectively treated gingival recessions. LDEE combined with TUN resulted in significantly greater KT width compared to DEE + TUN.
1. Introduction
Gingival recession (GR), defined as the apical displacement of the gingival margin leading to root surface exposure, is a common clinical finding with multifactorial etiology. It arises from a combination of anatomical predispositions (e.g., thin gingival biotype, tooth malposition, and alveolar bone dehiscence), mechanical trauma (notably from improper brushing techniques), and chronic inflammation associated with dental biofilm [1,2,3,4,5,6,7]. GR becomes more prevalent with age and is reported more frequently in male patients [2]. Clinically, it may result in dentin hypersensitivity, compromised aesthetics, and increased susceptibility to root caries, all of which can significantly affect oral health-related quality of life. Management of GR requires a comprehensive approach involving behavioral modifications, plaque control, and—when indicated—periodontal plastic surgery to restore the gingival margin and increase soft tissue volume [4,7,8,9,10,11,12,13,14,15,16,17,18].
Microsurgical techniques have gained popularity due to their precision and tissue preservation, particularly in the context of minimally invasive interventions. Among these, erbium family lasers (Er:YAG, Er,Cr:YSGG) have shown promise due to their affinity for water-rich soft tissues, allowing for controlled ablation with minimal thermal damage and enhanced healing outcomes [19,20,21,22,23,24,25]. Historically, free gingival grafts (FGGs) were the first approach used for recession coverage but were limited by suboptimal aesthetics and discomfort at the donor site [26,27,28,29,30,31,32]. The introduction of subepithelial connective tissue grafts (SCTGs) significantly improved clinical results, especially in Miller Class I and II recessions, achieving over 90% root coverage in favorable cases [6,33,34,35,36]. These outcomes were further optimized by the use of coronally advanced flaps (CAF) [37], supraperiosteal envelope techniques (SET) [38], and tunnel techniques (TUN) [39,40], with TUN now favored for its minimal invasiveness, enhanced vascularization, and better integration of grafts [41,42,43].
Palatal connective tissue remains the gold standard for grafting, typically harvested between the canine and first molar while avoiding neurovascular structures [44,45,46]. Two primary harvesting strategies are recognized: (1) SCTG obtained via trap-door or single-incision under a partial-thickness flap, and (2) deepithelialized gingival grafts (DGG), derived from full-thickness grafts with subsequent epithelial removal using scalpels, burs, or lasers [47,48,49,50,51,52]. Among these, the Zucchelli technique is the most widely adopted for scalpel-based deepithelialization [25,50,52]. Compared to SCTG, DGGs are histologically denser and contain less glandular or adipose tissue, which may enhance their volume stability [44,53]. While acellular dermal matrix allografts (ADMAs) offer a donor-site-free alternative, autogenous grafts remain superior in keratinized tissue gain and healing dynamics [54,55,56].
The present study aimed to evaluate the clinical efficacy of laser-assisted deepithelialization of free gingival grafts using an Er,Cr:YSGG laser in the treatment of multiple gingival recessions. Specifically, the study assessed complete root coverage (CRC), mean root coverage (MRC), and changes in clinical parameters such as recession depth (RD), keratinized tissue width (KT), gingival thickness (GT), probing depth (PD), and clinical attachment level (CAL) at 3- and 6-month follow-ups. In addition, gingival aesthetics were evaluated using the Pink Esthetic Score (PES), with results compared between laser- and scalpel-deepithelialized grafts.
2. Materials and Methods
2.1. Study Design
This study was a single-center, single-blinded, split-mouth randomized clinical trial (RCT) structured according to the CONSORT statement (Figure 1). It evaluated the use of laser deepithelialization of the free gingival graft to cover gingival recessions. In the study group, the connective tissue graft (SCTG) was harvested by deepithelializing the free gingival graft using the Er,Cr:YSGG laser (LDGG), while in the control group, the traditional deepithelialization technique with a 15C scalpel (DGG) was used. The prepared grafts were then used to cover gingival recessions using the tunnel technique (TUN). A sample of palatal mucosa subjected to laser treatment was stained with hematoxylin and eosin (H&E) and examined histopathologically under 40× magnification.
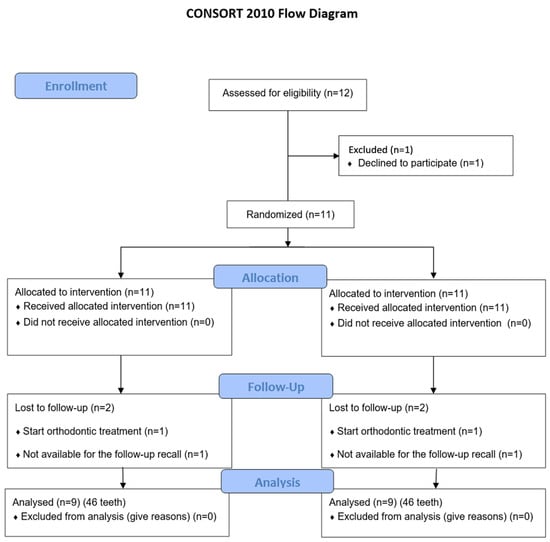
Figure 1.
Flowchart of treated subjects according to CONSORT 2010.
A sample of palatal mucosa subjected to Er,Cr:YSGG laser deepithelialization was fixed in 10% buffered formalin immediately after harvesting. Following fixation, the tissue was dehydrated, embedded in paraffin, sectioned at 5 µm, and stained with hematoxylin and eosin (H&E). Histological evaluation was performed under 40× magnification using a light microscope (Leica DM500, Leica Microsystems, Wetzlar, Germany). The aim was to assess the depth and uniformity of epithelial removal, the integrity of the connective tissue, and the presence of thermal damage. A black ink marker was used to indicate tissue orientation. The analysis confirmed clear epithelial removal without signs of carbonization, coagulation, or connective tissue necrosis.
2.2. Sample Size
The study included 46 teeth with gingival recession (23 in the LDGG group and 23 in the DEE group), treated in 9 participants—2 men and 7 women—aged 19 to 51 years (mean age: 36.1 ± 8.46 years). The study group included 2 lateral incisors (8.7%), 9 canines (39.1%), 9 first premolars (39.1%), and 3 s premolars (13%). The control group included 1 lateral incisor (4.3%), 7 canines (30.4%), 9 first premolars (39.1%), and 6 s premolars (26.1%). All patients were treated between February 2021 and August 2022. The procedures were performed in full accordance with the principles of the Declaration of Helsinki (1975, revised in 2013), and the study design was approved by the District Medical Chamber in Bielsko-Biała (approval no. 2021/1/28/3). The trial was registered at the official website ClinicalTrials.gov (identifier: NCT07064304).
The sample size was calculated using G*Power software (Version 3.1.9.7, Kiel University, Kiel, Germany). Assuming an effect size (Cohen’s d) of 0.8 for the primary outcome (keratinized tissue width, KT), a two-tailed test, a significance level of α = 0.05, and a statistical power (1 − β) of 0.80, the minimum number of gingival recessions required per group was 21. Therefore, the total number of 46 treated recessions (23 per group) in the split-mouth design was deemed sufficient to detect clinically meaningful differences.
Patients were recruited through periodontal consultations at the dental center during the same period. Each eligible participant received comprehensive verbal and written explanations of the study objectives, procedures, and potential risks, and informed consent was obtained prior to inclusion. Participants were consecutively enrolled based on strict eligibility criteria, and no financial or therapeutic incentives were offered to avoid selection bias. The study was performed in Bielsko-Biala, where it received ethical approval and supervision from the District Medical Chamber (approval no. 2021/1/28/3), and was carried out as part of a master’s thesis within the European Master’s Degree in Oral Laser Applications (EMDOLA) at Wroclaw Medical University in Poland. The study complied with all ethical and methodological standards expected of university-based research and followed the Declaration of Helsinki.
All patients agreed to participate in the study and signed a written informed consent according to the above-mentioned principles. The patients were recruited based on the following inclusion criteria:
- age over 18;
- presence of at least two teeth with gingival recession RT1 [11] in the jaw in incisors, canines, premolars;
- buccal gingival recession defects ≥2–4 mm in depth;
- no periodontal inflammation (CPITN < 2);
- proper oral hygiene API < 15;
- probing depths < 3 mm;
- detectable cementoenamel junction (CEJ);
- no history of previous periodontal plastic surgery at the affected sites.
Exclusion criteria were as follows:
- smoker, also e-cigarettes;
- systemic diseases affecting periodontal status or healing (e.g., diabetes mellitus, immunodeficiency, osteoporosis, cardiovascular disease);
- intake of medications;
- presence of caries lesions or restorations in the cervical area;
- pregnancy and lactation.
2.3. Initial Therapy and Clinical Measurements
After the inclusion in this study, all patients were scheduled on a prophylaxis appointment during which they had scaling and professional tooth cleaning and were also instructed to use the roll technique in order to minimize mechanical trauma. Surgical treatment of the recession defects was not scheduled until the patient could demonstrate an adequate standard of supragingival plaque control. A single calibrated examiner, who was masked to the treatment allocation, performed all clinical measurements. Clinical measurements were recorded at the baseline, and at 3 and 6 months after surgeries.
The following parameters were assessed:
- recession depth (RD) measured at the mid-buccal aspect of the study tooth from the CEJ to the most apical extension of gingival margin using electronic periodontal probe PA-ON (Orangedental, Biberach, Germany); Figure 2;
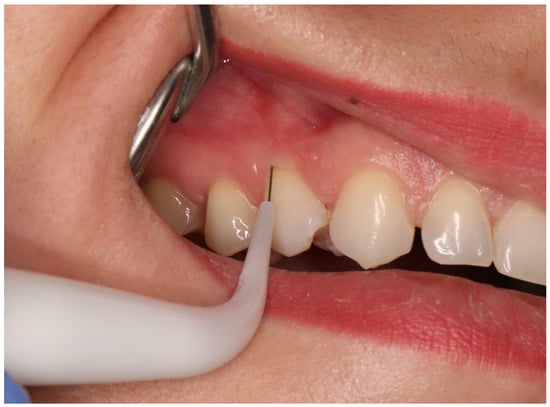 Figure 2. Electronic periodontal probe (PA-ON) examination.
Figure 2. Electronic periodontal probe (PA-ON) examination. - keratinized tissue (KT) measured from the most apical point of gingival margin to the muco-gingival junction (MGJ); the MGJ was identified by means of Lugol staining using a manual PCP-UNC 15 periodontal probe (Hu-Friedy, Chicago, IL, USA);
- gingival thickness (GT) measured at the mid-buccal aspect of the study tooth on a long axis, 2 mm apically from the gingival margin on CBCT slide (Kodak 8100 3D, Carestream/Trophy, Marne-la-Vallée, France), with a Field of View (FOV) of 5 cm × 4 cm, nominal beam of 73 kV, 12 mA and a voxel size of 150 µm. The measurement was valued by using Carestream 3D Suite (Carestream Health, Inc, Rochester, NY, USA);
- probing depth (PD) measured at the mesial, distal side, and mid-buccal aspect of the study tooth from the gingival margin to the bottom of the sulcus, PA-ON probe (Orangedental, Biberach, Germany); Figure 2;
- clinical attachment level (CAL) measured at the mid-buccal aspect of the study tooth from the CEJ to the bottom of the sulcus using electronic periodontal probe PA-ON (Orangedental, Biberach, Germany);
- approximal plaque index (API);
- community periodontal index of treatment needs (CPITN);
- mean root coverage (MRC), calculated as [(Baseline RD) − (6 months RD)/Baseline RD] × 100% [50];
- complete root coverage (CRC), calculated as the percentage of the teeth with recession defects having complete coverage achieved [(Teeth with CRC)/(All treated teeth)] × 100% [50];
- the graft area (GA), measured using the ImageJ (version 2.3.0/1.53t) Ver. G*Power (version 3.1.9.7) 1.53k computer program (https://imagej.nih.gov/ij accessed on 2 November 2024, NIH, Bethesda, MD, USA). Based on a 90-degree photo (Canon Eos 200, 135 mm + Yongnuo YN24EX flash ring) of the graft taken with a periodontal probe. Before the measurements, a calibration was performed, which consisted in marking a 15 mm reference section on periodontal probe PCP-UNC 15 (Hu-Friedy, Chicago, IL, USA);
- the pink aesthetics score (PES) developed by Furhauser et al. [57], assessed in the 3rd and 6th post-surgical month. It rates seven variables on a scale of 0 to 2 (mesial papilla, distal papilla, level of the gingival margin, marginal tissue contour, alveolar process contour, soft tissue color, soft tissue texture), yielding a maximum score of 14 points.
2.4. Randomization
Patients were assigned to one of two treatment groups using a computer-generated randomization table available at www.Randomizer.org (allocation ratio 1:1). The allocation was made secret through sealed, encoded opaque envelopes containing the treatment of a specific condition. The sealed envelope (containing treatment allocation) was opened during the procedure immediately before harvesting the graft.
2.5. Surgery
All surgical procedures were performed by the same oral surgeon in a microsurgical manner at 3× magnification (Heine, Germany). Immediately before the procedure, the root surfaces were cleaned with plastic brushes, and the contact points of the teeth were temporarily closed with a liquid, light-curing resin material (Filtek® Supreme Flowable, 3M ESPE, St. Paul, MN, USA) to prevent the suspension sutures from collapsing. After administering local anesthesia (Septanest 1:200,000, Septodont, Saint-Maur-des-Fosses Cedex, France), the exposed root surfaces were prepared using Gracey curettes (Hu-Friedy, Chicago, IL, USA). A modified tunnel technique (TUN) was performed at all recipient sites [58]. Initial incisions in the gingival fissure were made with a microsurgical blade. The partial thickness tunnel was created with tunnel knives (14,446.02, 14,439.00 Stoma Dentalsysteme GmbH & Co KG, Emmingen-Liptingen, Germany) and extended beyond the MGJ to achieve mobilization and tension-free displacement of the flap towards the crown. FGG was obtained from the area between the distal line angle of the maxillary strip and the distal line angle of the first maxillary molar, sized according to the needs of the procedure. The full-thickness gingival graft was harvested from the palate using a 15c scalpel. Four incisions were made on the palate mucosa—two horizontal and two vertical- so that a rectangle was created that corresponded to the graft design. A coronal horizontal incision was made approximately 2 mm from the gingival margin. The graft was harvested using incisions parallel to the mucosa surface at an appropriate depth, leaving the periosteum undamaged. The graft was kept on sterile gauze dipped in saline where the graft was prepared. First, the adipose tissue and submucosa were removed from the periosteal side of the graft using a 15c scalpel. On the study side (LDEE), deepithelialization was performed using Er,Cr:YSGG, 2780 nm laser (Waterlase iPluse, Biolase, Foothill Ranch, CA, USA) with an MZ6 tip and the power of 2.5 W, 30 Hz, pulse duration: 60 µs, Water—80%, Air—70%, energy per pulse: 83.3 mJ, fluence (energy density per pulse) 28.6 J/cm2. During the procedure, the tip was positioned at an angle of 30–40 degrees to the graft surface and with a slow movement (1 mm/s) in light contact. Movements were made line by line across the entire width of the graft. The epithelium was deepithelialized twice in this way. Finally, the blunt end of the scalpel was used to scrape off the free tissue remnants after ablation. For the control side (DEE), deepithelialization was performed with a 15c scalpel [50]. The blade was set parallel to the graft surface, and horizontal incisions were made to a depth of about 0.5 mm. If necessary, the light was perpendicular during the procedure so that it was possible to better assess the remaining epithelium, which reflects the light better compared to connective tissue. After deepithelialization, the grafts were then placed in the tunnel with mattress sutures on the mesial and distal sides. Vertical double-crossed sutures (5-0 monofilament suture, Dogsan sutures, Trabzon, Turkey) were used to stabilize the cheek soft tissue complex 1–2 mm coronally to the CEJ (Zuhr et al.) [51]. In the case of covering three adjacent recessions, the graft was additionally individually stabilized to the adjacent tooth, and in the case of two recessions, no additional stabilization was used.
2.6. Post-Surgical Protocol
All patients were prescribed a soft diet, avoiding mechanical injuries, brushing, and flossing at the surgical sites for 2 post-surgical weeks until the suture was removed. To achieve plaque control, 0.12% Oral chlorhexidine gluconate (Eludril, Pierre Fabre, Paris, France) was used 3 times a day for 1 min for the first 2 weeks. Patients were prescribed 100 mg nimesulide (Nimesil, Laboratori Guidotti S.p.A, Pisa, Italy) and instructed to take one dose at the end of surgical intervention and one 12 h after surgery for anti-inflammatory and analgesic treatment. They were also instructed to take additional pain medication should it be needed. Two weeks after surgery, sutures were removed, and plaque control was maintained using an ultra-soft toothbrush (5/100) and a roller technique. One month after the sutures were removed, patients were allowed to return to normal oral hygiene procedures. All patients had a hygienic appointment for the improvement of oral hygiene at 2, 4, and 6 weeks and 3 and 6 months after the procedure.
2.7. Statistical Analyses
The teeth were divided into two groups: the control (DEE) and the studied group (LDGG). Linear Mixed–Effect Models (LMM) with Gaussian identity were applied. Because control and studied teeth occurred in the same patient, an effect of patient considered as random effect while effect of group (control vs. studied group) and time (baseline—T0, 3 months post-op—T3, 6 months post-po.—T6, delta T0–T3 and delta T0–T6) were treated as fixed factors in particular variables: RD, CAL, KT, GT, PD, PES. Also, the interaction of these factors was included. The Wald and chi-square statistics were calculated, as well as the p-value of significance of the model. In particular time series between the control (DEE) and studied (LDEE) groups, Student’s t-test was used. For area of transplant (TA), Student’s paired t-tests were used. The normality of distribution was checked by means of the Shapiro–Wilk test. All statistics and visualizations were calculated in R language and environment (version 4.2.1, R Core Team, 2022) using libraries Stats, lm4, car, and ggplot2.
3. Results
The study included a total of 46 gingival recessions in nine patients (seven women and two men), aged between 19 and 51 years (mean age 36.1 ± 8.46), all of whom completed the 6-month follow-up period (Table 1). Figure 1 shows the CONSORT flowchart diagram reporting the numbers of participants, who were randomly assigned, given intended treatments, and analyzed for the primary outcome of the present study. A total of 46 teeth were included in the study, 23 in the study group (LDGG) and 23 in the control group (DGG). In the study group, there were two lateral incisors (8.7%), nine canines (39.1%), nine first premolars (39.1%), and three second premolars (13%). The control group included one lateral incisor (4.3%), seven canines (30.4%), nine first premolars (39.1%), and six second premolars (26.1%) (Table 1). All patients had good oral hygiene before surgeries and throughout the follow-up period, maintaining API levels of 11.61 ± 1.76 at baseline, 11.95 ± 2.27 3 months post-op, and 12.2 ± 2.12 6 months post-op.

Table 1.
Demographic data and the characteristics of study subjects.
There were no statistically significant differences between the groups in all clinical parameters assessed at the baseline (Table 2).

Table 2.
Clinical parameters (mean and SD) at baseline (T0), 3 months post-op. (T1) and 6 months post-op. (T2).
3.1. Recession Depth (RD)
In the case of RD in the LDGG group at baseline, the mean values were 2.60 ± 0.72 mm; 3 months after the surgery, the mean values were 0.08 ± 0.28 mm; while after 6 months, they were 0.13 ± 0.30 mm. Accordingly, in the DGG group, the mean values were 2.83 ± 0.90 mm at baseline, 0.11 ± 0.29 mm after 3 months, and 0.15 ± 0.35 mm after 6 months. The results obtained in both groups showed statistical significance as a function of time at the level of p < 0.001. On the other hand, the RD values between the groups did not show statistical significance in any follow-up period. Similarly, there were no statistically significant values between the groups in the case of a change in RD values at the 3-month baseline (LDGG—2.52 ± 0.67 mm, DGG—2.72 ± 0.79 mm) and at the 6-month baseline (LDGG—2.39 ± 0.67 mm, DGG—2.68 ± 0.83 mm).
3.2. Probing Depth (PD)
For PD measurements in the LDGG group, the following results were achieved: 1.66 ± 0.34 mm, 1.38 ± 0.44 mm, and 1.47 ± 0.43 mm at baseline, after 3 months, and after 6 months, respectively. In the DGG group, the following results were obtained: 1.51 ± 0.36 mm at baseline, 1.18 ± 0.33 mm after 3 months, and 1.28 ± 0.40 mm after 6 months. The change in time △T0–T1 was 0.28 ± 0.33 mm for LDGG and 0.33 ± 0.41 mm for DGG, while for △ T0–T2, it was 0.32 ± 0.46 mm for LDGG and 0.23 ± 0.44 mm for DGG. Statistically significant results were obtained only for the function of time in both groups. Comparisons of the remaining results as a function of time did not show statistical significance.
3.3. Keratinized Tissue (KT)
In the case of KT, statistically significant values (p < 0.05) were obtained between the groups, both after 3 months (3.73 ± 0.72 mm—LDGG, 3.21 ± 0.61 mm—DGG) and after 6 months (3.98 ± 0.76 mm—LDGG, 3.44 ± 0.74 mm—DGG). The increase in KT also showed statistical significance between the groups at the level of p < 0.05 in favor of LDGG, both in the T0–T1 period (1.39 ± 1.16—LDGG and 1.09 ± 1.08—DGG, p < 0.05) and in the T1–T2 period (1.65 ± 1.19—LDGG and 1.43 ± 1.27—DGG, p < 0.05). In each group, the change in KT was also statistically significant (p < 0.001) as a function of time (Table 3).

Table 3.
Change in clinical parameters at baseline—3 months post-op. (△T0–T1) and baseline—6 months post-op. (△T0–T2).
3.4. Gingival Thickness (GT)
For GT in the LDGG group, the results were 0.83 ± 0.17 at baseline, 2.17 ± 0.31 after 3 months, and 2.09 ± 0.28 after 6 months. The increase in GT in the LDGG group showed statistical significance at the level of p < 0.001. Similar statistical significance was obtained in the DGG group, where the results were as follows: at baseline—0.86 ± 0.28, after 3 months—2.19 ± 0.41, and after 6 months—1.98 ± 0.35. Although in the LDGG group, higher values were obtained both after 3 and 6 months in relation to the DGG group, the difference was not statistically significant. The comparison of the increase between the groups also showed no statistically significant values.
3.5. Clinical Attachment Level (CAL)
CAL values for the LDGG group were 4.26 ± 0.66 at baseline, 1.12 ± 0.47 after 3 months, and 1.24 ± 0.58 after 6 months. For the DGG group, they were 4.34 ± 0.91 at baseline, 1.04 ± 0.65 after 3 months, and 1.15 ± 0.76 after 6 months. In both groups, the reduction in CAL showed statistical significance (p < 0.001) over the 6-month follow-up period. The reduction in CAL after 3 months was 3.47 ± 0.7 for the LDGG group and 3.65 ± 0.92 for the DGG group, respectively, and showed no statistical significance between the groups (Table 4).

Table 4.
Values (mean and SD) of complete root coverage (CRC), mean root coverage (MRC), graft area (GA), and pink aesthetic score (PES) for LDGG and DGG site.
3.6. Mean (MRC) and Complete (CMC) Root Coverage
The mean root coverage (MRC) measured 6 months after surgery was 95% in the LDGG group and 94.8% in the DGG group. Complete root coverage (CRC) was achieved in 19 of 23 (82.6%) gingival defects treated in both the study and control groups. The graft area in the LDEE group was 103.88 ± 18.61 mm2, while in the DGG group, it was 103.45 ± 14.02 mm2, and the values did not show statistical significance between the groups.
3.7. Pink Aesthetic Score (PES)
The pink aesthetic score (PES) was assessed 3 and 6 months after surgery. In the LDGG group, the values were 12.5 ± 0.73 (after 3 months) and 13.2 ± 0.8 (after 6 months) out of a possible 14 points. In the DGG group, the results were 12.5 ± 0.9 and 13.3 ± 0.64 3 and 6 months after surgery, respectively.
3.8. Graft Area (GA)
The mean values obtained for the graft area were very similar: 103.88 ± 18.61 mm2 for the LDGG group and 103.45 ± 14.02 mm2 for the DEE group. The results showed no statistically significant difference between the groups.
3.9. Histopathological Analysis
The histological slide clearly demonstrated a distinct boundary between the deepithelialized connective tissue and the remaining full-thickness epithelium, confirming controlled and uniform epithelial removal. No signs of carbonization, thermal necrosis, or structural damage to the underlying connective tissue were observed, suggesting that the Er,Cr:YSGG laser preserves tissue viability while ensuring effective epithelial ablation. A black ink artifact was visible in the slide, used for specimen orientation, but did not obscure diagnostic interpretation (Figure 3).
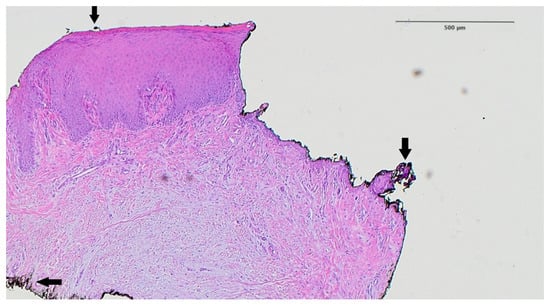
Figure 3.
Histological analysis of the palatal mucosa after deepithelialization with the Er,Cr:YSGG laser according to the methodology proposed in this study. The border between the deepithelialized and the full-thickness part is shown. Arrow—black artifact caused by ink used to mark the specimen (hematoxylin–eosin staining, magnification ×40).
4. Discussion
The aim of this study was to evaluate the clinical application of gingival grafts harvested from the palate after deepithelialization using an Er,Cr:YSGG laser (2780 nm), compared to gingival grafts deepithelialized using the traditional scalpel technique. The study was designed as a split-mouth randomized clinical trial (RCT). The study focused on the incisors, canines, and premolars of the maxilla due to the similar anatomical characteristics of the soft tissues and tooth roots in this region. A total of 46 gingival recessions in nine patients were treated using either laser-deepithelialized grafts (LDGG) or scalpel-deepithelialized grafts (DGG). After 6 months, both groups achieved comparable mean root coverage (MRC: 95% in LDGG vs. 94.8% in DGG) and complete root coverage (CRC: 82.6% in both). The LDGG group showed significantly greater keratinized tissue width at 3 and 6 months (p < 0.05), while other clinical parameters (RD, PD, CAL, GT) improved significantly over time in both groups without intergroup differences. Aesthetic outcomes (PES) and graft areas were similar. Currently, lasers are utilized in dentistry as an alternative to conventional surgical instruments, and they have major advantages such as the production of local homeostasis [59,60], reduced postoperative pain and edema [57], bacterial elimination [61,62], the possibility of contact-free incision [63], and the avoidance of the need for sutures [59]. However, the use of the scalpel in oral surgery has inherent problems, such as difficulty in the visualization of the surgical field due to hemorrhage and excessive blood loss [59,64], while the bur can cause thermal necrosis and particle release to the atmosphere [63]. In the above study, the Er,Cr:YSGG laser with a wavelength of 2.78 μm was used, which is very well suited for working on soft tissues due to effective water absorption [63,65,66]. Investigations have shown that this type of laser may provide straight, clean, and precise ablation and cause minimal thermal damage to the adjacent tissue [66,67].
The safety and effectiveness of erbium lasers when working on soft tissues is confirmed, among others, by Monteiro et al. [68], who showed that erbium lasers cause the least thermal damage to tissues in surgical margins, compared to CO2, diode, Nd:YAG, and electroscalpels. Similar observations were made by Grzech-Leśniak [69], who showed the effectiveness of deepithelialization using the Er:YAG laser without thermal damage to the graft on animal material.
There are only a few reports in the literature on the use of the Er,Cr:YSGG laser for the deepithelialization of gingival graft. One of them is the study conducted by Lin et al. [70], who in their publication describe examples of the use of gingival grafts deepithelialized by Er,Cr:YSGG (2780 nm) laser. LDGGs have been used to cover recession, cover soft tissue fenestration at the implant, and provide soft tissue augmentation at the alveolar process. All treatments were successful, and a satisfactory clinical effect was obtained. Moreover, the authors insisted that it is easier to remove epithelium completely from FGG using an Er,Cr:YSGG laser than using a 15 scalpel, although the complete removal of epithelialization is clear in their histological evidence. Another publication written by Fekrezad et al. [71] describes the use of the Er,Cr:YSGG laser for deepithelialization of the gingival graft used to cover gingival recessions. They state that due to high superficial absorption, low penetration into the tissue, good incision, and safety for bones, the Er,Cr:YSGG laser may be useful at every stage of the free gingival graft transplant procedure. Gursoy [25] carried out a randomized clinical trial that evaluated gingival grafts deepithelialized using an Er:YAG laser to cover gingival recessions. He achieved statistically significantly greater thickness of the keratinized gingiva (GT) and better aesthetics compared to the control group where DGG by a scalpel was used. These results are consistent with our observations, where a higher GT was achieved in the LDGG group, but the results were not statistically significant. Among lasers, erbium lasers seem to be the best choice for deepithelialization of gingival grafts, while in the literature, there are also articles describing the use of other lasers such as diode and CO2 lasers. Although they show greater thermal damage to tissues, satisfactory clinical effects can also be obtained. According to Kawamura et. al. [72], they assessed the mean thermal damage caused by the CO2 laser at 236.6 µm and the 808 nm diode laser at 261 µm. These results are much higher compared to the Er:YAG and Er,Cr:YSGG lasers, where the results were 17.9 µm and 37.7 µm, respectively.
An example of the use of a diode laser for deepithelialization is the randomized clinical trial where Ozcelik et al. [73] performed deepithelialization using an 810 nm diode laser and assessed the use of LDGG in covering recessions. They obtained very good clinical results comparable to the control group, where the gingival graft deepithelialization by a scalpel was applied. On the other hand, Yoshino [74] describes the use of gingival grafts deepithelialized using a CO2 laser (10,600 nm) to cover gingival recessions. He found that this method could obtain good-quality LDGGs and increased the width of the keratinized gingiva by 2.9 ± 0.3 mm after 12-month follow-ups.
Many techniques for obtaining connective tissue have been proposed and applied. Among them, trapdoor and single incision techniques for harvesting SCTG are now widely used. In these conventional techniques, the thickness of the connective tissue is determined by the morphology of the palatal soft tissues. In many clinical situations, it is not possible to obtain a connective tissue graft of adequate quality if the soft tissue of the palate is not thick enough to harvest the connective tissue, and the techniques are not recommended due to the risk of necrosis of the primary flap and/or a suboptimal composition of the graft, which may include glandular and adipose tissue instead of connective tissue [50,75]. According to Harris [53], a thickness of the palate mucosa exceeding or equal to 3 mm guarantees the histologically correct quality of the graft. With a thin palate mucosa, it is recommended to harvest FGG, from which the epithelium is then removed. The DGG technique requires a smaller thickness of the palate mucosa to obtain the appropriate quality of the graft and to leave the appropriate thickness of the soft tissue covering the bone [50].
Bertl et al. [44] investigated the composition of gingival grafts, assessing fibrous, adipose, and glandular tissue using various techniques to harvest grafts on fresh human cadavers. They concluded that CTG obtained from deepithelialized gingival grafts (DGG) contained significantly larger amounts of connective tissue and significantly smaller amounts of adipose/glandular tissue than CTG obtained using the split-flap method. Another RCT [50] compared the results for root coverage with the use of CTG obtained using the trapdoor method and CTG obtained by removing the epithelium from FGG by scalpel. There were no significant differences in root coverage or KT growth between the two types of CTG harvesting techniques. On the other hand, there was a statistically significant difference in gingival thickness in 12-month follow-ups in favor of DGG, explained by the fact that DGG could have more dense, fibrous connective tissue that is more stable and has less tendency to disappear. Additionally, Bakhishov in his research showed a statistically significant increase in CRC and MRC in the group with DGG compared to SCTG in 1-year follow-ups [76].
The above analyses indicate that, thanks to DGG, compared to SCGG, it is possible to introduce into the graft a part of connective tissue closer to the epithelium, which is denser, firmer, more stable, and probably more suitable for root covering [53]. However, if we want to take a step forward and develop the technique of deepithelialization of the gingival graft, clinical evaluation of new technologies will be necessary. Laser deepithelialization of gingival grafts is a relatively new method and does not yet have an adequate scientific base. Therefore, this randomized clinical trial was conducted to compare DEE and LDEE in covering gingival recessions. In 6-month follow-ups, similar results were obtained in terms of MCR and CRC, which proves that LDGG is as effective in covering gingival recessions as DGG with a scalpel, which so far has been considered to be the gold standard. Moreover, in the study group, a statistically significant higher value of KT was obtained compared to the control group, both during the 3- and 6-month follow-ups and by analyzing the increase in KT after 3 months and after 6 months. A higher value of GT was also observed in the LDGG group in the 3- and 6-month follow-ups, although this result was not statistically significant. Promising results were obtained, which may indicate that the use of the Er,Cr:YSGG laser will allow for a controlled and even more precise removal of the epithelium, leaving the most desirable part of the connective tissue located just under the epithelium [53]. Perhaps thanks to this, it will be possible to obtain better and more predictable clinical results.
Moreover, as we know, procedures in the field of periodontal plastic surgery require exceptional surgical skills and extensive clinical experience. Surgeon-dependent factors significantly influence outcomes and should not be underestimated [77,78,79]. Deepithelialization using a scalpel is a technically demanding procedure, especially in the hands of an inexperienced surgeon. Therefore, utilizing a laser as an easier method to learn, with reproducible and constant physical parameters, enables predictable outcomes and reduces surgeon-dependent errors [80,81,82,83,84,85]. In addition to surgical outcomes, it is important to consider patient-centered factors when evaluating the clinical value of new technologies. Although the present study did not collect data on postoperative discomfort, pain, or healing experience, previous investigations have shown that laser-assisted procedures are associated with reduced postoperative morbidity due to minimized thermal and mechanical trauma. Therefore, future clinical trials should include validated patient-reported outcome measures (PROMs) to better evaluate the subjective aspects of healing and overall patient satisfaction [86,87,88,89]. From a practical standpoint, the adoption of Er,Cr:YSGG lasers in periodontal procedures also requires evaluation in terms of cost-effectiveness, equipment accessibility, and clinician learning curve. The high initial investment, need for training in laser physics and safety, and variation in technique-specific outcomes could present barriers in general and specialist practices. However, the reproducibility of laser parameters, the minimally invasive nature of the procedure, and the potential for improved patient comfort and reduced complications may offset these challenges in selected cases—especially when graft precision and soft tissue preservation are critical to the outcome.
In this study, the pink aesthetic score (PES), according to Furhanser [57], was used to assess aesthetics. It was initially developed to evaluate the aesthetics of implants but was adopted by Zuhr and Hulzeler [49,51] to evaluate aesthetics following periodontal procedures and is particularly suitable for this purpose. This indicator is less commonly used than the method described by Cairo [79]; however, it offers a more comprehensive evaluation of soft tissue parameters, as highlighted in this study. The aesthetic effects obtained in both groups were comparable and considered excellent. Similarly, Gursoy et al. [25] achieved very good aesthetic results in recession coverage using LDGG harvested with an Er:YAG laser, which were even better compared to the traditional deepithelialized gingival graft (DGG) performed with a scalpel.
5. Conclusions
Within the limitations of this study, it can be concluded that both gingival grafts deepithelialized using an Er,Cr:YSGG laser and those deepithelialized with a scalpel effectively achieve coverage of gingival recessions. The clinical outcomes, expressed as mean root coverage (MRC) and complete root coverage (CRC), were comparable between both treatment groups. Notably, the use of laser-deepithelialized gingival grafts (LDGG) combined with the tunnel technique (TUN) resulted in greater keratinized tissue (KT) width compared to the scalpel-deepithelialized gingival graft (DGG) combined with TUN. Both approaches also demonstrated similarly favorable aesthetic outcomes at the 6-month follow-up evaluation. These preliminary findings suggest that laser-assisted deepithelialization may be a predictable and reproducible alternative to conventional techniques, particularly in cases where soft tissue precision and preservation are critical. However, further randomized clinical trials involving larger patient populations are needed to confirm these results. Future studies should also include patient-reported outcome measures (PROMs) and assess the feasibility of laser implementation in routine dental practice, considering factors such as cost-effectiveness, training requirements, and overall patient experience.
Author Contributions
Conceptualization, K.G.-L., J.M., A.B., methodology, K.G.-L., J.M., A.B.; software, K.G.-L., J.M., A.B.; validation, K.G.-L., J.M., A.B., R.W.; formal analysis, K.G.-L., J.M., A.B., R.W.; investigation, A.B.; resources, K.G.-L., J.M.; data curation, K.G.-L., J.M., A.B.; writing—original draft preparation, R.W., J.F.-R., Z.G.-L., K.G.-L.; writing—review and editing, R.W., J.F.-R., Z.G.-L., K.G.-L., J.M., J.A.S.; visualization, J.F.-R., R.W., K.G.-L.; supervision, K.G.-L., R.W., J.M., J.A.S.; project administration, A.B., R.W., J.A.S., J.M.; funding acquisition, K.G.-L., J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee No. 2021/1/28/3. (date of approval 28 January 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Imber, J.C.; Kasaj, A. Treatment of Gingival Recession: When and How? Int. Dent. J. 2021, 71, 178–187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhan, Y.; Wang, M.; Cao, X.; Liu, F. Effectiveness of acellular dermal matrix graft with a coronally advanced flap for the treatment of Miller Class I/II single gingival recession with thin gingival phenotype: Study protocol for a split-mouth randomised controlled trial. BMJ Open 2022, 12, e047703. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sarhan, S.; Ahmed, E.; Hussein, R.R.; Abou-Bakr, A. Prevalence, etiology and clinical characteristics of gingival recession in a sample of adult Egyptian dental patients: A cross sectional study. BMC Oral Health 2025, 25, 691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mythri, S.; Arunkumar, S.M.; Hegde, S.; Rajesh, S.K.; Munaz, M.; Ashwin, D. Etiology and occurrence of gingival recession—An epidemiological study. J. Indian Soc. Periodontol. 2015, 19, 671–675. [Google Scholar] [CrossRef]
- Fageeh, H.I.; Fageeh, H.N.; Bhati, A.K.; Thubab, A.Y.; Sharrahi, H.M.H.; Aljabri, Y.S.; Alotaibi, F.I. Assessing the Reliability of Miller’s Classification and Cairo’s Classification in Classifying Gingival Recession Defects: A Comparison Study. Medicina 2024, 60, 205. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rathore, P.; Manjunath, S.; Singh, R. Evaluating and comparing the efficacy of the microsurgical approach and the conventional approach for the periodontal flap surgical procedure: A randomized controlled trial. Dent. Med. Probl. 2024, 61, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Sarlati, F.; Moghaddas, O.; Shabahangfar, R.; Safari, S.; Valaei, N. Inter- and intra-examiner agreement of three classification systems of gingival recession. J. Adv. Periodontol. Implant Dent. 2019, 11, 1–6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Golob Deeb, J.; Reddy, N.; Kitten, T.; Carrico, C.K.; Grzech-Leśniak, K. Viability of bacteria associated with root caries after Nd:YAG laser application in combination with various antimicrobial agents: An in vitro study. Dent. Med. Probl. 2023, 60, 649–655. [Google Scholar] [CrossRef]
- Golob Deeb, J.; Smith, J.; Belvin, B.R.; Lewis, J.; Grzech-Leśniak, K. Er:YAG Laser Irradiation Reduces Microbial Viability When Used in Combination with Irrigation with Sodium Hypochlorite, Chlorhexidine, and Hydrogen Peroxide. Microorganisms 2019, 7, 612. [Google Scholar] [CrossRef]
- Sterczała, B.; Grzech-Leśniak, K.; Michel, O.; Trzeciakowski, W.; Dominiak, M.; Jurczyszyn, K. Assessment of Human Gingival Fibroblast Proliferation after Laser Stimulation In Vitro Using Different Laser Types and Wavelengths (1064, 980, 635, 450, and 405 nm)-Preliminary Report. J. Pers. Med. 2021, 11, 98. [Google Scholar] [CrossRef]
- Grzech-Leśniak, K.; Belvin, B.R.; Lewis, J.P.; Golob Deeb, J. Treatment with Nd:YAG Laser Irradiation Combined with Sodium Hypochlorite or Hydrogen Peroxide Irrigation on Periodontal Pathogens: An In Vitro Study. Photobiomodul. Photomed. Laser Surg. 2021, 39, 46–52. [Google Scholar] [CrossRef]
- Grzech-Leśniak, Z.; Pyrkosz, J.; Szwach, J.; Lelonkiewicz, M.; Pajączkowska, M.; Nowicka, J.; Matys, J.; Grzech-Leśniak, K. In vitro evaluation of the effect of Er:YAG laser with a fractional PS04 handpiece on microbial biofilm survival. Dent. Med. Probl. 2025. ahead of print. [Google Scholar] [CrossRef]
- Sanavi, F.; Weisgold, A.S.; Rose, L.F. Biologic width and its relation to periodontal biotypes. J. Esthetor. Dent. 1998, 10, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Zucchelli, G.; Mounssif, I. Periodontal plastic surgery. Periodontol. 2000 2015, 68, 333–368. [Google Scholar] [CrossRef]
- Mahajan, A. Mahajan’s modification of Miller’s classification for gingival recession. Dent. Hypotheses 2010, 1, 45–50. [Google Scholar] [CrossRef]
- Cairo, F.; Nieri, M.; Cincinelli, S.; Mervelt, J.; Pagliaro, U. The interproximal clinical attachment level to classify gingival recessions and predict root coverage outcomes: An explorative and reliability study. J. Clin. Periodontol. 2011, 38, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.D., Jr. A classification of marginal tissue recession. Int. J. Periodontics Restor. Dent. 1985, 5, 8–13. [Google Scholar]
- Darzi, A.; Smith, S.; Taffinder, N. Assessing operative skill. Needs to become more objective. BMJ 1999, 318, 887–888. [Google Scholar] [CrossRef]
- Burkhardt, R.; Lang, N.P. Coverage of localized gingival recessions: Comparison of micro- and macrosurgical techniques. J. Clin. Periodontol. 2005, 32, 287–293. [Google Scholar] [CrossRef]
- Dembicka-Mączka, D.; Kępa, M.; Fiegler-Rudol, J.; Grzech-Leśniak, Z.; Matys, J.; Grzech-Leśniak, K.; Wiench, R. Evaluation of the Disinfection Efficacy of Er: YAG Laser Light on Single-Species Candida Biofilms—An In Vitro Study. Dent. J. 2025, 13, 88. [Google Scholar] [CrossRef]
- Crespi, R.; Barone, A.; Covani, U. Er: YAG laser scaling of diseased root surfaces: A histologic study. J. Periodontol. 2006, 77, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Theodoro, L.H.; Haypek, P.; Bachmann, L.; Garcia, V.G.; Sampaio, J.E.; Zezell, D.M.; Eduardo, C.d.P. Effect of Er:YAG and diode laser irradiation on the root surface: Morphological and thermal analysis. J. Periodontol. 2003, 74, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Crespi Romanos, G.E.; Cassinelli, C.; Gherlone, E. Effects of Er:YAG laser and ultrasonic treatment on fibroblast attachment to root surfaces: An in vitro study. J. Periodontol. 2006, 77, 1217–1222. [Google Scholar] [CrossRef]
- Kensy, J.; Dobrzyński, M.; Wiench, R.; Grzech-Leśniak, K.; Matys, J. Fibroblasts Adhesion to Laser-Modified Titanium Surfaces-A Systematic Review. Materials 2021, 14, 7305. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, H.; Yarimoglu, E.; Kuru, B.; Ozkan Karaca, E.; Ince Kuka, G. Evaluation of the Effects of Er:YAG Laser for the De-Epithelialization of the Palatal Graft in the Treatment of Multiple Gingival Recessions: A Randomized Clinical Trial. Photobiomodul. Photomed. Laser Surg. 2019, 37, 715–721. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Hadilou, M.; Naserneysari, F.; Dolatabadi, A.; Tarzemany, R.; Vahed, N.; Nikniaz, L.; Fekrazad, R.; Gholami, L. Effect of photobiomodulation in secondary intention gingival wound healing-a systematic review and meta-analysis. BMC Oral Health 2021, 21, 258. [Google Scholar] [CrossRef]
- Lafzi, A.; Kadkhodazadeh, M.; Mojahedi, S.M.; Amid, R.; Shidfar, S.; Baghani, M.T. The Clinical Evaluation of the Effects of Low-Level Laser Therapy on the Donor and Recipient Sites of the Free Gingival Graft: A Case Series. J. Lasers Med. Sci. 2019, 10, 355–360. [Google Scholar] [CrossRef]
- Michalak, F.; Dominiak, M.; Grzech-Leśniak, Z.; Kiryk, J.; Grzech-Leśniak, K. Photobiomodulation in Medication-Related Osteonecrosis of the Jaw: Outcomes in Stage I and Its Adjunctive Role in Advanced Cases. Biomedicines 2025, 13, 1042. [Google Scholar] [CrossRef]
- El Mobadder, M.; Grzech-Lesniak, Z.; El Mobadder, W.; Rifai, M.; Ghandour, M.; Nammour, S. Management of Medication-Related Osteonecrosis of the Jaw with Photobiomodulation and Minimal Surgical Intervention. Dent. J. 2023, 11, 127. [Google Scholar] [CrossRef]
- Pini-Prato, G.P.; Cairo, F.; Nieri, M.; Franceschi, D.; Rotundo, R.; Cortellini, P. Coronally advanced flap versus connective tissue graft in the treatment of multiple gingival recessions: A split-mouth study with a 5-year follow-up. J. Clin. Periodontol. 2010, 37, 644–650. [Google Scholar] [CrossRef]
- Naziker, Y.; Ertugrul, A.S. Aesthetic evaluation of free gingival graft applied by partial de-epithelialization and free gingival graft applied by conventional method: A randomized controlled clinical study. Clin. Oral Investig. 2023, 27, 4029–4038. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, H.C.; Atkins, J.H. Freeutogenous gingival grafts. 1. Principles of successful grafting. Periodontics 1968, 6, 5–13. [Google Scholar] [PubMed]
- Bernimoulin, J.P.; Lüscher, B.; Mühlemann, H.R. Coronally repositioned periodontal flap. Clinical evaluation after one year. J. Clin. Periodontol. 1975, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.D., Jr. Root coverage using the free soft tissue autograft following citric acid application. III. A successful and predictalbe procedure in areas of deep-wide recession. Int. J. Periodontics Restor. Dent. 1985, 3, 14–36. [Google Scholar]
- Paolantonio, M.; Murro, C.D.; Cattabriga, A.; Cattabriga, M. Subpedicle connective tissue graft versus free gingival graft in the coverage of exposed root surfaces A 5-year clinical study. J. Clin. Periodontol. 1997, 24, 51–56. [Google Scholar] [CrossRef]
- Langer, B.; Langer, L. Subepithelial connective tissue graft technique for root coverage. J. Periodontol. 1985, 56, 715–720. [Google Scholar] [CrossRef]
- Zucchelli, G.; De Sanctis, M. Treatment of multiple recession-type defects in patients with esthetic demands. J. Periodontol. 2000, 71, 1506–1514. [Google Scholar] [CrossRef]
- Allen, A.L. Use of the supraperiosteal envelope in soft tissue grafting for root coverage. II. Clinical results. Int. J. Periodontics Restor. Dent. 1994, 14, 302–315. [Google Scholar]
- Azzi, R.; Etienne, D.; Carranza, F. Surgical reconstruction of the interdental papilla. Int. J. Periodontics Restor. Dent. 1998, 18, 466–473. [Google Scholar]
- Zabalegui, I.; Sicilia, A.; Cambra, J.; Gil, J.; Sanz, M. Treatment of multiple adjacent gingival recessions with the tunnel subepithelial connective tissue graft: A clinical report. Int. J. Periodontics Restor. Dent. 1999, 19, 199–206. [Google Scholar]
- Tatakis, D.N.; Chambrone, L.; Allen, E.P.; Langer, B.; McGuire, M.K.; Richardson, C.R.; Zabalegui, I.; Zadeh, H.H. Periodontal soft tissue root coverage procedures: A consensus report from the AAP Regeneration Workshop. J. Periodontol. 2015, 86 (Suppl. S2), S52–S55. [Google Scholar] [CrossRef] [PubMed]
- Tavelli, L.; Barootchi, S.; Nguyen, T.V.; Tattan, M.; Ravidà, A.; Wang, H.L. Efficacy of tunnel technique in the treatment of localized and multiple gingival recessions: A systematic review and me-ta-analysis. J. Periodontol. 2018, 89, 1075–1090. [Google Scholar] [CrossRef] [PubMed]
- Aroca, S.; Molnár, B.; Windisch, P.; Gera, I.; Salvi, G.E.; Nikolidakis, D.; Sculean, A. Treatment of multiple adjacent Miller class I and II gingival recessions with a Modified Coronally Advanced Tunnel (MCAT) technique and a collagen matrix or palatal connective tissue graft: A randomized, controlled clinical trial. J. Clin. Periodontol. 2013, 40, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Bertl, K.; Pifl, M.; Hirtler, L.; Rendl, B.; Nürnberger, S.; Stavropoulos, A.; Ulm, C. Relative Composition of Fibrous Connective and Fatty/Glandular Tissue in Connective Tissue Grafts Depends on the Harvesting Technique but not the Donor Site of the Hard Palate. J. Periodontol. 2015, 86, 1331–1339. [Google Scholar] [CrossRef]
- Reiser, G.M.; Bruno, J.F.; EMahan, P.; Larkin, L.H. The subepithelial connective tissue graft palatal donor site: Anatomic considerations for surgeons. Int. J. Periodontics Restor. Dent. 1996, 16, 130–137. [Google Scholar]
- Kuriakose, A.; Raju, S. Assessment of thickness of palatal mucosal donor site and its association with age and gender. J. Indian Soc. Periodontol. 2012, 16, 370–374. [Google Scholar] [CrossRef]
- Fiegler-Rudol, J.; Kapłon, K.; Kotucha, K.; Moś, M.; Skaba, D.; Kawczyk-Krupka, A.; Wiench, R. Hypocrellin-Mediated PDT: A Systematic Review of Its Efficacy, Applications, and Outcomes. Int. J. Mol. Sci. 2025, 26, 4038. [Google Scholar] [CrossRef]
- Edel, A. Clinical evaluation of free connective tissue grafts used to increase the width of keratinised gingiva. J. Clin. Periodontal. 1974, 1, 185–196. [Google Scholar] [CrossRef]
- Hürzeler, M.B.; Weng, D. A single-incision technique to harvest subepithelial connective tissue grafts from the palate. Int. J. Periodontics Restor. Dent. 1999, 19, 279–287. [Google Scholar]
- Zucchelli, G.; Mele, M.; Stefanini, M.; Mazzotti, C.; Marzadori, M.; Montebugnoli, L.; De Sanctis, M. Patient morbidity and root coverage outcome after subepithelial connective tissue and de—Epithelialized grafts: A comparative randomized—Controlled clinical trial. J. Clin. Periodontol. 2010, 37, 728–738. [Google Scholar] [CrossRef]
- Zuhr, O.; Fickl, S.; Wachtel, H.; Bolz, W.; Hürzeler, M.B. Covering of gingival recessions with a modified microsurgical tunnel technique: Case report. Int. J. Periodontics Restor. Dent. 2007, 27, 457–463. [Google Scholar]
- de Mattos, P.M.; Papalexiou, V.; Tramontina, V.A.; Kim, S.H.; Luczyszyn, S.M.; Bettega, P.V.C.; Johann, A.C.B.R. Evaluation of 2 techniques of epithelial removal in subepithelial connective tissue graft surgery: A comparative histological study. J. Periodontal Implant Sci. 2020, 50, 2–13. [Google Scholar] [CrossRef]
- Harris, R. Histologic evaluation of connective tissue grafts in humans. Int. J. Periodontics Restor. Dent. 2003, 23, 575–583. [Google Scholar]
- Novaes, A.B., Jr.; Grisi, D.C.; Molina, G.O.; Souza, S.L.; Taba, M., Jr.; Grisi, M.F. Comparative 6-month clinical study of a subepithelial connective tissue graft and acellular dermal matrix graft for the treatment of gingival recession. J. Periodontol. 2001, 72, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Tal, H.; Moses, O.; Zohar, R.; Meir, H.; Nemcovsky, C. Root coverage of advanced gingival recession: A comparative study between acellular dermal matrix allograft and subepithelial connective tissue grafts. J. Periodontol. 2002, 73, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Paolantonio, M.; Dolci, M.; Esposito, P.; D’Archivio, D.; Lisanti, L.; Di Luccio, A.; Perinetti, G. Subpedicle acellular dermal matrix graft and autogenous connective tissue graft in the treatment of gingival recessions: A comparative 1-year clinical study. J. Periodontol. 2002, 73, 1299–1307. [Google Scholar] [CrossRef]
- Fürhauser, R.; Florescu, D.; Benesch, T.; Haas, R.; Mailath, G.; Watzek, G. Evaluation of soft tissue around single-tooth implant crowns: The pink esthetic score. Clin. Oral Implants Res. 2005, 16, 639–644. [Google Scholar] [CrossRef]
- Aroca, S.; Keglevich, T.; Nikolidakis, D.; Gera, I.; Nagy, K.; Azzi, R.; Etienne, D. Treatment of class III multiple gingival recessions: A randomized-clinical trial. J. Clin. Periodontol. 2010, 37, 88–97. [Google Scholar] [CrossRef]
- D’Arcangelo, C.; Di Maio, F.D.N.; Prosperi, G.D.; Conte, E.; Baldi, M.; Caputi, S. A preliminary study of healing of diode laser versus scalpel incisions in rat oral tissue: A comparison of clinical, histological, and immunohistochemical results. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 103, 764–773. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C. Matsumoto K In vivo study of thehealing processes that occur in the jaws of rabbits following perforation by an Er,Cr:YSGG laser. Lasers Med. Sci. 2005, 20, 21–27. [Google Scholar] [CrossRef]
- Schwarz, F.; Sculean, A.; Georg, T.; Reich, E. Periodontal treatment with an Er:YAG laser compare to scaling and root planing. A controlled clinical study. J. Periodontol. 2001, 72, 361–367. [Google Scholar] [CrossRef]
- Folwaczny, M.; Mehl, A.; Aggstaller, H.; Hickel, R. Antimicrobial effects of 2,94 micron Er:YAG laser radiation on root surfaces: An in vitro study. J. Clin. Periodontol. 2002, 29, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ishizaki, N.; Suzuki, N.; Kimura, Y.; Matsumoto, K. Morphological changes of bovine mandibular bone irradiated by Er,Cr:YSGG laser: An in vitro study. J. Clin. Laser Med. Surg. 2002, 20, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Arashiro, D.S.; Rapley, J.W.; Cobb, C.M.; Killoy, W.J. Histologic evaluation of porcine skin incisions produced by CO2 laser electrosurgery and scalpel. Int. J. Periodontics Restor. Dent. 1996, 16, 479–491. [Google Scholar]
- Lewandrowski, K.U.; Lorente, C.; Schomacker, K.T.; Fiotte, T.J.; Wilkes, J.W.; Deutsch, T.F. Use of the Er:YAG laser for improved plating in maxillofacial surgery: Comparison of bone healing in laser and drill osteotomies. Lasers Surg. Med. 1996, 19, 40–45. [Google Scholar] [CrossRef]
- Aoki, A.; Sasaki, K.M.; Watanabe, H.; Ishikawa, I. Lasers in nonsurgical periodontal therapy. Periodontol. 2000 2004, 36, 59–97. [Google Scholar] [CrossRef]
- Cercadillo-Ibarguren, I.; España Tost, A.J.; Arnabat Domínguez, J.; Valmaseda Castellón, E.; Berini Aytés, L.; Gay Escoda, C. Histologic evaluation of thermal damage produced on soft tissues by CO2, Er,Cr:YSGG and diode lasers. Med. Oral Patol. Oral Cir. Bucal 2010, 15, 912–918. [Google Scholar] [CrossRef]
- Monteiro, L.; Delgado, M.L.; Garcês, F.; Machado, M.; Ferreira, F.; Martins, M.; Salazar, F.; Pacheco, J.J. A histological evaluation of the surgical margins from human oral fibrous-epithelial lesions excised with CO2 laser, Diode laser, Er:YAG laser, Nd: Laser, electrosurgical scalpel and cold scalpel. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e271–e280. [Google Scholar] [CrossRef]
- Grzech-Leśniak, K.; Matys, J.; Jurczyszyn, K.; Ziółkowski, P.; Dominiak, M.; Junior, A.B.; Romeo, U. Histological and Thermometric Examination of Soft Tissue De-Epithelialization Using Digitally Controlled Er:YAG Laser Handpiece: An Ex Vivo Study. Photomed Laser Surg. 2018, 36, 313–319. [Google Scholar] [CrossRef]
- Lin, J.C.; Nevins, M.; Kim, D.M. Laser de-epithelialization of autogenous gingival graft for root coverage and soft tissue augmentation procedures. Int. J. Periodontics Restor. Dent. 2018, 38, 405–411. [Google Scholar] [CrossRef]
- Fekrazad, R.; Chiniforush, N.; Kalhori, K. All done procedure by laser in free gingival graft treatment: A case series study. J. Cosmet. Laser Ther. 2019, 21, 4–10. [Google Scholar] [CrossRef]
- Kawamura, R.; Mizutani, K.; Lin, T.; Kakizaki, S.; Mimata, A.; Watanabe, K.; Saito, N.; Meinzer, W.; Iwata, T.; Izumi, Y.; et al. Ex Vivo Evaluation of Gingival Ablation with Various Laser Systems and Electroscalpel. Photobiomodul. Photomed Laser Surg. 2020, 38, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Ozcelik, O.; Seydaoglu, G.; Haytac, C.M. Diode laser for harvesting de—Epithelialized palatal graft in the treatment of gingival recession defects: A randomized clinical trial. J. Clin. Periodontol. 2016, 43, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, H.; Hasuike, A.; Sanjo, N.; Sato, D.; Kubota, T.; Nagashima, H.; Sato, S. CO2 Laser De-epithelization Technique for Subepithelial Connective Tissue Graft: A Study of 21 Recessions. In Vivo 2020, 34, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.J. A comparison of two techniques for obtaining a connective tissue graft from the palate. Int. J. Periodontics Restor. Dent. 1997, 17, 260–271. [Google Scholar]
- Bakhishov, H.; Isler, S.C.; Bozyel, B.; Yıldırım, B.; Tekindal, M.A.; Ozdemir, B. De-epithelialized gingival graft versus subepithelial connective tissue graft in the treatment of multiple adjacent gingival recessions using the tunnel technique: 1-year results of a randomized clinical trial. J. Clin. Periodontol. 2021, 48, 970–983. [Google Scholar] [CrossRef]
- Cortellini, P.; Tonetti, M.S. Improved wound stability with a modified minimally invasive surgical technique in the regenerative treatment of isolated interdental intrabony defects. J. Clin. Periodontol. 2009, 36, 157–163. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Jepsen, S.; Bouchard, P.; Cairo, F.; Eickholz, P.; Graziani, F.; Herrera, D.; Jepsen, S.; Jung, R.; Machtei, E.; et al. Clinical efficacy of periodontal plastic surgery procedures: Consensus report of Group 2 of the 10th European Workshop on Periodontology. J. Clin. Periodontal. 2014, 41, S36–S43. [Google Scholar] [CrossRef]
- Cairo, F.; Rotundo, R.; Miller, P.D., Jr.; Pini Prato, G.P. Root coverage esthetic score: A system to evaluate the esthetic outcome of the treatment of gingival recession through evaluation of clinical cases. J. Periodontal. 2009, 80, 705–710. [Google Scholar] [CrossRef]
- Parker, S.; Grzech-Leśniak, K.; Cronshaw, M.; Matys, J.; Jr, A.B.; Nammour, S. Full operating parameter recording as an essential component of the reproducibility of laser-tissue interaction and treatments. Adv. Clin. Exp. Med. 2024, 33, 653–656. [Google Scholar] [CrossRef]
- Matys, J.; Grzech-Lesniak, K.; Dominiak, M. Assessment of an Impact of a Diode Laser Mode with Wavelength of 980 nm on a Temperature Rise Measured by Means of k-02 Thermocouple: Preliminary Results. Dent. Med. Probl. 2016, 53, 345–351. [Google Scholar] [CrossRef]
- Kolberg-Babrzyńska, I.; Grzech-Leśniak, K.; Kiryk, J.; Dominiak, M.; Matys, J. Effects of endodontic retreatment by conventional therapy compared to combined therapy with an Er:YAG laser and photobiomodulation: A randomized clinical trial. Dent. Med. Probl. 2025. [Google Scholar] [CrossRef] [PubMed]
- Grzech-Leśniak, Z.; Pyrkosz, J.; Szwach, J.; Kosidło, P.; Matys, J.; Wiench, R.; Pajączkowska, M.; Nowicka, J.; Dominiak, M.; Grzech-Leśniak, K. Antibacterial Effects of Er:YAG Laser Irradiation on Candida-Streptococcal Biofilms. Life 2025, 15, 474. [Google Scholar] [CrossRef] [PubMed]
- Grzech-Leśniak, Z.; Szwach, J.; Lelonkiewicz, M.; Migas, K.; Pyrkosz, J.; Szwajkowski, M.; Kosidło, P.; Pajączkowska, M.; Wiench, R.; Matys, J.; et al. Effect of Nd:YAG Laser Irradiation on the Growth of Oral Biofilm. Microorganisms 2024, 12, 2231. [Google Scholar] [CrossRef]
- Deeb, J.G.; Grzech-Leśniak, K.; Weaver, C.; Matys, J.; Bencharit, S. Retrieval of Glass Fiber Post Using Er:YAG Laser and Conventional Endodontic Ultrasonic Method: An In Vitro Study. J. Prosthodont. 2019, 28, 1024–1028. [Google Scholar] [CrossRef]
- Alfawal, A.M.H.; Hajeer, M.Y.; Ajaj, M.A.; Hamadah, O.; Brad, B.; Latifeh, Y. Evaluation of patient-centered outcomes associated with the acceleration of canine retraction by using minimally invasive surgical procedures: A randomized clinical controlled trial. Dent. Med. Probl. 2020, 57, 285–293. [Google Scholar] [CrossRef]
- Gonçalves, R.C.G.; Cardoso, R.B.; Bauer, J.; Santos, V.M.D.; Jabur, R.O.; Bortoluzzi, M.C. Exploring the relationship between anxiety, patient characteristics and pain outcomes in oral surgery under local anesthesia: The measurement problem. Dent. Med. Probl. 2024, 61, 515–523. [Google Scholar] [CrossRef]
- Kumar, G.; Jena, S.; Manila, N.; Fareed, M.; Karobari, M.I. Incidence of postoperative pain after single-visit and multiple-visit root canal therapy: A systematic review. BMC Oral Health 2025, 25, 47. [Google Scholar] [CrossRef]
- Elmsmari, F.; Shujaie, H.; Alzaabi, R.; González, J.A.; Aljafarawi, T.; Olivieri, J.G.; Jurado, C.A.; Afrashtehfar, K.I. Lasers efficacy in pain management after primary and secondary endodontic treatment: A systematic review and meta-analysis of randomized clinical trials. Sci. Rep. 2024, 14, 26028. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).