Analysis of the Effect of Pupil Size and Corneal Aberration on the Optical Performance of Premium Intraocular Lenses
Abstract
1. Introduction
2. Materials and Methods
2.1. Intraocular Lenses
2.2. Surface Measurement Method
2.3. Pupil Sizes and Eye Models
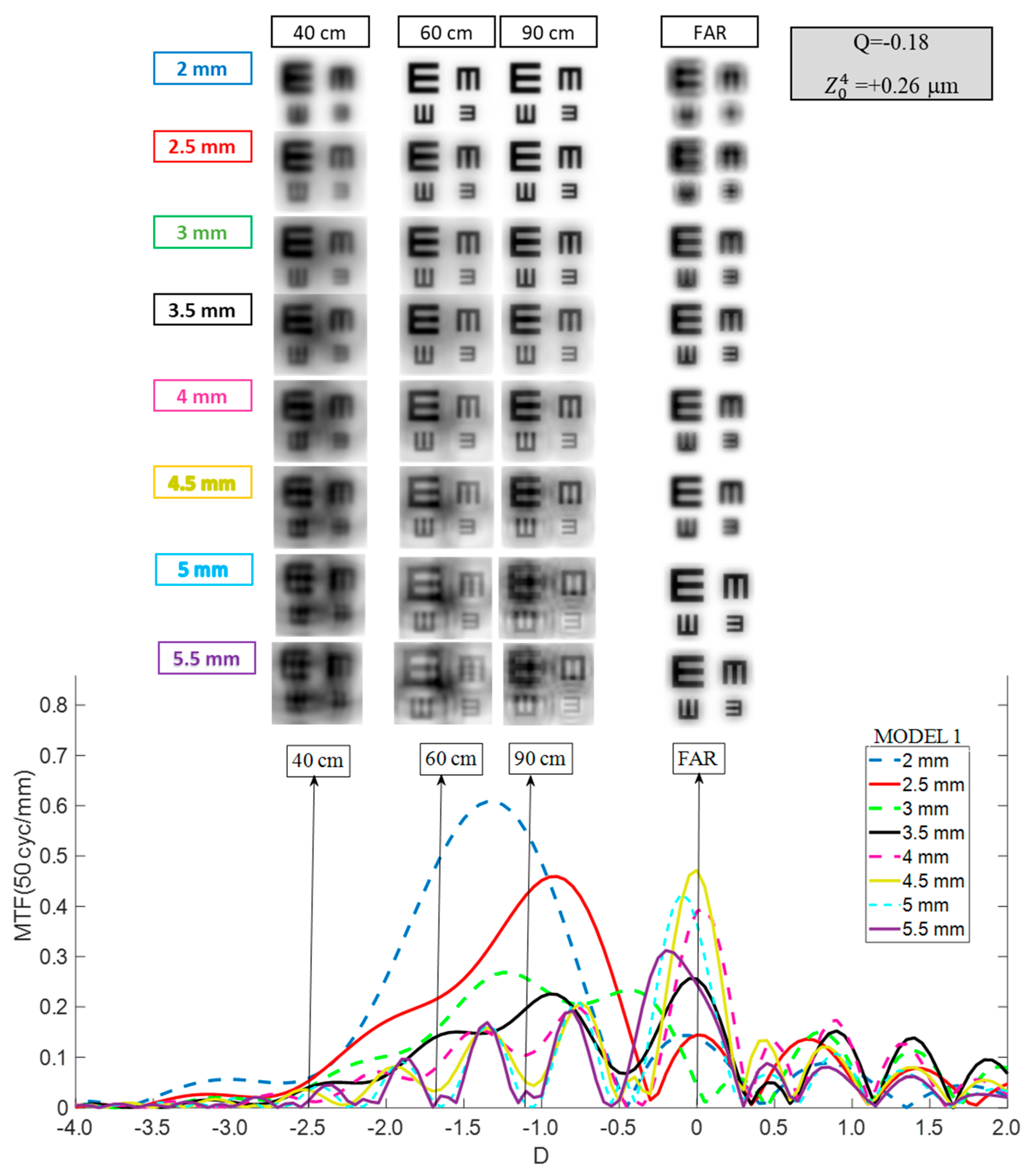
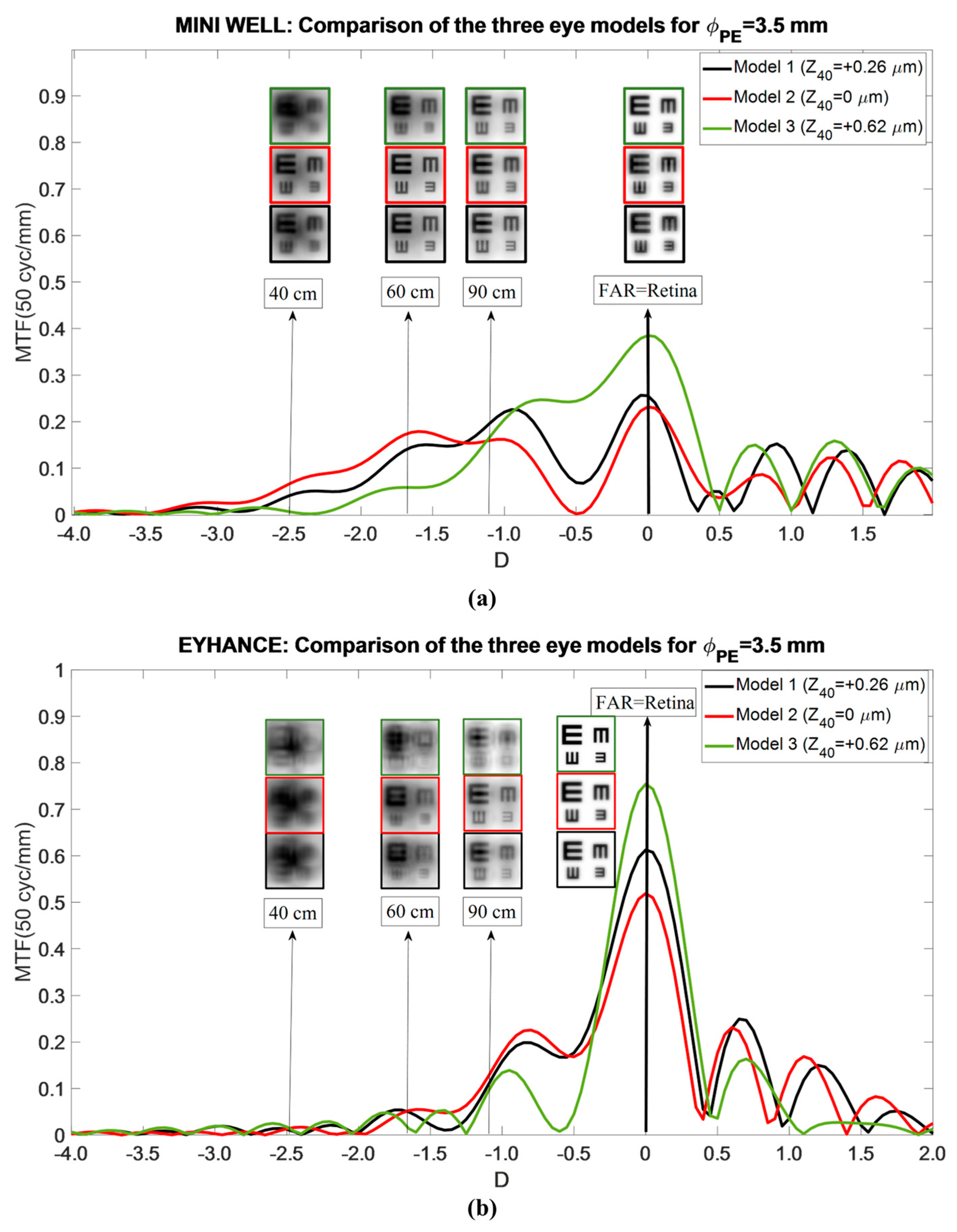
- Model 1: Mean population aberrated cornea. This model represents a typical corneal profile. The first corneal surface had an asphericity (Q) of −0.18 for 6 mm of entrance pupil. This configuration resulted in a primary SA () of +0.26 μm for a 6 mm entrance pupil.
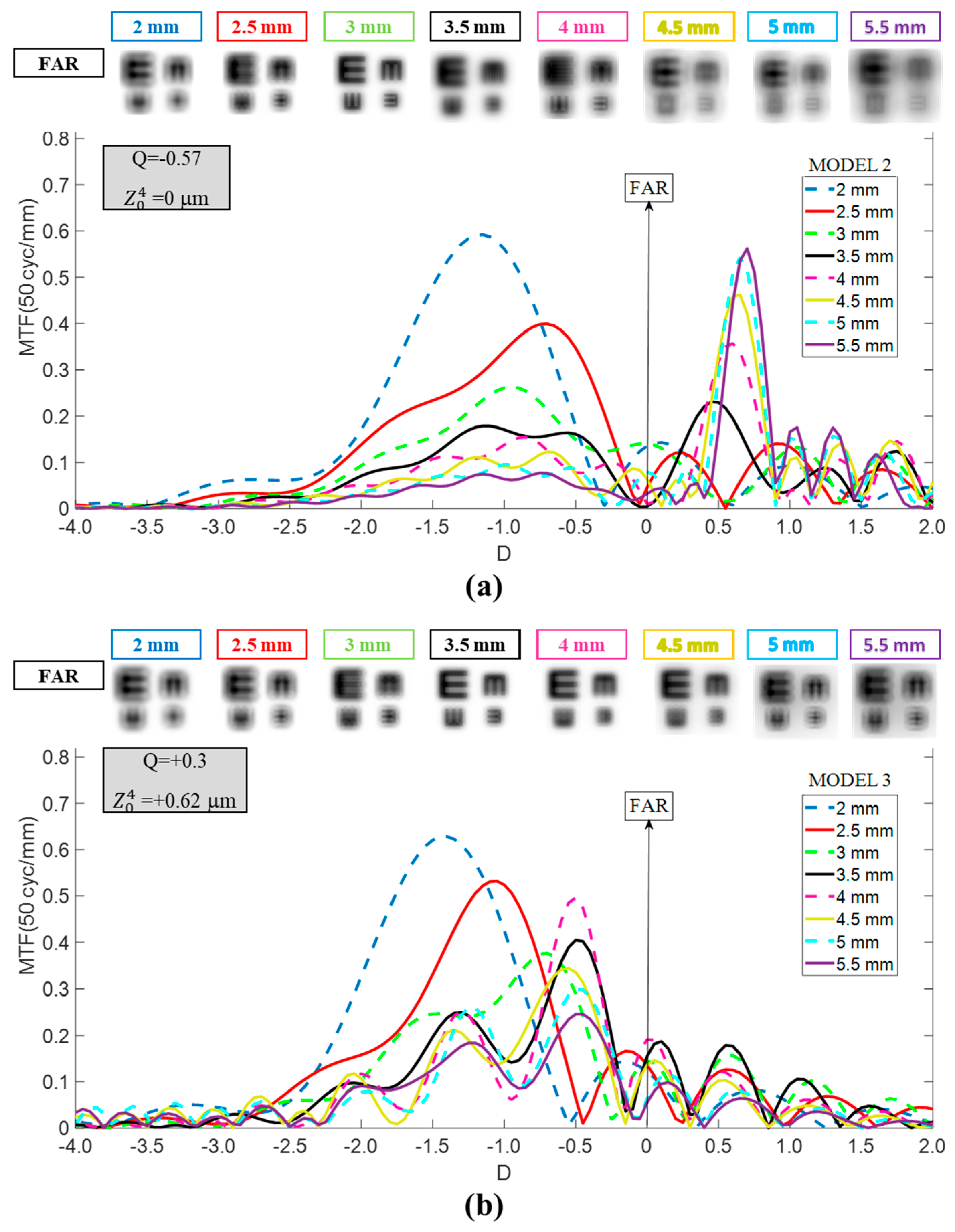
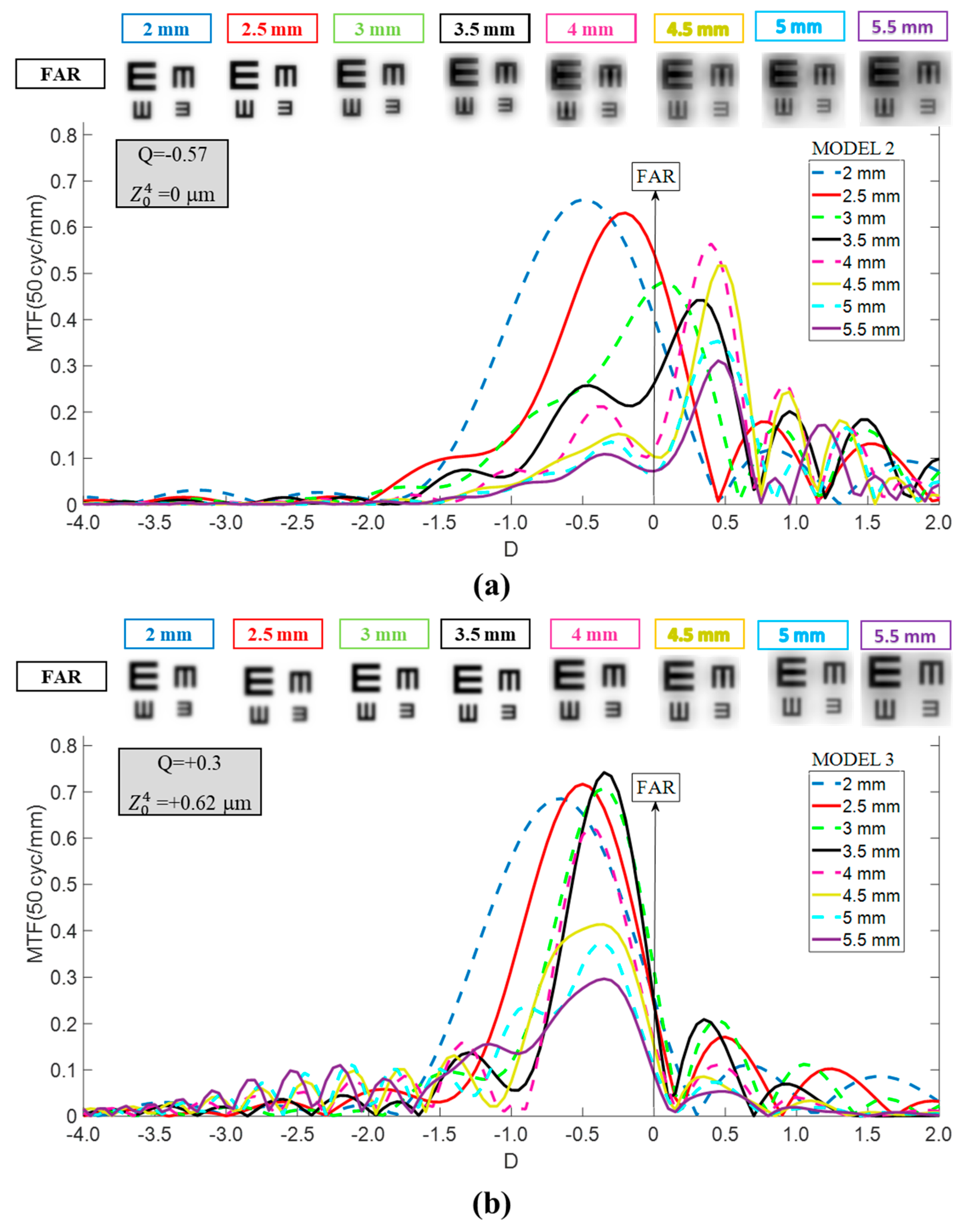
- Model 2: Aberration-free cornea. Designed to isolate IOL performance, this model eliminated corneal SA. The first corneal surface was modified to a more prolate asphericity (Q = −0.57), producing zero SA ( = 0 μm) for a 6 mm pupil entrance. This configuration also represents patients with naturally more prolate corneas or those who have undergone hyperopic LASIK. For reference, a +4.00 D hyperopic correction typically induces an asphericity change (ΔQ) of approximately −0.40, depending on the laser system and ablation profile [14,15].
- Model 3: Myopic LASIK cornea. To complete the analysis, a third model was generated to represent patients with a more oblate cornea than normal, similar to the changes induced by myopic LASIK surgery. An asphericity change (ΔQ) of +0.48 was assumed (Q = +0.3), corresponding to a correction between −4 D and −5 D [16]. This configuration resulted in a value of +0.62 μm for a 6 mm entrance pupil.
2.4. Through-Object MTF Curve Calculation Process
3. Results
3.1. Mini Well
3.2. Tecnis Eyhance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IOL | Intraocular Lens |
| SA | Spherical Aberration |
| EDOF | Extended Depth of Focus |
| MTF | Modulation Transfer Function |
| TO MTF | Through-Object Modulation Transfer Function |
| DOF | Depth of Focus |
| VA | Visual Acuity |
| CDVA | Distance-Corrected Visual Acuity |
| DCIV | Distance-Corrected at Intermediate Distance Visual Acuity |
| DCNVA | Distance-Corrected at Near Distance Visual Acuity |
References
- ISO 11979–2; Standardization IOf. Ophthalmic Implants–Intraocular Lenses—Part 2: Optical Properties and Test Methods. International Organization for Standardization: Geneva, Switzerland, 2014.
- Tandogan, T.; Son, H.S.; Choi, C.Y.; Knorz, M.C.; Auffarth, G.U.; Khoramnia, R. Laboratory Evaluation of the Influence of Decentration and Pupil Size on the Optical Performance of a Monofocal, Bifocal, and Trifocal Intraocular Lens. J. Refract. Surg. 2017, 33, 808–812. [Google Scholar] [CrossRef]
- Ouchi, M.; Shiba, T. Diffractive multifocal intraocular lens implantation in eyes with a small-diameter pupil. Sci. Rep. 2018, 8, 11686. [Google Scholar] [CrossRef]
- Lasta, M.; Miháltz, K.; Kovács, I.; Vécsei-Marlovits, P.V. Effect of Spherical Aberration on the Optical Quality after Implantation of Two Different Aspherical Intraocular Lenses. J. Ophthalmol. 2017, 2017, 8039719. [Google Scholar] [CrossRef]
- Holladay, J.T.; Kozhaya, K.; Koch, D.D.; Wang, L. Impact of spherical aberration on visual quality and depth of focus. J. Cataract. Refract. Surg. 2024, 50, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, M.J.; Kim, K.H. In vitro optical performance of multifocal and extended depth-of-focus intraocular lenses in spherical aberration conditions. J. Cataract. Refract. Surg. 2022, 48, 616–622. [Google Scholar] [CrossRef]
- Kiel, M.; Grabitz, S.D.; Hopf, S.; Koeck, T.; Wild, P.S.; Schmidtmann, I.; Lackner, K.J.; Münzel, T.; Beutel, M.E.; Pfeiffer, N.; et al. Distribution of Pupil Size and Associated Factors: Results from the Population-Based Gutenberg Health Study. J. Ophthalmol. 2022, 2022, 9520512. [Google Scholar] [CrossRef]
- Lago, C.M.; de Castro, A.; Marcos, S. Computational simulation of the optical performance of an EDOF intraocular lens in post-LASIK eyes. J. Cataract. Refract. Surg. 2023, 49, 1153–1159. [Google Scholar] [CrossRef]
- Ruiz-Alcocer, J.; Martínez-Alberquilla, I.; Rementería-Capelo, L.A.; De Gracia, P.; Lorente-Velázquez, A. Changes in Optical Quality Induced by Tilt and Decentration of a Trifocal IOL and a Novel Extended Depth of Focus IOL in Eyes with Corneal Myopic Ablations. J. Refract. Surg. 2021, 37, 532–537. [Google Scholar] [CrossRef]
- Camps, V.J.; Miret, J.J.; García, C.; Tolosa, A.; Piñero, D.P. Simulation of the Effect of Different Presbyopia-Correcting Intraocular Lenses With Eyes With Previous Laser Refractive Surgery. J. Refract. Surg. 2018, 34, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Miret, J.J.; Camps, V.J.; Garcia, C.; Caballero, M.T.; Gonzalez-Leal, J.M. Analysis and comparison of monofocal, extended depth of focus and trifocal intraocular lens profiles. Sci. Rep. 2022, 12, 8654. [Google Scholar] [CrossRef] [PubMed]
- Miret, J.J.; Camps, V.J.; García, C.; Caballero, M.T.; Gonzalez-Leal, J.M. Analysis of the optical performance of intraocular lenses using profilometric measurements. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 263, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Liou, H.L.; Brennan, N.A. Anatomically accurate, finite model eye for optical modeling. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 1997, 14, 1684–1695. [Google Scholar] [CrossRef]
- Chen, C.C.; Izadshenas, A.; Rana, M.A.A.; Azar, D.T. Corneal asphericity after hyperopic laser in situ keratomileusis. J. Cataract. Refract. Surg. 2002, 28, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Bottos, K.M.; Leite, M.T.; Aventura-Isidro, M.; Bernabe-Ko, J.; Wongpitoonpiya, N.; Ong-Camara, N.H.; Purcell, T.L.; Schanzlin, D.J. Corneal asphericity and spherical aberration after refractive surgery. J. Cataract. Refract. Surg. 2011, 37, 1109–1115. [Google Scholar] [CrossRef]
- Kwon, Y.; Bott, S. Postsurgery corneal asphericity and spherical aberration due to ablation efficiency reduction and corneal remodelling in refractive surgeries. Eye 2009, 23, 1845–1850. [Google Scholar] [CrossRef]
- Lee, Y.; Łabuz, G.; Son, H.; Yildirim, T.M.; Khoramnia, R.; Auffarth, G.U. Assessment of the image quality of extended depth-of-focus intraocular lens models in polychromatic light. J. Cataract. Refract. Surg. 2020, 46, 108–115. [Google Scholar] [CrossRef]
- Azor, J.A.; Vega, F.; Armengol, J.; Millan, M.S. Optical Assessment and Expected Visual Quality of Four Extended Range of Vision Intraocular Lenses. J. Refract. Surg. 2022, 38, 688–697. [Google Scholar] [CrossRef]
- Schmid, R.; Fuchs, C.; Luedtke, H.; Borkenstein, A.F. Depth of focus of four novel extended range of vision intraocular lenses. Eur. J. Ophthalmol. 2023, 33, 257–261. [Google Scholar] [CrossRef]
- Wang, L.; Dai, E.; Koch, D.D.; Nathoo, A. Optical aberrations of the human anterior cornea. J. Cataract. Refract. Surg. 2003, 29, 1514–1521. [Google Scholar] [CrossRef]
- Al-Somali, A.; Abouollo, H.M.; Al-Jindan, M.; Alothman, A.; Alhataln, H. Corneal asphericity and its related factors. Med. Hypothesis Discov. Innov. Ophthalmol. 2023, 12, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Kohnen, T.; Mahmoud, K.; Bühren, J. Comparison of corneal higher-order aberrations induced by myopic and hyperopic LASIK. Ophthalmology 2005, 112, 1692. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, R.; Curatolo, M.C. A New Extended Depth of Focus Intraocular Lens Based on Spherical Aberration. J. Refract. Surg. 2017, 33, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Vicent, A.; Esteve-Taboada, J.J.; Del Aguila-Carrasco, A.J.; Ferrer-Blasco, T.; Montes-Mico, R. In vitro optical quality comparison between the Mini WELL Ready progressive multifocal and the TECNIS Symfony. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Kozhaya, K.; Kenny, P.I.; Esfandiari, S.; Wang, L.; Weikert, M.P.; Koch, D.D. Effect of spherical aberration on visual acuity and depth of focus in pseudophakic eyes. J. Cataract. Refract. Surg. 2024, 50, 24–29. [Google Scholar] [CrossRef]
- Chow, S.S.W.; Chan, T.C.Y.; Ng, A.L.K.; Kwok, A.K.H. Outcomes of presbyopia-correcting intraocular lenses after laser in situ keratomileusis. Int. Ophthalmol. 2019, 39, 1199–1204. [Google Scholar] [CrossRef]
- Li, Q.; Wang, F.; Wu, Z.; Liu, Z.; Zhan, C.; Chen, B.; Sima, J.; Stieger, K.; Li, S. Trifocal diffractive intraocular lens implantation in patients after previous corneal refractive laser surgery for myopia. BMC Ophthalmol. 2020, 20, 293. [Google Scholar] [CrossRef]
- Vrijman, V.; van der Linden, J.W.; van der Meulen, I.J.; Mourits, M.P.; Lapid-Gortzak, R. Multifocal intraocular lens implantation after previous corneal refractive laser surgery for myopia. J. Cataract. Refract. Surg. 2017, 43, 909–914. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, T.; Lu, Y. Effect of Decentration on the Optical Quality of Monofocal, Extended Depth of Focus, and Bifocal Intraocular Lenses. J. Refract. Surg. 2019, 35, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.J.; Galvis, V.; Salazar, M.; Villamizar, S.J.; Leal, N.; Tello, A. Presentation of a New Method for Quantitative Determination of Trifocal Intraocular Lens Decentration. Cesk Slov. Oftalmol. 2025, 81, 1–12. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miret, J.J.; Camps, V.J.; García, C.; Caballero, M.T.; Sempere-Molina, A.; Gonzalez-Leal, J.M. Analysis of the Effect of Pupil Size and Corneal Aberration on the Optical Performance of Premium Intraocular Lenses. J. Clin. Med. 2025, 14, 5336. https://doi.org/10.3390/jcm14155336
Miret JJ, Camps VJ, García C, Caballero MT, Sempere-Molina A, Gonzalez-Leal JM. Analysis of the Effect of Pupil Size and Corneal Aberration on the Optical Performance of Premium Intraocular Lenses. Journal of Clinical Medicine. 2025; 14(15):5336. https://doi.org/10.3390/jcm14155336
Chicago/Turabian StyleMiret, Juan J., Vicente J. Camps, Celia García, Maria T. Caballero, Antonio Sempere-Molina, and Juan M. Gonzalez-Leal. 2025. "Analysis of the Effect of Pupil Size and Corneal Aberration on the Optical Performance of Premium Intraocular Lenses" Journal of Clinical Medicine 14, no. 15: 5336. https://doi.org/10.3390/jcm14155336
APA StyleMiret, J. J., Camps, V. J., García, C., Caballero, M. T., Sempere-Molina, A., & Gonzalez-Leal, J. M. (2025). Analysis of the Effect of Pupil Size and Corneal Aberration on the Optical Performance of Premium Intraocular Lenses. Journal of Clinical Medicine, 14(15), 5336. https://doi.org/10.3390/jcm14155336






