Venous Thromboembolism Prophylaxis in the Neurocritically Ill Population
Abstract
1. Introduction
Considerations for Choice of VTE Chemoprophylaxis Agent
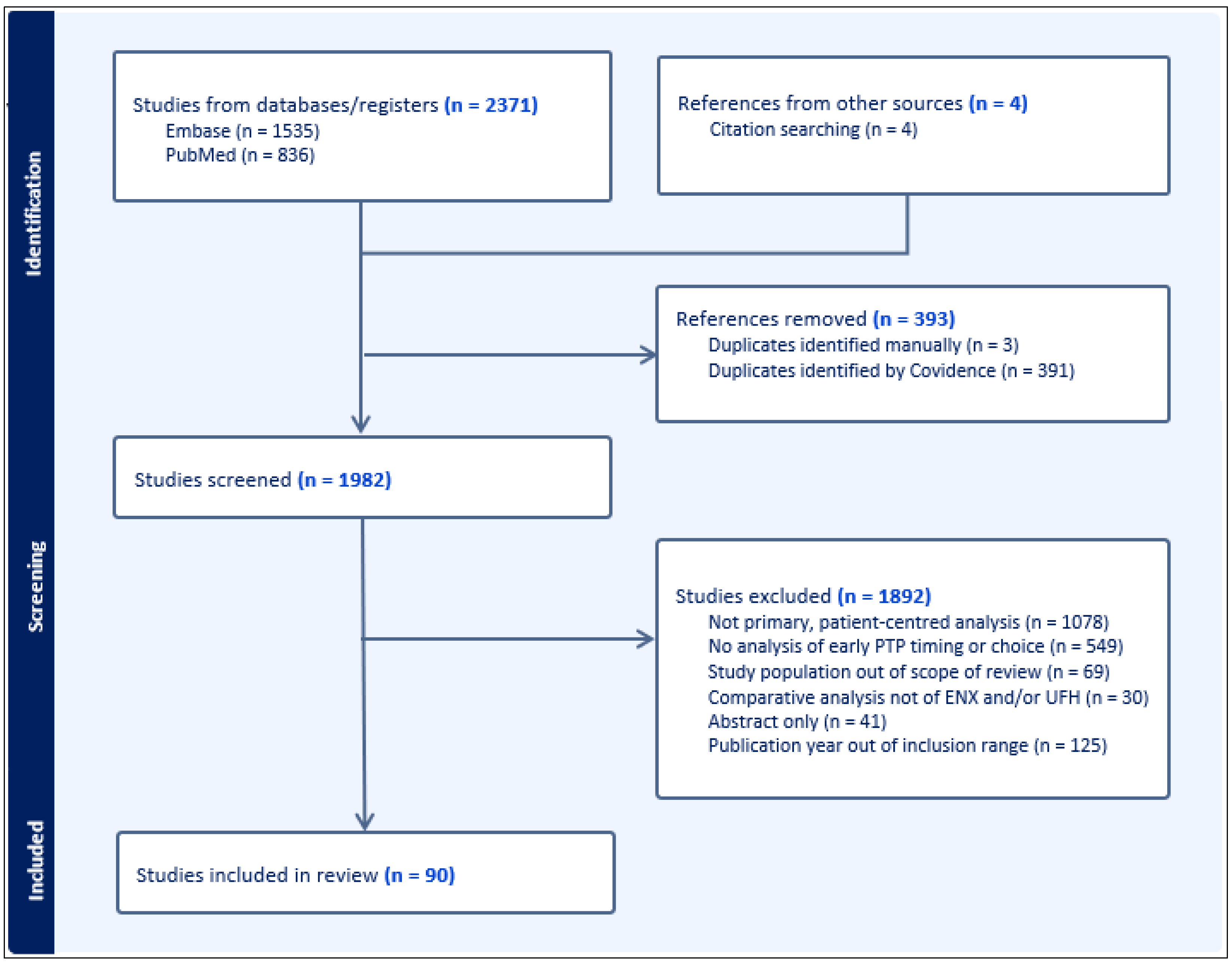
2. Methods
3. Results
4. Discussion
4.1. Traumatic Brain Injury
4.1.1. Timing of PTP and Outcomes
4.1.2. Choice of Agent for PTP and Outcomes
4.1.3. Interpretation and Considerations
4.2. Intracranial Hemorrhage
4.2.1. Timing of PTP and Outcomes
4.2.2. Interpretation and Considerations
4.3. Non-Traumatic Subarachnoid Hemorrhage
4.3.1. Timing of PTP and Outcomes
4.3.2. Choice of Agent for PTP and Outcomes
4.3.3. Interpretation and Considerations
4.4. Spinal Cord Injury or Spinal Surgery
4.4.1. Timing of PTP and Outcomes
4.4.2. Choice of Agent for PTP and Outcomes
4.4.3. Interpretation and Considerations
4.5. Neurosurgical Intervention
4.5.1. Timing of PTP and Outcomes
4.5.2. Choice of Agent for PTP and Outcomes
4.5.3. Interpretation and Considerations
4.6. PTP Interruption and VTE Risk
5. Limitations of Existing Data
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIS | Abbreviated injury scale |
| CT | Computer tomography |
| DVT | Deep vein thrombosis |
| ENX | Enoxaparin |
| EVD | External ventricular drain |
| GCS | Glasgow coma scale |
| GOS | Glasgow outcome scale |
| HE | Hematoma expansion |
| ICH | Intracranial hemorrhage |
| ICP | Intracranial pressure |
| IDH1 | Isocitrate dehydrogenase |
| IVC | Infravena cava |
| NCS | Neurocritical care society |
| NI | Neurosurgical intervention |
| OR | Odds Ratio |
| PCB | Placebo |
| PE | Pulmonary embolism |
| PTP | Pharmacologic thromboprophylaxis |
| RCT | Randomized control trial |
| RTOR | Return to OR |
| SAH | Subarachnoid hemorrhage |
| SCI | Spinal cord injury |
| TBI | Traumatic brain injury |
| UFH | Unfractionated heparin |
| VEGF | Vascular endothelial growth factor |
| VTE | Venous thromboembolism |
References
- Gao, X.; Zeng, L.; Wang, H.; Zeng, S.; Tian, J.; Chen, L.; Peng, T. Prevalence of Venous Thromboembolism in Intensive Care Units: A Meta-Analysis. J. Clin. Med. 2022, 11, 6691. [Google Scholar] [CrossRef] [PubMed]
- Sauro, K.M.; Soo, A.; Kramer, A.; Couillard, P.; Kromm, J.; Zygun, D.; Niven, D.J.; Bagshaw, S.M.; Stelfox, H.T. Venous Thromboembolism Prophylaxis in Neurocritical Care Patients: Are Current Practices, Best Practices? Neurocrit. Care 2019, 30, 355–363. [Google Scholar] [CrossRef]
- Viarasilpa, T.; Panyavachiraporn, N.; Jordan, J.; Marashi, S.M.; van Harn, M.; Akioyamen, N.O.; Kowalski, R.G.; Mayer, S.A. Venous Thromboembolism in Neurocritical Care Patients. J. Intensive Care Med. 2020, 35, 1226–1234. [Google Scholar] [CrossRef]
- Sahle, B.W.; Pilcher, D.; Peter, K.; McFadyen, J.D.; Litton, E.; Bucknall, T. Mortality data from omission of early thromboprophylaxis in critically ill patients highlights the importance of an individualised diagnosis-related approach. Thromb. J. 2023, 21, 59. [Google Scholar] [CrossRef]
- Bagot, C.N.; Arya, R. Virchow and his triad: A question of attribution. Br. J. Haematol. 2008, 143, 180–190. [Google Scholar] [CrossRef]
- Nyquist, P.; Bautista, C.; Jichici, D.; Burns, J.; Chhangani, S.; DeFilippis, M.; Goldenberg, F.D.; Kim, K.; Liu-DeRyke, X.; Mack, W.; et al. Prophylaxis of Venous Thrombosis in Neurocritical Care Patients: An Evidence-Based Guideline: A Statement for Healthcare Professionals from the Neurocritical Care Society. Neurocrit. Care 2016, 24, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Nyquist, P.; Jichici, D.; Bautista, C.; Burns, J.; Chhangani, S.; DeFilippis, M.; Goldenberg, F.D.; Kim, K.; Liu-DeRyke, X.; Mack, W.; et al. Prophylaxis of Venous Thrombosis in Neurocritical Care Patients: An Executive Summary of Evidence-Based Guidelines: A Statement for Healthcare Professionals From the Neurocritical Care Society and Society of Critical Care Medicine. Crit. Care Med. 2017, 45, 476–479. [Google Scholar] [CrossRef]
- American College of Surgeons. Best Practices Guidelines—The Management of Traumatic Brain Injury; American College of Surgeons: Chicago, IL, USA, 2024. [Google Scholar]
- Carney, N.; Totten, A.M.; O’REilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Ley, E.J.; Brown, C.V.; Moore, E.E.; Sava, J.A.; Peck, K.A.; Ciesla, D.J.; Sperry, J.L.; Rizzo, A.G.; Rosen, N.G.; Brasel, K.J.; et al. Updated guidelines to reduce venous thromboembolism in trauma patients: A Western Trauma Association critical decisions algorithm. J. Trauma Acute Care Surg. 2020, 89, 971–981. [Google Scholar] [CrossRef]
- Hoh, B.L.; Ko, N.U.; Amin-Hanjani, S.; Chou, S.H.-Y.; Cruz-Flores, S.; Dangayach, N.S.; Derdeyn, C.P.; Du, R.; Hänggi, D.; Hetts, S.W.; et al. 2023 Guideline for the Management of Patients With Aneurysmal Subarachnoid Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 2023, 54, e314–e370. [Google Scholar] [CrossRef]
- Consortium for Spinal Cord Medicine. Prevention of Venous Thromboembolism in Individuals with Spinal Cord Injury: Clinical Practice Guidelines for Health Care Providers, 3rd ed.; Consortium for Spinal Cord Medicine; Paralyzed Veterans of America: Washington, DC, USA, 2016; Volume 22, pp. 209–240. [Google Scholar]
- Alexander, K.M.; Butts, C.C.; Lee, Y.-L.L.; Kutcher, M.E.; Polite, N.; Haut, E.R.; Spain, D.; Berndtson, A.E.; Costantini, T.W.; Simmons, J.D. Survey of venous thromboembolism prophylaxis in trauma patients: Current prescribing practices and concordance with clinical practice guidelines. Trauma Surg. Acute Care Open 2023, 8, e001070. [Google Scholar] [CrossRef] [PubMed]
- Fareed, J.; Hoppensteadt, D.; Walenga, J.; Iqbal, O.; Ma, Q.; Jeske, W.; Sheikh, T. Pharmacodynamic and pharmacokinetic properties of enoxaparin: Implications for clinical practice. Clin. Pharmacokinet. 2003, 42, 1043–1057. [Google Scholar] [CrossRef]
- Martel, N.; Lee, J.; Wells, P.S. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: A meta-analysis. Blood 2005, 106, 2710–2715. [Google Scholar] [CrossRef] [PubMed]
- Ostadal, P.; Alan, D.; Vejvoda, J.; Segethova, J.; Kruger, A. Anti-Xa activity of enoxaparin and nadroparin in patients with acute coronary syndrome. Exp. Clin. Cardiol. 2008, 13, 175–178. [Google Scholar] [PubMed]
- Langlois, J.A.; Rutland-Brown, W.; Wald, M.M. The Epidemiology and Impact of Traumatic Brain Injury: A Brief Overview. J. Head Trauma Rehabil. 2006, 21, 375–378. [Google Scholar] [CrossRef]
- Benjamin, E.; Recinos, G.; Aiolfi, A.; Inaba, K.; Demetriades, D. Pharmacological Thromboembolic Prophylaxis in Traumatic Brain Injuries: Low Molecular Weight Heparin Is Superior to Unfractionated Heparin. Ann. Surg. 2017, 266, 463–469. [Google Scholar] [CrossRef]
- Jakob, D.A.; Müller, M.; Lewis, M.; Wong, M.D.; Exadaktylos, A.K.; Demetriades, D. Risk factors for thromboembolic complications in isolated severe head injury. Eur. J. Trauma Emerg. Surg. 2024, 50, 185–195. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Chien, C.-Y.; Matsushima, K.; Schellenberg, M.; Inaba, K.; Moore, E.E.; Sauaia, A.; Knudson, M.M.; Martin, M.J.; The CLOTT Study Group. Early venous thromboembolism prophylaxis in patients with trauma intracranial hemorrhage: Analysis of the prospective multicenter Consortium of Leaders in Traumatic Thromboembolism study. J. Trauma Acute Care Surg. 2023, 95, 649–656. [Google Scholar] [CrossRef]
- Cole, K.L.; Nguyen, S.; Gelhard, S.; Hardy, J.; Cortez, J.; Nunez, J.M.; Menacho, S.T.; Grandhi, R. Factors Associated with Venous Thromboembolism Development in Patients with Traumatic Brain Injury. Neurocrit. Care 2024, 40, 568–576. [Google Scholar] [CrossRef]
- Jakob, D.A.; Lewis, M.; Benjamin, E.R.; Mitchao, D.P.; Exadaktylos, A.K.; Demetriades, D. Timing of venous thromboembolic pharmacological prophylaxis in traumatic combined subdural and subarachnoid hemorrhage. Am. J. Surg. 2022, 223, 1194–1199. [Google Scholar] [CrossRef]
- Filiberto, D.M.; Byerly, S.; Lenart, E.K.; Fischer, P.E.; Kerwin, A.J. Body Mass Index and Pharmacologic Venous Thromboembolism Prophylaxis in Traumatic Brain Injury. J. Surg. Res. 2023, 291, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Jakob, D.A.; Benjamin, E.R.; Recinos, G.; Cremonini, C.; Lewis, M.; Demetriades, D. Venous thromboembolic pharmacological prophylaxis in severe traumatic acute subdural hematomas: Early prophylaxis is effective and safe. Am. J. Surg. 2022, 223, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Al-Dorzi, H.M.; Al-Yami, G.; Al-Daker, F.; Alqirnas, M.Q.; Alhamadh, M.S.; Khan, R. The association of timing of pharmacological prophylaxis and venous thromboembolism in patients with moderate-to-severe traumatic brain injury: A retrospective cohort study. Ann. Thorac. Med. 2022, 17, 102–109. [Google Scholar] [CrossRef]
- Byrne, J.P.; Mason, S.A.; Gomez, D.; Hoeft, C.; Subacius, H.; Xiong, W.; Neal, M.; Pirouzmand, F.; Nathens, A.B. Timing of Pharmacologic Venous Thromboembolism Prophylaxis in Severe Traumatic Brain Injury: A Propensity-Matched Cohort Study. J. Am. Coll. Surg. 2016, 223, 621–631.e5. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.R.; Carmichael, H.; Zangara, T.; Dunn, J.; Schroeppel, T.J.; Campion, E.; Goodman, M.; Hosokawa, P.; Sauaia, A.; Moore, E.E.; et al. A Stitch in Time Saves Clots: Venous Thromboembolism Chemoprophylaxis in Traumatic Brain Injury. J. Surg. Res. 2021, 258, 289–298. [Google Scholar] [CrossRef]
- Farooqui, A.; Hiser, B.; Barnes, S.L.; Litofsky, N.S. Safety and efficacy of early thromboembolism chemoprophylaxis after intracranial hemorrhage from traumatic brain injury. J. Neurosurg. 2013, 119, 1576–1582. [Google Scholar] [CrossRef]
- Hollfelder, E.K.; Rappaport, S.; Cheng, J.; Patel, J.H. Retrospective evaluation of chemical venous thromboembolism prophylaxis in traumatic brain injury. Surg. Pract. Sci. 2023, 13, 100168. [Google Scholar] [CrossRef]
- Scudday, T.; Brasel, K.; Webb, T.; Codner, P.; Somberg, L.; Weigelt, J.; Herrmann, D.; Peppard, W. Safety and efficacy of prophylactic anticoagulation in patients with traumatic brain injury. J. Am. Coll. Surg. 2011, 213, 148–153, discussion 153–154. [Google Scholar] [CrossRef]
- Shulkosky, M.M.; Han, E.J.; Wahl, W.L.; Hecht, J.P. Effects of Early Chemoprophylaxis in Traumatic Brain Injury and Risk of Venous Thromboembolism. Am. Surg. 2023, 89, 2513–2519. [Google Scholar] [CrossRef]
- Ang, D.; Pierre, K.; Armstrong, J.; Dunne, J.; Flaherty, S.; Gonzalez, E.; McKenney, M.; Offner, P.; Plurad, D.; Liu, H.; et al. Timing and Type of Venous Thromboembolic Chemoprophylaxis Is Associated with Acute Traumatic Brain Injury Outcomes. Neurotrauma Rep. 2022, 3, 511–521. [Google Scholar] [CrossRef]
- Phelan, H.A.; Wolf, S.E.; Norwood, S.H.; Aldy, K.; Brakenridge, S.C.; Eastman, A.L.; Madden, C.J.; Nakonezny, P.A.; Yang, L.; Chason, D.P.; et al. A randomized, double-blinded, placebo-controlled pilot trial of anticoagulation in low-risk traumatic brain injury: The Delayed Versus Early Enoxaparin Prophylaxis I (DEEP I) study. J. Trauma Acute Care Surg. 2012, 73, 1434–1441. [Google Scholar] [CrossRef]
- Ratnasekera, A.M.D.; Kim, D.; Seng, S.S.; Jacovides, C.; Kaufman, E.J.M.; Sadek, H.M.A.-B.; Perea, L.L.D.; Monaco, C.D.; Shnaydman, I.M.; Lee, A.B.J.; et al. Early VTE prophylaxis in severe traumatic brain injury: A propensity score weighted EAST multicenter study. J. Trauma Acute Care Surg. 2023, 95, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Elkbuli, A.; Patel, H.; Breeding, T.; Nasef, H.; Chin, B.; Wright, D.-D.; Zito, T.; Poulin, S.R.; Rhodes-Lyons, H.X. Racial Distribution and Associated Outcomes for Patients With and Without Severe-Isolated Traumatic Brain Injuries Following Venous Thromboembolism Prophylaxis. Am. Surg. 2024, 90, 969–977. [Google Scholar] [CrossRef]
- Rivas, L.; Vella, M.; Ju, T.; Fernandez-Moure, J.S.; Sparks, A.; Seamon, M.J.; Sarani, B. Early Chemoprophylaxis Against Venous Thromboembolism in Patients With Traumatic Brain Injury. Am. Surg. 2022, 88, 187–193. [Google Scholar] [CrossRef]
- Stormann, P.; Osinloye, W.; Freiman, T.M.; Seifert, V.; Marzi, I.; Lustenberger, T. Early Chemical Thromboprophylaxis Does not Increase the Risk of Intracranial Hematoma Progression in Patients with Isolated Severe Traumatic Brain Injury. World J. Surg. 2019, 43, 2804–2811. [Google Scholar] [CrossRef] [PubMed]
- Stormann, P.; Osinloye, W.; Verboket, R.D.; Schindler, C.R.; Woschek, M.; Marzi, I.; Lustenberger, T. Early start of thromboprophylaxis does not increase risk of intracranial hematoma progression in multiply injured patients with traumatic brain injury. Brain Inj. 2022, 36, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Byrne, J.P.; Geerts, W.; Mason, S.A.; Gomez, D.; Hoeft, C.; Murphy, R.; Neal, M.; Nathens, A.B. Effectiveness of low-molecular-weight heparin versus unfractionated heparin to prevent pulmonary embolism following major trauma: A propensity-matched analysis. J. Trauma Acute Care Surg. 2017, 82, 252–262. [Google Scholar] [CrossRef]
- Kim, J.; Gearhart, M.M.; Zurick, A.; Zuccarello, M.; James, L.; Luchette, F.A. Preliminary report on the safety of heparin for deep venous thrombosis prophylaxis after severe head injury. J. Trauma 2002, 53, 38–42, discussion 43. [Google Scholar] [CrossRef]
- Koehler, D.M.; Shipman, J.; Davidson, M.A.; Guillamondegui, O. Is early venous thromboembolism prophylaxis safe in trauma patients with intracranial hemorrhage. J. Trauma 2011, 70, 324–329. [Google Scholar] [CrossRef]
- Meyer, R.M.; Larkin, M.B.; Szuflita, N.S.; Neal, C.J.; Tomlin, J.M.; Armonda, R.A.; Bailey, J.A.; Bell, R.S. Early venous thromboembolism chemoprophylaxis in combat-related penetrating brain injury. J. Neurosurg. 2017, 126, 1047–1055. [Google Scholar] [CrossRef]
- Reiff, D.A.; Haricharan, R.N.; Bullington, N.M.; Griffin, R.L.; McGwin, G.; Rue, L.W. Traumatic brain injury is associated with the development of deep vein thrombosis independent of pharmacological prophylaxis. J. Trauma 2009, 66, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
- Saadi, R.; Brandt, K.; Madlinger, R.; Nerenberg, S.F. Assessment of the Use of Pharmacologic Venous Thromboembolism Prophylaxis in Post-Traumatic Brain Injury Patients. J. Pharm. Pract. 2021, 34, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Hachem, L.D.; Mansouri, A.; Scales, D.C.; Geerts, W.; Pirouzmand, F. Anticoagulant prophylaxis against venous thromboembolism following severe traumatic brain injury: A prospective observational study and systematic review of the literature. Clin. Neurol. Neurosurg. 2018, 175, 68–73. [Google Scholar] [CrossRef]
- Depew, A.J.; Hu, C.K.; Nguyen, A.C.; Driessen, N. Thromboembolic prophylaxis in blunt traumatic intracranial hemorrhage: A retrospective review. Am. Surg. 2008, 74, 906–911. [Google Scholar] [CrossRef]
- Johnson, P.L.; Dualeh, S.H.A.; Ward, A.L.; Jean, R.A.; Aubry, S.T.; Chapman, A.J.; Curtiss, W.J.; Joseph, J.R.; Scott, J.W.; Hemmila, M.R. Association of timing and agent for venous thromboembolism prophylaxis in patients with severe traumatic brain injury on venous thromboembolism events, mortality, neurosurgical intervention, and discharge disposition. J. Trauma Acute Care Surg. 2024, 97, 590–603. [Google Scholar] [CrossRef]
- Levy, A.S.; Salottolo, K.; Bar-Or, R.B.; Offner, P.; Mains, C.; Sullivan, M.B.; Bar-Or, D.M. Pharmacologic Thromboprophylaxis Is a Risk Factor for Hemorrhage Progression in a Subset of Patients With Traumatic Brain Injury. J. Trauma 2010, 68, 886–894. [Google Scholar] [CrossRef]
- Elkbuli, A.; Watts, E.; Patel, H.; Chin, B.; Wright, D.-D.; Inouye, M.; Nunez, D.; Rhodes, H.X. National Analysis of Outcomes for Adult Trauma Patients With Isolated Severe Blunt Traumatic Brain Injury Following Venous Thromboembolism Prophylaxis. J. Surg. Res. 2024, 300, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Maragkos, G.A.; Cho, L.D.; Legome, E.; Wedderburn, R.; Margetis, K. Delayed Cranial Decompression Rates After Initiation of Unfractionated Heparin versus Low-Molecular-Weight Heparin in Traumatic Brain Injury. World Neurosurg. 2022, 164, e1251–e1261. [Google Scholar] [CrossRef]
- Condon, F.; Grigorian, A.; Russell, D.; Demetriades, D. Venous thromboembolism chemoprophylaxis in geriatric trauma patients with isolated severe traumatic brain injury. Eur. J. Trauma Emerg. Surg. 2024, 50, 197–203. [Google Scholar] [CrossRef]
- Ratnasekera, A.M.; Seng, S.S.; Kim, D.; Ji, W.; Jacovides, C.L.; Kaufman, E.J.; Sadek, H.M.; Perea, L.L.; Poloni, C.M.; Shnaydman, I.; et al. Propensity weighted analysis of chemical venous thromboembolism prophylaxis agents in isolated severe traumatic brain injury: An EAST sponsored multicenter study. Injury 2024, 55, 111523. [Google Scholar] [CrossRef]
- Minshall, C.T.; Eriksson, E.A.; Leon, S.M.; Doben, A.R.; McKinzie, B.P.; Fakhry, S.M. Safety and efficacy of heparin or enoxaparin prophylaxis in blunt trauma patients with a head abbreviated injury severity score >2. J. Trauma 2011, 71, 396–399, discussion 399–400. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Zhang, X.; Chen, H. Patients with venous thromboembolism after spontaneous intracerebral hemorrhage: A review. Thromb. J. 2021, 19, 93. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.M.; Ziai, W.C.; Cordonnier, C.; Dowlatshahi, D.; Francis, B.; Goldstein, J.N.; Hemphill, J.C.; Johnson, R.; Keigher, K.M.; Mack, W.J.; et al. 2022 Guideline for the Management of Patients With Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 2022, 53, e282–e361. [Google Scholar] [CrossRef]
- Ji, R.; Wang, L.; Liu, X.; Liu, Y.; Wang, D.; Wang, W.; Zhang, R.; Jiang, R.; Jia, J.; Feng, H.; et al. A novel risk score to predict deep vein thrombosis after spontaneous intracerebral hemorrhage. Front. Neurol. 2022, 13, 930500. [Google Scholar] [CrossRef] [PubMed]
- Tetri, S.; Hakala, J.; Juvela, S.; Saloheimo, P.; Pyhtinen, J.; Rusanen, H.; Savolainen, E.-R.; Hillbom, M. Safety of low-dose subcutaneous enoxaparin for the prevention of venous thromboembolism after primary intracerebral haemorrhage. Thromb. Res. 2008, 123, 206–212. [Google Scholar] [CrossRef]
- Orken, D.N.; Kenangil, G.; Ozkurt, H.; Guner, C.; Gundogdu, L.; Basak, M.; Forta, H. Prevention of Deep Venous Thrombosis and Pulmonary Embolism in Patients With Acute Intracerebral Hemorrhage. Neurologist 2009, 15, 329–331. [Google Scholar] [CrossRef]
- Muñoz-Venturelli, P.; Wang, X.; Lavados, P.M.; Stapf, C.; Robinson, T.; Lindley, R.; Heeley, E.; Delcourt, C.; Chalmers, J.; Anderson, C.S.; et al. Prophylactic heparin in acute intracerebral hemorrhage: A propensity score-matched analysis of the INTERACT2 study. Int. J. Stroke 2016, 11, 549–556. [Google Scholar] [CrossRef]
- Wang, T.-F.; Milligan, P.E.; Wong, C.A.; Deal, E.N.; Thoelke, M.S.; Gage, B.F. Efficacy and safety of high-dose thromboprophylaxis in morbidly obese inpatients. Thromb. Haemost. 2014, 111, 88–93. [Google Scholar] [CrossRef]
- Frisoli, F.A.; Shinseki, M.; Nwabuobi, L.; Zeng, X.L.; Adrados, M.; Kanter, C.; Frangos, S.G.; Huang, P.P. Early Venous Thromboembolism Chemoprophylaxis After Traumatic Intracranial Hemorrhage. Neurosurgery 2017, 81, 1016–1020. [Google Scholar] [CrossRef]
- Ianosi, B.; Gaasch, M.; Rass, V.; Huber, L.; Hackl, W.; Kofler, M.; Schiefecker, A.J.; Addis, A.; Beer, R.; Rhomberg, P.; et al. Early thrombosis prophylaxis with enoxaparin is not associated with hematoma expansion in patients with spontaneous intracerebral hemorrhage. Eur. J. Neurol. 2019, 26, 333–341. [Google Scholar] [CrossRef]
- Kananeh, M.F.; Fonseca-Paricio, M.J.; Liang, J.W.; Sullivan, L.T.; Sharma, K.; Shah, S.O.; Vibbert, M.D. Ultra-Early Venous Thromboembolism (VTE) Prophylaxis in Spontaneous Intracerebral Hemorrhage (sICH). J. Stroke Cerebrovasc. Dis. 2021, 30, 105476. [Google Scholar] [CrossRef]
- Wu, T.C.; Kasam, M.; Harun, N.; Hallevi, H.; Bektas, H.; Acosta, I.; Misra, V.; Barreto, A.D.; Gonzales, N.R.; Lopez, G.A.; et al. Pharmacological deep vein thrombosis prophylaxis does not lead to hematoma expansion in intracerebral hemorrhage with intraventricular extension. Stroke 2011, 42, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Huhtakangas, J.; Juvela, S.; Bode, M.; Tatlisumak, T.; Savolainen, M.; Numminen, H.; Ollikainen, J.; Luostarinen, L.; Kupila, L.; et al. Early vs. late enoxaparin for the prevention of venous thromboembolism in patients with ICH: A double blind placebo controlled multicenter study. Clin. Neurol. Neurosurg. 2021, 202, 106534. [Google Scholar] [CrossRef]
- Laurent, D.; Bardhi, O.; Kubilis, P.; Corliss, B.; Adamczak, S.; Geh, N.; Dodd, W.; Vaziri, S.; Busl, K.; Fox, W.C. Early chemoprophylaxis for deep venous thrombosis does not increase the risk of hematoma expansion in patients presenting with spontaneous intracerebral hemorrhage. Surg. Neurol. Int. 2021, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Paciaroni, M.; Agnelli, G.; Venti, M.; Alberti, A.; Acciarresi, M.; Caso, V. Efficacy and safety of anticoagulants in the prevention of venous thromboembolism in patients with acute cerebral hemorrhage: A meta-analysis of controlled studies. J. Thromb. Haemost. 2011, 9, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Li, J.; Xu, L.; Deng, S.; Wang, Z. Safety of Prophylactic Heparin in the Prevention of Venous Thromboembolism After Spontaneous Intracerebral Hemorrhage: A Meta-analysis. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2020, 81, 253–260. [Google Scholar] [CrossRef]
- Etminan, N.; Chang, H.S.; Hackenberg, K.; De Rooij, N.K.; Vergouwen, M.D.; Rinkel, G.J.; Algra, A. Worldwide Incidence of Aneurysmal Subarachnoid Hemorrhage According to Region, Time Period, Blood Pressure, and Smoking Prevalence in the Population: A Systematic Review and Meta-analysis. JAMA Neurol. 2019, 76, 588–597. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Rinkel, G.J.; Rothwell, P.M. Time trends in outcome of subarachnoid hemorrhage: Population-based study and systematic review. Neurology 2010, 74, 1494–1501. [Google Scholar] [CrossRef]
- Muehlschlegel, S. Subarachnoid Hemorrhage. Contin. Lifelong Learn. Neurol. 2018, 24, 1623–1657. [Google Scholar] [CrossRef]
- Pan, J.; Bonow, R.H.; Temkin, N.; Robinson, E.F.; Sekhar, L.N.; Levitt, M.R.; Lele, A.V. Incidence and Risk Model of Venous Thromboembolism in Patients with Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2023, 172, e418–e427. [Google Scholar] [CrossRef]
- Kshettry, V.R.; Rosenbaum, B.P.; Seicean, A.; Kelly, M.L.; Schiltz, N.K.; Weil, R.J. Incidence and risk factors associated with in-hospital venous thromboembolism after aneurysmal subarachnoid hemorrhage. J. Clin. Neurosci. 2014, 21, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Siironen, J.; Juvela, S.; Varis, J.; Porras, M.; Poussa, K.; Ilveskero, S.; Hernesniemi, J.; Lassila, R. No effect of enoxaparin on outcome of aneurysmal subarachnoid hemorrhage: A randomized, double-blind, placebo-controlled clinical trial. J. Neurosurg. 2003, 99, 953–959. [Google Scholar] [CrossRef]
- Wurm, G.; Tomancok, B.; Nussbaumer, K.; Adelwöhrer, C.; Holl, K. Reduction of ischemic sequelae following spontaneous subarachnoid hemorrhage: A double-blind, randomized comparison of enoxaparin versus placebo. Clin. Neurol. Neurosurg. 2004, 106, 97–103. [Google Scholar] [CrossRef]
- Hantsche, A.; Wilhelmy, F.; Kasper, J.; Wende, T.; Hamerla, G.; Rasche, S.; Meixensberger, J.; Lindner, D. Early prophylactic anticoagulation after subarachnoid hemorrhage decreases systemic ischemia and improves outcome. Clin. Neurol. Neurosurg. 2021, 207, 106809. [Google Scholar] [CrossRef] [PubMed]
- Kilgore, C.B.; Nair, S.K.; Ran, K.R.; Caplan, J.M.; Jackson, C.M.; Gonzalez, L.F.; Huang, J.; Tamargo, R.J.; Xu, R. Venous thromboembolism in aneurysmal subarachnoid hemorrhage: Risk factors and timing of chemoprophylaxis. Clin. Neurol. Neurosurg. 2023, 231, 107822. [Google Scholar] [CrossRef]
- Manoel, A.L.d.O.; Turkel-Parrella, D.; Germans, M.; Kouzmina, E.; Almendra, P.d.S.; Marotta, T.; Spears, J.; Abrahamson, S. Safety of early pharmacological thromboprophylaxis after subarachnoid hemorrhage. Can. J. Neurol. Sci. 2014, 41, 554–561. [Google Scholar] [CrossRef]
- Ukpabi, C.; Sadan, O.; Shi, Y.; Greene, K.N.; Samuels, O.; Mathew, S.; Joy, J.; Mei, Y.; Asbury, W. Pharmacologic Venous Thromboembolism Prophylaxis in Patients with Nontraumatic Subarachnoid Hemorrhage Requiring an External Ventricular Drain. Neurocrit. Care 2024, 41, 779–787. [Google Scholar] [CrossRef]
- Amidei, C.B.; Salmaso, L.; Bellio, S.; Saia, M. Epidemiology of traumatic spinal cord injury: A large population-based study. Spinal Cord 2022, 60, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Godat, L.N.; Haut, E.R.; Moore, E.E.; Knudson, M.M.; Costantini, T.W. Venous thromboembolism risk after spinal cord injury: A secondary analysis of the CLOTT study. J. Trauma Acute Care Surg. 2023, 94, 23–29. [Google Scholar] [CrossRef]
- Wei, B.; Zhou, H.; Liu, G.; Zheng, Y.; Zhang, Y.; Hao, C.; Wang, Y.; Kang, H.; Lu, X.; Yuan, Y.; et al. Risk factors for venous thromboembolism in patients with spinal cord injury: A systematic review and meta-analysis. J. Spinal Cord Med. 2023, 46, 181–193. [Google Scholar] [CrossRef]
- Chang, R.; Scerbo, M.H.; Schmitt, K.M.; Adams, S.D.; Choi, T.J.; Wade, C.E.; Holcomb, J.B. Early chemoprophylaxis is associated with decreased venous thromboembolism risk without concomitant increase in intraspinal hematoma expansion after traumatic spinal cord injury. J. Trauma Acute Care Surg. 2017, 83, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, A.M.; Tsolinas, R.; Alazzeh, M.; Haefeli, J.; Talbott, J.F.; Ferguson, A.R.; Bresnahan, J.C.; Beattie, M.S.; Manley, G.T.; Whetstone, W.D.; et al. Safety and effectiveness of early chemical deep venous thrombosis prophylaxis after spinal cord injury: Pilot prospective data. Neurosurg. Focus 2017, 43, E21. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, M.; Khan, M.; O’kEeffe, T.; Pollack, N.; Hamidi, M.; Kulvatunyou, N.; Sakran, J.V.; Gries, L.; Joseph, B. Optimal timing of initiation of thromboprophylaxis in spine trauma managed operatively: A nationwide propensity-matched analysis of trauma quality improvement program. J. Trauma Acute Care Surg. 2018, 85, 387–392. [Google Scholar] [CrossRef]
- Lui, A.; Park, C.; Chryssikos, T.; Radabaugh, H.; Patel, A.; Aabedi, A.A.; Ferguson, A.R.; Espin, A.T.; Mummaneni, P.V.; Dhall, S.S.; et al. Safety and comparative efficacy of initiating low-molecular-weight heparin within 24 hours of injury or surgery for venous thromboembolism prophylaxis in patients with spinal cord injury: A prospective TRACK-SCI registry study. Neurosurg. Focus 2023, 55, E17. [Google Scholar] [CrossRef] [PubMed]
- Ahlquist, S.; Park, H.Y.; Kelley, B.; Holly, L.; Shamie, A.N.; Park, D.Y. Venous Thromboembolism Chemoprophylaxis Within 24 Hours of Surgery for Spinal Cord Injury: Is It Safe and Effective? Neurospine 2020, 17, 407–416. [Google Scholar] [CrossRef]
- Dhillon, E.S.; Khanna, R.; Cloney, M.; Roberts, H.; Cybulski, G.R.; Koski, T.R.; Smith, Z.A.; Dahdaleh, N.S. Timing and risks of chemoprophylaxis after spinal surgery: A single-center experience with 6869 consecutive patients. J. Neurosurg. Spine 2017, 27, 681–693. [Google Scholar] [CrossRef]
- Dornbush, C.; Maly, C.; Bartschat, N.; Lilienthal, M.; Galet, C.; Skeete, D.A.; Igram, C. Chemoprophylaxis Timing Is Not Associated With Postoperative Bleeding After Spinal Trauma Surgery. Clin. Neurol. Neurosurg. 2023, 225, 107590. [Google Scholar] [CrossRef]
- Halim, T.A.; Chhabra, H.S.; Arora, M.; Kumar, S. Pharmacological prophylaxis for deep vein thrombosis in acute spinal cord injury: An Indian perspective. Spinal Cord 2014, 52, 547–550. [Google Scholar] [CrossRef]
- Hamidi, M.; Asmar, S.; Bible, L.; Hanna, K.; Castanon, L.; Avila, M.; Ditillo, M.; Joseph, B. Early Thromboprophylaxis in Operative Spinal Trauma Does Not Increase Risk of Bleeding Complications. J. Surg. Res. 2021, 258, 119–124. [Google Scholar] [CrossRef]
- Lambrechts, M.J.; Toci, G.R.; Issa, T.Z.; Narayanan, R.; Lee, Y.; Schaefer, J.; Hilibrand, A.S.; Vaccaro, A.R.; Harrop, J.S.; Schroeder, G.D.; et al. Immediate vs delayed venous thromboembolism prophylaxis following spine surgery: Increased rate of unplanned reoperation for postoperative hematoma with immediate prophylaxis. Spine J. 2024, 24, 2019–2025. [Google Scholar] [CrossRef]
- Cox, J.B.; Weaver, K.J.; Neal, D.W.; Jacob, R.P.; Hoh, D.J. Decreased incidence of venous thromboembolism after spine surgery with early multimodal prophylaxis: Clinical article. J. Neurosurg. Spine 2014, 21, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, R.A.; Chavarria-Aguilar, M.; Cockerham, W.T.; Lewis, P.L.; Barker, D.E.; Durham, R.M.; Ciraulo, D.L.; Richart, C.M. Routine prophylactic vena cava filtration is not indicated after acute spinal cord injury. J. Trauma 2002, 52, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Fiasconaro, M.; Poeran, J.; Liu, J.; Wilson, L.A.; Memtsoudis, S.G. Venous thromboembolism and prophylaxis therapy after elective spine surgery: A population-based study. Can. J. Anesth. 2021, 68, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Lowery, A.; Patel, A.; Ames, R.; Ramsey, F.; Slattery, B.; Pazionis, T. Prevalence of Venous Thromboembolism Following Acute Spinal Cord Injury in an Urban Inner City Hospital. Int. J. Spine Surg. 2021, 15, 562–569. [Google Scholar] [CrossRef]
- Spinal Cord Injury Thromboprophylaxis Investigators. Prevention of venous thromboembolism in the acute treatment phase after spinal cord injury: A randomized, multicenter trial comparing low-dose heparin plus intermittent pneumatic compression with enoxaparin. J. Trauma 2003, 54, 1116–1124, discussion 1125–1126. [Google Scholar] [CrossRef]
- Neifert, S.N.B.; Chapman, E.K.B.; Rothrock, R.J.; Gilligan, J.; Yuk, F.; McNeill, I.T.; Rasouli, J.J.; Gal, J.S.; Caridi, J.M. Lower Mortality and Morbidity with Low-Molecular-Weight Heparin for Venous Thromboembolism Prophylaxis in Spine Trauma. Spine 2020, 45, 1613–1618. [Google Scholar] [CrossRef]
- Liu, D.; Song, D.; Ning, W.; Zhang, X.; Chen, S.; Zhang, H. Efficacy and safety of prophylaxis for venous thromboembolism in brain neoplasm patients undergoing neurosurgery: A systematic review and Bayesian network meta-analysis. J. Thromb. Thrombolysis 2023, 55, 710–720. [Google Scholar] [CrossRef]
- Riedl, J.; Ay, C. Venous Thromboembolism in Brain Tumors: Risk Factors, Molecular Mechanisms, and Clinical Challenges. Semin. Thromb. Hemost. 2019, 45, 334–341. [Google Scholar] [CrossRef]
- Agnelli, G.; Piovella, F.; Buoncristiani, P.; Severi, P.; Pini, M.; D’ANgelo, A.; Beltrametti, C.; Damiani, M.; Andrioli, G.C.; Pugliese, R.; et al. Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of venous thromboembolism after elective neurosurgery. N. Engl. J. Med. 1998, 339, 80–85. [Google Scholar] [CrossRef]
- Byrne, J.P.; Witiw, C.D.; Schuster, J.M.; Pascual, J.L.; Cannon, J.W.; Martin, N.D.; Reilly, P.M.; Nathens, A.B.; Seamon, M.J. Association of Venous Thromboembolism Prophylaxis After Neurosurgical Intervention for Traumatic Brain Injury With Thromboembolic Complications, Repeated Neurosurgery, and Mortality. JAMA Surg. 2022, 157, e215794. [Google Scholar] [CrossRef]
- Al Tannir, A.H.; Golestani, S.; Tentis, M.; Murphy, P.B.; Schramm, A.T.; Peschman, J.; Dodgion, C.; Holena, D.; Miranda, S.; Carver, T.W.; et al. Early venous thromboembolism chemoprophylaxis in traumatic brain injury requiring neurosurgical intervention: Safe and effective. Surgery 2024, 175, 1439–1444. [Google Scholar] [CrossRef] [PubMed]
- Shafiei, M.; Sabouri, M.; Aminmansour, B.; Mahmoodkhani, M.; Sourani, A.; Salehi, I.; Foroughi, M. Enoxaparin initiation after chronic subdural hematoma evacuation, a randomized clinical trial on timing and outcomes. Surg. Pract. 2023, 27, 153–162. [Google Scholar] [CrossRef]
- Algattas, H.; Talentino, S.E.; Eichar, B.; Williams, A.A.; Murphy, J.M.; Zhang, X.; Garcia, R.M.; Newhouse, D.; Jaman, E.; Safonova, A.; et al. Venous Thromboembolism Anticoagulation Prophylaxis Timing in Patients Undergoing Craniotomy for Tumor. Neurosurg. Pract. 2021, 2, 2–8. [Google Scholar] [CrossRef]
- Briggs, R.G.; Lin, Y.-H.; Dadario, N.B.; Young, I.M.; Conner, A.K.; Xu, W.; Tanglay, O.; Kim, S.J.; Fonseka, R.D.; Bonney, P.A.; et al. Optimal timing of post-operative enoxaparin after neurosurgery: A single institution experience. Clin. Neurol. Neurosurg. 2021, 207, 106792. [Google Scholar] [CrossRef]
- Jiang, S.H.; Hukamdad, M.; Gould, A.; Bhaskara, M.; Chiu, R.G.; Sadeh, M.; Mehta, A.I. Effect of perioperative anticoagulant prophylaxis in patients with traumatic subdural hematoma and a history of anticoagulant use: A propensity-matched National Trauma Data Bank analysis. Neurosurg. Focus 2023, 55, E3. [Google Scholar] [CrossRef]
- Tanweer, O.; Boah, A.; Huang, P. Risks for hemorrhagic complications after placement of external ventricular drains with early chemical prophylaxis against venous thromboembolisms. J. Neurosurg. 2013, 119, 1309–1313. [Google Scholar] [CrossRef]
- Cage, T.A.; Lamborn, K.R.; Ware, M.L.; Frankfurt, A.; Chakalian, L.; Berger, M.S.; McDermott, M.W. Adjuvant enoxaparin therapy may decrease the incidence of postoperative thrombotic events though does not increase the incidence of postoperative intracranial hemorrhage in patients with meningiomas. J. Neurooncol. 2009, 93, 151–156. [Google Scholar] [CrossRef][Green Version]
- Hallan, D.R.; Sciscent, B.; Rizk, E. A Retrospective Comparative Cohort Study of Craniotomy and Prophylactic Enoxaparin Timing. Cureus 2022, 14, e23867. [Google Scholar] [CrossRef]
- Kandula, V.; Shah, P.V.; Thirunavu, V.M.; Yerneni, K.; Karras, C.; Abecassis, Z.A.; Hopkins, B.; Bloch, O.; Potts, M.B.; Jahromi, B.S.; et al. Low-molecular-weight Heparin (enoxaparin) versus unfractionated heparin for venous thromboembolism prophylaxis in patients undergoing craniotomy. Clin. Neurol. Neurosurg. 2022, 223, 107482. [Google Scholar] [CrossRef]
- Bell, J.S.; Florence, T.J.; Phillips, H.W.; Patel, K.; Macaluso, N.J.; Villanueva, P.G.; Naik, P.K.; Kim, W. Comparison of the Safety of Prophylactic Anticoagulants After Intracranial Surgery. Neurosurgery 2021, 89, 527–536. [Google Scholar] [CrossRef]
- Farr, S.; Toor, H.; Patchana, T.; Podkovik, S.; Wiginton, J.G.; Sweiss, R.; Wacker, M.R.; Miulli, D.E. Risks, Benefits, and the Optimal Time to Resume Deep Vein Thrombosis Prophylaxis in Patients with Intracranial Hemorrhage. Cureus 2019, 11, e5827. [Google Scholar] [CrossRef] [PubMed]
- Catapano, J.S.; Koester, S.W.; Parikh, P.P.; Rumalla, K.; Stonnington, H.O.; Singh, R.; Winkler, E.A.; Graffeo, C.S.; Rudy, R.F.; Srinivasan, V.M.; et al. Association between external ventricular drain removal or replacement and prophylactic anticoagulation in patients with aneurysmal subarachnoid hemorrhage: A propensity-adjusted analysis. Acta Neurochir. 2023, 165, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Salottolo, K.; Offner, P.; Levy, A.S.; Mains, C.W.; Slone, D.S.; Bar-Or, D. Interrupted pharmocologic thromboprophylaxis increases venous thromboembolism in traumatic brain injury. J. Trauma 2011, 70, 19–24, discussion 25–26. [Google Scholar] [CrossRef] [PubMed]
| Guideline (Publication Year) | Recommendations | |
|---|---|---|
| Timing | Agent | |
| aSAH | ||
| NCS 2016 * | ≥24 h after aneurysm securement by open surgical approach or by endovascular coiling | UFH recommended for PTP |
| NCS/SCCM 2017 ** | ≥24 h after aneurysm securement by open surgical approach or by endovascular coiling | UFH recommended for PTP |
| AHA/ASA 2023 † | After aneurysm securement by open surgical approach or by endovascular coiling | No recommendation |
| ICH | ||
| NCS 2016 * | Within 48 h of hospital admission with stable hematoma and no ongoing coagulopathy | LMWH or UFH |
| NCS/SCCM 2017 ** | Within 48 h of hospital admission with stable hematoma and no ongoing coagulopathy | LMWH or UFH |
| AHA/ASA 2022 ‡ | At 24–48 h from ICH onset | LMWH or UFH |
| Neurosurgical Intervention | ||
| NCS 2016 * | Standard Elective Spine Surgery | |
| No recommendation | LMWH (combined with mechanical specifically with increased risk of VTE), with UFH only as an alternative to other methods because of increased risk of bleeding | |
| Complicated Spinal Surgery | ||
| No recommendation | LMWH or UFH | |
| Elective Craniotomy (with or without glioma resection) | ||
| Within 24 h after craniotomy | LMWH or UFH | |
| Elective Intracranial/Intra-arterial Procedures | ||
| Immediate | LMWH or UFH | |
| NCS/SCCM 2017 ** | Standard Elective Spine Surgery | |
| No recommendation | LMWH (combined with mechanical specifically with increased risk of VTE), with UFH only as an alternative to other methods because of increased risk of bleeding | |
| Complicated Spinal Surgery | ||
| No recommendation | LMWH or UFH | |
| Elective Craniotomy (with or without glioma resection) | ||
| Within 24 h after craniotomy | LMWH or UFH | |
| Elective Intracranial/Intra-arterial Procedures | ||
| Immediate | LMWH or UFH | |
| SCI | ||
| NCS 2016 * | Early as possible, within 72 h of injury; as soon as bleeding is controlled | LMWH or adjusted dose UFH |
| Consortium for Spinal Cord Medicine 2016 ¥ | After there is no evidence of active bleeding | LMWH recommended for PTP; UFH recommended against as low-dose or adjusted dose |
| NCS/SCCM 2017 ** | Early as possible, within 72 h of injury; as soon as bleeding is controlled | LMWH or adjusted dose UFH |
| WTA 2020 †† | Within 24 h with moderate–high-risk VTE and stabilization of spinal cord injury | LMWH 30 mg q12 h (with CrCL ≥ 30 mL/min), preferable to UFH |
| TBI | ||
| NCS 2016 * | Within 24 h of TBI or within 24 h after craniotomy; within 24–48 h in patients with TBI and ICH or 24 h after craniotomy | LMWH or UFH, |
| NCS/SCCM 2017 ** | Within 24 h of TBI or within 24 h after craniotomy; within 24–48 h in patients with TBI and ICH or 24 h after craniotomy | LMWH or UFH, |
| Brain Trauma Foundation 2017‡‡ | No recommendation | LMWH or low-dose UFH; however, there is noted to be an increased risk of expansion of ICH |
| WTA 2020 †† | Within 24 h with moderate–high-risk VTE and no TBI progression on follow-up CT | LMWH, ENX 30 mg q12 h (with CrCL ≥ 30 mL/min), preferable to UFH |
| American College of Surgeons 2024 ¥¥ | Within 24 h with low-risk nonoperative TBI and no TBI progression on follow-up CT; within 24–48 h with moderate–severe risk nonoperative TBI and no TBI progression on follow-up CT; within 24–48 h after craniotomy/craniectomy for TBI and no ICH progression on postoperative CT | LMWH preferred over UFH |
| UFH | Enoxaparin | |
|---|---|---|
| Bioavailability | Variable | 100% |
| Mean MW (range) (kDa) | 16 (4–30) | 4.5 (mostly 2–8) |
| Proportion with both anti-Xa and anti-IIa activity | 95% | <30% |
| Metabolism | RES primarily in liver and spleen | Hepatic (desulfation and/or depolymerization to lower weight molecules with very low potency) |
| Excretion | Urine (small amounts as unchanged drug); elimination of therapeutic doses occurs rapidly via nonrenal mechanisms | Urine (clearance decreased by 30% and AUC increased 65% with CrCL < 30 mL/min) |
| Pharmacokinetics | First-order (with time and dose dependence of anti-Xa/anti-IIa effects) | Mixed-order kinetic behavior |
| Half-life elimination, plasma | 1–2 h | 4.5–7 h (based on anti-Xa activity) (duration 40 mg dose ~ 12 h based on anti-Xa activity) |
| Usual subcutaneous prophylaxis dosing interval | 5000 units q8h-q12h | 30–40 mg q12h-q24h |
| Maximum neutralization by protamine (%) | 100 | 60–75 |
| Risk of HIT | 2.60% | 0.20% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banerjee, O.; Rodrigues, R.; Adkins, L.; Busl, K.M. Venous Thromboembolism Prophylaxis in the Neurocritically Ill Population. J. Clin. Med. 2025, 14, 4434. https://doi.org/10.3390/jcm14134434
Banerjee O, Rodrigues R, Adkins L, Busl KM. Venous Thromboembolism Prophylaxis in the Neurocritically Ill Population. Journal of Clinical Medicine. 2025; 14(13):4434. https://doi.org/10.3390/jcm14134434
Chicago/Turabian StyleBanerjee, Oyshik, Roysten Rodrigues, Lauren Adkins, and Katharina M. Busl. 2025. "Venous Thromboembolism Prophylaxis in the Neurocritically Ill Population" Journal of Clinical Medicine 14, no. 13: 4434. https://doi.org/10.3390/jcm14134434
APA StyleBanerjee, O., Rodrigues, R., Adkins, L., & Busl, K. M. (2025). Venous Thromboembolism Prophylaxis in the Neurocritically Ill Population. Journal of Clinical Medicine, 14(13), 4434. https://doi.org/10.3390/jcm14134434






