Clinical Management of Cerebral Amyloid Angiopathy
Abstract
1. Introduction
2. Pathophysiology and Progression of the Sporadic CAA
3. Diagnosis
3.1. Clinical Manifestations
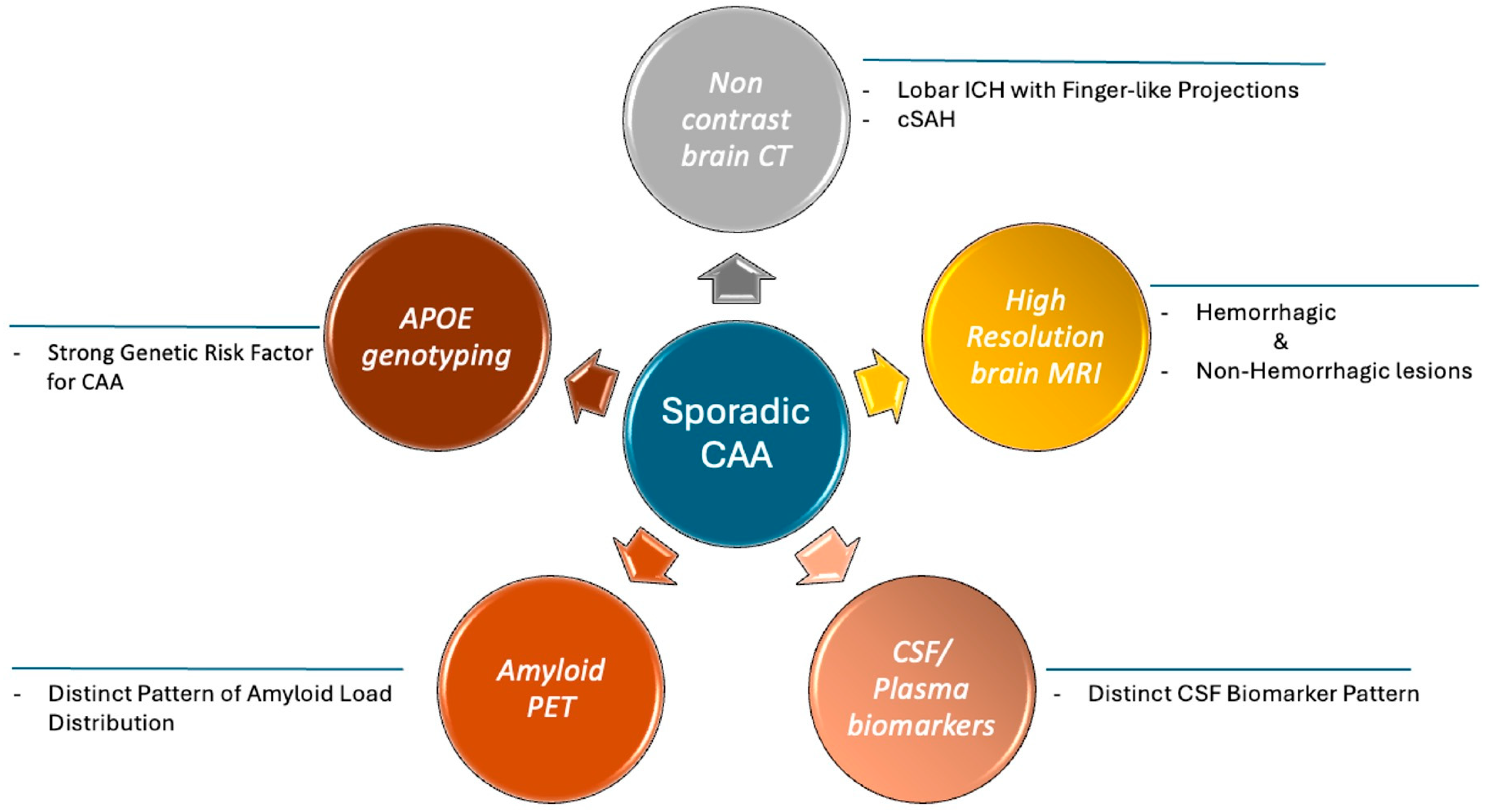
3.2. Neuroimaging Findings
3.3. Cerebrospinal Fluid and Plasma Biomarkers
3.4. PET Findings
3.5. Apolipoprotein E Gene
4. Management
4.1. Management of Cognitive Impairment
4.2. Management of Intracerebral Hemorrhage
4.3. Management of Transient Focal Neurological Episodes
4.4. Management of Acute Ischemic Stroke: Intravenous Thrombolysis
4.5. Management of Acute Ischemic Stroke: Mechanical Thrombectomy
4.6. Secondary Prevention and Left Atrial Appendage Occlusion
5. CAA Mimics
6. Rare or Early-Onset Types of CAA
6.1. Cerebral Amyloid Angiopathy-Related Inflammation (CAA-ri)
6.2. Hereditary CAA
6.3. Iatrogenic CAA
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Charidimou, A.; Boulouis, G.; Gurol, M.E.; Ayata, C.; Bacskai, B.J.; Frosch, M.P.; Viswanathan, A.; Greenberg, S.M. Emerging concepts in sporadic cerebral amyloid angiopathy. Brain 2017, 140, 1829–1850. [Google Scholar] [CrossRef]
- Boulouis, G.; Charidimou, A.; Greenberg, S.M. Sporadic Cerebral Amyloid Angiopathy: Pathophysiology, Neuroimaging Features, and Clinical Implications. Semin. Neurol. 2016, 36, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Koemans, E.A.; Chhatwal, J.P.; van Veluw, S.J.; van Etten, E.S.; van Osch, M.J.P.; van Walderveen, M.A.A.; Sohrabi, H.R.; Kozberg, M.G.; Shirzadi, Z.; Terwindt, G.M.; et al. Progression of cerebral amyloid angiopathy: A pathophysiological framework. Lancet Neurol. 2023, 22, 632–642. [Google Scholar] [CrossRef]
- Malhotra, K.; Zompola, C.; Theodorou, A.; Katsanos, A.H.; Shoamanesh, A.; Gupta, H.; Beshara, S.; Goyal, N.; Chang, J.; Tayal, A.H.; et al. Prevalence, Characteristics, and Outcomes of Undetermined Intracerebral Hemorrhage: A Systematic Review and Meta-Analysis. Stroke 2021, 52, 3602–3612. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Boulouis, G.; Charidimou, A.; Roongpiboonsopit, D.; Jessel, M.J.; Pasi, M.; Reijmer, Y.D.; Fotiadis, P.; Ayres, A.; Merrill, E.; et al. Dementia incidence and predictors in cerebral amyloid angiopathy patients without intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 2018, 38, 241–249. [Google Scholar] [CrossRef]
- Chan, E.; Bonifacio, G.B.; Harrison, C.; Banerjee, G.; Best, J.G.; Sacks, B.; Harding, N.; Del Rocio Hidalgo Mas, M.; Jäger, H.R.; Cipolotti, L.; et al. Domain-specific neuropsychological investigation of CAA with and without intracerebral haemorrhage. J. Neurol. 2023, 270, 6124–6132. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, K.; Theodorou, A.; Katsanos, A.H.; Zompola, C.; Shoamanesh, A.; Boviatsis, E.; Paraskevas, G.P.; Spilioti, M.; Cordonnier, C.; Werring, D.J.; et al. Prevalence of Clinical and Neuroimaging Markers in Cerebral Amyloid Angiopathy: A Systematic Review and Meta-Analysis. Stroke 2022, 53, 1944–1953. [Google Scholar] [CrossRef]
- Knudsen, K.A.; Rosand, J.; Karluk, D.; Greenberg, S.M. Clinical diagnosis of cerebral amyloid angiopathy: Validation of the Boston criteria. Neurology 2001, 56, 537–539. [Google Scholar] [CrossRef]
- Linn, J.; Halpin, A.; Demaerel, P.; Ruhland, J.; Giese, A.D.; Dichgans, M.; van Buchem, M.A.; Bruckmann, H.; Greenberg, S.M. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 2010, 74, 1346–1350. [Google Scholar] [CrossRef]
- Charidimou, A.; Boulouis, G.; Frosch, M.P.; Baron, J.C.; Pasi, M.; Albucher, J.F.; Banerjee, G.; Barbato, C.; Bonneville, F.; Brandner, S.; et al. The Boston criteria version 2.0 for cerebral amyloid angiopathy: A multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol. 2022, 21, 714–725. [Google Scholar] [CrossRef]
- Auriel, E.; Charidimou, A.; Gurol, M.E.; Ni, J.; Van Etten, E.S.; Martinez-Ramirez, S.; Boulouis, G.; Piazza, F.; DiFrancesco, J.C.; Frosch, M.P.; et al. Validation of Clinicoradiological Criteria for the Diagnosis of Cerebral Amyloid Angiopathy-Related Inflammation. JAMA Neurol. 2016, 73, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, A.; Tsantzali, I.; Stefanou, M.I.; Sacco, S.; Katsanos, A.H.; Shoamanesh, A.; Karapanayiotides, T.; Koutroulou, I.; Stamati, P.; Werring, D.J.; et al. CSF and plasma biomarkers in cerebral amyloid angiopathy: A single-center study and a systematic review/meta-analysis. Eur. Stroke J. 2024, 23969873241260538. [Google Scholar] [CrossRef] [PubMed]
- Charidimou, A.; Farid, K.; Baron, J.C. Amyloid-PET in sporadic cerebral amyloid angiopathy: A diagnostic accuracy meta-analysis. Neurology 2017, 89, 1490–1498. [Google Scholar] [CrossRef]
- Charidimou, A.; Farid, K.; Tsai, H.H.; Tsai, L.K.; Yen, R.F.; Baron, J.C. Amyloid-PET burden and regional distribution in cerebral amyloid angiopathy: A systematic review and meta-analysis of biomarker performance. J. Neurol. Neurosurg. Psychiatry 2018, 89, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, G.; Collinge, J.; Fox, N.C.; Lashley, T.; Mead, S.; Schott, J.M.; Werring, D.J.; Ryan, N.S. Clinical considerations in early-onset cerebral amyloid angiopathy. Brain 2023, 146, 3991–4014. [Google Scholar] [CrossRef]
- Theodorou, A.; Palaiodimou, L.; Malhotra, K.; Zompola, C.; Katsanos, A.H.; Shoamanesh, A.; Boviatsis, E.; Dardiotis, E.; Spilioti, M.; Sacco, S.; et al. Clinical, Neuroimaging, and Genetic Markers in Cerebral Amyloid Angiopathy-Related Inflammation: A Systematic Review and Meta-Analysis. Stroke 2023, 54, 178–188. [Google Scholar] [CrossRef]
- Attems, J.; Jellinger, K.; Thal, D.R.; Van Nostrand, W. Review: Sporadic cerebral amyloid angiopathy. Neuropathol. Appl. Neurobiol. 2011, 37, 75–93. [Google Scholar] [CrossRef]
- Charidimou, A.; Gang, Q.; Werring, D.J. Sporadic cerebral amyloid angiopathy revisited: Recent insights into pathophysiology and clinical spectrum. J. Neurol. Neurosurg. Psychiatry 2012, 83, 124–137. [Google Scholar] [CrossRef]
- Herzig, M.C.; Van Nostrand, W.E.; Jucker, M. Mechanism of cerebral beta-amyloid angiopathy: Murine and cellular models. Brain Pathol. 2006, 16, 40–54. [Google Scholar] [CrossRef]
- Weller, R.O.; Djuanda, E.; Yow, H.Y.; Carare, R.O. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009, 117, 1–14. [Google Scholar] [CrossRef]
- Wardlaw, J.M. Blood-brain barrier and cerebral small vessel disease. J. Neurol. Sci. 2010, 299, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Doubal, F.N.; MacLullich, A.M.; Ferguson, K.J.; Dennis, M.S.; Wardlaw, J.M. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke 2010, 41, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Voumvourakis, K.; Tsivgoulis, G.; Papathanasiou, M.A.; Simitsi, A.; Stefanis, L.; Papageorgiou, S.G. Teaching NeuroImages: MRI-visible Virchow-Robin perivascular spaces in cerebral small-vessel disease. Neurology 2014, 83, e119–e120. [Google Scholar] [CrossRef] [PubMed]
- Corbo, R.M.; Scacchi, R. Apolipoprotein E (APOE) allele distribution in the world. Is APOE*4 a ‘thrifty’ allele? Ann. Hum. Genet. 1999, 63, 301–310. [Google Scholar] [CrossRef]
- Hu, H.; Wan, S.; Hu, Y.; Wang, Q.; Li, H.; Zhang, N. Deciphering the role of APOE in cerebral amyloid angiopathy: From genetic insights to therapeutic horizons. Ann. Med. 2025, 57, 2445194. [Google Scholar] [CrossRef]
- Grinberg, L.T.; Thal, D.R. Vascular pathology in the aged human brain. Acta Neuropathol. 2010, 119, 277–290. [Google Scholar] [CrossRef]
- Dotti, C.G.; De Strooper, B. Alzheimer’s dementia by circulation disorders: When trees hide the forest. Nat. Cell Biol. 2009, 11, 114–116. [Google Scholar] [CrossRef]
- Olichney, J.M.; Hansen, L.A.; Hofstetter, C.R.; Grundman, M.; Katzman, R.; Thal, L.J. Cerebral infarction in Alzheimer’s disease is associated with severe amyloid angiopathy and hypertension. Arch. Neurol. 1995, 52, 702–708. [Google Scholar] [CrossRef]
- Reijmer, Y.D.; Fotiadis, P.; Martinez-Ramirez, S.; Salat, D.H.; Schultz, A.; Shoamanesh, A.; Ayres, A.M.; Vashkevich, A.; Rosas, D.; Schwab, K.; et al. Structural network alterations and neurological dysfunction in cerebral amyloid angiopathy. Brain 2015, 138, 179–188. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Nandigam, R.N.; Delgado, P.; Betensky, R.A.; Rosand, J.; Viswanathan, A.; Frosch, M.P.; Smith, E.E. Microbleeds versus macrobleeds: Evidence for distinct entities. Stroke 2009, 40, 2382–2386. [Google Scholar] [CrossRef]
- Xiong, L.; Davidsdottir, S.; Reijmer, Y.D.; Shoamanesh, A.; Roongpiboonsopit, D.; Thanprasertsuk, S.; Martinez-Ramirez, S.; Charidimou, A.; Ayres, A.M.; Fotiadis, P.; et al. Cognitive Profile and its Association with Neuroimaging Markers of Non-Demented Cerebral Amyloid Angiopathy Patients in a Stroke Unit. J. Alzheimer’s Dis. 2016, 52, 171–178. [Google Scholar] [CrossRef]
- Case, N.F.; Charlton, A.; Zwiers, A.; Batool, S.; McCreary, C.R.; Hogan, D.B.; Ismail, Z.; Zerna, C.; Coutts, S.B.; Frayne, R.; et al. Cerebral Amyloid Angiopathy Is Associated With Executive Dysfunction and Mild Cognitive Impairment. Stroke 2016, 47, 2010–2016. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; van Veluw, S.J.; Bounemia, N.; Charidimou, A.; Pasi, M.; Boulouis, G.; Reijmer, Y.D.; Giese, A.K.; Davidsdottir, S.; Fotiadis, P.; et al. Cerebral Cortical Microinfarcts on Magnetic Resonance Imaging and Their Association With Cognition in Cerebral Amyloid Angiopathy. Stroke 2018, 49, 2330–2336. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Charidimou, A.; Pasi, M.; Boulouis, G.; Pongpitakmetha, T.; Schirmer, M.D.; Singh, S.; Benson, E.; Gurol, E.M.; Rosand, J.; et al. Predictors for Late Post-Intracerebral Hemorrhage Dementia in Patients with Probable Cerebral Amyloid Angiopathy. J. Alzheimer’s Dis. 2019, 71, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Brenowitz, W.D.; Nelson, P.T.; Besser, L.M.; Heller, K.B.; Kukull, W.A. Cerebral amyloid angiopathy and its co-occurrence with Alzheimer’s disease and other cerebrovascular neuropathologic changes. Neurobiol. Aging 2015, 36, 2702–2708. [Google Scholar] [CrossRef]
- Biffi, A.; Greenberg, S.M. Cerebral amyloid angiopathy: A systematic review. J. Clin. Neurol. 2011, 7, 1–9. [Google Scholar] [CrossRef]
- Theodorou, A.; Athanasaki, A.; Melanis, K.; Pachi, I.; Sterpi, A.; Koropouli, E.; Bakola, E.; Chondrogianni, M.; Stefanou, M.I.; Vasilopoulos, E.; et al. Cognitive Impairment in Cerebral Amyloid Angiopathy: A Single-Center Prospective Cohort Study. J. Clin. Med. 2024, 13, 7427. [Google Scholar] [CrossRef]
- Smith, E.E.; Charidimou, A.; Ayata, C.; Werring, D.J.; Greenberg, S.M. Cerebral Amyloid Angiopathy-Related Transient Focal Neurologic Episodes. Neurology 2021, 97, 231–238. [Google Scholar] [CrossRef]
- Safouris, A.; Gazagnes, M.D.; Triantafyllou, N.; Tsivgoulis, G. Cerebral amyloid angiopathy-associated microbleed mimicking transient ischemic attack. J. Neurol. Sci. 2015, 351, 198–199. [Google Scholar] [CrossRef]
- Theodorou, A.; Chondrogianni, M.; Bakola, E.; Kaloudi, G.; Foska, A.; Michalakakou, S.; Melanis, K.; Paraskevas, G.P.; Tsivgoulis, G. Cortical Superficial Siderosis and Transient Focal Neurological Episode Preceding Lobar Hemorrhage in Cerebral Amyloid Angiopathy. Stroke 2023, 54, e48–e51. [Google Scholar] [CrossRef]
- Charidimou, A.; Smith, E.E. Cardiovascular Management in Asymptomatic (Silent) Cerebral Microbleeds and Suspected Cerebral Amyloid Angiopathy. Stroke 2024, 55, 1101–1112. [Google Scholar] [CrossRef]
- Pasi, M.; Marini, S.; Morotti, A.; Boulouis, G.; Xiong, L.; Charidimou, A.; Ayres, A.M.; Lee, M.J.; Biffi, A.; Goldstein, J.N.; et al. Cerebellar Hematoma Location: Implications for the Underlying Microangiopathy. Stroke 2018, 49, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Rosand, J.; Muzikansky, A.; Kumar, A.; Wisco, J.J.; Smith, E.E.; Betensky, R.A.; Greenberg, S.M. Spatial clustering of hemorrhages in probable cerebral amyloid angiopathy. Ann. Neurol. 2005, 58, 459–462. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Samarasekera, N.; Lerpiniere, C.; Humphreys, C.; McCarron, M.O.; White, P.M.; Nicoll, J.A.R.; Sudlow, C.L.M.; Cordonnier, C.; Wardlaw, J.M.; et al. The Edinburgh CT and genetic diagnostic criteria for lobar intracerebral haemorrhage associated with cerebral amyloid angiopathy: Model development and diagnostic test accuracy study. Lancet Neurol. 2018, 17, 232–240. [Google Scholar] [CrossRef]
- Sembill, J.A.; Knott, M.; Xu, M.; Roeder, S.S.; Hagen, M.; Sprügel, M.I.; Mrochen, A.; Borutta, M.; Hoelter, P.; Engelhorn, T.; et al. Simplified Edinburgh CT Criteria for Identification of Lobar Intracerebral Hemorrhage Associated With Cerebral Amyloid Angiopathy. Neurology 2022, 98, e1997–e2004. [Google Scholar] [CrossRef] [PubMed]
- Charidimou, A.; Frosch, M.P.; Al-Shahi Salman, R.; Baron, J.C.; Cordonnier, C.; Hernandez-Guillamon, M.; Linn, J.; Raposo, N.; Rodrigues, M.; Romero, J.R.; et al. Advancing diagnostic criteria for sporadic cerebral amyloid angiopathy: Study protocol for a multicenter MRI-pathology validation of Boston criteria v2.0. Int. J. Stroke 2019, 14, 956–971. [Google Scholar] [CrossRef] [PubMed]
- Khurram, A.; Kleinig, T.; Leyden, J. Clinical associations and causes of convexity subarachnoid hemorrhage. Stroke 2014, 45, 1151–1153. [Google Scholar] [CrossRef]
- Forman, R.; Conners, J.J.; Song, S.Y.; John, S.; Garg, R.; Harris, J.; Lee, V.H. The Spectrum of Nontraumatic Convexity Subarachnoid Hemorrhage. J. Stroke Cerebrovasc. Dis. 2019, 28, 104473. [Google Scholar] [CrossRef]
- Stanton, J.E.D.; Chandratheva, A.; Wilson, D.; Hostettler, I.C.; Islam, S.; Werring, D.J. Clinical features distinguish cerebral amyloid angiopathy-associated convexity subarachnoid haemorrhage from suspected TIA. J. Neurol. 2020, 267, 133–137. [Google Scholar] [CrossRef]
- Charidimou, A.; Boulouis, G.; Fotiadis, P.; Xiong, L.; Ayres, A.M.; Schwab, K.M.; Gurol, M.E.; Rosand, J.; Greenberg, S.M.; Viswanathan, A. Acute convexity subarachnoid haemorrhage and cortical superficial siderosis in probable cerebral amyloid angiopathy without lobar haemorrhage. J. Neurol. Neurosurg. Psychiatry 2018, 89, 397–403. [Google Scholar] [CrossRef]
- Charidimou, A.; Meegahage, R.; Fox, Z.; Peeters, A.; Vandermeeren, Y.; Laloux, P.; Baron, J.C.; Jäger, H.R.; Werring, D.J. Enlarged perivascular spaces as a marker of underlying arteriopathy in intracerebral haemorrhage: A multicentre MRI cohort study. J. Neurol. Neurosurg. Psychiatry 2013, 84, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Charidimou, A.; Boulouis, G.; Haley, K.; Auriel, E.; van Etten, E.S.; Fotiadis, P.; Reijmer, Y.; Ayres, A.; Vashkevich, A.; Dipucchio, Z.Y.; et al. White matter hyperintensity patterns in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 2016, 86, 505–511. [Google Scholar] [CrossRef]
- van den Brink, H.; Zwiers, A.; Switzer, A.R.; Charlton, A.; McCreary, C.R.; Goodyear, B.G.; Frayne, R.; Biessels, G.J.; Smith, E.E. Cortical Microinfarcts on 3T Magnetic Resonance Imaging in Cerebral Amyloid Angiopathy. Stroke 2018, 49, 1899–1905. [Google Scholar] [CrossRef]
- Ishikawa, H.; Ii, Y.; Shindo, A.; Tabei, K.I.; Umino, M.; Ito, A.O.; Matsuura, K.; Taniguchi, A.; Matsuyama, H.; Niwa, A.; et al. Cortical Microinfarcts Detected by 3-Tesla Magnetic Resonance Imaging: Differentiation Between Cerebral Amyloid Angiopathy and Embolism. Stroke 2020, 51, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Pasi, M.; Pongpitakmetha, T.; Charidimou, A.; Singh, S.D.; Tsai, H.H.; Xiong, L.; Boulouis, G.; Warren, A.D.; Rosand, J.; Frosch, M.P.; et al. Cerebellar Microbleed Distribution Patterns and Cerebral Amyloid Angiopathy. Stroke 2019, 50, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.H.; Pasi, M.; Tsai, L.K.; Chen, Y.F.; Chen, Y.W.; Tang, S.C.; Gurol, M.E.; Yen, R.F.; Jeng, J.S. Superficial Cerebellar Microbleeds and Cerebral Amyloid Angiopathy: A Magnetic Resonance Imaging/Positron Emission Tomography Study. Stroke 2020, 51, 202–208. [Google Scholar] [CrossRef]
- Das, A.S.; Abramovitz Fouks, A.; Gökçal, E.; Rotschild, O.; Pasi, M.; Regenhardt, R.W.; Goldstein, J.N.; Viswanathan, A.; Rosand, J.; Greenberg, S.M.; et al. Characterizing the underlying microangiopathy of deep cerebellar intracerebral hemorrhage. J. Neurol. 2025, 272, 167. [Google Scholar] [CrossRef]
- Charidimou, A.; Friedrich, J.O.; Greenberg, S.M.; Viswanathan, A. Core cerebrospinal fluid biomarker profile in cerebral amyloid angiopathy: A meta-analysis. Neurology 2018, 90, e754–e762. [Google Scholar] [CrossRef]
- Charidimou, A.; Boulouis, G. Core CSF Biomarker Profile in Cerebral Amyloid Angiopathy: Updated Meta-Analysis. Neurology 2024, 103, e209795. [Google Scholar] [CrossRef]
- Margraf, N.G.; Jensen-Kondering, U.; Weiler, C.; Leypoldt, F.; Maetzler, W.; Philippen, S.; Bartsch, T.; Flüh, C.; Röcken, C.; Möller, B.; et al. Cerebrospinal Fluid Biomarkers in Cerebral Amyloid Angiopathy: New Data and Quantitative Meta-Analysis. Front. Aging Neurosci. 2022, 14, 783996. [Google Scholar] [CrossRef]
- Verbeek, M.M.; Kremer, B.P.; Rikkert, M.O.; Van Domburg, P.H.; Skehan, M.E.; Greenberg, S.M. Cerebrospinal fluid amyloid beta(40) is decreased in cerebral amyloid angiopathy. Ann. Neurol. 2009, 66, 245–249. [Google Scholar] [CrossRef]
- De Kort, A.M.; Kuiperij, H.B.; Marques, T.M.; Jäkel, L.; van den Berg, E.; Kersten, I.; van Berckel-Smit, H.E.P.; Duering, M.; Stoops, E.; Abdo, W.F.; et al. Decreased Cerebrospinal Fluid Amyloid β 38, 40, 42, and 43 Levels in Sporadic and Hereditary Cerebral Amyloid Angiopathy. Ann. Neurol. 2023, 93, 1173–1186. [Google Scholar] [CrossRef] [PubMed]
- van Etten, E.S.; Verbeek, M.M.; van der Grond, J.; Zielman, R.; van Rooden, S.; van Zwet, E.W.; van Opstal, A.M.; Haan, J.; Greenberg, S.M.; van Buchem, M.A.; et al. β-Amyloid in CSF: Biomarker for preclinical cerebral amyloid angiopathy. Neurology 2017, 88, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.F.; Tsai, H.H.; Liu, C.J.; Lee, B.C.; Tsai, Y.C.; Yen, R.F.; Jeng, J.S.; Tsai, L.K. Plasma Phosphorylated Tau 217 as a Discriminative Biomarker for Cerebral Amyloid Angiopathy. Eur. J. Neurol. 2025, 32, e70066. [Google Scholar] [CrossRef] [PubMed]
- Farid, K.; Charidimou, A.; Baron, J.C. Amyloid positron emission tomography in sporadic cerebral amyloid angiopathy: A systematic critical update. NeuroImage Clin. 2017, 15, 247–263. [Google Scholar] [CrossRef]
- Kuo, P.Y.; Tsai, H.H.; Lee, B.C.; Chiang, P.T.; Liu, C.J.; Chen, Y.F.; Jeng, J.S.; Yen, R.F.; Tsai, L.K. Differences in lobar microbleed topography in cerebral amyloid angiopathy and hypertensive arteriopathy. Sci. Rep. 2024, 14, 3774. [Google Scholar] [CrossRef]
- Tsai, H.H.; Liu, C.J.; Lee, B.C.; Chen, Y.F.; Yen, R.F.; Jeng, J.S.; Tsai, L.K. Cerebral tau pathology in cerebral amyloid angiopathy. Brain Commun. 2024, 6, fcae086. [Google Scholar] [CrossRef]
- Okine, D.N.; Knopman, D.S.; Mosley, T.H.; Wong, D.F.; Johansen, M.C.; Walker, K.A.; Jack, C.R., Jr.; Kantarci, K.; Pike, J.R.; Graff-Radford, J.; et al. Cerebral Microbleed Patterns and Cortical Amyloid-beta: The ARIC-PET Study. Stroke 2023, 54, 2613–2620. [Google Scholar] [CrossRef]
- Jo, S.; Cheong, E.N.; Kim, N.; Oh, J.S.; Shim, W.H.; Kim, H.J.; Lee, S.J.; Lee, Y.; Oh, M.; Kim, J.S.; et al. Role of White Matter Abnormalities in the Relationship Between Microbleed Burden and Cognitive Impairment in Cerebral Amyloid Angiopathy. J. Alzheimer’s Dis. 2022, 86, 667–678. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, J.; Wang, L.; Li, X.; Wang, Z.; Lin, M.; Jin, W.; Zhu, M.; Xu, B. Diagnostic Utility of Integrated(11)C-Pittsburgh Compound B Positron Emission Tomography/Magnetic Resonance for Cerebral Amyloid Angiopathy: A Pilot Study. Front. Aging Neurosci. 2021, 13, 721780. [Google Scholar] [CrossRef]
- Planton, M.; Pariente, J.; Nemmi, F.; Albucher, J.F.; Calviere, L.; Viguier, A.; Olivot, J.M.; Salabert, A.S.; Payoux, P.; Peran, P.; et al. Interhemispheric distribution of amyloid and small vessel disease burden in cerebral amyloid angiopathy-related intracerebral hemorrhage. Eur. J. Neurol. 2020, 27, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Planton, M.; Saint-Aubert, L.; Raposo, N.; Payoux, P.; Salabert, A.S.; Albucher, J.F.; Olivot, J.M.; Peran, P.; Pariente, J. Florbetapir Regional Distribution in Cerebral Amyloid Angiopathy and Alzheimer’s Disease: A PET Study. J. Alzheimer’s Dis. 2020, 73, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Jang, Y.K.; Kim, H.J.; Werring, D.J.; Lee, J.S.; Choe, Y.S.; Park, S.; Lee, J.; Kim, K.W.; Kim, Y.; et al. Clinical significance of amyloid beta positivity in patients with probable cerebral amyloid angiopathy markers. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1287–1298. [Google Scholar] [CrossRef]
- Tsai, H.H.; Pasi, M.; Tsai, L.K.; Chen, Y.F.; Lee, B.C.; Tang, S.C.; Fotiadis, P.; Huang, C.Y.; Yen, R.F.; Jeng, J.S.; et al. Microangiopathy underlying mixed-location intracerebral hemorrhages/microbleeds: A PiB-PET study. Neurology 2019, 92, e774–e781. [Google Scholar] [CrossRef] [PubMed]
- Raposo, N.; Planton, M.; Peran, P.; Payoux, P.; Bonneville, F.; Lyoubi, A.; Albucher, J.F.; Acket, B.; Salabert, A.S.; Olivot, J.M.; et al. Florbetapir imaging in cerebral amyloid angiopathy-related hemorrhages. Neurology 2017, 89, 697–704. [Google Scholar] [CrossRef]
- Tsai, H.H.; Tsai, L.K.; Chen, Y.F.; Tang, S.C.; Lee, B.C.; Yen, R.F.; Jeng, J.S. Correlation of Cerebral Microbleed Distribution to Amyloid Burden in Patients with Primary Intracerebral Hemorrhage. Sci. Rep. 2017, 7, 44715. [Google Scholar] [CrossRef]
- Gurol, M.E.; Becker, J.A.; Fotiadis, P.; Riley, G.; Schwab, K.; Johnson, K.A.; Greenberg, S.M. Florbetapir-PET to diagnose cerebral amyloid angiopathy: A prospective study. Neurology 2016, 87, 2043–2049. [Google Scholar] [CrossRef]
- Baron, J.C.; Farid, K.; Dolan, E.; Turc, G.; Marrapu, S.T.; O’Brien, E.; Aigbirhio, F.I.; Fryer, T.D.; Menon, D.K.; Warburton, E.A.; et al. Diagnostic utility of amyloid PET in cerebral amyloid angiopathy-related symptomatic intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 2014, 34, 753–758. [Google Scholar] [CrossRef]
- Gurol, M.E.; Viswanathan, A.; Gidicsin, C.; Hedden, T.; Martinez-Ramirez, S.; Dumas, A.; Vashkevich, A.; Ayres, A.M.; Auriel, E.; van Etten, E.; et al. Cerebral amyloid angiopathy burden associated with leukoaraiosis: A positron emission tomography/magnetic resonance imaging study. Ann. Neurol. 2013, 73, 529–536. [Google Scholar] [CrossRef]
- Ly, J.V.; Donnan, G.A.; Villemagne, V.L.; Zavala, J.A.; Ma, H.; O’Keefe, G.; Gong, S.J.; Gunawan, R.M.; Saunder, T.; Ackerman, U.; et al. 11C-PIB binding is increased in patients with cerebral amyloid angiopathy-related hemorrhage. Neurology 2010, 74, 487–493. [Google Scholar] [CrossRef]
- Johnson, K.A.; Gregas, M.; Becker, J.A.; Kinnecom, C.; Salat, D.H.; Moran, E.K.; Smith, E.E.; Rosand, J.; Rentz, D.M.; Klunk, W.E.; et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann. Neurol. 2007, 62, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Vinters, H.V.; Gilbert, J.J. Cerebral amyloid angiopathy: Incidence and complications in the aging brain. II. The distribution of amyloid vascular changes. Stroke 1983, 14, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Sin, M.K.; Cheng, Y.; Ahmed, A.; Roseman, J.M.; Dowling, N.M.; Zamrini, E. Cerebral Amyloid Angiopathy, Dementia, and Alzheimer Neuropathologic Changes: Findings From the ACT Autopsy Cohort. Neurology 2024, 103, e210009. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Seo, S.W.; Kim, C.; Kim, G.H.; Noh, H.J.; Kim, S.T.; Kwak, K.C.; Yoon, U.; Lee, J.M.; Lee, J.W.; et al. Pathogenesis of cerebral microbleeds: In vivo imaging of amyloid and subcortical ischemic small vessel disease in 226 individuals with cognitive impairment. Ann. Neurol. 2013, 73, 584–593. [Google Scholar] [CrossRef]
- Chawla, A.; Boisvert, W.A.; Lee, C.H.; Laffitte, B.A.; Barak, Y.; Joseph, S.B.; Liao, D.; Nagy, L.; Edwards, P.A.; Curtiss, L.K.; et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell 2001, 7, 161–171. [Google Scholar] [CrossRef]
- Tzourio, C.; Arima, H.; Harrap, S.; Anderson, C.; Godin, O.; Woodward, M.; Neal, B.; Bousser, M.G.; Chalmers, J.; Cambien, F.; et al. APOE genotype, ethnicity, and the risk of cerebral hemorrhage. Neurology 2008, 70, 1322–1328. [Google Scholar] [CrossRef]
- Eisenberg, D.T.; Kuzawa, C.W.; Hayes, M.G. Worldwide allele frequencies of the human apolipoprotein E gene: Climate, local adaptations, and evolutionary history. Am. J. Phys. Anthropol. 2010, 143, 100–111. [Google Scholar] [CrossRef]
- Castellano, J.M.; Kim, J.; Stewart, F.R.; Jiang, H.; DeMattos, R.B.; Patterson, B.W.; Fagan, A.M.; Morris, J.C.; Mawuenyega, K.G.; Cruchaga, C.; et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 2011, 3, 89ra57. [Google Scholar] [CrossRef]
- Montagne, A.; Nation, D.A.; Sagare, A.P.; Barisano, G.; Sweeney, M.D.; Chakhoyan, A.; Pachicano, M.; Joe, E.; Nelson, A.R.; D’Orazio, L.M.; et al. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature 2020, 581, 71–76. [Google Scholar] [CrossRef]
- Bales, K.R.; Liu, F.; Wu, S.; Lin, S.; Koger, D.; DeLong, C.; Hansen, J.C.; Sullivan, P.M.; Paul, S.M. Human APOE isoform-dependent effects on brain beta-amyloid levels in PDAPP transgenic mice. J. Neurosci. 2009, 29, 6771–6779. [Google Scholar] [CrossRef]
- Rannikmäe, K.; Samarasekera, N.; Martînez-Gonzâlez, N.A.; Al-Shahi Salman, R.; Sudlow, C.L. Genetics of cerebral amyloid angiopathy: Systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2013, 84, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Biffi, A.; Anderson, C.D.; Jagiella, J.M.; Schmidt, H.; Kissela, B.; Hansen, B.M.; Jimenez-Conde, J.; Pires, C.R.; Ayres, A.M.; Schwab, K.; et al. APOE genotype and extent of bleeding and outcome in lobar intracerebral haemorrhage: A genetic association study. Lancet Neurol. 2011, 10, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Hostettler, I.C.; Seiffge, D.; Wong, A.; Ambler, G.; Wilson, D.; Shakeshaft, C.; Banerjee, G.; Sharma, N.; Jäger, H.R.; Cohen, H.; et al. and Cerebral Small Vessel Disease Markers in Patients With Intracerebral Hemorrhage. Neurology 2022, 99, e1290–e1298. [Google Scholar] [CrossRef]
- Rannikmäe, K.; Kalaria, R.N.; Greenberg, S.M.; Chui, H.C.; Schmitt, F.A.; Samarasekera, N.; Al-Shahi Salman, R.; Sudlow, C.L. APOE associations with severe CAA-associated vasculopathic changes: Collaborative meta-analysis. J. Neurol. Neurosurg. Psychiatry 2014, 85, 300–305. [Google Scholar] [CrossRef]
- Paradela, R.S.; Justo, A.F.O.; Paes, V.R.; Leite, R.E.P.; Pasqualucci, C.A.; Grinberg, L.T.; Naslavsky, M.S.; Zatz, M.; Nitrini, R.; Jacob-Filho, W.; et al. Association between APOE-ε4 allele and cognitive function is mediated by Alzheimer’s disease pathology: A population-based autopsy study in an admixed sample. Acta Neuropathol. Commun. 2023, 11, 205. [Google Scholar] [CrossRef] [PubMed]
- Salloway, S.; Chalkias, S.; Barkhof, F.; Burkett, P.; Barakos, J.; Purcell, D.; Suhy, J.; Forrestal, F.; Tian, Y.; Umans, K.; et al. Amyloid-Related Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients With Early Alzheimer Disease. JAMA Neurol. 2022, 79, 13–21. [Google Scholar] [CrossRef]
- Salloway, S.; Wojtowicz, J.; Voyle, N.; Lane, C.A.; Klein, G.; Lyons, M.; Rossomanno, S.; Mazzo, F.; Bullain, S.; Barkhof, F.; et al. Amyloid-Related Imaging Abnormalities (ARIA) in Clinical Trials of Gantenerumab in Early Alzheimer Disease. JAMA Neurol. 2025, 82, 19–29. [Google Scholar] [CrossRef]
- Charidimou, A.; Zonneveld, H.I.; Shams, S.; Kantarci, K.; Shoamanesh, A.; Hilal, S.; Yates, P.A.; Boulouis, G.; Na, H.K.; Pasi, M.; et al. and cortical superficial siderosis in CAA: Meta-analysis and potential mechanisms. Neurology 2019, 93, e358–e371. [Google Scholar] [CrossRef]
- Kantarci, K.; Lowe, V.; Przybelski, S.A.; Weigand, S.D.; Senjem, M.L.; Ivnik, R.J.; Preboske, G.M.; Roberts, R.; Geda, Y.E.; Boeve, B.F.; et al. APOE modifies the association between Aβ load and cognition in cognitively normal older adults. Neurology 2012, 78, 232–240. [Google Scholar] [CrossRef]
- van Dort, R.; Kaushik, K.; Rasing, I.; van der Zwet, R.G.J.; Schipper, M.R.; van der Grond, J.; van Rooden, S.; van Zwet, E.W.; Terwindt, G.M.; Middelkoop, H.A.M.; et al. Cognition in (pre)symptomatic Dutch-type hereditary and sporadic cerebral amyloid angiopathy. Alzheimer’s Dement. 2024, 20, 7518–7528. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and Management of Dementia: Review. JAMA 2019, 322, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.E.; Cieslak, A.; Barber, P.; Chen, J.; Chen, Y.W.; Donnini, I.; Edwards, J.D.; Frayne, R.; Field, T.S.; Hegedus, J.; et al. Therapeutic Strategies and Drug Development for Vascular Cognitive Impairment. J. Am. Heart Assoc. 2017, 6, e005568. [Google Scholar] [CrossRef] [PubMed]
- Battle, C.E.; Abdul-Rahim, A.H.; Shenkin, S.D.; Hewitt, J.; Quinn, T.J. Cholinesterase inhibitors for vascular dementia and other vascular cognitive impairments: A network meta-analysis. Cochrane Database Syst. Rev. 2021, 2, CD013306. [Google Scholar] [CrossRef] [PubMed]
- Paterson, R.W.; Abdi, Z.; Haines, A.; Schott, J.M. Significant cognitive improvement with cholinesterase inhibition in AD with cerebral amyloid angiopathy. Clin. Neurol. Neurosurg. 2016, 144, 64–66. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Ziai, W.C.; Cordonnier, C.; Dowlatshahi, D.; Francis, B.; Goldstein, J.N.; Hemphill, J.C.; Johnson, R.; Keigher, K.M.; Mack, W.J.; et al. 2022 Guideline for the Management of Patients With Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 2022, 53, e282–e361. [Google Scholar] [CrossRef]
- Shoamanesh, A.; Patrice Lindsay, M.; Castellucci, L.A.; Cayley, A.; Crowther, M.; de Wit, K.; English, S.W.; Hoosein, S.; Huynh, T.; Kelly, M.; et al. Canadian stroke best practice recommendations. Int. J. Stroke 2021, 16, 321–341. [Google Scholar] [CrossRef]
- Sprügel, M.I.; Kuramatsu, J.B.; Gerner, S.T.; Sembill, J.A.; Beuscher, V.D.; Hagen, M.; Roeder, S.S.; Lücking, H.; Struffert, T.; Dörfler, A.; et al. Antiplatelet Therapy in Primary Spontaneous and Oral Anticoagulation-Associated Intracerebral Hemorrhage. Stroke 2018, 49, 2621–2629. [Google Scholar] [CrossRef] [PubMed]
- Law, Z.K.; Desborough, M.; Roberts, I.; Al-Shahi Salman, R.; England, T.J.; Werring, D.J.; Robinson, T.; Krishnan, K.; Dineen, R.; Laska, A.C.; et al. Outcomes in Antiplatelet-Associated Intracerebral Hemorrhage in the TICH-2 Randomized Controlled Trial. J. Am. Heart Assoc. 2021, 10, e019130. [Google Scholar] [CrossRef]
- Hanger, H.C.; Geddes, J.A.; Wilkinson, T.J.; Lee, M.; Baker, A.E. Warfarin-related intracerebral haemorrhage: Better outcomes when reversal includes prothrombin complex concentrates. Intern. Med. J. 2013, 43, 308–316. [Google Scholar] [CrossRef]
- Connolly, S.J.; Crowther, M.; Eikelboom, J.W.; Gibson, C.M.; Curnutte, J.T.; Lawrence, J.H.; Yue, P.; Bronson, M.D.; Lu, G.; Conley, P.B.; et al. Full Study Report of Andexanet Alfa for Bleeding Associated with Factor Xa Inhibitors. N. Engl. J. Med. 2019, 380, 1326–1335. [Google Scholar] [CrossRef]
- Demchuk, A.M.; Yue, P.; Zotova, E.; Nakamya, J.; Xu, L.; Milling, T.J.; Ohara, T.; Goldstein, J.N.; Middeldorp, S.; Verhamme, P.; et al. Hemostatic Efficacy and Anti-FXa (Factor Xa) Reversal With Andexanet Alfa in Intracranial Hemorrhage: ANNEXA-4 Substudy. Stroke 2021, 52, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Pollack, C.V.; Reilly, P.A.; van Ryn, J.; Eikelboom, J.W.; Glund, S.; Bernstein, R.A.; Dubiel, R.; Huisman, M.V.; Hylek, E.M.; Kam, C.W.; et al. Idarucizumab for Dabigatran Reversal—Full Cohort Analysis. N. Engl. J. Med. 2017, 377, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.; Chan, A.; Atallah, S.; Derry, K.; Baje, M.; Zimmermann, L.L.; Martin, R.; Groysman, L.; Stern-Nezer, S.; Minokadeh, A.; et al. Treatment of adults with intracranial hemorrhage on apixaban or rivaroxaban with prothrombin complex concentrate products. J. Thromb. Thrombolysis 2021, 51, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Arima, H.; Heeley, E.; Delcourt, C.; Huang, Y.; Wang, J.; Stapf, C.; Robinson, T.; Woodward, M.; Chalmers, J.; et al. Magnitude of blood pressure reduction and clinical outcomes in acute intracerebral hemorrhage: Intensive blood pressure reduction in acute cerebral hemorrhage trial study. Hypertension 2015, 65, 1026–1032. [Google Scholar] [CrossRef]
- Moullaali, T.J.; Wang, X.; Martin, R.H.; Shipes, V.B.; Robinson, T.G.; Chalmers, J.; Suarez, J.I.; Qureshi, A.I.; Palesch, Y.Y.; Anderson, C.S. Blood pressure control and clinical outcomes in acute intracerebral haemorrhage: A preplanned pooled analysis of individual participant data. Lancet Neurol. 2019, 18, 857–864. [Google Scholar] [CrossRef]
- Li, Q.; Warren, A.D.; Qureshi, A.I.; Morotti, A.; Falcone, G.J.; Sheth, K.N.; Shoamanesh, A.; Dowlatshahi, D.; Viswanathan, A.; Goldstein, J.N. Ultra-Early Blood Pressure Reduction Attenuates Hematoma Growth and Improves Outcome in Intracerebral Hemorrhage. Ann. Neurol. 2020, 88, 388–395. [Google Scholar] [CrossRef]
- Shah, M.; Birnbaum, L.; Rasmussen, J.; Sekar, P.; Moomaw, C.J.; Osborne, J.; Vashkevich, A.; Woo, D. Effect of Hyperosmolar Therapy on Outcome Following Spontaneous Intracerebral Hemorrhage: Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) Study. J. Stroke Cerebrovasc. Dis. 2018, 27, 1061–1067. [Google Scholar] [CrossRef]
- Sun, S.; Li, Y.; Zhang, H.; Wang, X.; She, L.; Yan, Z.; Lu, G. The effect of mannitol in the early stage of supratentorial hypertensive intracerebral hemorrhage: A systematic review and meta-analysis. World Neurosurg. 2018, 124, 386–396. [Google Scholar] [CrossRef]
- Middleton, S.; McElduff, P.; Ward, J.; Grimshaw, J.M.; Dale, S.; D’Este, C.; Drury, P.; Griffiths, R.; Cheung, N.W.; Quinn, C.; et al. Implementation of evidence-based treatment protocols to manage fever, hyperglycaemia, and swallowing dysfunction in acute stroke (QASC): A cluster randomised controlled trial. Lancet 2011, 378, 1699–1706. [Google Scholar] [CrossRef]
- Yogendrakumar, V.; Lun, R.; Khan, F.; Salottolo, K.; Lacut, K.; Graham, C.; Dennis, M.; Hutton, B.; Wells, P.S.; Fergusson, D.; et al. Venous thromboembolism prevention in intracerebral hemorrhage: A systematic review and network meta-analysis. PLoS ONE 2020, 15, e0234957. [Google Scholar] [CrossRef]
- Sandset, E.C.; Anderson, C.S.; Bath, P.M.; Christensen, H.; Fischer, U.; Gąsecki, D.; Lal, A.; Manning, L.S.; Sacco, S.; Steiner, T.; et al. European Stroke Organisation (ESO) guidelines on blood pressure management in acute ischaemic stroke and intracerebral haemorrhage. Eur. Stroke J. 2021, 6, XLVIII. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Zusman, B.E.; Shutter, L.A.; Choxi, R.; Yassin, A.; Antony, A.; Thirumala, P.D. The Prevalence and Impact of Status Epilepticus Secondary to Intracerebral Hemorrhage: Results from the US Nationwide Inpatient Sample. Neurocrit. Care 2018, 28, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Vespa, P.M.; O’Phelan, K.; Shah, M.; Mirabelli, J.; Starkman, S.; Kidwell, C.; Saver, J.; Nuwer, M.R.; Frazee, J.G.; McArthur, D.A.; et al. Acute seizures after intracerebral hemorrhage: A factor in progressive midline shift and outcome. Neurology 2003, 60, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Pan, C.; Guo, W.; Bai, S.; Nie, H.; Feng, Y.; Li, G.; Deng, H.; Ma, Y.; Zhu, S.; et al. Efficacy and safety of four interventions for spontaneous supratentorial intracerebral hemorrhage: A network meta-analysis. J. Neurointerv. Surg. 2020, 12, 598–604. [Google Scholar] [CrossRef]
- Sondag, L.; Schreuder, F.H.B.M.; Boogaarts, H.D.; Rovers, M.M.; Vandertop, W.P.; Dammers, R.; Klijn, C.J.M.; Dutch ICH Surgery Trial Study Group, p.o.t.C.c. Neurosurgical Intervention for Supratentorial Intracerebral Hemorrhage. Ann. Neurol. 2020, 88, 239–250. [Google Scholar] [CrossRef]
- Raposo, N.; Charidimou, A.; Roongpiboonsopit, D.; Onyekaba, M.; Gurol, M.E.; Rosand, J.; Greenberg, S.M.; Goldstein, J.N.; Viswanathan, A. Convexity subarachnoid hemorrhage in lobar intracerebral hemorrhage: A prognostic marker. Neurology 2020, 94, e968–e977. [Google Scholar] [CrossRef]
- Charidimou, A.; Boulouis, G.; Greenberg, S.M.; Viswanathan, A. Cortical superficial siderosis and bleeding risk in cerebral amyloid angiopathy: A meta-analysis. Neurology 2019, 93, e2192–e2202. [Google Scholar] [CrossRef]
- Islam, M.M.; Poly, T.N.; Walther, B.A.; Yang, H.C.; Lin, M.C.; Li, Y.C. Risk of Hemorrhagic Stroke in Patients Exposed to Nonsteroidal Anti-Inflammatory Drugs: A Meta-Analysis of Observational Studies. Neuroepidemiology 2018, 51, 166–176. [Google Scholar] [CrossRef]
- Ungprasert, P.; Matteson, E.L.; Thongprayoon, C. Nonaspirin Nonsteroidal Anti-Inflammatory Drugs and Risk of Hemorrhagic Stroke: A Systematic Review and Meta-Analysis of Observational Studies. Stroke 2016, 47, 356–364. [Google Scholar] [CrossRef]
- Goldstein, L.B.; Toth, P.P.; Dearborn-Tomazos, J.L.; Giugliano, R.P.; Hirsh, B.J.; Peña, J.M.; Selim, M.H.; Woo, D.; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; and Stroke Council. Aggressive LDL-C Lowering and the Brain: Impact on Risk for Dementia and Hemorrhagic Stroke: A Scientific Statement From the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2023, 43, e404–e442. [Google Scholar] [CrossRef]
- Amarenco, P.; Bogousslavsky, J.; Callahan, A.; Goldstein, L.B.; Hennerici, M.; Rudolph, A.E.; Sillesen, H.; Simunovic, L.; Szarek, M.; Welch, K.M.; et al. High-dose atorvastatin after stroke or transient ischemic attack. N. Engl. J. Med. 2006, 355, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.C.; Leung, W.C.; Ho, C.; Chiu, M.W.; Leung, I.Y.; Wong, Y.K.; Roxanna, L.K.; Sum, C.H.; Lui, D.T.; Cheung, R.T.; et al. Association of LDL-cholesterol <1.8 mmol/L and statin use with the recurrence of intracerebral hemorrhage. Int. J. Stroke 2024, 19, 695–704. [Google Scholar] [CrossRef]
- Palaiodimou, L.; Theodorou, A.; Lachanis, S.; Paraskevas, G.P.; Papathanasiou, M.; Zompola, C.; Voumvourakis, K.I.; Tsivgoulis, G. Stopping “transient ischemic attacks” by antiplatelet withdrawal. Neurol. Res. Pract. 2021, 3, 19. [Google Scholar] [CrossRef]
- Tsivgoulis, G.; Zand, R.; Katsanos, A.H.; Turc, G.; Nolte, C.H.; Jung, S.; Cordonnier, C.; Fiebach, J.B.; Scheitz, J.F.; Klinger-Gratz, P.P.; et al. Risk of Symptomatic Intracerebral Hemorrhage After Intravenous Thrombolysis in Patients With Acute Ischemic Stroke and High Cerebral Microbleed Burden: A Meta-analysis. JAMA Neurol. 2016, 73, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Charidimou, A.; Turc, G.; Oppenheim, C.; Yan, S.; Scheitz, J.F.; Erdur, H.; Klinger-Gratz, P.P.; El-Koussy, M.; Takahashi, W.; Moriya, Y.; et al. Microbleeds, Cerebral Hemorrhage, and Functional Outcome After Stroke Thrombolysis. Stroke 2017, 48, 2084–2090. [Google Scholar] [CrossRef] [PubMed]
- Berge, E.; Whiteley, W.; Audebert, H.; De Marchis, G.M.; Fonseca, A.C.; Padiglioni, C.; de la Ossa, N.P.; Strbian, D.; Tsivgoulis, G.; Turc, G. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur. Stroke J. 2021, 6, I. [Google Scholar] [CrossRef]
- Schlemm, L.; Braemswig, T.B.; Boutitie, F.; Vynckier, J.; Jensen, M.; Galinovic, I.; Simonsen, C.Z.; Cheng, B.; Cho, T.H.; Fiehler, J.; et al. Cerebral Microbleeds and Treatment Effect of Intravenous Thrombolysis in Acute Stroke: An Analysis of the WAKE-UP Randomized Clinical Trial. Neurology 2022, 98, e302–e314. [Google Scholar] [CrossRef]
- Gattringer, T.; Eppinger, S.; Beitzke, M.; Wuensch, G.; Niederkorn, K.; Deutschmann, H.; Fazekas, F.; Enzinger, C. Cortical Superficial Siderosis and Risk of Bleeding after Thrombolysis for Ischemic Stroke. Cerebrovasc. Dis. 2015, 40, 191–197. [Google Scholar] [CrossRef]
- Prats-Sánchez, L.; Camps-Renom, P.; Sotoca-Fernández, J.; Delgado-Mederos, R.; Martínez-Domeño, A.; Marín, R.; Almendrote, M.; Dorado, L.; Gomis, M.; Codas, J.; et al. Remote Intracerebral Hemorrhage After Intravenous Thrombolysis: Results From a Multicenter Study. Stroke 2016, 47, 2003–2009. [Google Scholar] [CrossRef]
- Weller, J.M.; Hattingen, E.; Petzold, G.C.; Bode, F.J. Successful mechanical thrombectomy in stroke with thrombolysis-associated intracerebral hemorrhage-a case report. J. Stroke Cerebrovasc. Dis. 2019, 28, 285–287. [Google Scholar] [CrossRef]
- Weller, J.M.; Enkirch, S.J.; Bogs, C.; Braemswig, T.B.; Deb-Chatterji, M.; Keil, F.; Kindler, C.; Maywald, S.; Schirmer, M.D.; Stösser, S.; et al. Endovascular Treatment for Acute Stroke in Cerebral Amyloid Angiopathy. Stroke 2021, 52, e581–e585. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.S.; Duckwiler, G.R.; Jahan, R.; Tateshima, S.; Gonzalez, N.R.; Szeder, V.; Saver, J.L.; Kim, D.; Ali, L.K.; Starkman, S.; et al. Mechanical thrombectomy for acute ischemic stroke with cerebral microbleeds. J. Neurointerv. Surg. 2016, 8, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Shoamanesh, A.; Pearce, L.A.; Bazan, C.; Catanese, L.; McClure, L.A.; Sharma, M.; Marti-Fabregas, J.; Anderson, D.C.; Kase, C.S.; Hart, R.G.; et al. Microbleeds in the Secondary Prevention of Small Subcortical Strokes Trial: Stroke, mortality, and treatment interactions. Ann. Neurol. 2017, 82, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Shoamanesh, A.; Hart, R.G.; Connolly, S.J.; Kasner, S.E.; Smith, E.E.; Martí-Fàbregas, J.; Liu, Y.Y.; Uchiyama, S.; Mikulik, R.; Veltkamp, R.; et al. Microbleeds and the Effect of Anticoagulation in Patients With Embolic Stroke of Undetermined Source: An Exploratory Analysis of the NAVIGATE ESUS Randomized Clinical Trial. JAMA Neurol. 2021, 78, 11–20. [Google Scholar] [CrossRef]
- Chabriat, H.; Maeder, P.; Gass, A.; Michel, P.; Bracoud, L.; Hennerici, M. Results of the PERFORM magnetic resonance imaging study. J. Neurol. 2013, 260, 3071–3076. [Google Scholar] [CrossRef]
- Martí-Fàbregas, J.; Camps-Renom, P.; Best, J.G.; Ramos-Pachon, A.; Guasch-Jiménez, M.; Martinez-Domeño, A.; Guisado-Alonso, D.; Gómez-Ansón, B.M.; Ambler, G.; Wilson, D.; et al. Stroke Risk and Antithrombotic Treatment During Follow-up of Patients With Ischemic Stroke and Cortical Superficial Siderosis. Neurology 2023, 100, e1267–e1281. [Google Scholar] [CrossRef]
- Shoamanesh, A.; Catanese, L.; Sakai, O.; Pikula, A.; Kase, C.S. Diffusion-weighted imaging hyperintensities in intracerebral hemorrhage: Microinfarcts or microbleeds? Ann. Neurol. 2013, 73, 795–796. [Google Scholar] [CrossRef]
- van Veluw, S.J.; Lauer, A.; Charidimou, A.; Bounemia, N.; Xiong, L.; Boulouis, G.; Fotiadis, P.; Ayres, A.; Gurol, M.E.; Viswanathan, A.; et al. Evolution of DWI lesions in cerebral amyloid angiopathy: Evidence for ischemia. Neurology 2017, 89, 2136–2142. [Google Scholar] [CrossRef]
- Ii, Y.; Maeda, M.; Ishikawa, H.; Ito, A.; Matsuo, K.; Umino, M.; Shindo, A.; Kida, H.; Satoh, M.; Niwa, A.; et al. Cortical microinfarcts in patients with multiple lobar microbleeds on 3 T MRI. J. Neurol. 2019, 266, 1887–1896. [Google Scholar] [CrossRef]
- Yao, X.; Abraham, N.S.; Sangaralingham, L.R.; Bellolio, M.F.; McBane, R.D.; Shah, N.D.; Noseworthy, P.A. Effectiveness and Safety of Dabigatran, Rivaroxaban, and Apixaban Versus Warfarin in Nonvalvular Atrial Fibrillation. J. Am. Heart Assoc. 2016, 5, e003725. [Google Scholar] [CrossRef]
- Connolly, S.J.; Eikelboom, J.; Joyner, C.; Diener, H.C.; Hart, R.; Golitsyn, S.; Flaker, G.; Avezum, A.; Hohnloser, S.H.; Diaz, R.; et al. Apixaban in patients with atrial fibrillation. N. Engl. J. Med. 2011, 364, 806–817. [Google Scholar] [CrossRef]
- Charidimou, A.; Perosa, V.; Frosch, M.P.; Scherlek, A.A.; Greenberg, S.M.; van Veluw, S.J. Neuropathological correlates of cortical superficial siderosis in cerebral amyloid angiopathy. Brain 2020, 143, 3343–3351. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.; Barnes, G.D. Direct Oral Anticoagulants Versus Vitamin K Antagonist in Elderly Patients With Atrial Fibrillation: Sometimes Less Is More, but Sometimes More Is More. J. Am. Heart Assoc. 2023, 12, e032127. [Google Scholar] [CrossRef]
- Hayes, K.N.; Zhang, T.; Kim, D.H.; Daiello, L.A.; Lee, Y.; Kiel, D.P.; Berry, S.D.; Zullo, A.R. Benefits and Harms of Standard Versus Reduced-Dose Direct Oral Anticoagulant Therapy for Older Adults With Multiple Morbidities and Atrial Fibrillation. J. Am. Heart Assoc. 2023, 12, e029865. [Google Scholar] [CrossRef]
- Fandler-Höfler, S.; Obergottsberger, L.; Ambler, G.; Eppinger, S.; Wünsch, G.; Kneihsl, M.; Seiffge, D.; Banerjee, G.; Wilson, D.; Nash, P.; et al. Association of the Presence and Pattern of MRI Markers of Cerebral Small Vessel Disease With Recurrent Intracerebral Hemorrhage. Neurology 2023, 101, e794–e804. [Google Scholar] [CrossRef] [PubMed]
- Al-Shahi Salman, R.; Stephen, J.; Tierney, J.F.; Lewis, S.C.; Newby, D.E.; Parry-Jones, A.R.; White, P.M.; Connolly, S.J.; Benavente, O.R.; Dowlatshahi, D.; et al. Effects of oral anticoagulation in people with atrial fibrillation after spontaneous intracranial haemorrhage (COCROACH): Prospective, individual participant data meta-analysis of randomised trials. Lancet Neurol. 2023, 22, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Shoamanesh, A.; Committee, E.-A.S. Anticoagulation in patients with cerebral amyloid angiopathy. Lancet 2023, 402, 1418–1419. [Google Scholar] [CrossRef]
- Seiffge, D.J.; Cancelloni, V.; Räber, L.; Paciaroni, M.; Metzner, A.; Kirchhof, P.; Fischer, U.; Werring, D.J.; Shoamanesh, A.; Caso, V. Secondary stroke prevention in people with atrial fibrillation: Treatments and trials. Lancet Neurol. 2024, 23, 404–417. [Google Scholar] [CrossRef]
- Schrag, M.; Mac Grory, B.; Nackenoff, A.; Eaton, J.; Mistry, E.; Kirshner, H.; Yaghi, S.; Ellis, C.R. Left Atrial Appendage Closure for Patients with Cerebral Amyloid Angiopathy and Atrial Fibrillation: The LAA-CAA Cohort. Transl. Stroke Res. 2021, 12, 259–265. [Google Scholar] [CrossRef]
- Blanc, C.; Blanc, G.; Boveda, S.; Calvière, L.; Combes, N.; Viguier, A.; Mondoly, P.; Albucher, J.F.; Gollion, C.; Fabry, V.; et al. Left Atrial Appendage Closure in Patients With Atrial Fibrillation and Coexisting Cerebral Amyloid Angiopathy. Stroke 2021, 52, e792–e793. [Google Scholar] [CrossRef]
- Sharma, R.; Dearaugo, S.; Infeld, B.; O’Sullivan, R.; Gerraty, R.P. Cerebral amyloid angiopathy: Review of clinico-radiological features and mimics. J. Med. Imaging Radiat. Oncol. 2018, 62, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Psychogios, K.; Xiromerisiou, G.; Kargiotis, O.; Safouris, A.; Fiolaki, A.; Bonakis, A.; Paraskevas, G.P.; Giannopoulos, S.; Tsivgoulis, G. Hereditary cerebral amyloid angiopathy mimicking CADASIL syndrome. Eur. J. Neurol. 2021, 28, 3866–3869. [Google Scholar] [CrossRef] [PubMed]
- Salvarani, C.; Brown, R.D.; Calamia, K.T.; Christianson, T.J.; Huston, J.; Meschia, J.F.; Giannini, C.; Miller, D.V.; Hunder, G.G. Primary central nervous system vasculitis: Comparison of patients with and without cerebral amyloid angiopathy. Rheumatology (Oxford) 2008, 47, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Ruano, L.; Branco, M.; Samões, R.; Taipa, R.; Melo Pires, M. The etiology of spontaneous intracerebralhemorrhage: Insights from a neuropathological series. Clin. Neuropathol. 2018, 37, 16–21. [Google Scholar] [CrossRef]
- Guey, S.; Lesnik Oberstein, S.A.J.; Tournier-Lasserve, E.; Chabriat, H. Hereditary Cerebral Small Vessel Diseases and Stroke: A Guide for Diagnosis and Management. Stroke 2021, 52, 3025–3032. [Google Scholar] [CrossRef]
- Guey, S.; Chabriat, H. Monogenic causes of cerebral small vessel disease and stroke. Handb. Clin. Neurol. 2024, 204, 273–287. [Google Scholar] [CrossRef]
- Mancuso, M.; Arnold, M.; Bersano, A.; Burlina, A.; Chabriat, H.; Debette, S.; Enzinger, C.; Federico, A.; Filla, A.; Finsterer, J.; et al. Monogenic cerebral small-vessel diseases: Diagnosis and therapy. Consensus recommendations of the European Academy of Neurology. Eur. J. Neurol. 2020, 27, 909–927. [Google Scholar] [CrossRef]
- Riech, S.; Kallenberg, K.; Moerer, O.; Hellen, P.; Bärtsch, P.; Quintel, M.; Knauth, M. The Pattern of Brain Microhemorrhages After Severe Lung Failure Resembles the One Seen in High-Altitude Cerebral Edema. Crit. Care Med. 2015, 43, e386–e389. [Google Scholar] [CrossRef]
- Chan, M.S.; Roebuck, D.J.; Yuen, M.P.; Li, C.K.; Chan, Y.L. MR imaging of the brain in patients cured of acute lymphoblastic leukemia--the value of gradient echo imaging. AJNR Am. J. Neuroradiol. 2006, 27, 548–552. [Google Scholar]
- Lee, H.Y.; Chang, K.H.; Kim, J.H.; Na, D.G.; Kwon, B.J.; Lee, K.W.; Park, S.H. Serial MR imaging findings of acute hemorrhagic leukoencephalitis: A case report. AJNR Am. J. Neuroradiol. 2005, 26, 1996–1999. [Google Scholar]
- Yamamoto, A.; Kikuchi, Y.; Homma, K.; O’uchi, T.; Furui, S. Characteristics of intravascular large B-cell lymphoma on cerebral MR imaging. AJNR Am. J. Neuroradiol. 2012, 33, 292–296. [Google Scholar] [CrossRef] [PubMed]
- McMillion, L.; Melton, D.M.; Erickson, J.C. Teaching neuroimage: Primary cerebral amyloidoma mimicking CNS neoplasm. Neurology 2008, 71, e68. [Google Scholar] [CrossRef] [PubMed]
- Bakola, E.; Katsanos, A.H.; Palaiodimou, L.; Theodorou, A.; Stefanou, M.I.; Chondrogianni, M.; Andreadou, E.; Papadopoulou, M.; Konstantakos, V.; Voumvourakis, K.; et al. Delayed recurrent enhancing white matter lesions complicating coiling of intracranial aneurysm. Eur. J. Neurol. 2021, 28, 2388–2391. [Google Scholar] [CrossRef]
- Bakola, E.; Papagiannopoulou, G.; Palaiodimou, L.; Lagios, K.; Archontakis, E.; Theodorou, A.; Katsanos, A.H.; Triantafyllou, S.; Zouvelou, V.; Lachanis, S.; et al. Delayed Leucoencephalopathy as a Complication after Endovascular Therapy of Intracranial Aneurysms-A Case Series. J. Clin. Med. 2023, 12, 496. [Google Scholar] [CrossRef]
- Vanacker, P.; Nelissen, N.; Van Laere, K.; Thijs, V.N. Images in neurology. Scattered cerebral microbleeds due to cardiac myxoma. Arch. Neurol. 2009, 66, 796–797. [Google Scholar] [CrossRef]
- Kaufmann, J.; Buecke, P.; Meinel, T.; Beyeler, M.; Scutelnic, A.; Kaesmacher, J.; Mujanović, A.; Dobrocky, T.; Arsany, H.; Peters, N.; et al. Frequency of ischaemic stroke and intracranial haemorrhage in patients with reversible cerebral vasoconstriction syndrome (RCVS) and posterior reversible encephalopathy syndrome (PRES)—A systematic review. Eur. J. Neurol. 2024, 31, e16246. [Google Scholar] [CrossRef]
- Pachi, I.; Theodorou, A.; Velonakis, G.; Bakola, E.; Chondrogianni, M.; Akrivaki, A.; Palialexis, K.; Spiliopoulos, S.; Tsivgoulis, G. Challenges in diagnosis and management of cerebral venous thrombosis as underlying cause of lobar intracerebral hemorrhage. J. Stroke Cerebrovasc. Dis. 2024, 33, 107759. [Google Scholar] [CrossRef]
- Cuvinciuc, V.; Viguier, A.; Calviere, L.; Raposo, N.; Larrue, V.; Cognard, C.; Bonneville, F. Isolated acute nontraumatic cortical subarachnoid hemorrhage. AJNR Am. J. Neuroradiol. 2010, 31, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Eng, J.A.; Frosch, M.P.; Choi, K.; Rebeck, G.W.; Greenberg, S.M. Clinical manifestations of cerebral amyloid angiopathy-related inflammation. Ann. Neurol. 2004, 55, 250–256. [Google Scholar] [CrossRef]
- Corovic, A.; Kelly, S.; Markus, H.S. Cerebral amyloid angiopathy associated with inflammation: A systematic review of clinical and imaging features and outcome. Int. J. Stroke 2018, 13, 257–267. [Google Scholar] [CrossRef]
- Charidimou, A. Diagnosing Cerebral Amyloid Angiopathy-Related Inflammation. Neurology 2024, 103, e209647. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.K.; Anderson, N.E.; Hutchinson, D.; Synek, B.; Barber, P.A. Cerebral amyloid angiopathy related inflammation: Three case reports and a review. J. Neurol. Neurosurg. Psychiatry 2011, 82, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Ueda, M.; Fukushima, W.; Tamaoka, A.; Shoji, M.; Ando, Y.; Yamada, M. Nationwide survey on cerebral amyloid angiopathy in Japan. Eur. J. Neurol. 2019, 26, 1487–1493. [Google Scholar] [CrossRef]
- Theodorou, A.; Palaiodimou, L.; Papagiannopoulou, G.; Kargiotis, O.; Psychogios, K.; Safouris, A.; Bakola, E.; Chondrogianni, M.; Kotsali-Peteinelli, V.; Melanis, K.; et al. Clinical Characteristics, Neuroimaging Markers, and Outcomes in Patients with Cerebral Amyloid Angiopathy: A Prospective Cohort Study. J. Clin. Med. 2023, 12, 5591. [Google Scholar] [CrossRef]
- Grangeon, L.; Quesney, G.; Verdalle-Cazes, M.; Coulette, S.; Renard, D.; Wacongne, A.; Allou, T.; Olivier, N.; Boukriche, Y.; Blanchet-Fourcade, G.; et al. Different clinical outcomes between cerebral amyloid angiopathy-related inflammation and non-inflammatory form. J. Neurol. 2022, 269, 4972–4984. [Google Scholar] [CrossRef]
- Szalardy, L.; Fakan, B.; Maszlag-Torok, R.; Ferencz, E.; Reisz, Z.; Radics, B.L.; Csizmadia, S.; Szpisjak, L.; Annus, A.; Zadori, D.; et al. Identifying diagnostic and prognostic factors in cerebral amyloid angiopathy-related inflammation: A systematic analysis of published and seven new cases. Neuropathol. Appl. Neurobiol. 2024, 50, e12946. [Google Scholar] [CrossRef]
- Theodorou, A.; Tsibonakis, A.; Pateras, I.S.; Kaloudi, G.; Bakola, E.; Chondrogianni, M.; Andreadou, E.; Panayiotides, I.G.; Tsivgoulis, G. Multiple cerebral microinfarcts: An uncommon presentation of Cerebral Amyloid Angiopathy-related inflammation. Neurol. Res. Pract. 2023, 5, 28. [Google Scholar] [CrossRef]
- Theodorou, A.; Lachanis, S.; Alexopoulos, P.; Palaiodimou, L.; Kollia, N.; Voumvourakis, K.; Tsivgoulis, G. Teaching NeuroImages: Acute convexity subarachnoid hemorrhage: An underrecognized presentation of CAA-ri. Neurology 2019, 93, e524–e525. [Google Scholar] [CrossRef] [PubMed]
- Panteleienko, L.; Banerjee, G.; Mallon, D.H.; Harvey, V.; Oliver, R.; Hotton, G.; Knight, W.; Datta, S.; Zandi, M.S.; Jäger, H.R.; et al. Sulcal Hyperintensity as an Early Imaging Finding in Cerebral Amyloid Angiopathy-Related Inflammation. Neurology 2024, 103, e210084. [Google Scholar] [CrossRef]
- Martín-Jiménez, P.; Sánchez-Tornero, M.; Llamas-Velasco, S.; Guerrero-Molina, M.P.; González-Sánchez, M.; Herrero-San Martín, A.; Blanco-Palmero, V.; Calleja-Castaño, P.; Francisco-Gonzalo, J.; Hilario, A.; et al. Cerebral amyloid angiopathy-related inflammation: Clinical features and treatment response in a case series. Neurologia (Engl. Ed.) 2021. [Google Scholar] [CrossRef]
- Shams, S.; Martola, J.; Cavallin, L.; Granberg, T.; Shams, M.; Aspelin, P.; Wahlund, L.O.; Kristoffersen-Wiberg, M. SWI or T2*: Which MRI sequence to use in the detection of cerebral microbleeds? The Karolinska Imaging Dementia Study. AJNR Am. J. Neuroradiol. 2015, 36, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Martucci, M.; Sarria, S.; Toledo, M.; Coscojuela, P.; Vert, C.; Siurana, S.; Auger, C.; Rovira, A. Cerebral amyloid angiopathy-related inflammation: Imaging findings and clinical outcome. Neuroradiology 2014, 56, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Antolini, L.; DiFrancesco, J.C.; Zedde, M.; Basso, G.; Arighi, A.; Shima, A.; Cagnin, A.; Caulo, M.; Carare, R.O.; Charidimou, A.; et al. Spontaneous ARIA-like Events in Cerebral Amyloid Angiopathy-Related Inflammation: A Multicenter Prospective Longitudinal Cohort Study. Neurology 2021, 97, e1809–e1822. [Google Scholar] [CrossRef] [PubMed]
- Bangad, A.; Abbasi, M.; Payabvash, S.; de Havenon, A. Imaging of Amyloid-beta-related Arteritis. Neuroimaging Clin. N. Am. 2024, 34, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Piazza, F.; Greenberg, S.M.; Savoiardo, M.; Gardinetti, M.; Chiapparini, L.; Raicher, I.; Nitrini, R.; Sakaguchi, H.; Brioschi, M.; Billo, G.; et al. Anti-amyloid β autoantibodies in cerebral amyloid angiopathy-related inflammation: Implications for amyloid-modifying therapies. Ann. Neurol. 2013, 73, 449–458. [Google Scholar] [CrossRef]
- Zedde, M.; Pascarella, R.; Piazza, F. CAA-ri and ARIA: Two Faces of the Same Coin? AJNR Am. J. Neuroradiol. 2023, 44, E13–E14. [Google Scholar] [CrossRef]
- Regenhardt, R.W.; Thon, J.M.; Das, A.S.; Thon, O.R.; Charidimou, A.; Viswanathan, A.; Gurol, M.E.; Chwalisz, B.K.; Frosch, M.P.; Cho, T.A.; et al. Association Between Immunosuppressive Treatment and Outcomes of Cerebral Amyloid Angiopathy-Related Inflammation. JAMA Neurol. 2020, 77, 1261–1269. [Google Scholar] [CrossRef]
- Plotzker, A.S.; Henson, R.L.; Fagan, A.M.; Morris, J.C.; Day, G.S. Clinical and Paraclinical Measures Associated with Outcome in Cerebral Amyloid Angiopathy with Related Inflammation. J. Alzheimer’s Dis. 2021, 80, 133–142. [Google Scholar] [CrossRef]
- Raghavan, P.; Looby, S.; Bourne, T.D.; Wintermark, M. Cerebral amyloid angiopathy-related inflammation: A potentially reversible cause of dementia with characteristic imaging findings. J. Neuroradiol. 2016, 43, 11–17. [Google Scholar] [CrossRef]
- Rempe, T.; Sollero, C.E.V.; Rodriguez, E.; Viswanathan, V.T.; Carlson, A.; Rees, J.; Tuna, I.S.; Kresak, J.; Gyang, T.V. Corticosteroids lead to short-term improvement in cerebral amyloid angiopathy-related inflammation. J. Neuroimmunol. 2020, 348, 577377. [Google Scholar] [CrossRef]
- Kloppenborg, R.P.; Richard, E.; Sprengers, M.E.; Troost, D.; Eikelenboom, P.; Nederkoorn, P.J. Steroid responsive encephalopathy in cerebral amyloid angiopathy: A case report and review of evidence for immunosuppressive treatment. J. Neuroinflamm. 2010, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Cancelloni, V.; Rufa, A.; Battisti, C.; De Stefano, N.; Mastrocinque, E.; Garosi, G.; Venezia, D.; Chiarotti, I.; Cerase, A. Diagnosis, treatment, and follow-up of patients with cerebral amyloid angiopathy-related inflammation. Neurol. Sci. 2022, 43, 6381–6387. [Google Scholar] [CrossRef] [PubMed]
- van Etten, E.S.; Gurol, M.E.; van der Grond, J.; Haan, J.; Viswanathan, A.; Schwab, K.M.; Ayres, A.M.; Algra, A.; Rosand, J.; van Buchem, M.A.; et al. Recurrent hemorrhage risk and mortality in hereditary and sporadic cerebral amyloid angiopathy. Neurology 2016, 87, 1482–1487. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Wolfe, M.S. Presenilin: Running with scissors in the membrane. Cell 2007, 131, 215–221. [Google Scholar] [CrossRef]
- Kim, S.H.; Wang, R.; Gordon, D.J.; Bass, J.; Steiner, D.F.; Lynn, D.G.; Thinakaran, G.; Meredith, S.C.; Sisodia, S.S. Furin mediates enhanced production of fibrillogenic ABri peptides in familial British dementia. Nat. Neurosci. 1999, 2, 984–988. [Google Scholar] [CrossRef]
- Palsdottir, A.; Snorradottir, A.O.; Thorsteinsson, L. Hereditary cystatin C amyloid angiopathy: Genetic, clinical, and pathological aspects. Brain Pathol. 2006, 16, 55–59. [Google Scholar] [CrossRef]
- Kiuru-Enari, S.; Haltia, M. Hereditary gelsolin amyloidosis. Handb. Clin. Neurol. 2013, 115, 659–681. [Google Scholar] [CrossRef]
- Liz, M.A.; Coelho, T.; Bellotti, V.; Fernandez-Arias, M.I.; Mallaina, P.; Obici, L. A Narrative Review of the Role of Transthyretin in Health and Disease. Neurol. Ther. 2020, 9, 395–402. [Google Scholar] [CrossRef]
- Bornebroek, M.; Haan, J.; Maat-Schieman, M.L.; Van Duinen, S.G.; Roos, R.A. Hereditary cerebral hemorrhage with amyloidosis-Dutch type (HCHWA-D): I--A review of clinical, radiologic and genetic aspects. Brain Pathol. 1996, 6, 111–114. [Google Scholar] [CrossRef]
- Nochlin, D.; Bird, T.D.; Nemens, E.J.; Ball, M.J.; Sumi, S.M. Amyloid angiopathy in a Volga German family with Alzheimer’s disease and a presenilin-2 mutation (N141I). Ann. Neurol. 1998, 43, 131–135. [Google Scholar] [CrossRef]
- Canevelli, M.; Piscopo, P.; Talarico, G.; Vanacore, N.; Blasimme, A.; Crestini, A.; Tosto, G.; Troili, F.; Lenzi, G.L.; Confaloni, A.; et al. Familial Alzheimer’s disease sustained by presenilin 2 mutations: Systematic review of literature and genotype-phenotype correlation. Neurosci. Biobehav. Rev. 2014, 42, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Blöndal, H.; Guomundsson, G.; Benedikz, E.; Jóhannesson, G. Dementia in hereditary cystatin C amyloidosis. Prog. Clin. Biol. Res. 1989, 317, 157–164. [Google Scholar] [PubMed]
- Palsdottir, A.; Helgason, A.; Palsson, S.; Bjornsson, H.T.; Bragason, B.T.; Gretarsdottir, S.; Thorsteinsdottir, U.; Olafsson, E.; Stefansson, K. A drastic reduction in the life span of cystatin C L68Q carriers due to life-style changes during the last two centuries. PLoS Genet. 2008, 4, e1000099. [Google Scholar] [CrossRef] [PubMed]
- Bevers, M.B.; McGuone, D.; Jerath, N.U.; Musolino, P.L. Leptomeningeal transthyretin-type amyloidosis presenting as acute hydrocephalus and subarachnoid hemorrhage. J. Clin. Neurosci. 2016, 29, 203–205. [Google Scholar] [CrossRef]
- Kaushik, K.; van Etten, E.S.; Siegerink, B.; Kappelle, L.J.; Lemstra, A.W.; Schreuder, F.H.B.M.; Klijn, C.J.M.; Peul, W.C.; Terwindt, G.M.; van Walderveen, M.A.A.; et al. Iatrogenic Cerebral Amyloid Angiopathy Post Neurosurgery: Frequency, Clinical Profile, Radiological Features, and Outcome. Stroke 2023, 54, 1214–1223. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Taniguchi, Y.; Sakai, K.; Kitamoto, T.; Takao, M.; Murayama, S.; Iwasaki, Y.; Yoshida, M.; Shimizu, H.; Kakita, A.; et al. Significant association of cadaveric dura mater grafting with subpial Aβ deposition and meningeal amyloid angiopathy. Acta Neuropathol. 2016, 132, 313–315. [Google Scholar] [CrossRef]
- Ritchie, D.L.; Adlard, P.; Peden, A.H.; Lowrie, S.; Le Grice, M.; Burns, K.; Jackson, R.J.; Yull, H.; Keogh, M.J.; Wei, W.; et al. Amyloid-β accumulation in the CNS in human growth hormone recipients in the UK. Acta Neuropathol. 2017, 134, 221–240. [Google Scholar] [CrossRef]
- Jaunmuktane, Z.; Quaegebeur, A.; Taipa, R.; Viana-Baptista, M.; Barbosa, R.; Koriath, C.; Sciot, R.; Mead, S.; Brandner, S. Evidence of amyloid-β cerebral amyloid angiopathy transmission through neurosurgery. Acta Neuropathol. 2018, 135, 671–679. [Google Scholar] [CrossRef]
- Jaunmuktane, Z.; Mead, S.; Ellis, M.; Wadsworth, J.D.; Nicoll, A.J.; Kenny, J.; Launchbury, F.; Linehan, J.; Richard-Loendt, A.; Walker, A.S.; et al. Evidence for human transmission of amyloid-β pathology and cerebral amyloid angiopathy. Nature 2015, 525, 247–250. [Google Scholar] [CrossRef]
- Banerjee, G.; Samra, K.; Adams, M.E.; Jaunmuktane, Z.; Parry-Jones, A.R.; Grieve, J.; Toma, A.K.; Farmer, S.F.; Sylvester, R.; Houlden, H.; et al. Iatrogenic cerebral amyloid angiopathy: An emerging clinical phenomenon. J. Neurol. Neurosurg. Psychiatry 2022, 93, 693–700. [Google Scholar] [CrossRef]
- Fandler-Höfler, S.; Kaushik, K.; Storti, B.; Pikija, S.; Mallon, D.; Ambler, G.; Damavandi, P.T.; Panteleienko, L.; Canavero, I.; van Walderveen, M.A.A.; et al. Clinical-radiological presentation and natural history of iatrogenic cerebral amyloid angiopathy. J. Neurol. Neurosurg. Psychiatry 2025. [Google Scholar] [CrossRef] [PubMed]
- Muir, R.T.; Callum, J.L.; Yu, A.Y.X.; Kapral, M.K.; Swartz, R.H.; Black, S.E.; MacIntosh, B.J.; Fergusson, D.A.; Kleinman, S.; Demchuk, A.D.; et al. Beta-Amyloid Related Neurodegenerative and Neurovascular Diseases: Potential Implications for Transfusion Medicine. Transfus. Med. Rev. 2024, 38, 150858. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, K.; Wermer, M.J.H.; van Etten, E.S. Cerebral amyloid angiopathy decades after red blood cell transfusions: A report of two cases from a prospective cohort. Eur. J. Neurol. 2024, 31, e16277. [Google Scholar] [CrossRef]
- Zhao, J.; Rostgaard, K.; Lauwers, E.; Dahlén, T.; Ostrowski, S.R.; Erikstrup, C.; Pedersen, O.B.; de Strooper, B.; Lemmens, R.; Hjalgrim, H.; et al. Intracerebral Hemorrhage Among Blood Donors and Their Transfusion Recipients. JAMA 2023, 330, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Jensen-Kondering, U. Spatial colocalization of imaging markers in iatrogenic cerebral amyloid angiopathy with the site of surgery: A metaanalysis. J. Neurol. Sci. 2024, 458, 122931. [Google Scholar] [CrossRef]
- Panteleienko, L.; Mallon, D.; Htet, C.M.M.; Lizak, N.; Zandi, M.; Banerjee, G.; Werring, D.J. Cerebral Amyloid Angiopathy-Related Inflammation in Iatrogenic Cerebral Amyloid Angiopathy. Eur. J. Neurol. 2025, 32, e70198. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Aparicio, H.J.; Furie, K.L.; Goyal, M.S.; Hinman, J.D.; Kozberg, M.; Leonard, A.; Fisher, M.J.; Council, A.H.A.S.; Nursing, C.o.C.a.S.; et al. Vascular Neurology Considerations for Antiamyloid Immunotherapy: A Science Advisory From the American Heart Association. Stroke 2025, 56, e30–e38. [Google Scholar] [CrossRef]
- Voigt, S.; Koemans, E.A.; Rasing, I.; van Etten, E.S.; Terwindt, G.M.; Baas, F.; Kaushik, K.; van Es, A.C.G.M.; van Buchem, M.A.; van Osch, M.J.P.; et al. Minocycline for sporadic and hereditary cerebral amyloid angiopathy (BATMAN): Study protocol for a placebo-controlled randomized double-blind trial. Trials 2023, 24, 378. [Google Scholar] [CrossRef]
- Bax, F.; Warren, A.; Fouks, A.A.; van den Brink, H.; van Veluw, S.J.; Kozberg, M.G.; Greenberg, S.M. Minocycline in Severe Cerebral Amyloid Angiopathy: A Single-Center Cohort Study. J. Am. Heart Assoc. 2024, 13, e033464. [Google Scholar] [CrossRef]




| |
| 1. Definite CAA | Full post-mortem examination demonstrating the following: • Presentation with spontaneous ICH, TFNEs, cSAH, or CI/Dementia • Severe CAA with vasculopathy • Absence of other diagnostic lesion |
| 2. Probable CAA with supporting pathology | Clinical data and pathologic tissue (evacuated hematoma or cortical biopsy) demonstrating the following: • Presentation with spontaneous ICH, TFNEs, cSAH, or CI/Dementia • Some degree of CAA in specimen • Absence of other diagnostic lesion |
| 3. Probable CAA | Clinical data and MRI demonstrating the following: • Age ≥ 50 years • Presentation with spontaneous ICH, TFNEs, or CI/Dementia • ≥2 of the following strictly lobar hemorrhagic lesions on T2*-weighted MRI, in any combination: ICH, CMB, cSS/cSAH foci OR • 1 lobar hemorrhagic lesion + 1 white matter feature (Severe CSO-PVS or WMH-MS) • Absence of any deep hemorrhagic lesions (ICH, CMB) on T2*weighted-MRI • Absence of other cause of hemorrhagic lesions * • Hemorrhagic lesion in cerebellum not counted as either lobar or deep hemorrhagic lesion |
| 4. Possible CAA | Clinical data and MRI demonstrating the following: • Age ≥ 50 years • Presentation with spontaneous ICH, TFNEs, or CI/Dementia • Absence of other cause of hemorrhage * • 1 strictly lobar hemorrhagic lesion on T2*-weighted MRI: ICH, CMB, cSS/cSAH focus OR • 1 white matter feature (Severe CSO-PVS or WMH-MS) • Absence of any deep hemorrhagic lesions (ICH, CMB) on T2*-weighted MRI • Absence of other cause of hemorrhagic lesions * • Hemorrhagic lesion in cerebellum not counted as either lobar or deep hemorrhagic lesion |
| |
| 1. Definite CAA-ri | Definitive diagnosis of CAA-ri requires brain biopsy. |
| 2. Probable CAA-ri | 1. Age > 40 y 2. Presence of ≥1 of the following clinical features: headache, decrease in consciousness, behavioral change, or focal neurological signs and seizures; the presentation is not directly attributable to an acute ICH 3. MRI shows unifocal or multifocal WMH lesions (corticosubcortical or deep) that are asymmetric and extend to the immediately subcortical white matter; the asymmetry is not due to past ICH 4. Presence of ≥1 of the following corticosubcortical hemorrhagic lesions: cerebral macrobleed, cerebral microbleed, or cortical superficial siderosis 5. Absence of neoplastic, infectious, or other cause |
| 3. Possible CAA-ri | 1. Age ≥ 40 y 2. Presence of ≥1 of the following clinical features: headache, decrease in consciousness, behavioral change, or focal neurological signs and seizures; the presentation is not directly attributable to an acute ICH 3. MRI shows WMH lesions that extend to the immediately subcortical white matter 4. Presence of ≥1 of the following corticosubcortical hemorrhagic lesions: cerebral macrobleed, cerebral microbleed, or cortical superficial siderosis 5. Absence of neoplastic, infectious, or other cause |
| Conditions Mimicking CAA, CAA-ri, Iatrogenic CAA, and Hereditary CAA | |
|---|---|
|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodorou, A.; Fanouraki, S.; Bakola, E.; Papagiannopoulou, G.; Palaiodimou, L.; Chondrogianni, M.; Stefanou, M.-I.; Stavrinou, L.; Athanasaki, A.; Psychogios, K.; et al. Clinical Management of Cerebral Amyloid Angiopathy. J. Clin. Med. 2025, 14, 4259. https://doi.org/10.3390/jcm14124259
Theodorou A, Fanouraki S, Bakola E, Papagiannopoulou G, Palaiodimou L, Chondrogianni M, Stefanou M-I, Stavrinou L, Athanasaki A, Psychogios K, et al. Clinical Management of Cerebral Amyloid Angiopathy. Journal of Clinical Medicine. 2025; 14(12):4259. https://doi.org/10.3390/jcm14124259
Chicago/Turabian StyleTheodorou, Aikaterini, Stella Fanouraki, Eleni Bakola, Georgia Papagiannopoulou, Lina Palaiodimou, Maria Chondrogianni, Maria-Ioanna Stefanou, Lampis Stavrinou, Athanasia Athanasaki, Klearchos Psychogios, and et al. 2025. "Clinical Management of Cerebral Amyloid Angiopathy" Journal of Clinical Medicine 14, no. 12: 4259. https://doi.org/10.3390/jcm14124259
APA StyleTheodorou, A., Fanouraki, S., Bakola, E., Papagiannopoulou, G., Palaiodimou, L., Chondrogianni, M., Stefanou, M.-I., Stavrinou, L., Athanasaki, A., Psychogios, K., Kargiotis, O., Safouris, A., Velonakis, G., Paraskevas, G. P., & Tsivgoulis, G. (2025). Clinical Management of Cerebral Amyloid Angiopathy. Journal of Clinical Medicine, 14(12), 4259. https://doi.org/10.3390/jcm14124259







