An Artificial Intelligence Algorithm for Early Detection of Left Ventricular Systolic Dysfunction in Patients with Normal Sinus Rhythm
Abstract
1. Introduction
1.1. Subclinical Cardiac Dysfunction and Preventive Strategies
1.2. Deep Learning-Based Electrocardiographic Analysis for LVSD Detection
1.3. Study Objective and Clinical Gap
2. Materials and Methods
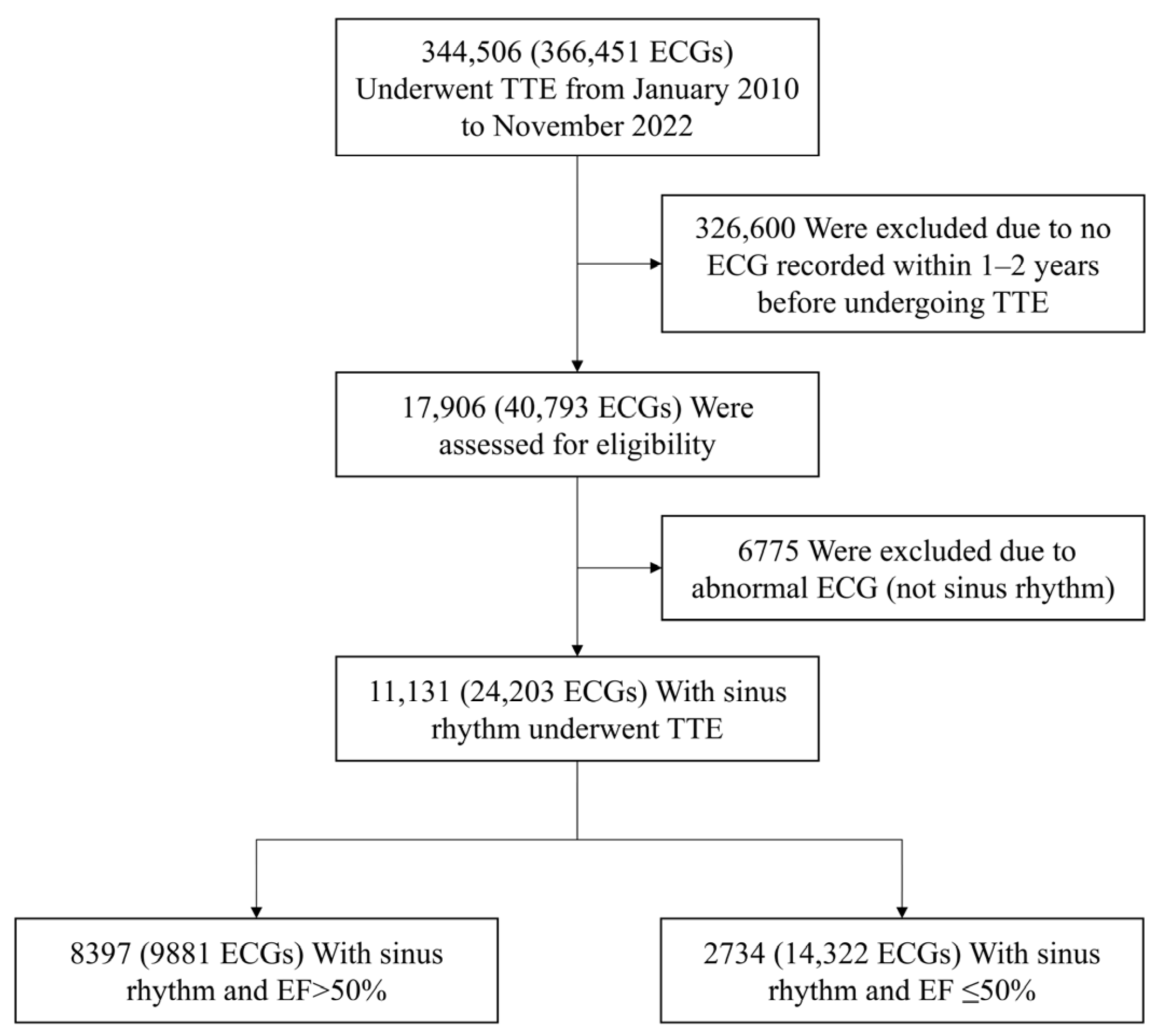
2.1. Study Population
2.2. ECG Acquisition
2.3. Algorithm Development
2.4. Outcomes
2.5. Statistical Analysis
2.6. Use of Generative AI Tools
3. Results
3.1. Baseline Characteristics of the Study Population
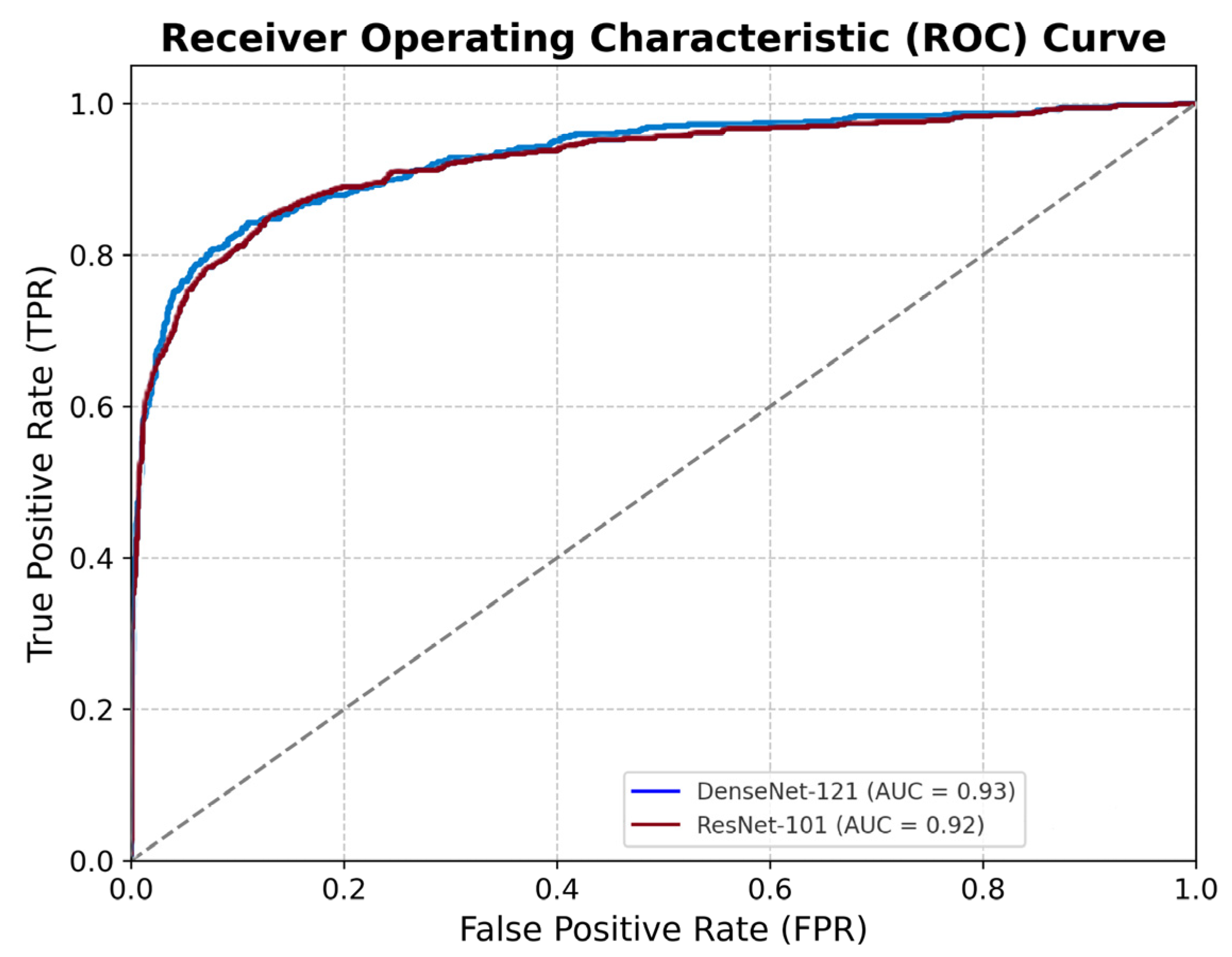
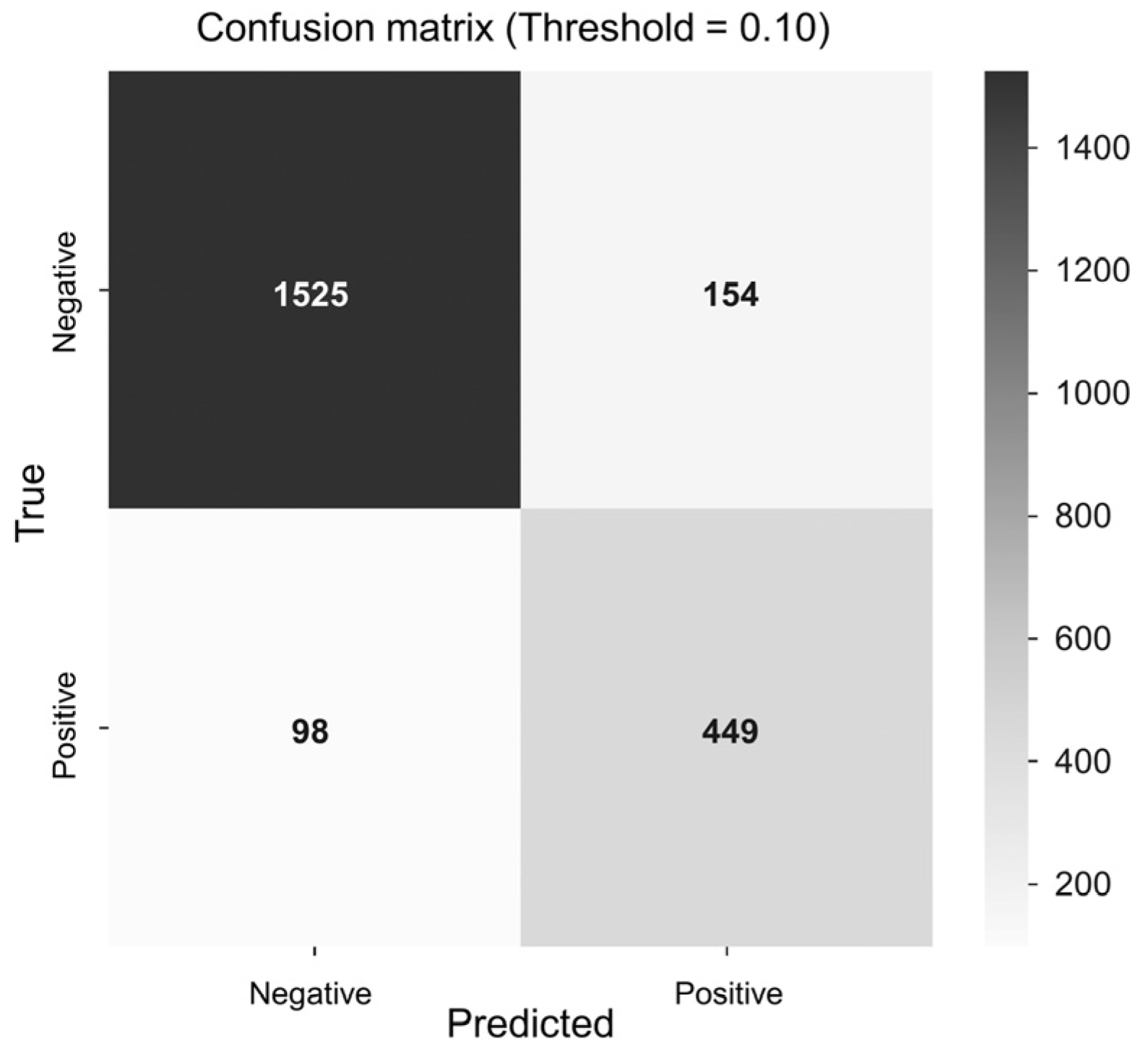
3.2. Model Performance
3.3. Association with Echocardiographic LVSD
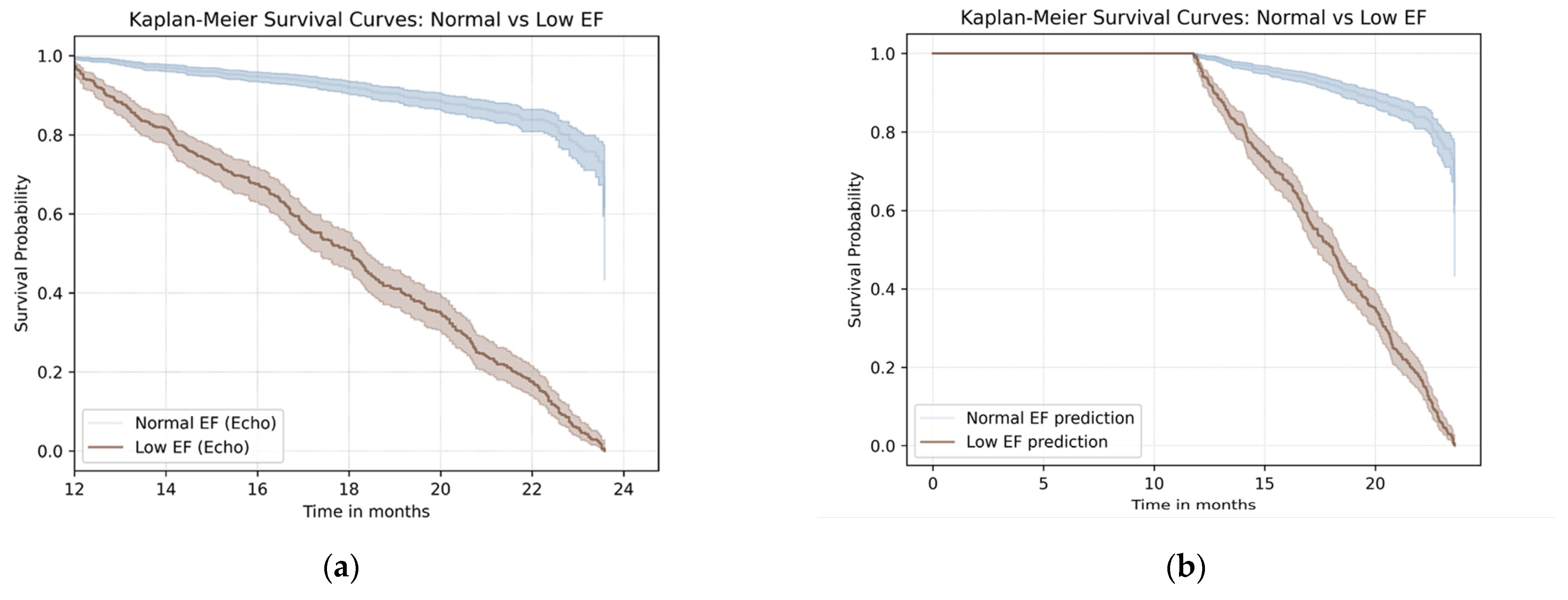
3.4. Survival Outcomes Based on Echocardiographic and AI Classification
4. Discussion
4.1. Early Prediction of LVSD Using AI-Enabled ECG
4.2. AI-ECG and Subclinical Myocardial Remodeling
4.3. Clinical Implications of AI-ECG for Population Screening
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| LVSD | Left ventricular systolic dysfunction |
| ECG | Electrocardiogram |
| EF | Ejection fraction |
| CI | Confidence interval |
| AF | Atrial fibrillation |
| TTE | Transthoracic echocardiography |
| CNN | Convolutional neural network |
| AUC | Area under curve |
| ROC | Receiver operating characteristic |
| PPV | Positive predictive value |
| NPV | Negative predictive value |
References
- Jakubiak, G.K. Cardiac Troponin Serum Concentration Measurement Is Useful Not Only in the Diagnosis of Acute Cardiovascular Events. J. Pers. Med. 2024, 14, 230. [Google Scholar] [CrossRef]
- Wang, T.J.; Evans, J.C.; Benjamin, E.J.; Levy, D.; LeRoy, E.C.; Vasan, R.S. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation 2003, 108, 977–982. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; McDonald, K.; Maisel, A.S. Screening for asymptomatic left ventricular dysfunction using B-type natriuretic Peptide. Congest. Heart Fail. 2008, 14, 5–8. [Google Scholar] [CrossRef]
- Vasan, R.S.; Benjamin, E.J.; Larson, M.G.; Leip, E.P.; Wang, T.J.; Wilson, P.W.; Levy, D. Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction: The Framingham heart study. Jama 2002, 288, 1252–1259. [Google Scholar] [CrossRef]
- Goetze, J.P.; Mogelvang, R.; Maage, L.; Scharling, H.; Schnohr, P.; Sogaard, P.; Rehfeld, J.F.; Jensen, J.S. Plasma pro-B-type natriuretic peptide in the general population: Screening for left ventricular hypertrophy and systolic dysfunction. Eur. Heart J. 2006, 27, 3004–3010. [Google Scholar] [CrossRef]
- Redfield, M.M.; Rodeheffer, R.J.; Jacobsen, S.J.; Mahoney, D.W.; Bailey, K.R.; Burnett, J.C., Jr. Plasma brain natriuretic peptide to detect preclinical ventricular systolic or diastolic dysfunction: A community-based study. Circulation 2004, 109, 3176–3181. [Google Scholar] [CrossRef] [PubMed]
- Macheret, F.; Boerrigter, G.; McKie, P.; Costello-Boerrigter, L.; Lahr, B.; Heublein, D.; Sandberg, S.; Ikeda, Y.; Cataliotti, A.; Bailey, K.; et al. Pro-B-type natriuretic peptide1–108 circulates in the general community: Plasma determinants and detection of left ventricular dysfunction. J. Am. Coll. Cardiol. 2011, 57, 1386–1395. [Google Scholar] [CrossRef]
- Cersosimo, A.; Zito, E.; Pierucci, N.; Matteucci, A.; La Fazia, V.M. A Talk with ChatGPT: The Role of Artificial Intelligence in Shaping the Future of Cardiology and Electrophysiology. J. Pers. Med. 2025, 15, 205. [Google Scholar] [CrossRef] [PubMed]
- Attia, Z.I.; Kapa, S.; Lopez-Jimenez, F.; McKie, P.M.; Ladewig, D.J.; Satam, G.; Pellikka, P.A.; Enriquez-Sarano, M.; Noseworthy, P.A.; Munger, T.M.; et al. Screening for cardiac contractile dysfunction using an artificial intelligence–enabled electrocardiogram. Nat. Med. 2019, 25, 70–74. [Google Scholar] [CrossRef]
- Attia, Z.I.; Kapa, S.; Yao, X.; Lopez-Jimenez, F.; Mohan, T.L.; Pellikka, P.A.; Carter, R.E.; Shah, N.D.; Friedman, P.A.; Noseworthy, P.A. Prospective validation of a deep learning electrocardiogram algorithm for the detection of left ventricular systolic dysfunction. J. Cardiovasc. Electrophysiol. 2019, 30, 668–674. [Google Scholar] [CrossRef]
- König, S.; Hohenstein, S.; Nitsche, A.; Pellissier, V.; Leiner, J.; Stellmacher, L.; Hindricks, G.; Bollmann, A. Artificial intelligence-based identification of left ventricular systolic dysfunction from 12-lead electrocardiograms: External validation and advanced application of an existing model. Eur. Heart J.-Digit. Health 2023, 5, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Rushlow, D.R.; Inselman, J.W.; McCoy, R.G.; Thacher, T.D.; Behnken, E.M.; Bernard, M.E.; Rosas, S.L.; Akfaly, A.; Misra, A.; et al. Artificial intelligence–enabled electrocardiograms for identification of patients with low ejection fraction: A pragmatic, randomized clinical trial. Nat. Med. 2021, 27, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Attia, Z.I.; Dugan, J.; Rideout, A.; Maidens, J.N.; Venkatraman, S.; Guo, L.; Noseworthy, P.A.; Pellikka, P.A.; Pham, S.L.; Kapa, S.; et al. Automated detection of low ejection fraction from a one-lead electrocardiogram: Application of an AI algorithm to an electrocardiogram-enabled Digital Stethoscope. Eur. Heart J.-Digit. Health 2022, 3, 373–379. [Google Scholar] [CrossRef]
- Khunte, A.; Sangha, V.; Oikonomou, E.K.; Dhingra, L.S.; Aminorroaya, A.; Mortazavi, B.J.; Coppi, A.; Brandt, C.A.; Krumholz, H.M.; Khera, R. Detection of left ventricular systolic dysfunction from single-lead electrocardiography adapted for portable and wearable devices. Npj Digit. Med. 2023, 6, 124. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Kang, S.; Lee, H.S.; Lee, M.S.; Son, J.M.; Kwon, J.-m.; Lee, H.S.; Choi, Y.Y.; Kim, S.R.; Cho, D.-H.; et al. Deep learning algorithm for predicting left ventricular systolic dysfunction in atrial fibrillation with rapid ventricular response. Eur. Heart J.-Digit. Health 2024, 5, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Adedinsewo, D.; Carter, R.E.; Attia, Z.; Johnson, P.; Kashou, A.H.; Dugan, J.L.; Albus, M.; Sheele, J.M.; Bellolio, F.; Friedman, P.A.; et al. Artificial Intelligence-Enabled ECG Algorithm to Identify Patients with Left Ventricular Systolic Dysfunction Presenting to the Emergency Department with Dyspnea. Circ. Arrhythm. Electrophysiol. 2020, 13, e008437. [Google Scholar] [CrossRef]
- Kashou, A.H.; Medina-Inojosa, J.R.; Noseworthy, P.A.; Rodeheffer, R.J.; Lopez-Jimenez, F.; Attia, I.Z.; Kapa, S.; Scott, C.G.; Lee, A.T.; Friedman, P.A.; et al. Artificial Intelligence-Augmented Electrocardiogram Detection of Left Ventricular Systolic Dysfunction in the General Population. Mayo Clin. Proc. 2021, 96, 2576–2586. [Google Scholar] [CrossRef]
- Sangha, V.; Nargesi, A.A.; Dhingra, L.S.; Khunte, A.; Mortazavi, B.J.; Ribeiro, A.H.; Banina, E.; Adeola, O.; Garg, N.; Brandt, C.A.; et al. Detection of Left Ventricular Systolic Dysfunction from Electrocardiographic Images. Circulation 2023, 148, 765–777. [Google Scholar] [CrossRef]
- Attia, Z.I.; Harmon, D.M.; Behr, E.R.; Friedman, P.A. Application of artificial intelligence to the electrocardiogram. Eur. Heart J. 2021, 42, 4717–4730. [Google Scholar] [CrossRef]
- Christopoulos, G.; Graff-Radford, J.; Lopez, C.L.; Yao, X.; Attia, Z.I.; Rabinstein, A.A.; Petersen, R.C.; Knopman, D.S.; Mielke, M.M.; Kremers, W.; et al. Artificial Intelligence-Electrocardiography to Predict Incident Atrial Fibrillation: A Population-Based Study. Circ. Arrhythmia Electrophysiol. 2020, 13, e009355. [Google Scholar] [CrossRef]
- Liu, W.T.; Hsieh, P.H.; Lin, C.S.; Fang, W.H.; Wang, C.H.; Tsai, C.S.; Hung, Y.J.; Hsieh, C.B.; Lin, C.; Tsai, D.J. Opportunistic Screening for Asymptomatic Left Ventricular Dysfunction With the Use of Electrocardiographic Artificial Intelligence: A Cost-Effectiveness Approach. Can. J. Cardiol. 2024, 40, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.S.; Thao, V.; Borah, B.J.; Attia, I.Z.; Medina Inojosa, J.; Kapa, S.; Carter, R.E.; Friedman, P.A.; Lopez-Jimenez, F.; Yao, X.; et al. Cost Effectiveness of an Electrocardiographic Deep Learning Algorithm to Detect Asymptomatic Left Ventricular Dysfunction. Mayo Clin. Proc. 2021, 96, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, L.S.; Aminorroaya, A.; Sangha, V.; Pedroso, A.F.; Asselbergs, F.W.; Brant, L.C.C.; Barreto, S.M.; Ribeiro, A.L.P.; Krumholz, H.M.; Oikonomou, E.K.; et al. Heart failure risk stratification using artificial intelligence applied to electrocardiogram images: A multinational study. Eur. Heart J. 2025, 46, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
| Number of Patients | ECG No. | Age, Years | Male (%) | Female (%) | Unknown Sex (%) | |
|---|---|---|---|---|---|---|
| Normal EF (EF > 50%) | ||||||
| Total population | 8397 | 9582 | 56.2 ± 11.3 | 4316 (51.4) | 3776 (45.0) | 305 (3.6) |
| Training group (70%) | 5877 | 6906 | 56.3 ± 11.2 | 3025 (51.5) | 2631 (44.8) | 221 (3.8) |
| Validation group (10%) | 840 | 996 | 55.2 ± 11.4 | 417 (49.6) | 390 (46.4) | 33 (3.9) |
| Test group (20%) | 1680 | 1680 | 56.2 ± 11.5 | 874 (52.0) | 755 (44.9) | 51 (3.0) |
| LVSD (EF ≤ 50%) | ||||||
| Total population | 2734 | 14,322 | 67.8 ± 12.1 | 1870 (68.4) | 722 (26.4) | 142 (5.2) |
| Training group (70%) | 1913 | 10,115 | 62.5 ± 13.1 | 1314 (68.7) | 501 (26.2) | 98 (5.1) |
| Validation group (10%) | 274 | 1298 | 55.2 ± 11.4 | 196 (71.5) | 63 (23.0) | 15 (5.5) |
| Test group (20%) | 547 | 2909 | 56.2 ± 11.5 | 360 (65.8) | 158 (28.9) | 29 (5.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Lee, H.J.; Song, S.-H.; Woo, K.; Kim, J.; Kim, J.; Kim, J.Y.; Park, S.-J.; On, Y.K.; Park, K.-M. An Artificial Intelligence Algorithm for Early Detection of Left Ventricular Systolic Dysfunction in Patients with Normal Sinus Rhythm. J. Clin. Med. 2025, 14, 4257. https://doi.org/10.3390/jcm14124257
Park S, Lee HJ, Song S-H, Woo K, Kim J, Kim J, Kim JY, Park S-J, On YK, Park K-M. An Artificial Intelligence Algorithm for Early Detection of Left Ventricular Systolic Dysfunction in Patients with Normal Sinus Rhythm. Journal of Clinical Medicine. 2025; 14(12):4257. https://doi.org/10.3390/jcm14124257
Chicago/Turabian StylePark, Seongjin, Hyo Jin Lee, Sung-Hee Song, KyungChang Woo, Jiwon Kim, Juwon Kim, Ju Youn Kim, Seung-Jung Park, Young Keun On, and Kyoung-Min Park. 2025. "An Artificial Intelligence Algorithm for Early Detection of Left Ventricular Systolic Dysfunction in Patients with Normal Sinus Rhythm" Journal of Clinical Medicine 14, no. 12: 4257. https://doi.org/10.3390/jcm14124257
APA StylePark, S., Lee, H. J., Song, S.-H., Woo, K., Kim, J., Kim, J., Kim, J. Y., Park, S.-J., On, Y. K., & Park, K.-M. (2025). An Artificial Intelligence Algorithm for Early Detection of Left Ventricular Systolic Dysfunction in Patients with Normal Sinus Rhythm. Journal of Clinical Medicine, 14(12), 4257. https://doi.org/10.3390/jcm14124257






