Abstract
Background: The objective of the present systematic study was to analyze and characterize the gastric vein (GV) variations to understand their significance within clinical contexts, particularly in gastric and liver surgeries and managing conditions associated with the portal vein system. Methods: We conducted a systematic review, examining various databases, including Medline, Scopus, Web of Science, Google Scholar, CINAHL, and EMBASE, up to April 2025. Two independent authors conducted the literature search, selected pertinent studies, and extracted relevant data. The methodological quality of the studies was evaluated utilizing the Assessment Tool for Anatomical Studies (AQUA). The pooled prevalence was estimated through the application of a random effects model. Results: Among the 279 articles reviewed, 11 studies were ultimately incorporated into the systematic analysis, encompassing 47,993 subjects. The pooled prevalence of GV variants was determined to be 8.32%, revealing considerable heterogeneity (I2 = 98.92%). A subgroup analysis showed a greater prevalence of GV variants in diagnostic imaging studies than in cadaveric studies, with a higher frequency observed in males than in females. Conclusions: The morphological variability of the GVs holds clinical significance, as it may significantly impact the management of abdominal disorders, particularly during surgical and endovascular interventions. This study emphasizes the necessity of thorough preoperative evaluations to identify these variations, thereby minimizing surgical complications and enhancing therapeutic outcomes for patients suffering from gastric and portal vein system disorders. Integrating advanced imaging techniques into clinical practice may facilitate improved surgical and therapeutic planning.
1. Introduction
The gastric veins (GVs) play a crucial role in the stomach’s circulatory system by serving as the primary veins that drain blood from this organ and surrounding structures into the hepatic portal vein (HPV). The right and left gastric veins (RGV and LGV) anastomose at the lesser curvature of the stomach, draining into the lateral surface of the HPV, on both sides [1]. They are positioned and oriented parallel to the gastric arteries, right and left gastric arteries, and inferior to the lesser omentum [2,3]. The LGV, which runs alongside the LGA, passes anteriorly to the celiac artery and is positioned between the common hepatic artery and splenic artery. The LGV drains the upper section of the lesser curvature in the corpus, cardia, and lower esophagus via esophageal tributary veins. This drainage is crucial for forming gastroesophageal ulcers [4,5,6].
Other GVs and gastroepiploic veins (GEVs) originate along the greater curvature, extending to the left before draining into the SV. The right GEV originates in the pyloric region, runs along the greater curvature to the right, and empties into the superior mesenteric vein (SMV). These veins collect blood from the greater curvature and the greater omentum, channeling their flows into the portal vein system (PVS) to enable hepatic detoxification of substances absorbed from the stomach [6,7]. The present systematic review will specifically focus on analyzing the LGV and RGV, concentrating on subjects through imaging and cadaver studies, searching for subjects with and without GV variants, with the presence of symptoms being key. Clinically, the LGV and RGV are intricately associated with the HPV, establishing them as crucial pathways in portal vein (PV) circulation [7,8].
The aberrant LGV (ALGV) crosses the gastrohepatic ligament, entering the II and III hepatic segments and directly communicating with the left portal veins (LPVs) [8]. The ALGV drains the LGV and has three types: Type 1 acts as an aberrant LPV (ALPV); Type 2 partially anastomoses intrahepatically to the LPV; Type 3 completely anastomoses intrahepatically with the LPV [9,10]. The LGV drainage may vary, typically into the HPV and the SV or intrahepatically into the PVS [9,10,11]. Following Lee’s classification (2019), the LGV has four types: (1) Type I crosses the common hepatic artery into the PVS; (2) Type II drains anteriorly to the celiac artery; (3) Type III crosses the splenic artery to drain into the HPV; (4) Type IV drains directly into the liver or proximal PV. The ARGV, extending about 6 cm through the hepatic ligament, aligns with the LPV [12]. ARGVs that drain into the second liver or peripheral PV branch have a prevalence of approximately 1.5%, while ALGV is a rare variant with a prevalence of less than 1.0% [13].
These morphologies have crucial clinical implications. Variants like ALGV help reduce gastric variceal bleeding in cirrhotic patients [10]. ARGV maintains a hepatic flow, aiding in the placement of a transjugular intrahepatic portosystemic shunt (TIPS) when HPV recanalization is impossible [14]. LGV can lead to atrophy in the second hepatic segment and facilitate metastasis of gastric tumors to the left hepatic lobe [15]. Preoperative analysis of LGV is essential to identify its location and reduce surgical risks, while understanding interportal venous communication is vital for treating esophageal varices and planning embolization [16]. The current study aims to explore GV morphological variants using systematic and statistical methods to assess the occurrence of clinically significant variants. Abdominal vein variations can be very complex, and if not identified quickly, they may hinder diagnostic and surgical processes. Therefore, detecting possible morphological variants through imaging is crucial to facilitate the precise diagnosis and management of conditions or surgical issues related to the GV and adjacent regions. Additionally, when considering conditions like portal hypertension, a detailed knowledge of the stomach’s vascular anatomy is vital to mitigate complications associated with heightened blood flow through the PV. Ultimately, we propose that diagnostic studies aimed at improving our understanding of these variants and their clinical consequences could significantly enhance our anatomical and clinical insights into the subject.
2. Materials and Methods
2.1. Protocol and Registration
We were guided by the PRISMA statement [17] in carrying out this evidence-based systematic review. The registration number in the Systematic Reviews Registry (PROSPERO) is CRD42024574548 (9 August 2024).
2.2. Electronic Search
We explored various databases in January to identify the most relevant studies for our research question. These included MEDLINE (via PubMed), Google Scholar, Web of Science (WOS), CINAHL, EMBASE, and Scopus, covering the timeframe from their inception until July 2024. Our search strategy utilized a blend of terms: “gastric veins” (Not Mesh), “gastric drainage” (Not Mesh), “portal system” (Not Mesh), “variations gastric veins” (Not Mesh), “clinical anatomy” (Not Mesh), and “anatomical variation” (Not Mesh), incorporating the Boolean operators AND, OR, and NOT (Supplementary Table S1).
2.3. Eligibility Criteria
The eligibility criteria for this review included studies that examined variations in the morphology of the GV and their correlation with specific clinical conditions. Studies were deemed eligible if they met the following requirements: (1) Sample: dissections or images that demonstrated GV variations; (2) Results: reported prevalence of subjects with GV variants and their associations with abdominal pathologies; (3) Studies: this systematic review considered research articles of both retrospective and prospective observational designs, published in English in peer-reviewed journals, and indexed in relevant databases. To determine exclusion criteria, we applied the following filters: (1) Sample: studies conducted on animals; (2) studies that focused on variants unrelated to the hepatic region or its drainage area; (3) studies that included letters to the editor or commentary.
2.4. Study Selection
Three authors independently analyzed the studies to select them thoroughly. Initially, two authors (Valenzuela JJ and Salgado C) reviewed the titles and abstracts of the references retrieved from the database searches. For the selected studies, we obtained the full text of the references that any authors deemed potentially relevant. A third reviewer (Caceres C) was involved if a consensus could not be reached. To assess the reliability and the risk of bias among the observers, we performed the kappa agreement test between the authors, which yielded a score of 0.80, interpreted as indicating good agreement (Supplementary Table S2).
2.5. Data Collection Process
Two authors (Nova P and Orellana M) independently extracted data on the outcomes of each study. The data extracted from the included studies were as follows: (a) authors and year of publication, (b) total sample size and age, (c) prevalence, (d) variant characteristics, (e) regional geography, (f) sex of the sample, and (g) clinical considerations.
2.6. Assessment of the Methodological Quality of the Included Studies
To assess the bias in the included studies, we utilized the verification table for anatomical studies (AQUA) developed by the International Working Group on Evidence-Based Anatomy (IEBA) (Tomaszewski et al., 2016) [18]. Two reviewers (Valenzuela JJ and Nova P) independently examined the five domains outlined by the AQUA tool and then collaborated to build the table and construct the bias graph.
2.7. Statistical Methods
The data was analyzed using R statistical software (version 2025.05.0+496) (https://posit.co/download/rstudio-desktop/, accessed on 1 January 2025) to determine the prevalence of GV morphological variants. Summary data were combined using the DerSimonian–Laird model and a Freeman–Tukey double arcsine transformation. A random effects model was employed due to the high heterogeneity in the prevalence of GV variants. The heterogeneity among the included studies was assessed using the chi2 test and the I2 statistic. A p-value of 0.10 for the chi2 test was deemed significant, following Cochrane collaboration guidelines [18]. The I2 statistic values were interpreted within a 95% confidence interval (CI) as follows: 0–40% indicated no significant heterogeneity, 30–60% suggested moderate heterogeneity, 50–90% indicated substantial heterogeneity, and 75–100% reflected considerable heterogeneity [19]. To investigate small study effects (where smaller studies may yield different results compared to larger ones), a DOI plot incorporating the LFK index was produced [20,21].
2.8. Subgroup Analysis
We performed the same statistical analyses across each subgroup to reduce biases that might result in underestimating or overestimating the subgroup results. We also included prevalence rates for each group and qualitative assessments of their clinical implications. Ultimately, we categorized the subgroups into three classifications: imaging studies, patients, and cadaveric specimens, allowing for a thorough individual analysis for each category.
3. Results
3.1. Selection of the Articles
The search yielded 279 articles from various databases, aligning with our research team’s criteria and search terms. The filtration process focused on these articles’ titles and/or abstracts. From the initial pool of 31 articles included [8,9,10,11,12,13,14,16,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42], 11 studies were selected for inclusion [9,10,11,13,29,30,31,34,38,40,43]. These articles were chosen for their comprehensive survey of the sample, detailed statistical data for each variant, and transparent methodology (Figure 1).
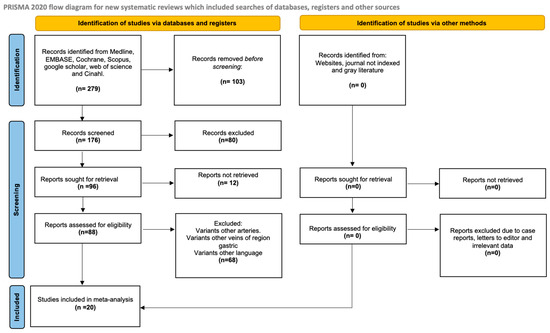
Figure 1.
Search diagram of the methodology process.
3.2. Characteristics of the Included Studies
Thirty-one studies included 47,993 subjects. Eleven studies contributed to the calculated prevalence. Geographic distribution: 17 studies from Asia, 11 from Europe, 1 from Oceania, 2 from the Americas, and 0 from Africa. Sex was reported for 3857 subjects: 2618 men and 1239 women. The mean age was 53.8 years (Table 1 and Table 2).

Table 1.
Characteristics of the included studies (ALGV—aberrant left gastric vein, CT—computed tomography, and CTA—computed tomography angiography).

Table 2.
Characteristics of the variants and clinical implications (APV = aberrant portal vein, PV = portal vein, LGV—left gastric vein, SV—splenic vein, and ALGV—aberrant left gastric vein).
3.3. Description of the Variants
GVs, especially LGV and RGV, are crucial for stomach venous drainage. The LGV collects blood from the upper stomach, including the cardia and body, ascends along the lesser curvature, and drains into the HPV. It runs near the LGA and connects to the esophagus and pylorus. The RGV drains blood from the lower stomach, including the pyloric antrum. It parallels the LGV and the lesser curvature and drains into the HPV, accompanying the right gastric artery. Understanding these veins’ pathways and significance is key to their clinical relevance.
Aberrant gastric veins (AGVs) may correspond to atypical venous vessels or morphological variants of the stomach that diverge from the standard venous paths. They can connect unusually with veins, such as the SV or mesenteric vein (MSV), thereby altering drainage patterns. For instance, ALGVs drain into branches of the HPV instead of directly, a pattern that applies to RGVs (Figure 2).
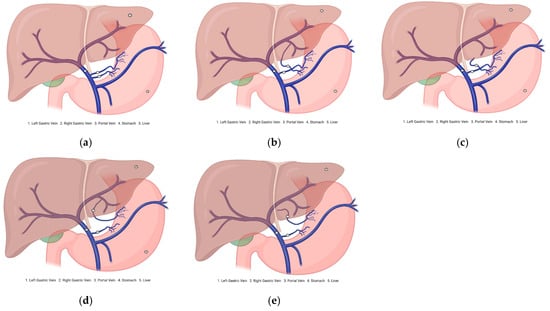
Figure 2.
Diagram of the gastric vein morphological variants. (a) Normal venous drainage of the stomach. (b) Aberrant right gastric vein drains into the portal vein branch. (c) Aberrant right gastric vein draining into the hepatic parenchyma. (d) Aberrant left gastric vein draining into the portal vein branch. (e) Aberrant left gastric vein draining into the hepatic parenchyma.
AGVs can create clinical challenges. Their unexpected locations complicate surgeries, such as gastrectomy and liver resection, where accurate anatomical knowledge is crucial. In portal hypertension, these veins can increase the risk of varices by acting as collateral pathways. Recognizing variations in imaging studies, including computed tomography angiography (CTA) and magnetic resonance angiography (MRA), is crucial for anticipating complications and enhancing patient outcomes. Furthermore, since GV morphological variations may also impact the effectiveness of treatments such as TIPS, a thorough understanding of individual venous anatomy is essential for customizing interventions. Consequently, comprehensive studies enhance the anatomical knowledge base and improve clinical practice by informing preoperative planning and management strategies. Therefore, identifying and documenting these variants in the present research can have significant implications for surgical safety and the management of gastrointestinal conditions.
3.3.1. Prevalence and Subgroups Analyzed
Four forest plots were created to calculate the prevalence of GV variants in the eleven studies [9,10,11,13,29,30,31,34,38,40,43] (Table 3). A prevalence of 8.32% (CI: 3.12–13.17) was estimated. The samples’ heterogeneity was 98.92%, indicating high variability, with a quite heterogeneous sample (Figure 3). The detection of publication bias was quantified using a DOI graph to visualize the asymmetry, with the LFK index showing a non-significant publication bias of 0.19 (Figure 4).

Table 3.
Prevalence of readable articles.
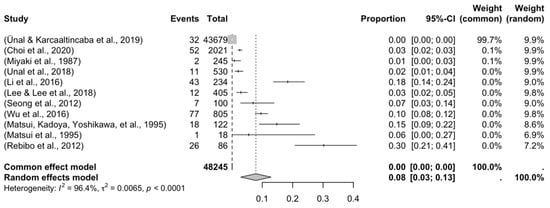
Figure 3.
Forest plot of the articles included in the meta-analysis.
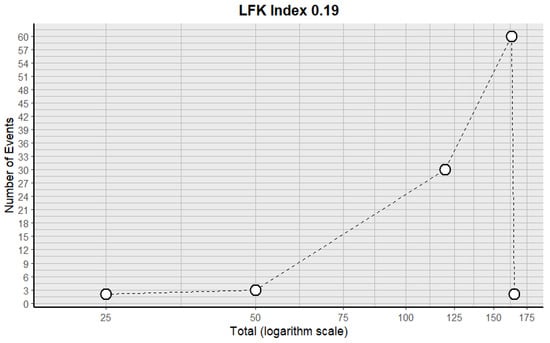
Figure 4.
DOI plot with the LFK index for the included studies.
Regarding the subgroup analysis, we grouped studies with a prevalence of no more than 50%. The first subgroup consisted of cadavers and images. In the cadaver subgroup, one study was included [34] with a prevalence of 0.082%. Among the imaging studies, 10 were included [9,10,11,13,29,30,31,34,38,40,43], revealing a prevalence of 8.18% (CI: 3.49–12.91) and a heterogeneity of 89.11%. This subgroup analysis demonstrated a significant difference, indicating a greater presence of diagnostic images (p = 0.0001).
The second subgroup analysis was for the continents from which the included studies were conducted. From Asia, eight studies were included [11,13,29,30,31,34,40,43], which presented a prevalence of 6.37% (CI: 4.19–8.98) and a heterogeneity of 88.12%. From Europe, three studies were included [9,10,38], presenting a prevalence of 2.11% (CI: 0.77-3.99) and a heterogeneity of 91.35%. Prevalence articles from America, Oceania, and Africa were not included. For this subgroup analysis, there was a significant difference in the presence of studies from Asia compared to Europe (p = 0.032).
Other subgroup analyses were performed on the sex of the subjects included. Seven studies showed males with the variant [11,13,29,30,31,40,43], which presented a prevalence of GV variants of 5.29% (CI 3.12–9.33); the heterogeneity of this comparison was 77.1%. Meanwhile, seven studies reported females with the variant [11,13,29,30,31,40,43], presenting a prevalence of 2.43% (CI 1.10–4.13) and a heterogeneity of 84.12%. For this subgroup analysis, there was a significant difference in favor of the greater presence of GV variants in males than in females (p = 0.024).
Further subgroup analyses were performed for the left gastric artery (LGA) and right gastric artery (RGA) variants. Ten studies showed LGV variants [9,10,11,13,29,30,31,38,40,43], with a prevalence of 8.18% (CI 7.01–10.12); the heterogeneity of this comparison was 77.1%. Meanwhile, three studies reported RGV variants [9,31,43], presenting a prevalence of 3.29% (CI 2.21–4.13) and a heterogeneity of 88.12%. For this subgroup analysis, there was a significant difference in favor of the greater presence of the LGV variants than the RGV variants (p = 0.012) (Table 4).

Table 4.
Subgroup analysis of the studies included in the systematic review analysis.
3.3.2. Risk of Bias in the Included Articles
Applying the AQUA checklist to the three reviewed studies allowed us to assess potential biases across the five domains: selection, performance, detection, attrition, and reporting bias. The low risk of bias suggests that these studies are reliable in providing accurate and clinically relevant information about GV morphological variations. The results are strong, enabling confident conclusions about the clinical implications of the identified variants. Figure 5 visually summarizes the assessment, highlighting the strengths of the included evidence in our evidence-based systematic review. We found a significant level of heterogeneity in the studies, primarily due to the variability in total sample sizes among studies that compare different GV variants. To address this issue, a random statistical model was employed. Additionally, a subgroup analysis was conducted, providing a more detailed examination with reduced heterogeneity. Therefore, while our results are robust, the high heterogeneity warrants caution in their interpretation.
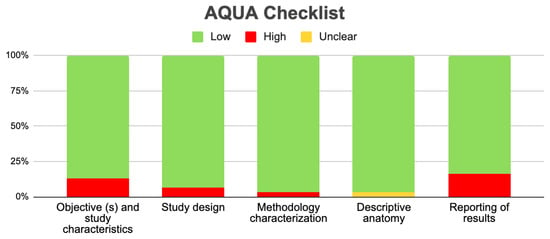
Figure 5.
Aqua chart checklist of the quality of the included studies.
4. Discussion
This systematic review investigated the GV morphological variants, with a primary emphasis on drainage variants and their relationship to the stomach. Our main finding reveals that, although these GV variants are rare in the population, they show considerable differences in their descriptions and drainage areas, which frequently complicates their understanding. The unfamiliarity with these variants can lead to difficulties in diagnosing various conditions affecting the stomach or adjacent structures, as well as intraoperative complications in different abdominal regions. Moreover, these morphological variants have been linked to a heightened risk of alterations in the venous drainage of tumors in the pyloric region and the lesser curvature of the stomach, highlighting the importance of understanding these variations.
The present systematic review compiles evidence on GV morphological variants, as no similar studies exist. Previous reviews mentioned GV variants superficially or indirectly. Frey (2022) [8] discussed the aspects of ALGV that are important for surgeons but lacked an in-depth examination of morphological variants. Meanwhile, Stefura (2018) [44] highlighted the venous trunk of Henle in portal circulation but only briefly touched on GV variants, underlining the novelty of this research. AGVs deviate from the stomach’s typical drainage, connecting abnormally to other veins, such as the SV or SMV. In portal hypertension, the AGVs can become prominent, increasing the risk of varicose veins and bleeding during abdominal surgery [27].
Regarding the quantitative results found in the present review, we discovered that the prevalence was linked to the description of a rare variant in the gastric region, defined as occurring in no more than 10% of the population. The studies analyzed for prevalence showed high heterogeneity; therefore, the reported results should be interpreted cautiously. On another note, we examined the studies that presented a quantifiable prevalence through subgroups, determining whether the sample studied consisted of cadavers or a collection of patient images. A significant difference was noted in favor of diagnosing or recognizing the variants through images, suggesting that this variant could be identified at some point in life due to a gastric alteration or surgery in the area. Regarding whether this variant produces symptoms on its own, we found no evidence to support this theory, leading us to believe that the diagnosis or discovery of this variant is random. The following subgroup analysis was by geographic region, including samples from Asia, Europe, America, Africa, or Oceania, where a statistically significant difference was found among the subjects from Asia. Two possible explanations could account for this finding: first, more studies have been conducted in that region; second, this variant may be more commonly associated with Asian populations. However, we could not find sufficient evidence to support these claims. In conclusion, our analysis indicates a significantly higher prevalence of GV variants among males. However, we lacked evidence to explain or substantiate this relationship. While these findings contribute to understanding the characteristics of GV variants, they should be interpreted cautiously, considering that some studies report a high overall prevalence. Additionally, this systematic review identifies several GV variants that are associated with significant clinical implications, particularly in abdominal conditions requiring surgical intervention [15]. These variants may facilitate surgical procedures, enhance venous drainage, or improve blood flow under certain physiological conditions. Such findings highlight the necessity of a thorough understanding of GV anatomy for clinicians, especially in surgical contexts where awareness of these variants can lead to better outcomes and fewer complications. Recognizing and documenting these morphological variations can also assist in preoperative planning and managing issues like portal hypertension [45]. According to Unal’s (2019) [9] classification, ALGV Type III signifies a complete anastomosis between the ALGV and the LPV. This variant preserves hepatic flow due to the anastomosis in patients with main HPV thrombosis, serving as a decompression pathway that significantly reduces the development and severity of extensive gastric variceal bleeding in cirrhotic patients with hypertensive gastropathy [27]. In instances of main HPV thrombosis, the ARGV can also sustain adequate hepatic flow, because the venous drainage of the lesser curvature of the stomach can operate independently of the main HPV, maintaining the patency of the intrahepatic portal system [24]. In the context of significant clinical procedures, portosystemic shunts, which can be total or intrahepatic, are employed. These shunts have defined indications and effects and are crucial in minimizing the risk of variceal bleeding, a potentially life-threatening complication in patients with portal hypertension. Total portosystemic shunts redirect all portal circulation to the vena cava, thus eliminating hepatoportal flow, which may affect liver function and increase the risk of encephalopathy [46]. The transjugular intrahepatic portosystemic shunt (TIPS) procedure, performed by interventional radiology, aims to lower the hepatic venous pressure gradient to below 12 mmHg. Using ultrasound (US), a catheter is inserted through the right internal jugular vein into the RHV, connecting it with the intrahepatic PV. The tract is dilated with an angioplasty balloon, and a stent is placed, resulting in a reduced portal pressure gradient and decreased blood flow to collaterals like esophageal and gastric varices [46]. The ARGV acts as an aberrant PV (APV) for TIPS patients whose main PV trunks cannot be recanalized. In cases of HPV thrombosis, when recanalization fails, this variant may be the only pathway for TIPS placement [14].
In addition, 14 studies were identified that described pathological considerations regarding GV variants [8,10,11,12,13,22,25,26,31,33,37,41,42]. Variceal bleeding is a common complication in patients with portal hypertension, directly linked to the LGV, as it is the primary source of blood supply for esophagogastric varices [11]. Such bleeding is more frequent in patients whose LGV venous diameter exceeds 5–6 mm, a parameter indicative of portal hypertension [11]. Furthermore, in 33.3% of patients with ALGV, atrophy of the second hepatic segment is observed, producing pseudolesions due to the imbalance in hepatic blood flow [8]. Additionally, ALGV provides a direct route for the periportal spread of gastric tumors through the gastrohepatic ligament, facilitating metastasis in the left hepatic lobe. An even lower survival rate is estimated in cases of gastric cancer with venous invasion facilitated by this variant [8]. The presence of ALGV was associated with HPV gas affecting only the left hepatic lobe due to drainage from the variant into the LPV in a patient with gastric pneumatosis secondary to a hiatal hernia [33]. Therefore, identifying variants in GV drainage through imaging is essential to prevent invasive procedures, enhance the understanding of related hepatic pseudolesions, and improve the monitoring of gastric cancer, particularly given the presence of these variants in cases of venous–hepatic metastasis [42].
Additionally, six imaging studies were identified [23,34,35,36,39,47]. The US study of the LGV termination defines its anatomical relationship with the adjacent vessels and drainage [34]. The CTA study of the ARGV is relevant when evaluating hepatic metastatic diseases, since hepatic vascular variants can be confused with more serious pathologies [47]. Three-dimensional CTA is essential for visualizing the peri-gastric anatomy preoperatively. Additionally, knowing about vascular anomalies, such as ALGV, in the liver vasculature is crucial for avoiding unnecessary invasive procedures and linking them to pseudolesions in the liver parenchyma [23]. Therefore, accurate imaging analysis is crucial for the differential diagnosis of different liver conditions. To optimize the clinical outcomes and reduce the perioperative risk, the integration of advanced imaging techniques such as CTA and magnetic resonance angiography (MRA) is strongly recommended for preoperative and diagnostic evaluation of gastric venous anatomy. CTA offers high spatial resolution and rapid acquisition, making it ideal for delineating venous drainage patterns, identifying anomalous venous pathways, and assessing their relationship with adjacent organs and vessels, particularly in patients with portal hypertension, hepatic malignancies, or those undergoing gastrectomy or bariatric procedures. In contrast, MRA, while slightly less spatially precise, provides excellent soft tissue contrast without ionizing radiation or iodinated contrast, which is advantageous in patients with renal dysfunction or requiring serial assessments. It is beneficial in assessing the flow characteristics, collateral formation, and chronic changes in venous morphology. Clinicians and surgeons should implement routine pre-interventional CTA or MRA in high-risk cases, including patients with suspected venous anomalies, those scheduled for laparoscopic gastric surgery, and individuals with cirrhosis or portal vein thrombosis, to enhance the anatomical precision and guide the intraoperative navigation. Moreover, adopting 3D reconstruction and vessel segmentation software (version 11.1.2) can facilitate multidisciplinary planning, enabling safer ligation, anastomosis, or shunt placement and reducing the likelihood of inadvertent vascular injury. Finally, seven studies have been identified that describe key surgical considerations in the management of patients with GV variants [14,16,28,29,31,38]. In this context, the gastrectomy procedure, primarily performed laparoscopically, is categorized into three types: atypical, subtotal, and total gastrectomy. Atypical gastrectomy is for resecting gastrointestinal stromal tumors with specific criteria. Subtotal gastrectomy partially removes the stomach, depending on pathology, possibly taking one-half, two-thirds, or three-fifths of it. An anastomosis connects the gastric stump to the duodenum (Pean Billroth I) or jejunum (Billroth II) to restore digestive continuity. Total gastrectomy removes the stomach and connects the jejunum with the esophagus or a Roux-en-Y loop [48]. The surgical technique adapts to the patient’s characteristics and the surgical team’s preferences. The procedure starts with the patient supine in the anti-Trendelenburg position, establishing pneumoperitoneum and placing five trocars, typically in the right and left anterior axillary line, left flank, and subxiphoid region. Next, the greater curvature is dissected, ligating the gastric vessels while preserving the gastroepiploic vessels for greater omentum irrigation. The lesser omentum is then sectioned, and the pyloric artery is ligated about 2–3 cm from the duodenum to enable the release and sectioning of the duodenal bulb, which is subsequently closed [49,50]. Depending on the objectives, gastrectomy can be partial or total, involving gastrojejunal anastomosis and stomach resection. The excised gastric portion is often not removed during gastric bypass; when needed, it is extracted via the umbilical route [48]. In the Type II variant [29], where the LGV runs anterior to the CA draining into the HPV, LGV injury during laparoscopic gastrectomy is 5. 5.8%, significantly lower than in variants I and III. Type II LGV is easily detectable without complete lymph node dissection. Injuries to LGVs with anterior drainage (Types Ia, II, and IIIa) were less common than with posterior drainage (Types Ip and IIIp) [29]. If the LGV is not visible anteriorly, surgeons must carefully avoid injuring it among the CHA or SA lymph nodes during standard gastrectomy, which involves re-sectioning at least two-thirds of the stomach [29]. In cases of ALGV and ALHA, the ALGV is divided at the root while preserving the ALHA, with only the branches extending into the stomach being divided to prioritize patient safety [28]. Pancreatectomy treats tumors in the pancreas or lower common bile duct, performed openly or laparoscopically, depending on the disease extent, involving resections of various pancreatic portions [51]. The open technique uses a supraumbilical incision to access the pancreas and surrounding structures, starting with greater omentum dissection, which separates the pancreatic isthmus from the portal vein. The trunk of Henle is ligated to prevent hemorrhage. Cephalic or total pancreatectomy requires cholecystectomy and bile duct mobilization to ensure adequate resection and reduce complications [51]. Confirming LGV anatomy is crucial before pancreatectomy, as damage can lead to ischemic gastric complications requiring additional gastrectomy. A portal phase abdominal CT scan with contrast visualizes the LGV and variants [38]. Pancreatectomy is a surgical procedure often performed for tumor pathologies affecting the pancreas or the lower common bile duct. It can be executed using open or laparoscopic techniques involving the resection of various portions of the pancreas: cephalic, left, medial, or total, depending on the extent of the disease [51]. The open technique involves a supraumbilical incision to access the pancreas and nearby structures, including the trunk of Henle, formed by the right gastroepiploic, middle colic, and inferior pancreaticoduodenal veins. Surgery begins with cutting the greater omentum outside the GEVs, freeing the pancreatic isthmus from the PV. Next, the trunk of Henle is ligated to prevent bleeding. For cephalic or total pancreatectomy, cholecystectomy and bile duct release are necessary for proper resection and to avoid complications [51]. Establishing the anatomy of the LGV is crucial before a pancreatectomy, as damage to this vein can lead to ischemic gastric complications and possible gastrectomy. Thus, a portal phase abdominal CT scan with contrast helps visualize the LGV and its variants [38]. When the LGV terminates at the SV or between the HPV and SV confluence, dissection must be cautious; during pancreatectomy, ligate the SV above its junction with the LGV [38]. In cases of venous resection, care should be taken to ensure that the LGV is connected to the HPV axis (either by repositioning the SV or by anastomosis directly to the HPV), as when the LGV terminates at the HPV, there is little risk of damage during a pancreatectomy [38] (Table 5 and Table 6).

Table 5.
Summary of clinical considerations (LGV—left gastric vein).

Table 6.
Summary subgroups: imaging, cadaver, or in vivo (surgery).
GV variants, particularly aberrant drainage patterns involving the LGV, short GVs, or posterior GVs, can significantly influence portal hemodynamics and predispose individuals toward various clinical complications. For instance, in patients with portal hypertension, atypical drainage of the LGV directly into the systemic circulation or the azygos system can serve as a collateral channel, exacerbating the formation of esophageal or gastric varices and increasing the risk of life-threatening upper gastrointestinal bleeding. Similarly, variations in which GVs drain into the SV or inferior mesenteric veins may alter regional pressure gradients, contributing to localized portal hypertensive gastropathy or even segmental colitis resulting from venous congestion. Furthermore, in surgical contexts, unrecognized variants may lead to accidental ligation or injury of ectopic GVs, resulting in intraoperative hemorrhage or postoperative ischemia of gastric tissue. These pathophysiological cascades underscore the clinical importance of preoperative imaging and thorough venous mapping in patients undergoing upper abdominal surgeries or interventional procedures for portal hypertension [52,53,54,55,56,57,58,59,60,61].
Our results aligned with past studies highlighting the significance of venous variants in gastrointestinal complications. Our analysis offers a detailed characterization of these variants and their clinical correlations, showcasing a comprehensive systematic review. Detecting variants via imaging is vital for diagnosing and managing related pathologies. Therefore, we advocate for the use of advanced imaging techniques in patients with gastrointestinal complications. Previous studies by Miyaki (1987) and Yamagami (1999) [34,47] emphasized the roles of US and CTA in assessing GV drainage, especially for aberrant variants. Future research ought to pursue longitudinal studies examining the long-term effects of these variants on gastrointestinal health, in conjunction with more extensive studies utilizing standardized classification methods. This study emphasizes the significance of GV variants in clinical complications, advocating for their consideration in clinical practice to enhance surgical planning and management for patients at risk of portal hypertension, thereby ultimately improving clinical and surgical outcomes.
5. Conclusions
Variants of abdominal blood vessels can be remarkably intricate, and if not identified promptly, they may complicate diagnostic and surgical procedures. Consequently, it is imperative to recognize these variants through diagnostic or pre-surgical imaging to ensure the accurate diagnosis and management of conditions or surgical complications that may involve the GV and adjacent areas. Furthermore, in the context of pathologies such as portal hypertension, a comprehensive understanding of the vascular anatomy of the stomach is essential to prevent complications that may arise from increased blood flow through the PV. To optimize the outcomes and reduce the perioperative risk, integrating advanced imaging techniques like CTA and magnetic resonance angiography (MRA) is recommended for the preoperative evaluation of gastric venous anatomy. Ultimately, we believe that diagnostic studies focused on enhancing our understanding of these variants and their clinical implications could significantly contribute to more comprehensive anatomical and clinical knowledge of the matter.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm14113630/s1, Table S1: Details of the search strategy. Table S2. Excluded studies and the reasons for their exclusion.
Author Contributions
Methodology, A.B.-M., C.S.-T., B.R.-O., J.S.-G. and J.J.V.-F.; Software, M.O.-D., A.B.-M. and C.C.-G.; Validation, C.C.-G., C.S.-T. and J.J.V.-F.; Formal analysis, P.N.-B., A.S.-S. and J.J.V.-F.; Investigation, G.T., P.N.-B., M.O.-D., J.J.V.-F. and G.O.-A.; Resources, A.B.-M. and M.K.; Data curation, P.N.-B., M.P., M.O.-D., A.S.-S. and A.B.-M.; Writing—original draft, A.S.-S., G.T. and M.P.; Supervision, G.T., M.K. and J.J.V.-F.; Project administration, J.S.-G.; Funding acquisition, J.J.V.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We would like to thank the “Article Processing Payment Support Program 2025, General Directorate of Research, Universidad Playa Ancha, Chile” for the payment of open access.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
HPV: hepatic portal vein, LGV: left gastric vein, RGV: right gastric vein, CA: celiac axis, SV: splenic vein, ALGV: aberrant left gastric vein, LPV: left portal vein, CHA: common hepatic artery, SA: splenic artery, ARGV: aberrant right gastric vein, TIPS: transjugular intrahepatic portosystemic shunt, ALHA: aberrant left hepatic artery, RGA: right gastric artery, and LGA: left gastric artery.
References
- Arviza, P.; Bombín, A.; Arrazola, J.; de Blas, C.S.; Talarico, E.F.; Bartolomé, A.M.P.; Gonzalez, A.V.; Gonzalez, L.E.; Rodriguez, C.S.; Munoz, M.D.; et al. Comparative anatomo-radiological study of intrahepatic venous vascularization in Spain. Ann. Anat. 2021, 237, 151740. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzade, M.; Akhlaghpoor, S.; Rouientan, H.; Hassanzadeh, S.; Ghorani, H.; Heidari-Foroozan, M.; Fathi, M.; Alemi, F.; Nouri, S.; Trinh, K.; et al. Splenic artery embolization for variceal bleeding in portal hypertension: A systematic review and meta-analysis. Emerg. Radiol. 2025, 32, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Akcali, A.; Ogan, B.; Buğra Sezen, C.; Metin, M. Giant left gastric artery aneurysm with intrathoracic extension. Saudi Med. J. 2025, 46, 199–201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cohen, D.L.; Bermont, A.; Richter, V.; Shirin, H. Real world management of esophageal ulcers: Analysis of their presentation, etiology, and outcomes. Acta Gastroenterol. Belg. 2021, 84, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Rouviere, H.; Delmas, A. Human Anatomy, Descriptive, Topographical and Functional. Trunk, 11th ed.; Masson Publishing: Issy-les-Moulineaux, France, 2005. [Google Scholar]
- Dalley, A.F., II; Agur, A.M.R. Moore’s Clinically Oriented Anatomy, 9th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2023. [Google Scholar]
- Loukas, M.; Louis, R.G., Jr.; Hullett, J.; Loiacano, M.; Skidd, P.; Wagner, T. An anatomical classification of the variations of the inferior phrenic vein. Surgical and radiologic anatomy. Surg. Radiol. Anat. 2005, 27, 566–574. [Google Scholar] [CrossRef]
- Frey, S.; De Mathelin, P.; Bachellier, P.; Addeo, P. Aberrant left gastric vein: What should surgeons know? Surg. Radiol. Anat. 2022, 44, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Unal, E.; Karcaaltincaba, M. Aberrant left gastric vein is associated with hepatic artery variations. Abdom. Radiol. 2019, 44, 3127–3132. [Google Scholar] [CrossRef] [PubMed]
- Unal, E.; Karaosmanoglu, A.D.; Ozmen, M.N.; Akata, D.; Karcaaltincaba, M. Computed Tomography-Based Diagnosis of Gastric Vein Invasion in Patients with Gastric Cancer. Eurasian J. Med. 2018, 50, 91–95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, C.Y.; Gao, B.L.; Song, B.; Fan, Q.Y.; Zhou, L.X.; Feng, P.Y.; Zhang, X.J.; Zhu, Q.F.; Xiang, C.; Peng, S.; et al. Evaluation of left gastric vein in Chinese healthy adults with multi-detector computed tomography. Postgrad. Med. 2016, 128, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Arhire, D.A.; Moraru, M.C.; Maxim, R.; Tarniceriu, C.C.; Partene Vicoleanu, S.A.; Haisan, A.; Nedelcu, I.; Nedelcu, A.H. Transient hepatic attenuation difference or fat sparing? Aberrant right gastric vein determining a pseudolesion at the border of the IInd/IIIrd liver segments. Review of developmental concepts. Folia Morphol. 2024, 83, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.W.; Chung, J.W.; Kim, H.C.; Choi, J.W.; Lee, M.; Hur, S.; Jae, H.J. Aberrant gastric venous drainage and associated atrophy of hepatic segment II: Computed tomography analysis of 2021 patients. Abdom. Radiol. 2020, 45, 2764–2771. [Google Scholar] [CrossRef] [PubMed]
- Bezzi, M.; Broglia, L.; Lemos, A.A.; Rossi, P. Transjugular intrahepatic portosystemic shunt in portal vein thrombosis: Role of the right gastric vein with anomalous insertion. Cardiovasc. Intervent Radiol. 1995, 18, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Brandner, G.; Parrado, R.H.; Dias, A.P.; Pullat, R.C.; Lancaster, W.P. Gastric Outlet Obstruction From Preduodenal Portal Vein in an Adult. Am. Surg. 2023, 89, 6212–6214. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, M. Aberrant left gastric vein directly draining into the liver. Clin Anat. 2000, 13, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tomaszewski, K.A.; Henry, B.M.; Kumar Ramakrishnan, P.; Roy, J.; Vikse, J.; Loukas, M.; Tubbs, R.S.; Walocha, J.A. Development of the Anatomical Quality Assurance (AQUA) checklist: Guidelines for reporting original anatomical studies. Clin. Anat. 2017, 30, 14–20. [Google Scholar] [CrossRef] [PubMed]
- von Hippel, P.T. The heterogeneity statistic I(2) can be biased in small meta-analyses. BMC Med. Res. Methodol. 2015, 15, 35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fu, R.; Gartlehner, G.; Grant, M.; Shamliyan, T.; Sedrakyan, A.; Wilt, T.J.; Griffith, L.; Oremus, M.; Raina, P.; Ismaila, A.; et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J. Clin. Epidemiol. 2011, 64, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Furuya-Kanamori, L.; Barendregt, J.J.; Doi, S.A.R. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int. J. Evid. Based Healthc. 2018, 16, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Caty, L.; Denève, E.; Fontaine, C.; Guillem, P. Concurrent aberrant right gastric vein directly draining into the liver and variations of the hepatic artery. Surg. Radiol. Anat. 2004, 26, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Deger, S.; Bozer, A. Liver Pseudotumor Due to Aberrant Left Gastric Vein: A Case Report. J. Belg. Soc. Radiol. 2023, 107, 82. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deneve, E.; Caty, L.; Fontaine, C.; Guillem, P. Simultaneous aberrant left and right gastric veins draining directly into the liver. Ann. Anat. 2003, 185, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Gabata, T.; Matsui, O.; Kadoya, M.; Ueda, K.; Kawamori, Y.; Yoshikawa, J.; Takashima, T. Aberrant gastric venous drainage in a focal spared area of segment IV in fatty liver: Demonstration with color Doppler sonography. Radiology 1997, 203, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Hiwatashi, A.; Yoshimitsu, K.; Honda, H.; Kuroiwa, T.; Irie, H.; Tajima, T.; Jimi, M.; Chijiiwa, K.; Masuda, K. Pseudolesion in segment II of the liver observed on CT during arterial portography caused by the aberrant left gastric venous drainage. Abdom. Imaging 1999, 24, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, K.; Sun, S.; Berst, M.J.; Heery, S.D.; Fajardo, L.L. Portal vein occlusion with aberrant left gastric vein functioning as a hepatopetal collateral pathway. J. Vasc. Interv. Radiol. 2004, 15, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Kuwada, K.; Kuroda, S.; Kikuchi, S.; Hori, N.; Kubota, T.; Nishizaki, M.; Kagawa, S.; Fujiwara, T. Strategic approach to concurrent aberrant left gastric vein and aberrant left hepatic artery in laparoscopic distal gastrectomy for early gastric cancer: A case report. Asian J. Endosc. Surg. 2015, 8, 454–456. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J. Anatomic variations in the left gastric vein and their clinical significance during laparoscopic gastrectomy. Surg. Endosc. 2019, 33, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
- Matsui, O.; Kadoya, M.; Takahashi, S.; Yoshikawa, J.; Gabata, T.; Takashima, T.; Kitagawa, K. Focal sparing of segment IV in fatty livers shown by sonography and CT: Correlation with aberrant gastric venous drainage. AJR Am. J. Roentgenol. 1995, 164, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Matsui, O.; Takahashi, S.; Kadoya, M.; Yoshikawa, J.; Gabata, T.; Takashima, T.; Kitagawa, K. Pseudolesion in segment IV of the liver at CT during arterial portography: Correlation with aberrant gastric venous drainage. Radiology 1994, 193, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Matsui, O.; Kadoya, M.; Yoshikawa, J.; Gabata, T.; Takahashi, S.; Ueda, K.; Kawamori, Y.; Takashima, T.; Nakanuma, Y. Aberrant gastric venous drainage in cirrhotic livers: Imaging findings in focal areas of liver parenchyma. Radiology 1995, 197, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Mittal, K.; Anandpara, K.; Dey, A.K.; Kedar, P.; Hira, P.; Kale, S. Left Aberrant Gastric Vein Causing Isolated Left Hepatic Portal Venous Gas Secondary to an Incarcerated Diaphragmatic Hernia. Pol. J. Radiol. 2015, 80, 364–367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miyaki, T.; Yamada, M.; Kumaki, K. Aberrant course of the left gastric vein in the human: Possibility of a persistent left portal vein. Cells Tissues Organs 1987, 130, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, G.C.; Fraum, T.J. Aberrant right gastric vein mimicking hepatic spread of prostate cancer on PSMA-PET/CT. Radiol. Case Rep. 2023, 18, 1140–1143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Natsume, T.; Shuto, K.; Kohno, T.; Ohira, G.; Tohma, T.; Sato, A.; Saito, H.; Ohta, T.; Kawahira, H.; Akai, T.; et al. Anatomic Variations of the Celiac Trunk and the Left Gastric Vein Assessing by Dual-Phase CT Angiography for Safety Laparoscopic Gastrectomy. J. Surg. Res. 2010, 158, 387–388. [Google Scholar] [CrossRef]
- Alfaro, A.J.; Herrera Ortiz, A.F.; Cardona Ortegón, J.D.; Varney, H.; Borrero León, R.; Torres, D.F.; Greiffenstein, J.; Dussan Tovar, C.A.; Aguirre, D.A. Aberrant Right and Left Gastric Veins as a Cause of Hepatic Pseudolesions: A Report of Three Cases. Cureus 2023, 15, e48455. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rebibo, L.; Chivot, C.; Fuks, D.; Sabbagh, C.; Yzet, T.; Regimbeau, J.M. Three-dimensional computed tomography analysis of the left gastric vein in a pancreatectomy. HPB 2012, 14, 414–421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roi, D.J. Ultrasound anatomy of the left gastric vein. Clin. Radiol. 1993, 47, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Seong, N.J.; Chung, J.W.; Kim, H.C.; Park, J.H.; Jae, H.J.; An, S.B.; Cho, B.H. Right gastric venous drainage: Angiographic analysis in 100 patients. Korean J. Radiol. 2012, 13, 53–60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Terayama, N.; Matsui, O.; Tatsu, H.; Gabata, T.; Kinoshita, A.; Hasatani, K. Focal sparing of fatty liver in segment II associated with aberrant left gastric vein. Br. J. Radiol. 2004, 77, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Unal, E.; Ozmen, M.N.; Akata, D.; Karcaaltincaba, M. Imaging of aberrant left gastric vein and associated pseudolesions of segments II and III of the liver and mimickers. Diagn. Interv. Radiol. 2015, 21, 105–110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, Y.; Chen, G.; Wu, P.; Zhu, J.; Peng, W.; Xing, C. CT imaging-based determination and classification of anatomic variations of left gastric vein. Surg. Radiol. Anat. 2017, 39, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Stefura, T.; Kacprzyk, A.; Droś, J.; Pędziwiatr, M.; Major, P.; Hołda, M.K. The venous trunk of henle (gastrocolic trunk): A systematic review and meta-analysis of its prevalence, dimensions, and tributary variations. Clin. Anat. 2018, 31, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Prado Neto, E.V.; Petroianu, A. Anatomical variations of portal venous system: Importance in surgical clinic. Arq. Bras. Cir. Dig. 2022, 35, e1666. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- García-Pagán, J.C. Derivación portosistémica, percutánea e intrahepática recubierta [Covered transjugular intrahepatic portosystemic shunts]. Gastroenterol. Hepatol. 2006, 29, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, T.; Nakamura, T.; Maeda, T. Aberrant gastric venous inflow to the left lobe of the liver parenchyma adjacent to the falciform ligament. Br. J. Radiol. 1999, 72, 903–905. [Google Scholar] [CrossRef] [PubMed]
- Saad, P.F.; Razuk, A.; Telles, G.J.; Park, J.H.; Esteves, F.P.; Caffaro, R.A. Trashepatic left gastric vein embolization in the treatment of recurrent hemorrhaging in patients with schistosomiasis previously submitted to non-derivative surgery. Arq. Gastroenterol. 2012, 49, 238–244. [Google Scholar] [CrossRef]
- Cheng, W.; Wang, K.Y.; Li, W.Q.; Li, Y.; Li, X.Y.; Ju, S. CT-based nomogram predicts esophageal gastric variceal bleeding in noncirrhotic portal hypertension caused by hepatic schistosomiasis. BMC Med. Inform. Decis. Mak. 2025, 25, 8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsuki, M.; Kani, H.; Tatsugami, F.; Yoshikawa, S.; Narumi, Y. Preoperative assessment of vascular anatomy around the stomach by 3D imaging using MDCT before laparoscopy-assisted gastrectomy. Am. J. Roentgenol. 2004, 183, 145–151. [Google Scholar] [CrossRef]
- Harada, S.; Yamamoto, A.; Jogo, A.; Kageyama, K.; Nakano, M.; Murai, K.; Matsushita, K.; Nishida, N.; Kaminou, T.; Miki, Y. Transhepatic Antegrade Gastric Variceal Sclerotherapy: Comparing Outcomes with and without Initial Efferent Vein Embolization. J. Vasc. Interv. Radiol. 2025, 36, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, M.S.; Kavanagh, P.V.; Chen, M.Y. Noninvasive assessment of portomesenteric venous thrombosis: Current concepts and imaging strategies. J. Comput. Assist. Tomogr. 2002, 26, 392–401. Available online: https://journals.lww.com/jcat/fulltext/2002/05000/Systolically_Gated_3D_Phase_Contrast_MRA_of.00014.aspx (accessed on 19 May 2025). [CrossRef]
- Murphy, D.J.; Aghayev, A.; Steigner, M.L. Vascular CT and MRI: A practical guide to imaging protocols. Insights Imaging 2018, 9, 905–921. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.I.; Ko, J.M.; Ahn, M.I.; Han, D.H.; Park, S.H. Dynamic CT and MRA findings of a case of portopulmonary venous anastomosis (PPVA) in a patient with portal hypertension: A case report and review of the literature. Acta Radiol. 2011, 52, 1044–1047. [Google Scholar] [CrossRef]
- Sahani, D.; Mehta, A.; Blake, M.; Prasad, S.; Harris, G. Preoperative hepatic vascular evaluation with CT and MR angiography: Implications for surgery. Radiographics 2004, 24, 1367–1380. [Google Scholar] [CrossRef]
- Young, V.; Rajeswaran, S. Management of portal hypertension in the pediatric population: A primer for the interventional radiologist. Semin. Interv. Radiol. 2018, 35, 174–184. [Google Scholar] [CrossRef]
- Laissy, J.P.; Trillaud, H.; Douek, P. MR angiography: Noninvasive vascular imaging of the abdomen. Abdom. Imaging 2002, 27, 553–569. [Google Scholar] [CrossRef]
- Malviya, K.K.; Verma, A. Importance of anatomical variation of the hepatic artery for complicated liver and pancreatic surgeries: A review emphasizing origin and branching. Diagnostics 2023, 13, 1233. [Google Scholar] [CrossRef] [PubMed]
- Patino, M.; Price, M.; Sahani, D. Computed tomography angiography of the hepatic, pancreatic, and splenic circulation. Radiol. Clin. N. Am. 2016, 54, 1173–1190. [Google Scholar] [CrossRef]
- Hoyos, S.; Vera, A.; Barragán, C.; Bejarano, D.; Lopez-Ruiz, A.M.; Grippi, F.; Mejia, A.; Molano, M.D.P. Surgical and interventional radiology management of vascular and biliary complications in liver transplantation: Narrative review. Dig. Dis. Interv. 2024, 8, 7–17. [Google Scholar] [CrossRef]
- Arkadopoulos, N.; Yiallourou, A.I.; Palialexis, C.; Stamatakis, E.; Kairi-Vassilatou, E.; Smyrniotis, V. Inferior vena cava obstruction and collateral circulation as unusual manifestations of hepatobiliary cystadenocarcinoma. Hepatobiliary & pancreatic diseases international. Hepatobiliary Pancreat Dis. Int. 2013, 12, 329–331. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).