Abstract
Thrombotic microangiopathy (TMA) has been observed in some patients receiving interferon beta (IFNβ) therapy for relapsing-remitting multiple sclerosis, but little is known about its clinical features and outcomes. We searched the literature to identify cases with IFNβ-related TMA and assessed their pattern of organ involvement, the presence of prodromal manifestations, the treatments used, and the outcomes. Thirty-five articles met the inclusion criteria, and data of 67 patients were collected. The median duration of IFNβ therapy before the diagnosis of TMA was 8 years, and 56/67 (84%) presented with acute kidney injury (AKI), of which 33 required acute dialysis. All but three patients had manifestations during the four weeks before TMA onset, including flu-like symptoms, headache, and worsening blood pressure control. In only two patients, ADAMTS13 activity was reduced, while 27% had low C3 levels. However, none showed causative genetic mutations associated with development of atypical hemolytic uremic syndrome. All patients discontinued IFNβ, 34 (55%) also received plasma exchange, and 12 (18%) received eculizumab. Complete renal recovery was achieved by 20 patients (30%), while 13 (20%) developed end-stage renal disease. Among those with AKI requiring dialysis, eculizumab therapy was associated with a significantly reduced risk of ESRD compared with plasma exchange. Therefore, TMA with features of aHUS mainly occurs after prolonged treatment with IFNβ and is preceded by prodromes, which may lead to an early diagnosis before life-threatening complications occur. Eculizumab appears beneficial in cases with severe kidney involvement, which supports a role of the complement system in the pathogenesis of these forms.
1. Introduction
Multiple sclerosis (MS) is the most frequent inflammatory demyelinating disease of the central nervous system, usually affecting young adults. The treatment of MS has substantially evolved over the last 20 years. Most immunosuppressive treatments have proven beneficial and have been approved mainly for the treatment of relapsing-remitting MS (RRMS) [1], while the management of the progressive forms remains challenging [2]. First-generation injectable disease-modifying therapies in RRMS include interferon beta (IFNβ) 1a or 1b and glatiramer acetate. Despite the well-known efficacy and long-term tolerability of IFNβ, a growing number of reports have recently associated long-term IFNβ therapy with the development of systemic adverse effects that affect multiple organs, including the kidneys, the skin, and the bone marrow [3,4]. The occurrence of such adverse effects is associated with both drug serum levels [5] and the duration of the treatment [6]. The kidney is particularly involved in IFNβ side effects with several patterns of toxicity ranging from isolated hypertension [6] to immune-mediated complications, such as podocytopathies, glomerulonephritis, acute tubular necrosis, (acute and chronic) tubulo-interstitial nephritis, or thrombotic microangiopathy (TMA) [7,8,9].
The occurrence of TMA has been described in a few patients under treatment with IFNβ, mostly as individual case reports or small case series [9,10,11]. Intriguingly, a recent study described a dose-dependent toxic effect of the IFNβ on the microvasculature [12], suggesting a role of this therapy in endothelial damage, which may result in TMA, thus challenging the hypothesis of a fortuitous association. Furthermore, a growing number of therapies has been associated with TMA, including calcineurin and mTOR inhibitors [13]. TMA is characterized by thrombocytopenia, microangiopathic hemolytic anemia and signs of ischemic damage in different organs (such as kidney involvement). Notably, it is increasingly recognized that TMA may present with hypertension or renal function impairment with no or mild thrombocytopenia and microangiopathic hemolytic anemia [13,14,15]. Awareness of the full spectrum of TMA is needed in order to recognize it in a timely manner and start treatment before serious complications or death occurs [16].
As the presentation and timing of TMA associated with IFNβ varies widely, it is possible that multiple pathophysiologic mechanisms are involved, such as (i) an increased expression of markers of platelet activation, which has been described in patients with MS [17]; (ii) the shift from the physiological antithrombotic profile of the endothelium to an exaggerated antiangiogenic and antifibrinolytic activity through the inhibition of the VEGF gene transcription, which may be promoted by type 1 IFNs [12]; (iii) activation of the thromboxane receptor and increased production of inflammatory mediators in response to IFNs [18]; (iv) dose-dependent microvascular pathological changes correlated with transcriptional activation of the IFN response through the type I interferon α/β receptor (IFNAR) [19]; (v) direct damage of endothelial cells through the induction of antibodies, such as anticardiolipin or anti-ADAMTS13 antibodies; (vi) the mutual activation of the complement and IFN pathways, resembling an “interferon-complement loop” [20]; (vii) IFNβ-induced podocyte loss and death together with the suppression of podocyte progenitor regeneration [21], leading to proteinuria, hypertension, and glomerulosclerosis [7,22,23], which can subsequently trigger a glomerulopathy-related TMA [24].
The estimated incidence of IFNβ-related TMA in patients with MS was reported to be 7.2 per 100,000 patient-years [25], but this figure is expected to increase as the number of MS patients treated with IFNβ for more than 6 to 10 years has been growing. The real incidence of IFN-related TMA remains undetermined, and it seems plausible that many cases are not recognized. Furthermore, its prognosis remains largely unclear. To improve our understanding of IFNβ-related TMA, we conducted a review of the literature and studied risk factors for its development, its clinical prodromes and presentation at onset, the efficacy of the available treatments, and the disease outcome, with particular reference to kidney failure.
2. Materials and Methods
2.1. Literature Review
The PubMed databases were searched in order to identify publications about INFβ-associated TMA in MS from 1999 to 2023, using the following medical subject heading terms: “thrombotic microangiopathy”, “interferon”, “interferon-beta”, “multiple sclerosis”, “haemolytic uremic syndrome”, and “thrombotic thrombocytopenic purpura”. We reviewed all articles written in English, and we selected those that provided individual data of patients with IFNβ-associated TMA. We collected information on drug dose, duration of therapy, manifestations in the week or months before hospital admission, laboratory tests (hemoglobin, platelet, serum creatinine, 24 h proteinuria, and C3 and C4 levels) at onset and throughout follow-up, genetic analysis, treatment performed (plasma exchange, eculizumab or other immunosuppressive drugs), and kidney outcome at last follow-up.
2.2. Author Survey
Moreover, we conducted a survey and asked all authors of the selected articles to provide additional information regarding genetic analysis (underlying causative mutations in complement genes), activation of serum complement pathways, renal biopsy features, clinical prodromes during weeks before TMA onset, response to therapy, and long-term outcome.
2.3. Definitions
- −
- Thrombotic microangiopathy (TMA) is defined as a disorder characterized by endothelial cell injury and activation with thrombotic occlusion of the small vessels that results in consumptive thrombocytopenia, microangiopathic hemolytic anemia and signs of ischemic damage in different organs [13]. The kidney is frequently involved as a result of endothelial cell injury and thrombosis of the glomerular capillaries. Laboratory findings suggestive of TMA include anemia, increased serum lactate dehydrogenase (LDH), haptoglobin consumption and schistocytes (erythrocyte fragmentation) on a blood film due to hemolysis, decreased platelet count, and kidney injury. If clinical features of TMA are observed, further testing of complement factor levels, such as C3 and C4, as well as CH50 and APH50 is recommended along with tight monitoring of blood pressure and fluid status. Negative results of ADAMTS13 activity and Shiga-toxin research allow one to rule out thrombotic thrombocytopenic purpura and typical hemolytic uremic syndrome, which are two distinct causes of TMA.
- −
- Malignant hypertension (MHT) is defined as a sudden and severe increase in systemic blood pressure (systolic pressure ≥ 180 mmHg and/or diastolic pressure ≥ 120 mmHg) associated with advanced bilateral hypertensive retinopathy and signs or symptoms of acute, ongoing organ damage.
- −
- Posterior reversible encephalopathy syndrome (PRES) is a clinico-radiological syndrome characterized clinically by visual disturbance, headache, seizure, and altered mental status and radiologically by white matter vasogenic edema affecting the posterior occipital and parietal brain regions, bilaterally.
2.4. Statistical Analysis
Finally, we performed a descriptive analysis and a multivariable logistic regression analysis including age at onset, malignant hypertension, anticomplement therapy (eculizumab), and plasmapheresis as variables in order to identify independent predictive parameters of renal failure. Categorical variables were reported as absolute frequencies and percentages, while continuous variables were reported as median value and interquartile range (IQR). A p-value < 0.05 was considered statistically significant. Statistical analysis was performed using the SPSS 22.0 software package (IBM, Armonk, NY, USA).
3. Results
3.1. Patients
We retrieved 52 publications and included 35 articles describing a total of 67 cases of IFNβ-related TMA (Table 1). Two were observational studies, and no randomized controlled trials were included. The remaining articles were individual case reports or small case series. All the cases are reported in Table 1.

Table 1.
Reported Cases of INFβ-related TMA in literature.
The main features of patients are presented in Table 2. The median age at diagnosis of INFβ-related TMA was 42 years (IQR 37–52), and the median length of therapy before TMA was 8 years (IQR 5–11.25 years). Forty-one (62%) received INFβ alpha isoform, while 26 (38%) received IFNβ beta isoform. The median dose was 88 μg per week (IQR 73–98). In the entire cohort, all patients had normal kidney function before starting IFNβ with no medical history except for cases with RRMS.

Table 2.
Characteristics of the study population.
3.2. Onset of TMA and Prodromal Manifestations
Forty-eight patients had data regarding manifestations in the previous weeks before the onset of TMA (Table 1). A wide range of symptoms were reported as possible prodromes in the previous month before TMA diagnosis. The main ones were (i) systemic symptoms (malaise, flu-like symptoms) in 23/48 (48%); (ii) neurological symptoms (headache, blurred vision) in 19/48 (40%); (iii) onset or progressive worsening of hypertension in 14/48 (30%); (iv) hemorrhagic diathesis (nose bleeding, petechial rash, etc.) in 7/48 (15%); (v) shortness of breath in 7/48 (15%); (vi) gastrointestinal symptoms (nausea, vomiting, and diarrhea) in 5/48 (10%); and (vii) proteinuria onset and progressive eGFR decline in 5/48 (10%) (Figure 1).
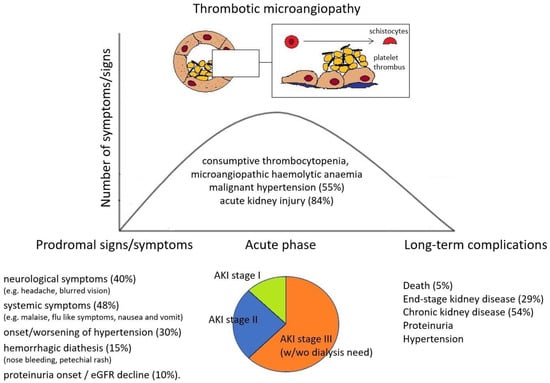
Figure 1.
Prodromes, clinical features, and outcomes in IFNβ-related TMA patients.
Only three patients (3/48, 6%) presented with a sudden onset of TMA without preceding clinical or laboratory manifestations. In all cases reported in the literature, the onset of IFNβ-related TMA was characterized by systemic involvement, while none had single-organ involvement, such as renal-limited TMA forms (without hemolytic anemia or thrombocytopenia) described in cases induced by VEGF and TK inhibitor [14,15]. Of the 26 patients with available data on anti-ADAMTS13 antibodies, two (8%) had positive anti-ADAMTS13 IgG. Their occurrence was likely due to IFNβ treatment, as suggested by several case reports describing anti-ADAMTS13 IgG after IFN-alpha therapy [33]. The presence of decreased C3 levels at TMA onset was found in 6/22 patients (27%), while predisposing genetic variants in complement regulatory genes were described in 6/25 (24%). In all published cases in which genetic analysis of complement factors was performed, causative mutations were identified in only one case (1/25, 4%) [44].
3.3. Organ Involvement
Most patients (56/67, 84%) presented with AKI with a median creatinine at onset of 3 mg/dL (IQR 2.5–4.8 mg/dL). Moreover, 9/67 (13%) had isolated urinary abnormalities without AKI, while two patients had no kidney involvement, only exclusive neurological involvement (2/67, 3%). Most patients with kidney involvement also exhibited an active urinary sediment with hematuria (25/42 patients, 59%) and proteinuria (41/45 patients, 91%). Proteinuria was in the nephrotic range in 31/41 (76%) patients. Among patients with AKI, 6 had stage 1 (11%), 17 had stage 2 (30%), and 33 had stage 3 (59%) according to AKI staging made by KDIGO guidelines (Figure 1). All patients with stage 3 AKI required renal replacement therapy. Furthermore, 34/62 (55%) presented with malignant hypertension (MHT), of whom 8 (23%) had posterior reversible encephalopathy syndrome (PRES). In general, neurological involvement was described in 21/62 (33%) patients. Extra-renal and extra-neurological organ involvement of TMA is reported in one patient with hepatic involvement and in 6/62 (10%) patients with moderate-to-severe heart disease.
3.4. Treatment and Outcome
In all patients, IFNβ was withdrawn. In addition to this, those cases with slower responses and more severe manifestations also received plasma exchange or plasmapheresis (34/62, 55%), corticosteroids (29/62, 47%), and/or eculizumab (12/62, 19%). In order to evaluate the efficacy of these adjunctive treatments for INFβ-related TMA and considering the absence of prospective studies, we performed a multivariate analysis on the 33 patients who experienced AKI stage 3 and had available follow-up data. In a multivariable logistic regression analysis including age at onset, malignant hypertension, eculizumab therapy, and plasmapheresis, eculizumab therapy was independently associated with kidney failure at last follow-up as a protective factor (OR 0.14, 95% CI 0.02–0.97, p = 0.04), while plasmapheresis did not show any significant association (Table 3).

Table 3.
Multivariable regression analysis of parameters associated with renal failure in patients with INFβ-related TMA and AKI stage 3 at onset included in the study cohort.
Considering only those patients who presented with AKI, 16/56 (28%) patients progressed to ESRD. Moreover, 30/56 (53%) patients developed CKD ≥ 3A stage, and 10/56 (18%) had a complete recovery of renal function. Of those without AKI at onset, 6/9 (66%) experienced a complete renal recovery (in terms of proteinuria and hypertension), 2/9 (22%) developed stage 2–3 CKD, and none progressed to ESRD. Overall, 3/67 patients (5%) died.
4. Discussion
Interferon therapy is associated with a wide range of renal manifestations, including isolated proteinuria; podocytopathies, particularly in patients with G1/G2 risk alleles in the APOL1 gene [56]; and TMA [7,8,9]. However, although several mechanisms have been proposed, it remains unclear how IFNβ determines endothelial damage underlying TMA.
Our literature review shows that INFβ-associated TMA most commonly occurs as a life-threatening condition and shares the features of atypical hemolytic uremic syndrome (aHUS), including severe hypertension and renal and neurological involvement [10]. Importantly, almost all cases published in the literature presented with prodromal manifestations likely related to endothelial damage due to interferon, such as hypertension, neurological and constitutional symptoms, and urinary abnormalities (Figure 1). These symptoms should be carefully considered in patients under INF therapy and should raise the suspicion of TMA before more severe complications (such as malignant hypertension, severe AKI, neurologic involvement, and hemolytic anemia) arise.
Furthermore, patients with INFβ-induced TMA showed a significant risk of progression to ESRD, particularly if they had AKI at onset. Conversely, patients with isolated proteinuria and/or hematuria without AKI reported a high incidence of complete remission after IFNβ discontinuation, significantly higher than that of the AKI group.
Endothelial damage due to IFN has been found to be dose dependent [12], supported by the finding that the median duration of IFN therapy before TMA onset was 8 years in the entire population. According to the classification criteria of drug-induced TMA (DITMA) [57], these INFβ-induced forms appear to be mainly “dose- and/or duration-related toxic reactions” rather than “immune-mediated reactions”. Consistently, chronic renal vascular histopathological abnormalities are frequently reported in numerous case reports of full-blown TMA [22,43].
The high prevalence of prodromal signs/symptoms supports the hypothesis that INF-mediated kidney damage is progressive and emphasizes the importance of the awareness of prodromal manifestations (“red flags”) of TMA that could guide clinicians to suspect it before the occurrence of AKI, neurological impairment, and other manifestations of full-blown TMA. In patients on IFNβ with new-onset proteinuria and/or hypertension, the immediate withdrawal of the drug may prevent the development of the severe complications of TMA.
INFβ-associated TMA is characterized by severe kidney involvement, and most patients had stage 3 AKI with variable degrees of proteinuria and/or hematuria. Blood pressure levels were also frequently elevated, and a significant proportion of patients experienced malignant hypertension and PRES, which are likely secondary to the severe endothelial damage in the kidneys. Furthermore, in view of the high frequency of central nervous system involvement, we also collected data on ADAMTS13 activity and anti-ADAMTS13 antibody measurements, which are performed to identify TTP [13,58]). In this cohort, positive anti-ADAMTS13 antibody and reduced ADAMTS13 activity were reported in only two patients. As those patients benefit from an early start of plasma exchange or plasmapheresis, the PLASMIC score should also be calculated while awaiting ADAMTS13 results [59].
As recommended for other TMA forms [13,60], complement activation should be evaluated by checking serum C3 and C4 levels. In patients with INFβ-related TMA, no patient presented decreased C4 levels, but 27% had reduced C3 activity. This suggests that the complement alternative pathway is overactivated in a subset of patients, as already shown in other forms of secondary TMA [61]. As suggested by a recent literature review [13], the presence of hypocomplementemia C3 in DITMA is associated with a higher deposition of complement protein in kidney tissue, serves as a major marker of complement activation, and could be used to predict a higher probability of response to complement inhibitor therapy. Among the 25 patients who had genetic testing, only one causative mutation associated with the development of TMA (being so defined as atypical hemolytic uremic syndrome), and only a minority of these showed variants in complement regulatory genes. Hence, similar to other DITMAs [62], in IFNβ-related TMA, the detrimental role of the complement system does not seem to be driven by a causative genetic defect. Rather, predisposing genetic variants in complement regulatory genes in combination with triggering environmental factors, such as IFNβ, may cause complement hyperactivation and consequently complement-mediated TMA (aHUS).
Although the cornerstone of INFβ-related TMA is withdrawal of the causative drug, this is frequently not sufficient to ameliorate kidney function and hematological parameters. Several patients in this cohort were also treated with adjunctive therapies, such as plasmapheresis, immunosuppressive drugs, and/or eculizumab. No prospective clinical trials have been performed to evaluate the effectiveness of those approaches in DITMA. However, among patients with severe IFNβ-related TMA and stage 3 AKI, eculizumab seems to be associated with a lower incidence of ESKD, which further support the role of the complement system in the pathogenesis of these forms. Therefore, eculizumab could represent an effective therapy for INFβ TMA forms, like that noted for other severe secondary TMA forms [11,63].
The retrospective nature of all included studies, the small number of reported patients (mostly as individual case reports or small case series), and the scarceness of comparative studies between different potential therapies limit the generalizability of our reported results.
5. Conclusions
INFβ-related TMA is a rare but severe adverse complication of prolonged INFβ therapy with a high risk of CKD and ESKD progression. The active surveillance of early signs of kidney involvement through the evaluation of urine, renal function, and blood pressure should be performed in all patients receiving long-term IFNβ therapy at their routine clinic appointments, and abnormalities should lead to promptly suspect TMA. An early withdrawal of the drug may prevent the development of the most serious manifestations of full-blown TMA, thus allowing for a better overall and renal prognosis. This form seems to be complement mediated. In cases of severe full-blown TMA and/or cases of incomplete response to drug withdrawal, eculizumab should be considered as a rescue therapy.
Author Contributions
Conceptualization, M.A. and G.T.; methodology, M.A., G.T. and T.M.; formal analysis, M.A. and T.M.; investigation T.M., S.L., E.P. and L.P.; data curation, M.A. and S.L.; writing—original draft preparation, M.A., G.T. and T.M.; writing—review and editing M.A., G.T., T.M., S.L., L.P. and M.P.A.; visualization, M.A.; supervision, M.A., G.T. and M.P.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All procedures performed in studies involving human participants were performed in accordance with the ethical standards of the Institutional Research Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Statement
Patient consent was waived due to the nature of this manuscript that is a review and did not directly enroll patients.
Data Availability Statement
The data underlying this article will be shared on reasonable request made to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zanghì, A.; D’Amico, E.; Patti, F. Immunosuppression in Relapsing Remitting Multiple Sclerosis: Moving towards Personalized Treatment. Expert Rev. Neurother. 2020, 20, 771–782. [Google Scholar] [CrossRef]
- Inojosa, H.; Proschmann, U.; Akgün, K.; Ziemssen, T. A Focus on Secondary Progressive Multiple Sclerosis (SPMS): Challenges in Diagnosis and Definition. J. Neurol. 2021, 268, 1210–1221. [Google Scholar] [CrossRef]
- Walther, E.U.; Hohlfeld, R. Multiple Sclerosis: Side Effects of Interferon Beta Therapy and Their Management. Neurology 1999, 53, 1622–1627. [Google Scholar] [CrossRef]
- Rommer, P.S.; Zettl, U.K. Managing the Side Effects of Multiple Sclerosis Therapy: Pharmacotherapy Options for Patients. Expert Opin. Pharmacother. 2018, 19, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Hegen, H.; Auer, M.; Deisenhammer, F. Pharmacokinetic Considerations in the Treatment of Multiple Sclerosis with Interferon-β. Expert Opin. Drug Metab. Toxicol. 2015, 11, 1803–1819. [Google Scholar] [CrossRef]
- De Jong, H.J.I.; Kingwell, E.; Shirani, A.; Cohen Tervaert, J.W.; Hupperts, R.; Zhao, Y.; Zhu, F.; Evans, C.; van der Kop, M.L.; Traboulsee, A.; et al. Evaluating the Safety of β-Interferons in MS: A Series of Nested Case-Control Studies. Neurology 2017, 88, 2310–2320. [Google Scholar] [CrossRef]
- Markowitz, G.S.; Nasr, S.H.; Stokes, M.B.; D’Agati, V.D. Treatment with IFN-{alpha}, -{beta}, or -{gamma} Is Associated with Collapsing Focal Segmental Glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 2010, 5, 607–615. [Google Scholar] [CrossRef]
- Gianassi, I.; Allinovi, M.; Caroti, L.; Cirami, L.C. Broad Spectrum of Interferon-Related Nephropathies-Glomerulonephritis, Systemic Lupus Erythematosus-like Syndrome and Thrombotic Microangiopathy: A Case Report and Review of Literature. World J. Nephrol. 2019, 8, 109–117. [Google Scholar]
- Dauvergne, M.; Buob, D.; Rafat, C.; Hennino, M.-F.; Lemoine, M.; Audard, V.; Chauveau, D.; Ribes, D.; Gall, E.C.-L.; Daugas, E.; et al. Renal Diseases Secondary to Interferon-β Treatment: A Multicentre Clinico-Pathological Study and Systematic Literature Review. Clin. Kidney J. 2021, 14, 2563–2572. [Google Scholar] [CrossRef]
- Hunt, D.; Kavanagh, D.; Drummond, I.; Weller, B.; Bellamy, C.; Overell, J.; Evans, S.; Jackson, A.; Chandran, S. Thrombotic Microangiopathy Associated with Interferon Beta. N. Engl. J. Med. 2014, 370, 1270–1271. [Google Scholar] [CrossRef]
- Allinovi, M.; Bellinvia, A.; Pesce, F.; Manani, S.M.; Razzolini, L.; Brezzi, B.; Protopapa, P.; Mantero, V.; Caroti, L.; Cirami, C.L.; et al. Safety and Efficacy of Eculizumab Therapy in Multiple Sclerosis: A Case Series. Brain Sci. 2021, 11, 1341. [Google Scholar] [CrossRef]
- Jia, H.; Thelwell, C.; Dilger, P.; Bird, C.; Daniels, S.; Wadhwa, M. Endothelial Cell Functions Impaired by Interferon in Vitro: Insights into the Molecular Mechanism of Thrombotic Microangiopathy Associated with Interferon Therapy. Thromb. Res. 2018, 163, 105–116. [Google Scholar] [CrossRef]
- Mazzierli, T.; Allegretta, F.; Maffini, E.; Allinovi, M. Drug-Induced Thrombotic Microangiopathy: An Updated Review of Causative Drugs, Pathophysiology, and Management. Front. Pharmacol. 2022, 13, 1088031. [Google Scholar] [CrossRef]
- Saba, E.S.; Cambron, J.C.; Go, R.S.; Leung, N.; Sridharan, M. Clinical Associations, Treatment, and Outcomes of Renal-Limited Thrombotic Microangiopathy. Blood 2018, 132, 4978. [Google Scholar] [CrossRef]
- Genest, D.S.; Patriquin, C.J.; Licht, C.; John, R.; Reich, H.N. Renal Thrombotic Microangiopathy: A Review. Am. J. Kidney Dis. 2023, 81, 591–605. [Google Scholar] [CrossRef]
- Tsai, H.-M. Atypical Hemolytic Uremic Syndrome: Beyond Hemolysis and Uremia. Am. J. Med. 2019, 132, 161–167. [Google Scholar] [CrossRef]
- Sheremata, W.A.; Jy, W.; Horstman, L.L.; Ahn, Y.S.; Alexander, J.S.; Minagar, A. Evidence of Platelet Activation in Multiple Sclerosis. J. Neuroinflamm. 2008, 5, 27. [Google Scholar] [CrossRef]
- Hanaoka, M.; Kubo, K.; Hayano, T.; Koizumi, T.; Kobayashi, T. Interferon-Alpha Elevates Pulmonary Blood Pressure in Sheep--the Role of Thromboxane Cascade. Eur. J. Pharmacol. 1999, 370, 145–151. [Google Scholar] [CrossRef]
- Kavanagh, D.; McGlasson, S.; Jury, A.; Williams, J.; Scolding, N.; Bellamy, C.; Gunther, C.; Ritchie, D.; Gale, D.P.; Kanwar, Y.S.; et al. Type I Interferon Causes Thrombotic Microangiopathy by a Dose-Dependent Toxic Effect on the Microvasculature. Blood 2016, 128, 2824–2833. [Google Scholar] [CrossRef]
- Jodele, S.; Medvedovic, M.; Luebbering, N.; Chen, J.; Dandoy, C.E.; Laskin, B.L.; Davies, S.M. Interferon-Complement Loop in Transplant-Associated Thrombotic Microangiopathy. Blood Adv. 2020, 4, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, A.; Angelotti, M.L.; Mulay, S.R.; Kulkarni, O.O.; Demleitner, J.; Dietrich, A.; Sagrinati, C.; Ballerini, L.; Peired, A.; Shankland, S.J.; et al. The Antiviral Cytokines IFN-α and IFN-β Modulate Parietal Epithelial Cells and Promote Podocyte Loss: Implications for IFN Toxicity, Viral Glomerulonephritis, and Glomerular Regeneration. Am. J. Pathol. 2013, 183, 431–440. [Google Scholar] [CrossRef]
- Modrego, P.J.; Gazulla, J. Arterial Hypertension Induced by Interferon Beta 1b in a Patient with Multiple Sclerosis. Mult. Scler. 2012, 18, 1655–1656. [Google Scholar] [CrossRef]
- Evans, R.; Rudd, P.; Bass, P.; Harber, M. Tip Variant Focal Segmental Glomerulosclerosis Associated with Interferon-β Treatment of Multiple Sclerosis. BMJ Case Rep. 2014, 2014, bcr2013203077. [Google Scholar] [CrossRef]
- Manenti, L.; Gnappi, E.; Vaglio, A.; Allegri, L.; Noris, M.; Bresin, E.; Pilato, F.P.; Valoti, E.; Pasquali, S.; Buzio, C. Atypical Haemolytic Uraemic Syndrome with Underlying Glomerulopathies. A Case Series and a Review of the Literature. Nephrol. Dial. Transplant. 2013, 28, 2246–2259. [Google Scholar] [CrossRef]
- Ben-Amor, A.-F.; Trochanov, A.; Fischer, T.Z. Cumulative Review of Thrombotic Microangiopathy, Thrombotic Thrombocytopenic Purpura, and Hemolytic Uremic Syndrome Reports with Subcutaneous Interferon β-1a. Adv. Ther. 2015, 32, 445–454. [Google Scholar] [CrossRef]
- Herrera, W.G.; Balizet, L.B.; Harberts, S.W.; Brown, S.T. Occurrence of a TTP-like Syndrome in Two Women Receiving Beta Interferon Therapy for Relapsing Multiple Sclerosis. Proc. Neurol. 1999, 52, A135. [Google Scholar]
- Serrano, A.; Xicoy, B.; Grifols, J.R.; Ribera, J.-M. Thrombotic thrombocytopenic purpura during treatment with interpheron. Med. Clin. 2007, 128, 276–277. [Google Scholar] [CrossRef] [PubMed]
- Li Cavoli, G.; Bono, L.; Tortorici, C.; Giammarresi, C.; Rotolo, U. Renal Thrombotic Microangiopathy Induced by β-Interferon. NDT Plus 2011, 4, 80. [Google Scholar] [CrossRef] [PubMed]
- Broughton, A.; Cosyns, J.-P.; Jadoul, M. Thrombotic Microangiopathy Induced by Long-Term Interferon-β Therapy for Multiple Sclerosis: A Case Report. Clin. Nephrol. 2011, 76, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Bensa, C.; Sohier, E.; Pillebout, L.; Galicier, L.; Gout, O. A Case of Thrombotic Microangiopathy Associated with Subcutaneous Beta-1a-Interferon Therapy. In Proceedings of the 27th Congress of the European Committee for Treatment and Research in Multiple Sclerosis, Amsterdam, The Netherlands, 19–22 October 2011. [Google Scholar]
- Olea, T.; Díaz-Mancebo, R.; Picazo, M.-L.; Martínez-Ara, J.; Robles, A.; Selgas, R. Thrombotic Microangiopathy Associated with Use of Interferon-Beta. Int. J. Nephrol. Renovasc. Dis. 2012, 5, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, M.; Pour Manshadi, S.M.Y.M.; Naderi, N.; Dehghan, A.; Azizi, S. Unusual Side Effects of Interferon Beta-1a in Patient with Multiple Sclerosis. Mater. Sociomed. 2012, 24, 203–205. [Google Scholar] [CrossRef]
- Nerrant, E.; Charif, M.; Ramay, A.-S.; Perrochia, H.; Patrier, L.; de Champfleur, N.M.; Renard, D.; Labauge, P. Hemolytic Uremic Syndrome: An Unusual Complication of Interferon-β Treatment in a MS Patient. J. Neurol. 2013, 260, 1915–1916. [Google Scholar] [CrossRef]
- Mahe, J.; Meurette, A.; Moreau, A.; Vercel, C.; Jolliet, P. Renal Thrombotic Microangiopathy Caused by Interferon Beta-1a Treatment for Multiple Sclerosis. Drug Des. Dev. Ther. 2013, 7, 723–728. [Google Scholar] [CrossRef]
- Orvain, C.; Augusto, J.-F.; Besson, V.; Marc, G.; Coppo, P.; Subra, J.-F.; Sayegh, J. Thrombotic Microangiopathy due to Acquired ADAMTS13 Deficiency in a Patient Receiving Interferon-Beta Treatment for Multiple Sclerosis. Int. Urol. Nephrol. 2014, 46, 239–242. [Google Scholar] [CrossRef]
- Vosoughi, R.; Marriott, J.J. Thrombotic Microangiopathy in Interferon Beta Treated Multiple Sclerosis Patients: Review of Literature and Report of Two New Cases. Mult. Scler. Relat. Disord. 2014, 3, 321–325. [Google Scholar] [CrossRef]
- Rubin, S.; Lacraz, A.; Galantine, V.; Gosse, P. Malignant Hypertension and Interferon-Beta: A Case Report. J. Hum. Hypertens. 2014, 28, 340–341. [Google Scholar] [CrossRef]
- Larochelle, C.; Grand’maison, F.; Bernier, G.P.; Latour, M.; Cailhier, J.-F.; Prat, A. Thrombotic Thrombocytopenic Purpura-Hemolytic Uremic Syndrome in Relapsing-Remitting Multiple Sclerosis Patients on High-Dose Interferon β. Mult. Scler. 2014, 20, 1783–1787. [Google Scholar] [CrossRef]
- Capobianco, M.; Piccoli, G.; Neve Vigotti, F.; Scapoli, P.; Deagostini, M.C.; Albera, C.; Roccatello, D.; Bertolotto, A. Interferon Beta-Related Nephropathy and Interstitial Lung Disease: A New Association and a Long-Term Warning. Mult. Scler. 2014, 20, 889–891. [Google Scholar] [CrossRef]
- Azkune Calle, I.; Sánchez Menoyo, J.L.; Ruiz Ojeda, J.; García Moncó, J.C.; Etxeguren Urkixo, I. Case Report of Thrombotic Microangiopathy Associated with Subcutaneous Interferon Beta-1a: An Emerging Complication? Neurologia 2016, 31, 508–509. [Google Scholar] [CrossRef] [PubMed]
- Nishio, H.; Tsukamoto, T.; Matsubara, T.; Okada, Y.; Takahashi, R.; Yanagita, M. Thrombotic Microangiopathy Caused by Interferon β-1b for Multiple Sclerosis: A Case Report. CEN Case Rep. 2016, 5, 179–183. [Google Scholar] [CrossRef][Green Version]
- Gerischer, L.M.; Siebert, E.; Janke, O.; Jungehuelsing, G.J.; Ruprecht, K. Favorable Outcome of Interferon-Beta Associated Thrombotic Microangiopathy Following Treatment with Corticosteroids, Plasma Exchange and Rituximab: A Case Report. Mult. Scler. Relat. Disord. 2016, 10, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Capobianco, M.; Vigotti, F.N.; Naretto, C.; Narioz, C.; Brocheriou, I.; Roccatello, D. The Case | The Young Philosopher with Multiple Sclerosis and Proteinuria. Kidney Int. 2016, 89, 961–963. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Milan Manani, S.; Virzì, G.M.; Gastaldon, F.; Proglio, M.; Brocca, A.; Ronco, C. Brief Review and a Clinical Case of Hemolytic Uremic Syndrome Associated with Interferon β Treatment. Blood Purif. 2017, 43, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Allinovi, M.; Cirami, C.L.; Caroti, L.; Antognoli, G.; Farsetti, S.; Amato, M.P.; Minetti, E.E. Thrombotic Microangiopathy Induced by Interferon Beta in Patients with Multiple Sclerosis: Three Cases Treated with Eculizumab. Clin. Kidney J. 2017, 10, 625–631. [Google Scholar] [CrossRef][Green Version]
- Omoto, S.; Utsumi, T.; Matsuno, H.; Terasawa, Y.; Iguchi, Y. Thrombotic Microangiopathy Presenting with Intestinal Involvement Following Long-Term Interferon-β1b Treatment for Multiple Sclerosis. Intern. Med. 2018, 57, 741–744. [Google Scholar] [CrossRef]
- Pérez, E.P.; Sánchez de La Nieta Garcıa, M.D.; López, L.G.; Hernández, F.R. Thrombotic Microangiopathy and Accelerated Hypertension after Treatment with Interferon Beta. Nefrologia 2018, 38, 564–565. [Google Scholar] [CrossRef]
- Etemadifar, M.; Sabeti, F.; Salari, M. Interferon Beta-1b-Induced Thrombotic Thrombocytopenic Purpura-Hemolytic Uremic Syndrome (TTP-HUS) in a Patient Treated for Multiple Sclerosis: A Case Report. Iran J. Neurol. 2018, 17, 91–94. [Google Scholar]
- Baghbanian, S.M.; Moghadasi, A.N. Thrombotic Microangiopathy Associated with Interferon-Beta Treatment in Patients with Multiple Sclerosis. Iran J. Neurol. 2018, 17, 89–90. [Google Scholar]
- Yam, C.; Fok, A.; Mclean, C.; Butler, E.; Kempster, P. Interferon-Beta in Multiple Sclerosis Causing Thrombotic Microangiopathy. Intern. Med. J. 2019, 49, 274–276. [Google Scholar] [CrossRef]
- Malekzadeh, M.M.; Alizadeh, R.; Aghsaeifard, Z.; Sahraian, M.A. Thrombotic Microangiopathy in Interferon-Beta-Treated Multiple Sclerosis Patient. Clin. Case Rep. 2020, 8, 1061–1064. [Google Scholar] [CrossRef]
- Parisi, M.; Manni, A.; Caputo, F.; Trojano, M.; Paolicelli, D. A Case Report of Late-Onset Atypical Hemolytic Uremic Syndrome during Interferon Beta in Multiple Sclerosis: Open Issues in Literature Review. Brain Behav. 2021, 11, e01930. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, M.; Stordeur, P.; Collart, F.; Dachy, B.; Pozdzik, A.; Mesquita, M.D.C.F.; Nortier, J. Interferon-β1a-Induced Thrombotic Microangiopathy: Possible Implication of the Alternative Pathway of the Complement. Kidney Int. Rep. 2022, 7, 1917–1921. [Google Scholar] [CrossRef] [PubMed]
- Mrabet, S.; Dahmane, R.; Raja, B.; Fradi, A.; Aicha, N.B.; Sahtout, W.; Azzabi, A.; Guedri, Y.; Zellama, D.; Achour, A.; et al. Thrombotic Microangiopathy due to Acquired Complement Factor I Deficiency in a Male Receiving Interferon-Beta Treatment for Multiple Sclerosis. Br. J. Clin. Pharmacol. 2023, 89, 1682–1685. [Google Scholar] [CrossRef] [PubMed]
- Akita, S.; Fujibayashi, K.; Ueno, E.-I.; Wakasa, M.; Kawai, Y.; Kajinami, K. Thrombotic Microangiopathy after a 15-Year Treatment with Interferon Beta-1b in a Patient with Multiple Sclerosis: A Case Report and Review of Literature. Intern. Med. 2023, 1846-23. [Google Scholar] [CrossRef]
- Friedman, D.J.; Pollak, M.R. APOL1 Nephropathy: From Genetics to Clinical Applications. Clin. J. Am. Soc. Nephrol. 2021, 16, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Al-Nouri, Z.L.; Reese, J.A.; Terrell, D.R.; Vesely, S.K.; George, J.N. Drug-Induced Thrombotic Microangiopathy: A Systematic Review of Published Reports. Blood 2015, 125, 616–618. [Google Scholar] [CrossRef] [PubMed]
- George, J.N.; Nester, C.M. Syndromes of Thrombotic Microangiopathy. N. Engl. J. Med. 2014, 371, 654–666. [Google Scholar] [CrossRef]
- Paydary, K.; Banwell, E.; Tong, J.; Chen, Y.; Cuker, A. Diagnostic Accuracy of the PLASMIC Score in Patients with Suspected Thrombotic Thrombocytopenic Purpura: A Systematic Review and Meta-analysis. Transfusion 2020, 60, 2047–2057. [Google Scholar] [CrossRef]
- Brocklebank, V.; Wood, K.M.; Kavanagh, D. Thrombotic Microangiopathy and the Kidney. Clin. J. Am. Soc. Nephrol. 2018, 13, 300–317. [Google Scholar] [CrossRef]
- Palma, L.M.P.; Sridharan, M.; Sethi, S. Complement in Secondary Thrombotic Microangiopathy. Kidney Int. Rep. 2021, 6, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Werion, A.; Storms, P.; Zizi, Y.; Beguin, C.; Bernards, J.; Cambier, J.-F.; Dahan, K.; Dierickx, D.; Godefroid, N.; Hilbert, P.; et al. Epidemiology, Outcomes, and Complement Gene Variants in Secondary Thrombotic Microangiopathies. Clin. J. Am. Soc. Nephrol. 2023, 18, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Caravaca-Fontan, F.; Praga, M. Complement Inhibitors Are Useful in Secondary Hemolytic Uremic Syndromes. Kidney Int. 2019, 96, 826–829. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).