The Short- and Long-Term Risk of Mortality in Intracranial Hemorrhage Patients with Tranexamic Acid Treatment in a Population-Based Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Source of Data
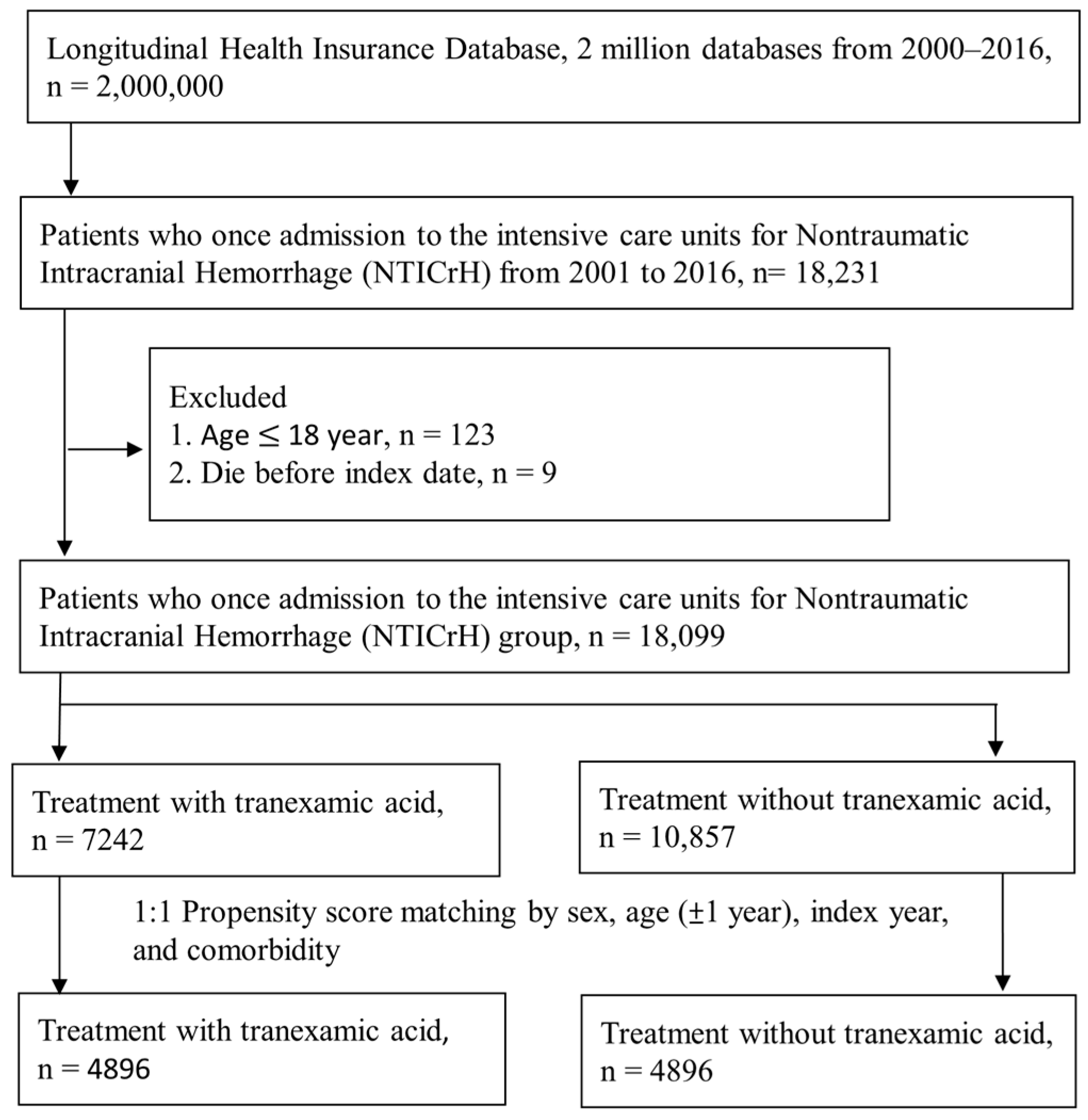
2.2. Study Population
2.3. Characteristics, Comorbidities, and Study Outcomes
2.4. Outcome Measurement
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
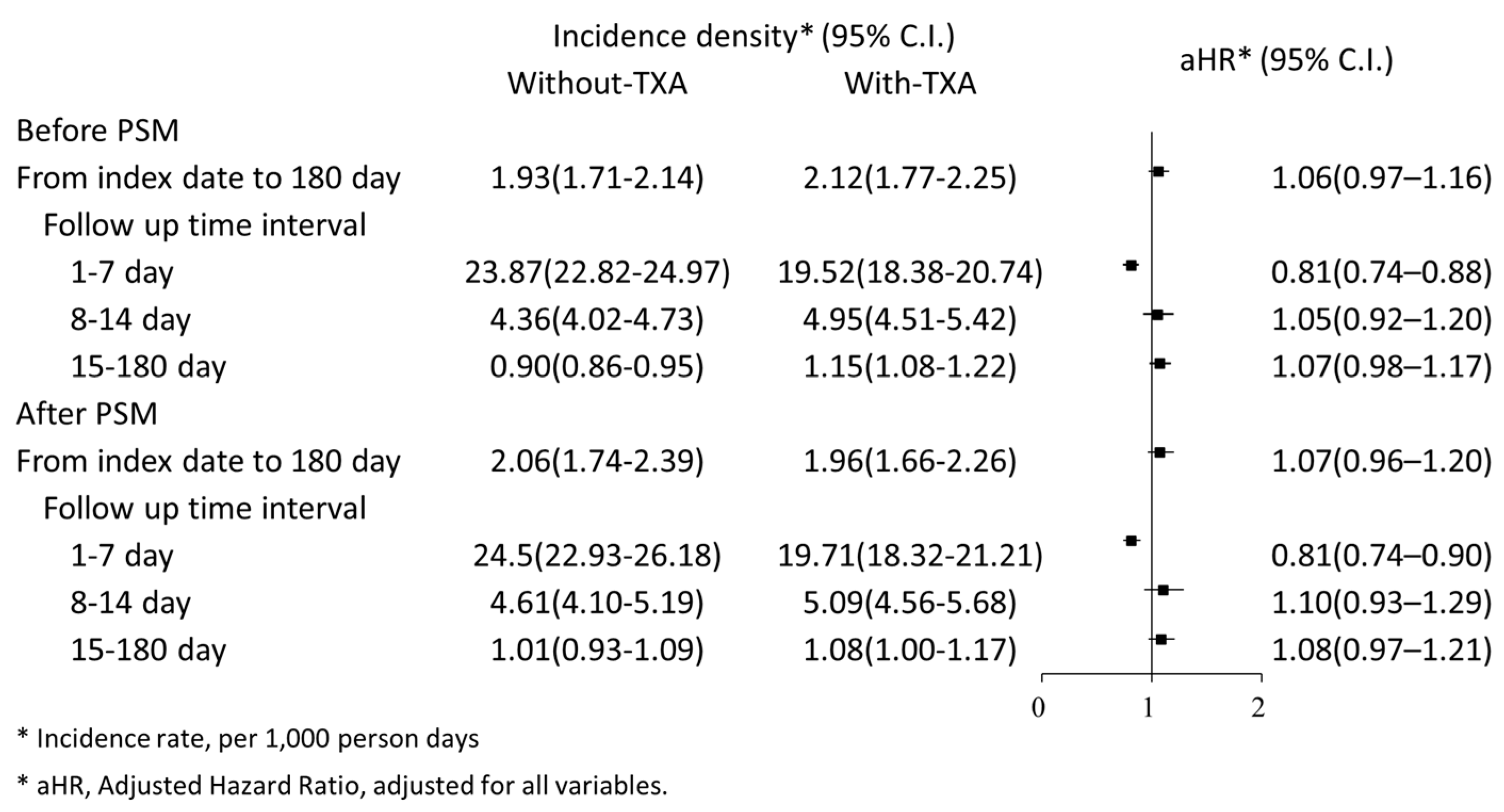
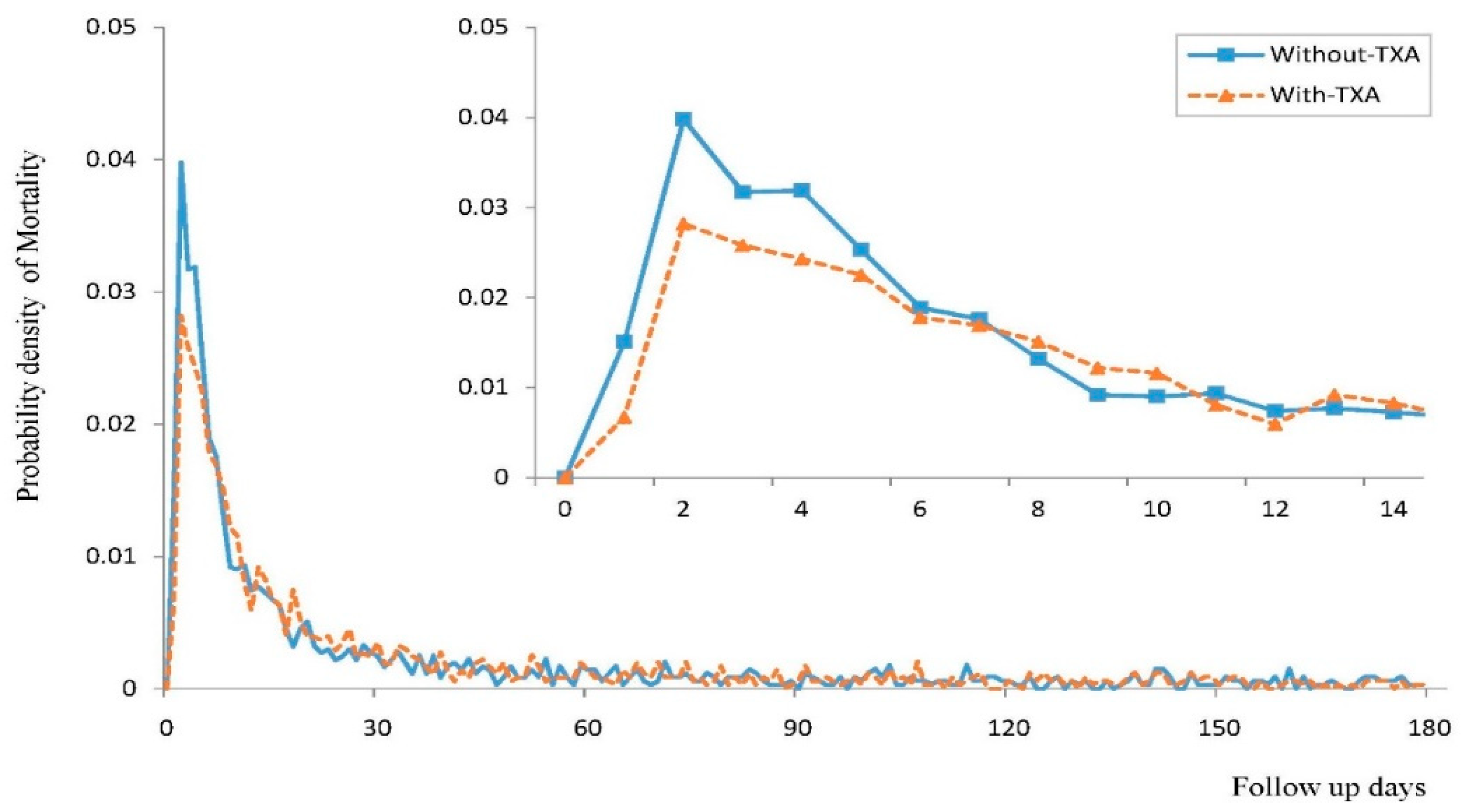
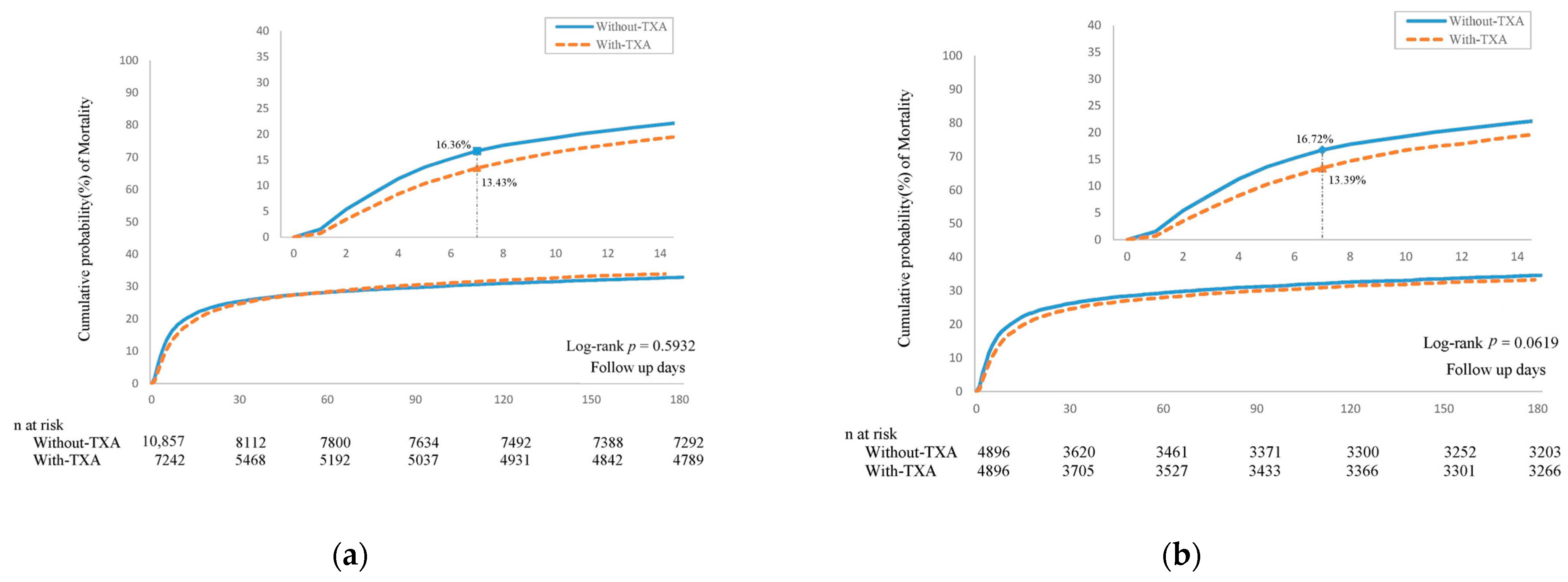
3.2. Risk of 180-Day Mortality in Patients Treated with TXA
3.3. Risk of 180-Day Mortality Compared between Early and Late TXA Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, S.M.; Broderick, J.; Hennerici, M.; Brun, N.C.; Diringer, M.N.; Mayer, S.A.; Begtrup, K.; Steiner, T. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006, 66, 1175–1181. [Google Scholar] [CrossRef]
- Dunn, C.J.; Goa, K.L. Tranexamic acid: A review of its use in surgery and other indications. Drugs 1999, 57, 1005–1032. [Google Scholar] [CrossRef]
- Roberts, I.; Shakur, H.; Afolabi, A.; Brohi, K.; Coats, T.; Dewan, Y.; Gando, S.; Guyatt, G.; Hunt, B.J.; Morales, C.; et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: An exploratory analysis of the CRASH-2 randomised controlled trial. Lancet 2011, 377, 1096–1101. [Google Scholar]
- Shakur, H.; Roberts, I.; Fawole, B.; Chaudhri, R.; El-Sheikh, M.; Akintan, A.; Qureshi, Z.; Kidanto, H.; Vwalika, B.; Abdulkadir, A.; et al. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): An international, randomised, double-blind, placebo-controlled trial. Lancet 2017, 389, 2105–2116. [Google Scholar] [CrossRef]
- Myles, P.S.; Smith, J.A.; Forbes, A.; Silbert, B.; Jayarajah, M.; Painter, T.; Cooper, D.J.; Marasco, S.; McNeil, J.; Bussières, J.S.; et al. Tranexamic Acid in Patients Undergoing Coronary-Artery Surgery. N. Engl. J. Med. 2017, 376, 136–148. [Google Scholar] [CrossRef]
- Devereaux, P.J.; Marcucci, M.; Painter, T.W.; Conen, D.; Lomivorotov, V.; Sessler, D.I.; Chan, M.T.V.; Borges, F.K.; Martínez-Zapata, M.J.; Wang, C.Y.; et al. Tranexamic Acid in Patients Undergoing Noncardiac Surgery. N. Engl. J. Med. 2022, 386, 1986–1997. [Google Scholar] [CrossRef]
- Bouillon-Minois, J.B.; Croizier, C.; Baker, J.S.; Pereira, B.; Moustafa, F.; Outrey, J.; Schmidt, J.; Peschanski, N.; Dutheil, F. Tranexamic acid in non-traumatic intracranial bleeding: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 15275. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wu, L.; Xue, R.; Ding, H. Efficacy and safety of tranexamic acid in aneurysmal subarachnoid hemorrhage: A meta-analysis of randomized controlled trials. Am. J. Emerg. Med. 2021, 50, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Sprigg, N.; Flaherty, K.; Appleton, J.P.; Al-Shahi Salman, R.; Bereczki, D.; Beridze, M.; Christensen, H.; Ciccone, A.; Collins, R.; Czlonkowska, A.; et al. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): An international randomised, placebo-controlled, phase 3 superiority trial. Lancet 2018, 391, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Meretoja, A.; Yassi, N.; Wu, T.Y.; Churilov, L.; Sibolt, G.; Jeng, J.S.; Kleinig, T.; Spratt, N.J.; Thijs, V.; Wijeratne, T.; et al. Tranexamic acid in patients with intracerebral haemorrhage (STOP-AUST): A multicentre, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2020, 19, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Polymeris, A.A.; Karwacki, G.M.; Siepen, B.M.; Schaedelin, S.; Tsakiris, D.A.; Stippich, C.; Guzman, R.; Nickel, C.H.; Sprigg, N.; Kägi, G.; et al. Tranexamic Acid for Intracerebral Hemorrhage in Patients on Non-Vitamin K Antagonist Oral Anticoagulants (TICH-NOAC): A Multicenter, Randomized, Placebo-Controlled, Phase 2 Trial. Stroke 2023, 54, 2223–2234. [Google Scholar] [CrossRef]
- Illanes, S.; Zhou, W.; Schwarting, S.; Heiland, S.; Veltkamp, R. Comparative effectiveness of hemostatic therapy in experimental warfarin-associated intracerebral hemorrhage. Stroke 2011, 42, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Christensen, H.; Cordonnier, C.; Korv, J.; Lal, A.; Ovesen, C.; Purrucker, J.C.; Toni, D.; Steiner, T. European Stroke Organisation Guideline on Reversal of Oral Anticoagulants in Acute Intracerebral Haemorrhage. Eur. Stroke J. 2019, 4, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.Y.; Su, C.C.; Shao, S.C.; Sung, S.F.; Lin, S.J.; Kao Yang, Y.H.; Lai, E.C. Taiwan’s National Health Insurance Research Database: Past and future. Clin. Epidemiol. 2019, 11, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Tien, N.I.; Lin, C.L.; Shen, H.Y.; Bau, D.T.; Lim, Y.P. Association of Antituberculosis Treatment and Lower Risk of Hyperlipidemia in Taiwanese Patients: A Population-Based Case-Control Study. In Vivo 2018, 32, 47–54. [Google Scholar] [PubMed]
- Rosner, B. Fundamentals of Biostatistics; Cengage Learning: Boston, MA, USA, 2015. [Google Scholar]
- van Asch, C.J.; Luitse, M.J.; Rinkel, G.J.; van der Tweel, I.; Algra, A.; Klijn, C.J. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis. Lancet Neurol. 2010, 9, 167–176. [Google Scholar] [CrossRef] [PubMed]
- González-Pérez, A.; Gaist, D.; Wallander, M.A.; McFeat, G.; García-Rodríguez, L.A. Mortality after hemorrhagic stroke: Data from general practice (The Health Improvement Network). Neurology 2013, 81, 559–565. [Google Scholar] [CrossRef]
- Fernando, S.M.; Qureshi, D.; Talarico, R.; Tanuseputro, P.; Dowlatshahi, D.; Sood, M.M.; Smith, E.E.; Hill, M.D.; McCredie, V.A.; Scales, D.C.; et al. Intracerebral Hemorrhage Incidence, Mortality, and Association With Oral Anticoagulation Use: A Population Study. Stroke 2021, 52, 1673–1681. [Google Scholar] [CrossRef]
- Sacco, S.; Marini, C.; Toni, D.; Olivieri, L.; Carolei, A. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke 2009, 40, 394–399. [Google Scholar] [CrossRef]
- Vergouwen, M.D.; Jong-Tjien-Fa, A.V.; Algra, A.; Rinkel, G.J. Time trends in causes of death after aneurysmal subarachnoid hemorrhage: A hospital-based study. Neurology 2016, 86, 59–63. [Google Scholar] [CrossRef]
- Abulhasan, Y.B.; Alabdulraheem, N.; Simoneau, G.; Angle, M.R.; Teitelbaum, J. Mortality after Spontaneous Subarachnoid Hemorrhage: Causality and Validation of a Prediction Model. World Neurosurg. 2018, 112, e799–e811. [Google Scholar] [CrossRef] [PubMed]
- Dowlatshahi, D.; Demchuk, A.M.; Flaherty, M.L.; Ali, M.; Lyden, P.L.; Smith, E.E. Defining hematoma expansion in intracerebral hemorrhage: Relationship with patient outcomes. Neurology 2011, 76, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zheng, J.; Guo, R.; Li, M.; Li, H.; Ma, L.; You, C. The accuracy of aneurysm size in predicting rebleeding after subarachnoid hemorrhage: A meta-analysis. Neurol. Sci. 2020, 41, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Ohkuma, H.; Tsurutani, H.; Suzuki, S. Incidence and significance of early aneurysmal rebleeding before neurosurgical or neurological management. Stroke 2001, 32, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Guo, X.M.; Li, R.L.; Zhao, K.; Bao, Q.J.; Yang, J.C.; Zhang, Q.; Yang, M.F. Tranexamic Acid for Acute Spontaneous Intracerebral Hemorrhage: A Meta-Analysis of Randomized Controlled Trials. Front. Neurol. 2021, 12, 761185. [Google Scholar] [CrossRef]
- Ren, J.; Qian, D.; Wu, J.; Ni, L.; Qian, W.; Zhao, G.; Huang, C.; Liu, X.; Zou, Y.; Xing, W. Safety and Efficacy of Tranexamic Acid in Aneurysmal Subarachnoid Hemorrhage: A Meta-Analysis of Randomized Controlled Trials. Front. Neurol. 2021, 12, 710495. [Google Scholar] [CrossRef]
- Post, R.; Germans, M.R.; Tjerkstra, M.A.; Vergouwen, M.D.I.; Jellema, K.; Koot, R.W.; Kruyt, N.D.; Willems, P.W.A.; Wolfs, J.F.C.; de Beer, F.C.; et al. Ultra-early tranexamic acid after subarachnoid haemorrhage (ULTRA): A randomised controlled trial. Lancet 2021, 397, 112–118. [Google Scholar] [CrossRef]
- Hillman, J.; Fridriksson, S.; Nilsson, O.; Yu, Z.; Saveland, H.; Jakobsson, K.E. Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: A prospective randomized study. J. Neurosurg. 2002, 97, 771–778. [Google Scholar] [CrossRef]
| Variables | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| Without-TXA | With-TXA | ASD | Without-TXA | With-TXA | ASD | |
| N | 10,857 | 7242 | 4896 | 4896 | ||
| Index year | 0.213 | 0.024 | ||||
| 2001–2005 | 3431 (31.6%) | 1794 (24.77%) | 1291 (26.37%) | 1266 (25.86%) | ||
| 2006–2010 | 3529 (32.5%) | 2206 (30.46%) | 1470 (30.02%) | 1505 (30.74%) | ||
| 2011–2016 | 3897 (35.89%) | 3242 (44.77%) | 2135 (43.61%) | 2125 (43.4%) | ||
| Sex | 0.055 | 0.007 | ||||
| Female | 4366 (40.21%) | 2718 (37.53%) | 1869 (38.17%) | 1886 (38.52%) | ||
| Male | 6491 (59.79%) | 4524 (62.47%) | 3027 (61.83%) | 3010 (61.48%) | ||
| Age | 0.062 | 0.043 | ||||
| 19–50 | 2357 (21.71%) | 1500 (20.71%) | 1007 (20.57%) | 999 (20.4%) | ||
| 51–60 | 2370 (21.83%) | 1612 (22.26%) | 1120 (22.88%) | 1089 (22.24%) | ||
| 61–70 | 2239 (20.62%) | 1486 (20.52%) | 992 (20.26%) | 1006 (20.55%) | ||
| 71–80 | 2395 (22.06%) | 1542 (21.29%) | 1043 (21.3%) | 1080 (22.06%) | ||
| >80 | 1496 (13.78%) | 1102 (15.22%) | 734 (14.99%) | 722 (14.75%) | ||
| Charlson Comorbidity Index | 0.062 | 0.042 | ||||
| 1 | 4470 (41.17%) | 2839 (39.2%) | 1976 (40.36%) | 1931 (39.44%) | ||
| 2 | 2377 (21.89%) | 1552 (21.43%) | 1067 (21.79%) | 1039 (21.22%) | ||
| 3 | 4010 (36.93%) | 2851 (39.37%) | 1853 (37.85%) | 1926 (39.34%) | ||
| Length of hospital stay | 0.094 | 0.089 | ||||
| 1–7 day | 3294 (30.34%) | 1884 (26.01%) | 1479 (30.21%) | 1295 (26.45%) | ||
| 8–14 day | 2219 (20.44%) | 1417 (19.57%) | 933 (19.06%) | 976 (19.93%) | ||
| >=15 day | 5344 (49.22%) | 3941 (54.42%) | 2484 (50.74%) | 2625 (53.62%) | ||
| Nasogastric tube feeding | 0.094 | 0.011 | ||||
| No | 3379 (31.12%) | 1945 (26.86%) | 1396 (28.51%) | 1371 (28%) | ||
| Yes | 7478 (68.88%) | 5297 (73.14%) | 3500 (71.49%) | 3525 (72%) | ||
| Ventilator usage | 0.142 | 0.014 | ||||
| No | 1135 (10.45%) | 471 (6.5%) | 392 (8.01%) | 374 (7.64%) | ||
| Yes | 9722 (89.55%) | 6771 (93.5%) | 4504 (91.99%) | 4522 (92.36%) | ||
| Intracranial pressure monitor | 0.050 | 0.006 | ||||
| No | 7719 (71.1%) | 4984 (68.82%) | 3434 (70.14%) | 3421 (69.87%) | ||
| Yes | 3138 (28.9%) | 2258 (31.18%) | 1462 (29.86%) | 1475 (30.13%) | ||
| Intracranial decompression operation | 0.065 | 0.005 | ||||
| No | 7985 (73.55%) | 5115 (70.63%) | 3558 (72.67%) | 3546 (72.43%) | ||
| Yes | 2872 (26.45%) | 2127 (29.37%) | 1338 (27.33%) | 1350 (27.57%) | ||
| Co-morbidity | ||||||
| Hypertension | 7961 (73.33%) | 5389 (74.41%) | 0.025 | 3635 (74.24%) | 3643 (74.41%) | 0.004 |
| Diabetes mellitus | 2826 (26.03%) | 1862 (25.71%) | 0.007 | 1289 (26.33%) | 1284 (26.23%) | 0.002 |
| Hyperlipidemia | 1478 (13.61%) | 938 (12.95%) | 0.019 | 648 (13.24%) | 661 (13.5%) | 0.008 |
| Kidney diseases | 1146 (10.56%) | 801 (11.06%) | 0.016 | 553 (11.29%) | 542 (11.07%) | 0.007 |
| Chronic pulmonary diseases | 1252 (11.53%) | 847 (11.7%) | 0.005 | 563 (11.5%) | 588 (12.01%) | 0.016 |
| Liver diseases | 1180 (10.87%) | 880 (12.15%) | 0.040 | 573 (11.7%) | 575 (11.74%) | 0.001 |
| Ischemic heart diseases | 1340 (12.34%) | 830 (11.46%) | 0.027 | 533 (10.89%) | 567 (11.58%) | 0.022 |
| Atrial fibrillation and flutter | 577 (5.31%) | 300 (4.14%) | 0.055 | 222 (4.53%) | 227 (4.64%) | 0.005 |
| Congestive heart failure | 948 (8.73%) | 592 (8.17%) | 0.020 | 413 (8.44%) | 414 (8.46%) | 0.001 |
| Malignancies | 721 (6.64%) | 513 (7.08%) | 0.018 | 355 (7.25%) | 326 (6.66%) | 0.023 |
| Rheumatic diseases | 104 (0.96%) | 72 (0.99%) | 0.004 | 46 (0.94%) | 48 (0.98%) | 0.004 |
| Dementia | 530 (4.88%) | 385 (5.32%) | 0.020 | 250 (5.11%) | 245 (5%) | 0.005 |
| Peripheral vascular diseases | 543 (5%) | 296 (4.09%) | 0.044 | 224 (4.58%) | 223 (4.55%) | 0.001 |
| Peptic ulcer disease | 1276 (11.75%) | 939 (12.97%) | 0.037 | 614 (12.54%) | 614 (12.54%) | 0.001 |
| Medication | ||||||
| NSAIDs | 8129 (74.87%) | 5430 (74.98%) | 0.002 | 3663 (74.82%) | 3664 (74.84%) | 0.001 |
| PPI | 4008 (36.92%) | 3122 (43.11%) | 0.127 | 2012 (41.09%) | 1970 (40.24%) | 0.017 |
| H2 blockers | 6527 (60.12%) | 5094 (70.34%) | 0.216 | 3302 (67.44%) | 3343 (68.28%) | 0.018 |
| Aspirin | 3906 (35.98%) | 2468 (34.08%) | 0.040 | 1698 (34.68%) | 1728 (35.29%) | 0.013 |
| Clopidogrel/Ticagrelor | 621 (5.72%) | 312 (4.31%) | 0.065 | 226 (4.62%) | 235 (4.8%) | 0.009 |
| Vitamin K and other hemostatics | 1810 (16.67%) | 4107 (56.71%) | 0.913 | 1789 (36.54%) | 1777 (36.29%) | 0.005 |
| Laxatives | 8828 (81.31%) | 6025 (83.2%) | 0.049 | 4025 (82.21%) | 4037 (82.46%) | 0.006 |
| Furosemide | 3623 (33.37%) | 2834 (39.13%) | 0.120 | 1810 (36.97%) | 1812 (37.01%) | 0.001 |
| Metoclopramide | 4489 (41.35%) | 3137 (43.32%) | 0.040 | 2071 (42.3%) | 2074 (42.36%) | 0.001 |
| Magnesium oxide | 3111 (28.65%) | 2110 (29.14%) | 0.011 | 1344 (27.45%) | 1352 (27.61%) | 0.004 |
| Antiepileptics | 5473 (50.41%) | 4170 (57.58%) | 0.144 | 2708 (55.31%) | 2696 (55.07%) | 0.005 |
| Antibiotics | 1728 (15.92%) | 877 (12.11%) | 0.110 | 635 (12.97%) | 623 (12.72%) | 0.007 |
| Alpha-blockers | 1160 (10.68%) | 869 (12%) | 0.041 | 570 (11.64%) | 565 (11.54%) | 0.003 |
| Beta-blockers | 6999 (64.47%) | 4769 (65.85%) | 0.029 | 3199 (65.34%) | 3183 (65.01%) | 0.007 |
| CCBs | 8736 (80.46%) | 6069 (83.8%) | 0.087 | 4071 (83.15%) | 4056 (82.84%) | 0.008 |
| ACEIs | 3230 (29.75%) | 1780 (24.58%) | 0.116 | 1271 (25.96%) | 1249 (25.51%) | 0.010 |
| ARBs | 3868 (35.63%) | 2988 (41.26%) | 0.116 | 1955 (39.93%) | 1965 (40.13%) | 0.004 |
| Variable | aHR (95% CI) | |
|---|---|---|
| 1–7 Day | 8–180 Day | |
| Study group | ||
| Without-TXA | Reference | Reference |
| With-TXA | 0.81 (0.74–0.90) | 1.09 (0.99–1.19) |
| Index year | ||
| 2001–2005 | Reference | Reference |
| 2006–2010 | 0.93 (0.80–1.08) | 0.96 (0.82–1.13) |
| 2011–2016 | 0.98 (0.84–1.14) | 1.05 (0.90–1.23) |
| Sex | ||
| Female | Reference | Reference |
| Male | 1.15 (1.04–1.28) | 0.95 (0.86–1.04) |
| Age | ||
| 19–50 | Reference | Reference |
| 51–60 | 0.79 (0.68–0.93) | 1.09 (0.93–1.29) |
| 61–70 | 0.85 (0.72–1.00) | 1.22 (1.03–1.44) |
| 71–80 | 1.07 (0.91–1.26) | 1.37 (1.17–1.61) |
| >80 | 1.08 (0.91–1.29) | 1.79 (1.51–2.12) |
| Nasogastric tube feeding (ref: no) | 2.65 (2.31–3.05) | 5.97 (4.84–7.36) |
| Ventilator usage (ref: no) | 2.50 (1.89–3.33) | 2.45 (1.70–3.52) |
| Intracranial pressure monitor (ref: no) | 0.73 (0.64–0.83) | 1.08 (0.97–1.19) |
| Intracranial decompression operation (ref: no) | 0.48 (0.41–0.56) | 0.70 (0.63–0.78) |
| Co-morbidity (ref: non) | ||
| Hypertension | 1.33 (1.17–1.51) | 0.89 (0.79–1.00) |
| Diabetes mellitus | 1.08 (0.96–1.21) | 1.29 (1.17–1.44) |
| Hyperlipidemia | 0.96 (0.81–1.13) | 0.84 (0.72–0.97) |
| Kidney diseases | 1.64 (1.42–1.89) | 1.90 (1.68–2.15) |
| Chronic pulmonary diseases | 0.94 (0.79–1.10) | 1.04 (0.91–1.18) |
| Liver diseases | 1.23 (1.06–1.42) | 1.29 (1.13–1.47) |
| Ischemic heart diseases | 1.40 (1.20–1.64) | 1.07 (0.92–1.23) |
| Atrial fibrillation and flutter | 1.00 (0.79–1.27) | 0.78 (0.63–0.95) |
| Congestive heart failure | 1.32 (1.11–1.58) | 1.24 (1.07–1.44) |
| Malignancies | 1.42 (1.19–1.70) | 1.69 (1.47–1.94) |
| Rheumatic diseases | 1.38 (0.89–2.13) | 1.51 (1.01–2.25) |
| Dementia | 0.95 (0.76–1.20) | 0.91 (0.75–1.09) |
| Peripheral vascular diseases | 0.93 (0.72–1.21) | 0.90 (0.73–1.12) |
| Peptic ulcer disease | 1.14 (0.98–1.33) | 1.06 (0.94–1.21) |
| Medication (ref: non) | ||
| NSAIDs | 0.83 (0.75–0.92) | 1.07 (0.95–1.20) |
| PPI | 0.96 (0.85–1.08) | 1.25 (1.13–1.39) |
| H2 blockers | 1.00 (0.90–1.12) | 1.04 (0.94–1.15) |
| Aspirin | 1.12 (1.00–1.25) | 1.06 (0.96–1.17) |
| Clopidogrel/Ticagrelor | 0.98 (0.76–1.25) | 0.97 (0.80–1.18) |
| Vitamin K and other hemostatic | 1.16 (1.05–1.29) | 1.15 (1.04–1.27) |
| Laxatives | 0.21 (0.19–0.24) | 0.58 (0.49–0.68) |
| Furosemide | 0.72 (0.63–0.81) | 1.65 (1.49–1.83) |
| Metoclopramide | 0.76 (0.67–0.85) | 1.30 (1.17–1.43) |
| Magnesium oxide | 0.57 (0.50–0.66) | 0.80 (0.72–0.89) |
| Antiepileptics | 0.92 (0.83–1.02) | 1.00 (0.90–1.10) |
| Antibiotics | 0.85 (0.70–1.02) | 1.10 (0.93–1.31) |
| Alpha-blockers | 0.80 (0.65–0.99) | 0.84 (0.73–0.98) |
| Beta-blockers | 1.02 (0.91–1.14) | 0.86 (0.78–0.96) |
| CCBs | 0.68 (0.60–0.76) | 0.77 (0.67–0.88) |
| ACEIs | 0.62 (0.54–0.71) | 0.91 (0.81–1.01) |
| ARBs | 0.55 (0.48–0.62) | 0.69 (0.62–0.77) |
| Incidence Density (95% CI) | HR * (95% CI) | aHR (95% CI) | ||
|---|---|---|---|---|
| Without-TXA | With-TXA | |||
| Early treatment | ||||
| From index date to 180 day | 1.82 (1.51–2.20) | 1.64 (1.42–1.90) | 1.06 (0.82–1.37) | 1.01 (0.78–1.33) |
| Follow up time interval | ||||
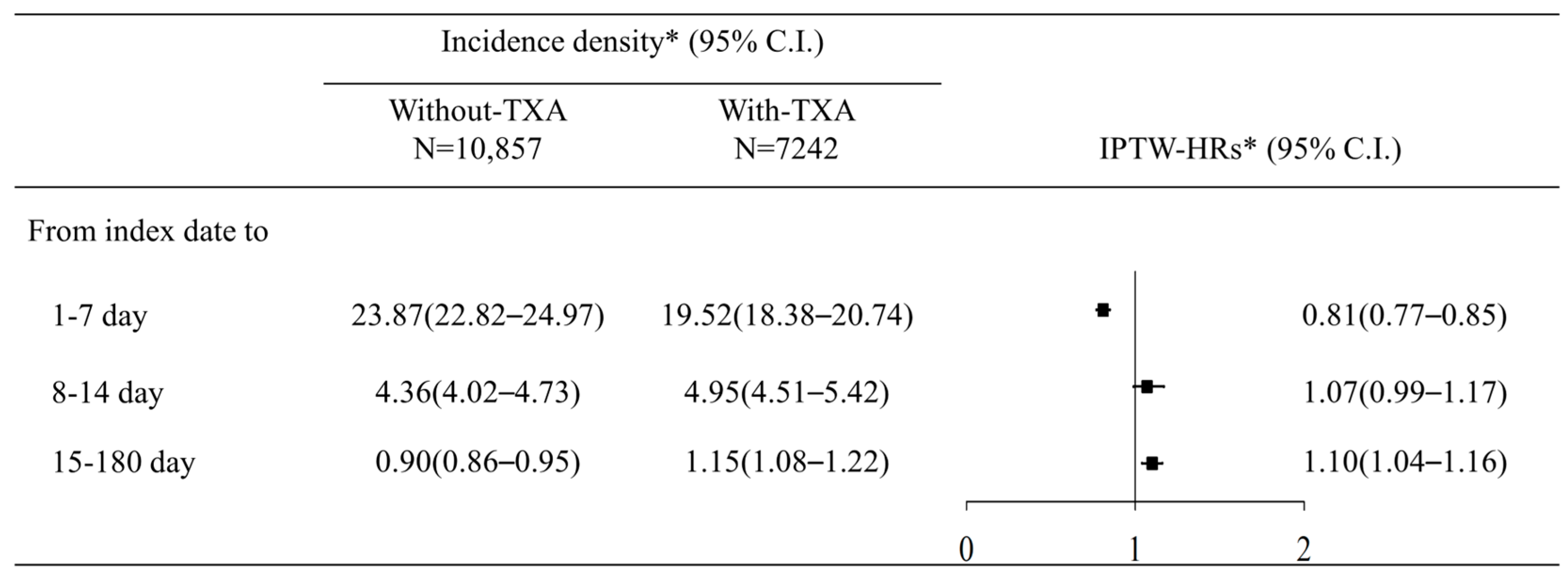
| 1–7 day | 25.08 (21.51–29.24) | 18.41 (16.16–20.96) | 0.74 (0.6–0.9) | 0.68 (0.55–0.84) |
| 8–14 day | 3.48 (2.53–4.78) | 4.2 (3.41–5.17) | 1.21 (0.83–1.77) | 1.14 (0.77–1.7) |
| 15–180 day | 0.77 (0.63–0.95) | 0.82 (0.71–0.95) | 1.07 (0.83–1.37) | 1.02 (0.78–1.33) |
| Late treatment | ||||
| From index date to 180 day | 1.95 (1.83–2.05) | 2.13 (1.98–2.29) | 1.32 (1.21–1.45) | 1.07 (0.97–1.18) |
| Follow up time interval | ||||
| 1–7 day | 23.76 (22.67–24.91) | 19.85 (18.54–21.25) | 0.84 (0.77–0.91) | 0.82 (0.74–0.9) |
| 8–14 day | 4.44 (4.08–4.82) | 5.17 (4.67–5.73) | 1.17 (1.02–1.33) | 1.04 (0.9–1.21) |
| 15–180 day | 0.92 (0.86–0.97) | 1.25 (1.17–1.34) | 1.35 (1.24–1.48) | 1.08 (0.98–1.19) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, C.-M.; Hu, S.-Y.; Liao, P.-L.; Huang, J.-Y.; Chou, M.-C.; Yang, S.-F.; Yeh, C.-B. The Short- and Long-Term Risk of Mortality in Intracranial Hemorrhage Patients with Tranexamic Acid Treatment in a Population-Based Cohort Study. J. Clin. Med. 2024, 13, 1597. https://doi.org/10.3390/jcm13061597
Chiu C-M, Hu S-Y, Liao P-L, Huang J-Y, Chou M-C, Yang S-F, Yeh C-B. The Short- and Long-Term Risk of Mortality in Intracranial Hemorrhage Patients with Tranexamic Acid Treatment in a Population-Based Cohort Study. Journal of Clinical Medicine. 2024; 13(6):1597. https://doi.org/10.3390/jcm13061597
Chicago/Turabian StyleChiu, Chien-Ming, Sung-Yuan Hu, Pei-Lun Liao, Jing-Yang Huang, Ming-Chih Chou, Shun-Fa Yang, and Chao-Bin Yeh. 2024. "The Short- and Long-Term Risk of Mortality in Intracranial Hemorrhage Patients with Tranexamic Acid Treatment in a Population-Based Cohort Study" Journal of Clinical Medicine 13, no. 6: 1597. https://doi.org/10.3390/jcm13061597
APA StyleChiu, C.-M., Hu, S.-Y., Liao, P.-L., Huang, J.-Y., Chou, M.-C., Yang, S.-F., & Yeh, C.-B. (2024). The Short- and Long-Term Risk of Mortality in Intracranial Hemorrhage Patients with Tranexamic Acid Treatment in a Population-Based Cohort Study. Journal of Clinical Medicine, 13(6), 1597. https://doi.org/10.3390/jcm13061597








