Abstract
(1) Background: The aging population is expected to triple by 2050. Executive functions decline with age, impacting daily tasks, and this is associated with neurodegenerative diseases. Aerobic and resistance exercises positively affect cognitive function in older adults by influencing growth markers. However, the modalities of exercise and the optimal parameters for maximum cognitive benefits remain unclear. (2) Methods: A meta-analysis of randomized clinical trials (RCTs) was conducted. The systematic search was on slowing cognitive decline and performed in the PubMed/MEDLINE and Cochrane Library databases. Articles were included if participants were ≥65 years, healthy, and performing resistance or aerobic exercise, and they were excluded if there was a combination of training and if they have neurological disease or cognitive impairment. (3) Results: The search strategy found a total of 1635 studies. After removing duplicates and assessing the inclusion and exclusion criteria, eight articles were included in the meta-analysis, with a total of 463 healthy older adults analyzed. No significant differences between the intervention groups and the control groups after the aerobic or resistance programs were found. (4) Conclusions: Aerobic exercise interventions improved executive function more than resistance training in older adults, but without statistically significant differences. This can serve as a guide to see, with caution, whether we need a multidisciplinary approach to be more effective in improving the cortical health of older adults.
1. Introduction
As the aging population is expected to almost triple by 2050 [1], there is a growing need for strategies to militate against common health issues associated with aging [2]. Especially age-related decline in cognitive performance has received an increment in experimental attention [3]. Cognitive decline has a negative impact on overall health, which imposes a heavy burden on families and health service systems [4].
Cognitive function refers to multiple mental abilities that include learning, thinking, reasoning, remembering, problem solving, decision making, and attention [5]. Executive function, the cognitive processes, primarily linked to the prefrontal cortex of the brain, which guide and regulate purposeful, goal-directed behavior, is one area of cognitive function that is known to decline because of the aging process [6,7]. Executive function is an umbrella for functions such as planning, working memory, inhibition, mental flexibility, and initiation and monitoring of action [8]. Because of the significance of cognitive function processes in performing daily tasks, cognitive dysfunction is associated with less functionality, which is also particularly associated with age-related neurodegenerative diseases such as Alzheimer’s [9].
From young to older adults, executive functions are associated with physical activity. The scientific literature [10,11,12] suggest that older adults use executive functions to support the execution of complex motor tasks because motor control may become less automated with age. Moreover, several studies [13,14] have shown that the older the age, the worse the score on cognitive tasks. This is relevant, as one study described that worse performance of executive functions was related to worse performance of daily living tasks [15].
To improve the decline in cognitive functions in older adults, some randomized controlled studies have shown that aerobic exercise and resistance exercise have positive effects on cognitive function in older adults [16]. It was also shown that exercise increases cerebral blood flow, reduces brain shrinkage, and improves brain tissue metabolism, leading to adaptive changes in brain function [16,17]. Previous meta-analyses [18] showed that exercise training interventions are an effective approach to improving executive function in older populations. More specifically, it has been shown that different training interventions such as resistance training and aerobic exercise improved executive function sub-domains such as working memory, cognitive flexibility, inhibition, and planning in older adults [8].
On a physiological level, exercise training stimulates growth markers that positively influence physical and cognitive function (e.g., insulin-like growth factor-1 [IGF-1]) in both the periphery and the central nervous systems [19]. As for the optimal dose, the benefits obtained depend on the intensity, frequency, type of exercise, and time, and all these parameters are still not completely clear at present [20]. As such, the precise implementation of these variables is critical to effectively elicit improvements in both physical and cognitive function [20].
Although there is a lot of literature on the effects of both aerobic and resistance exercises on physical and cognitive levels in older adults, many variables have been proposed regarding the type of exercise used, the dose, and the time. The objective of this review is to evaluate, through an analysis of the scientific literature, which exercise modality is most optimal to improve the executive functions of older adults.
2. Materials and Methods
This systematic review and meta-analysis were performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [21]. The protocol of this systematic review was published in the International Prospective Register of Systematic Reviews (PROSPERO: CRD42023467344).
2.1. Systematic Literature Search
We conducted a systematic literature search for studies included up to July 2023 in the electronic databases PubMed and Cochrane. Four domains (participants, intervention, outcomes, and study design) were used for the search strategy. The main terms selected were (1) participants: elderly OR older adults; (2) intervention: aerobic exercise OR aerobic training OR resistance training OR resistance exercise; (3) outcomes: cognitive functions OR executive functions OR cognitive abilities; and (4) study design: the search strategy proposed was used to include only RCT. The complete search strategies are available in the Supplementary Material S1.
2.2. Selection Criteria
The systematic review and meta-analysis included RCT with a sample of older adults ≥ 65 years old without cognitive impairment. Studies had to use as intervention, aerobic/cardiovascular (AE) or resistance exercise (RE). At least one of the following outcomes had to be reported in the primary articles to be included: cognitive function, executive function, or cognitive skills. We excluded articles that (1) had participants with neurological disease or cognitive impairment; (2) had interventions combining both AE and RE or other interventions (balance and pulmonary rehabilitation); and (3) were written in languages other than English. We also excluded review articles, book chapters, and study protocols.
2.3. Screening, Selection Process, and Data Extraction
After removing duplicate papers, the articles were independently screened by 2 reviewers (S.R.R. and N.S.A.) to identify appropriate articles by the titles and abstracts. We used Rayyan.ai software (https://rayyan.qcri.org, accessed on 15 August 2023) [22] to eliminate duplicate articles and for identifying relevant works by titles and abstracts. The criteria used by the two reviewers during the initial screening involved including articles only if the titles and abstracts were related to the topic studied and the specific population of older adults. Both reviewers (S.R.R. and N.S.A.) used a standardized form to independently extract data from each paper, where they included the following: author’s last name, year of publication, study design, type of intervention, comparison, study variables, and results.
2.4. Assessment of Methodological Quality and Risk of Bias
Risk of bias and methodological quality of the included trials were independently assessed by 2 authors (S.R.R. and N.L.L.) using the Cochrane Risk of Bias (RoB2), a widely accepted tool to evaluate the quality of an RCT in the biomedical field, proposed by the Cochrane Collaboration [23] and the Physiotherapy Evidence Database (PEDro) scale [24]. These are especially designed to assess the quality of physiotherapy trials based on random allocation, concealed allocation, baseline between-group similarity, blinding of participants, therapists and assessors, dropouts, intention-to-treat statistical analysis, between-group statistical comparison, point measures, and variability data [24]. Both authors (S.R.R. and N.L.L.) considered the combination of these 2 tools to increase the reliability of the assessment of the methodological quality.
2.5. Data Synthesis and Analysis
Review Manager 5 (Cochrane Collaboration, Oxford, UK) was used for all statistical analyses. If data were not reported in the paper, the authors were contacted by e-mail to obtain the necessary data to be included in the quantitative analysis. In this systematic review and meta-analysis, the mean difference was chosen as the effect size when studies used the same tool of measure. The standard mean difference (SMD) was chosen as the effect size when studies used different tools to measure the outcome. A 95% confidence Interval (CI) for all the effect sizes was used. The inverse of the variance (IV) statistical test was used for the quantitative analysis. In this systematic review and meta-analysis, a random effects model was used to determine the overall effect size because the number of included studies was small [25]. We considered p < 0.05 as statistically significant. Heterogeneity was calculated by the I2 statistic, being classified as low (I2 ≤ 25%), moderate (25 < I2 < 50%), or high (I2 ≥ 50%) [26].
3. Results
The search strategy found 1635 studies (PubMed: 1214 and Cochrane Library: 421). After removing duplicates, a total of 1424 studies were initially considered to be included in the study. After screening the titles and abstracts for not meeting the inclusion criteria, 1310 studies were excluded. A total of 114 studies remained in the study, and after assessing the full text, 8 studies met the inclusion criteria for the quantitative analysis, and 109 studies were rejected because the population, sample, or outcomes were not those studied in this study (Figure 1). One study was dropped from the meta-analysis for not presenting the required data after the authors had been contacted.
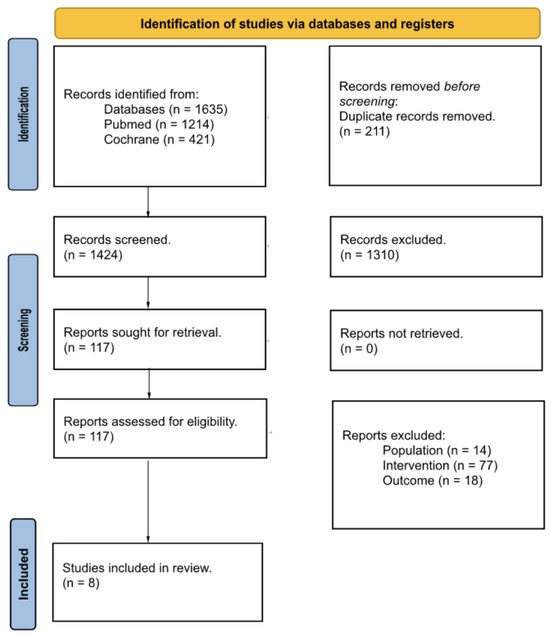
Figure 1.
PRISMA 2020 flow diagram.
3.1. Study Characteristics
In Table 1, we summarize the sample characteristics of all the articles included [27,28,29,30,31,32,33,34]. A total of 463 older adults, each over 65 years old, were included in this systematic review. We saw that the distribution was greater for women, which was 72,2% of the study population. The sample size varied between 16 [27] and 119 [28] individuals, with an average age between 65.8 [29] and 75.2 [28] years old. Regarding the types of the interventions, five studies included aerobic training in the experimental group, and four studies included resistance training in the experimental group. Only one study (Iuliano et al. 2015) included both interventions, aerobic training and resistance training. For the control group, six studies included a non-training group, one included an educational session [28], and another one included stretching exercises [30]. Finally, regarding the study variables, executive function was assessed with the Stroop Color Test in four studies [29,30,33,34], with the Stroop Task Performance in one study [27], with the Mini Mental State Exam in one study [31], with the Task Switching Test in one study [28], and with the Wisconsin Card Sorting Test (WCST) in one study [32].

Table 1.
Study characteristics.
3.2. PEDro Score
The average score for the PEDro scale can be found in Table 2. The score was 5/10, and was considered a “fair” methodological quality across the studies included in the systematic review. When we analyzed the main methodological problems, we found that 0/8 studies did not have their participants blinded, and 0/8 studies did not have their therapists blinded.

Table 2.
PEDro scale.
3.3. Risk of Bias Graph and Summary
Figure 2 shows the Rob 2 tool graph and summary. We found a risk of bias in terms of performance bias (5/8) and detection bias (5/8). We found a low risk of bias in terms of selection bias (7/8) and attrition bias, reporting bias, and other biases (8/8). (+) signs in green indicate a low risk of bias; (?) signs in yellow indicate unclear a risk of bias, and red marks indicate a high risk of bias.
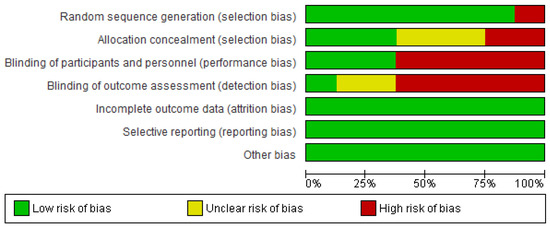
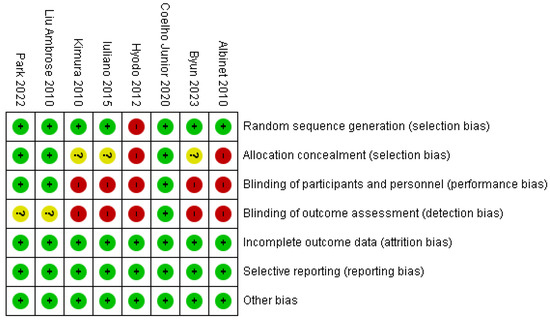
Figure 2.
Risk of bias graph and summary [27,28,29,30,31,32,33,34].
3.4. Effectiveness of Interventions
3.4.1. Resistance Exercise
Four articles [28,29,30,31] reported results about resistance exercise. Regarding all the studies, the study by Liu Ambrose et al. [30] focused on resistance training. They found, among their results, improvements in the Stroop performance compared with the control group. In the study conducted by Iuliano [29], the authors focused on high-intensity strength training, and no significant improvements were found between pre- and post-measures of the experimental group. In the study conducted by Kimura et al. [28], the authors focused on progressive resistance training. They found significant changes between pre- and post-intervention executive functions. Finally, for the study conducted by Coelho Junior et al. [31], the authors focused on resistance training, and found significant cognitive function improvements in comparison with the control group. Considering the four studies that focused on resistance exercise, there was a total sample of 286 participants. Figure 3 shows the comparison between resistance training and the control group. An analysis shows an overall SMD of 0.0 [ 95%CI −0.33, 0.43] and an overall effect of Z= 0.24 (p = 0.81). The heterogeneity was considered moderate (I2 = 54%).

Figure 3.
Effectiveness of resistance exercise on executive function [28,29,30,31].
3.4.2. Aerobic Exercise
Four articles [27,29,32,33,34] reported results about aerobic exercise. The only study that could not be incorporated into the meta-analysis was the Byun et al. study [33], because after requesting the data from the authors, no response was received, and the data could not be incorporated. The study conducted by Hyodo et al. [27] focused on an acute bout of moderate aerobic exercise. They found significant improvements in cognitive performance after the intervention program. In their study, Albinet et al. [32] focused on an aerobic training program to enhance aerobic endurance. They also found significant results in the aerobic group in comparison with the control group. On the other hand, for the study conducted by Iuliano et al. [29], the authors focused on a high-intensity cardiovascular training. They found no significant differences in the scores of the cognitive functions after the intervention for the aerobic exercise group. Finally, in the study conducted by Park et al. [34], the authors focused on aerobic stepping exercise. They found greater improvements in executive function than those in the control group. Considering the four studies, there was a total sample of 152 participants. Figure 4 shows an overall SMD of −0.81 [−1.77, −0.16] and an overall effect of Z = 1.64 (p = 0.10). The heterogeneity was considered high (I2 = 86%).

Figure 4.
Effectiveness of aerobic exercise on executive function [27,29,32,33,34].
4. Discussion
The objective of the study is to analyze the effects of two different types of exercise (AE or RE) on the executive function in older adults. This meta-analysis summarizes results from a total of eight studies and 463 participants, and shows the results obtained. After describing the results in the previous section, this systematic review and meta-analysis show no significant statistical differences in both aerobic and resistance exercises and their respective control groups in terms of improving executive function in older adults. Possible causes of this non-statistically significant result could include inadequate methodology, heterogeneity, and a small sample size.
4.1. Resistance Exercise
It is important to note that the heterogeneity between studies was moderate for resistance exercise (I2 = 54%). This percentage may be due to the difference in the years of publication between the different articles included. Furthermore, it may also be due to the difference in the sample size of the different articles included.
The result of our meta-analysis shows no significant differences between the resistance exercise and the control groups in terms of improving executive function in older adults. These findings differ from another study [35], where the authors showed significant interaction effects for resistance training with executive function, suggesting that this type of training has particularly pronounced effects on these domains of cognitive function. On the other hand, our results are similar to those of other studies [36,37,38,39], where they fail to show any consistent evidence for the benefits of resistance training on different domains of cognitive function.
First, the differences observed may have been because the studies did not use the same executive function measurements or scales and needed to include another measurement for the executive function. In the second place, they may be explained by the duration of the interventions. In this metanalysis, the articles that used resistance exercise as the type of intervention had an intervention duration of less than 6 months, except the study of Coelho-Junior et al. [31]. The duration of these interventions is a critical factor because exercise interventions of less than 6 months have demonstrated controversial findings on cortical function [40]. In fact, Gomes-Osman et al. [41] showed a meaningful relationship between the total length of target exercise interventions and cognitive gains in older adults with and without cognitive impairment, suggesting a potential “length threshold” to boost brain health.
4.2. Aerobic Exercise
For this type of intervention, it is important to highlight that heterogeneity was high (I2 = 86%). This may have occurred, firstly, due to the difference in year of publication and secondly, due to the difference in the sample and the timing of the interventions.
The results of our study show that the aerobic exercise group had better cognitive performance after the intervention, but the results were not statistically significant (p = 0.10) in comparison with the control groups. The present results are in line with the following study by Frost et al. [42], where they also found no statistically significant group effect on executive functions after a cycling program. This is because, as different studies have explained [43,44], aerobic exercise alone is not enough to produce significant changes in the executive function. Another factor that can explain our results is the duration of the interventions, taking into account that in our reviewed studies, Albinet et al. [32] carried out 12 weeks of intervention and Park et al. [34] carried out 5 weeks; perhaps a greater weekly duration of exercise is required to induce cognitive health [42]. Moreover, Coetsee et al. [44] also found that the accuracy on the two Stroop tasks did not change significantly after their interventions in any of the aerobic groups (moderate and high-intensity). These results relate to those of our study and could be due, as described by Kramer et al. [45] to the fact that aerobic exercise has a selective effect on cognition, without significantly modifying parameters of executive function.
On the other hand, the study by Smith et al. [46] differs from our results, after reviewing nineteen studies and obtaining significant results (p = 0.018) in their study in attention and processing speed, executive function, and memory. In the present study, it may have been the case that because the sample of studies analyzed with aerobic exercise was so small, we did not find statistically significant results with respect to executive function, even though we observed in the studies analyzed that there were positive changes in executive function after the interventions.
4.3. Need for New Therapeutic Strategies
Therapeutic strategies are an important factor to take into account when carrying out different exercise modalities in older adults and can explain our results of the program’s personalization [47].
The interplay between an individual’s intrinsic characteristics, behaviors, and environmental/ecological influences is crucial to achieving the optimal trajectory [47]. In our study, the fact of not having considered the intrinsic capacities of each individual to personalize the exercise may have been a factor in the results not being significant. As Gronwald et al. [48] said in their article, exercise prescriptions should be individualized, adjusted, and controlled like any other medical treatment.
Another critical factor is the type of therapeutic approach. As Kim et al. describe in their study [49], despite the importance of exercise therapy, some older adults tend to refuse exercise itself due to their malfunctioning body, which means that we need to devise new therapeutic approaches that achieve similar results. This is a relevant factor, because it could be that in the articles included in our study, many of the participants who carried out the aerobic or resistance exercise programs could not achieve the expected benefits due to their abilities, motivations or limitations, and another therapeutic approach would have been a better option to improve cognitive function.
A possible future line, as Heesterbeek et al. [50] describe in their study, to improve cognitive functioning in older adults with cognitive decline, can be body vibration exercise programs. They found that the vibratory stimulus with exercise can be a promising intervention, as it induces brain activation in the sensory cortex and increases blood circulation safely, influencing cognitive function positively through activated cerebrovascular circulation [49,50]. It could be a promising therapeutic tool for those older adults who cannot perform an aerobic or resistance exercise protocol due to their intrinsic abilities and motivational factors or because they are in a frail situation.
4.4. Limitations of the Study
It is important to mention the heterogeneity of the studies, as well as the intrinsic characteristics of the studies. These differences are mainly related to the number of participants, the type of interventions, and the duration of the interventions. Another consideration is that the articles analyzed on resistance training were evaluated with the MMSE scale, which is less sensitive to changes that may occur. Moreover, the small number of studies analyzed becomes another limitation. One more study constraint was not being able to obtain the data from the author of one of the studies and to add them to the meta-analysis.
5. Conclusions
After analyzing our results, we can conclude that aerobic exercise is a training modality that produces better changes regarding executive function compared to resistance exercise. Even so, the positive changes observed with aerobic exercise are not statistically significant. This can serve as a guide to see, with caution, the importance of aerobic exercise at the cognitive level and continue investigating whether we need a different approach to be more effective in improving the cortical health of older adults.
More clinical trials with a larger number of studies should be carried out to continue investigating the effects of exercise on a cognitive level and to investigate what types of interventions are most effective in improving the health of older adults.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13051301/s1.
Author Contributions
All review authors (S.R.-R., N.L.-L., J.R.-S., M.C.-V., C.L.-d.-C., G.R.O., S.O.W. and A.P.-B.) contributed to the development of the review. S.R.-R., M.C.-V. and N.L.-L. selected the final included studies and extracted data. S.R.-R. entered data into RevMan and carried out the meta-analysis. S.R.-R., N.L.-L., G.R.O., J.R.-S. and C.L.-d.-C. interpreted the results. S.R.-R., G.R.O. and N.L.-L. wrote the article. C.L.-d.-C., S.O.W., G.R.O. and A.P.-B. reviewed and verified the paper. As the contact author, N.L.-L. has overall responsibility for the review. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- United Nations. World Population Ageing 2009; Edited by Division DoEaSAP; United Nations: New York City, NY, USA, 2009. [Google Scholar]
- Van Uffelen, J.G.; Chin A Paw, M.J.; Hopman-Rock, M.; van Mechelen, W. The effects of exercise on cognition in older adults with and without cognitive decline: A systematic review. Clin. J. Sport Med. 2008, 18, 486–500. [Google Scholar] [CrossRef] [PubMed]
- Vieweg, J.; Leinen, P.; Verwey, W.B.; Shea, C.H.; Panzer, S. The Cognitive Status of Older Adults: Do Reduced Time Constraints Enhance Sequence Learning? J. Mot. Behav. 2020, 52, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.M.; Yae, K.; Covinsky, K.E. Cognitive impairment, depressive symptoms, and functional decline in older people. J. Am. Geriatr. Soc. 2002, 50, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.G.; Chacon, M.; Chaee, D.S. Chapter 2—Theories of Cognitive Aging and Work. In Work Across the Lifespan; Baltes, B.B., Rudolph, C.W., Zacher, H., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 17–45. [Google Scholar]
- Huang, C.; Lin, P.; Hung, C.; Chang, Y.; Hung, T. Type of physical exercise and inhibitory function in older adults: An event-related potential study. Psychol. Sport Exerc. 2014, 15, 205–211. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P. The nature and Organization of Individual Differences in executive functions: Four general conclusions. Curr. Dir. Psychol. Sci. 2012, 21, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wang, J.; Cai, X.; Zhang, X.; Zhang, J.; Peng, M.; Xiao, D.; Ouyang, H.; Yan, F. Effects of physical activity interventions on executive function in older adults with dementia: A meta-analysis of randomized controlled trials. Geriatr. Nurs. 2023, 51, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Martyr, A.; Clare, L. Executive function and activities of daily living in Alzheimer’s disease: A correlational meta-analysis. Dement. Geriatr. Cogn. Disord. 2012, 33, 189–203. [Google Scholar] [CrossRef]
- De Greeff, J.W.; Bosker, R.J.; Oosterlaan, J.; Visscher, C.; Hartman, E. Effects of Physical Activity on Executive Functions, Attention and Academic Performance in Preadolescent Children: A Meta-Analysis. J. Sci. Med. Sport 2018, 21, 501–507. [Google Scholar] [CrossRef]
- Van Impe, A.; Bruijn, S.M.; Coxon, J.P.; Wenderoth, N.; Sunaert, S.; Duysens, J.; Swinnen, S.P. Age-Related Neural Correlates of Cognitive Task Performance Under Increased Postural Load. Age 2013, 35, 2111–2124. [Google Scholar] [CrossRef]
- Park, D.; Reuter-Lorenz, P. The Adaptive Brain: Aging and Neurocognitive Scaffolding. Annu. Rev. Psychol. 2009, 60, 173–196. [Google Scholar] [CrossRef]
- Braga, P.L.G.; Henrique, J.S.; Almeida, S.S.; Arida, R.M.; Gomes da Silva, S. Factors affecting executive function performance of Brazilian elderly in the Stroop test. Braz. J. Med. Biol. Res. 2022, 55, e11917. [Google Scholar] [CrossRef] [PubMed]
- Keith, N.R.; Stump, T.E.; Clark, D.O. Developing a self-reported physical fitness survey. Med. Sci. Sport Exerc. 2012, 44, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Suchy, Y.; Gereau Mora, M.; DesRuisseaux, L.A.; Brothers, S.L. It’s complicated: Executive functioning moderates impacts of daily busyness on everyday functioning in community-dwelling older adults. J. Int. Neuropsychol. Soc. 2023, 29, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Sanders, L.M.J.; Hortobagyi, T.; Karssemeijer, E.G.A.; Van der Zee, E.A.; Scherder, E.J.A.; Van Heuvelen, M.J.G. Effects of low- and high-intensity physical exercise on physical and cognitive function in older persons with dementia: A randomized controlled trial. Alzheimers Res. Ther. 2020, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.H.; Lee, J.Y.; Song, W. Effects of resistance exercise training on cognitive function and physical performance in cognitive frailty: A randomized controlled trial. J. Nutr. Health Aging 2018, 22, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.T.; Etnier, J.L.; Chan, K.H.; Chiu, P.K.; Hung, T.M.; Chang, Y.K. Effects of Exercise Training Interventions on Executive Function in Older Adults: A Systematic Review and Meta-Analysis. Sports Med. 2020, 50, 1451–1467. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.A. Exercise builds brain health: Keyroles of growth factor cascades and inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef]
- Nagamatsu, L.S.; Flicker, L.; Kramer, A.F.; Voss, M.W.; Erickson, K.I.; Hsu, C.L.; Liu-Ambrose, T. Exercise is medicine for the body and the brain. Br. J. Sports Med. 2014, 48, 943–944. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 134, 103–112. [Google Scholar]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savovic, J.; Page, M.; Elbers, R.; Blencowe, N.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Cashin, A.; McAuley, J. Clinimetrics: Physiotherapy evidence database (PEDro) scale. J. Physiother. 2020, 66, 59. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.; Deeks, J.; Altman, D. Measuring inconsistency in metaanalyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, K.; Dan, I.; Suwabe, K.; Kyutoku, Y.; Yamada, Y.; Akahori, M.; Byun, K.; Kato, M.; Soya, H. Acute moderate exercise enhances compensatory brain activation in older adults. Neurobiol. Aging 2012, 33, 2621–2632. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Obuchi, S.; Arai, T.; Nagasawa, H.; Shiba, Y.; Watanabe, S.; Kojima, M. The influence of short-term strength training on health-related quality of life and executive cognitive function. J. Physiol. Anthropol. 2010, 29, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, E.; di Cagno, A.; Aquino, G.; Fiorilli, G.; Mignogna, P.; Calcagno, G.; Di Costanzo, A. Effects of different types of physical activity on the cognitive functions and attention in older people: A randomized controlled study. Exp. Gerontol. 2015, 70, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Liu-Ambrose, T.; Nagamatsu, L.S.; Graf, P.; Beattie, B.L.; Ashe, M.C.; Handy, T.C. Resistance training and executive functions: A 12-month randomized controlled trial. Arch. Intern. Med. 2010, 170, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Júnior, H.J.; Gonçalves, I.O.; Sampaio, R.A.C.; Sampaio, P.Y.S.; Lusa Cadore, E.; Calvani, R.; Picca, A.; Izquierdo, M.; Marzetti, E.; Uchida, M.C. Effects of Combined Resistance and Power Training on Cognitive Function in Older Women: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 3435. [Google Scholar] [CrossRef]
- Albinet, C.T.; Boucard, G.; Bouquet, C.A.; Audiffren, M. Increased heart rate variability and executive performance after aerobic training in the elderly. Eur. J. Appl. Physiol. 2010, 109, 617–624. [Google Scholar] [CrossRef]
- Byun, K.; Hyodo, K.; Suwabe, K.; Fukuie, T.; Ha, M.S.; Damrongthai, C.; Kuwamizu, R.; Koizumi, H.; Yassa, M.A.; Soya, H. Mild exercise improves executive function with increasing neural efficiency in the prefrontal cortex of older adults. Geroscience 2023, 46, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H. Effects of acute moderate-intensity aerobic exercise on executive function and prefrontal cortex activity in community-dwelling older adults: A single-blind, randomized controlled trial. Geriatr. Gerontol. Int. 2022, 22, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2018, 52, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.K.; Nien, Y.H.; Tsai, C.L.; Etnier, J.L. Physical activity and cognition in older adults: The potential of Tai Chi Chuan. J. Aging Phys. Activ. 2010, 18, 451–472. [Google Scholar] [CrossRef]
- Snowden, M.; Steinman, L.; Mochan, K.; Grodstein, F.; Prohaska, T.R.; Thurman, D.J.; Brown, D.R.; Laditka, J.N.; Soares, J.; Zweiback, D.J.; et al. Effect of exercise on cognitive performance in community-dwelling older adults: Review of intervention trials and recommendations for public health practice and research. J. Am. Geriatr. Soc. 2011, 59, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Angevaren, M.; Aufdemkampe, G.; Verhaar, H.J.; Aleman, A.; Vanhees, L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008, 3, CD005381. [Google Scholar]
- Kelly, M.E.; Loughrey, D.; Lawlor, B.A.; Robertson, I.H.; Walsh, C.; Brennan, S. The impact of exercise on the cognitive functioning of healthy older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2014, 16, 12–31. [Google Scholar] [CrossRef]
- Li, Z.; Peng, X.; Xiang, W.; Han, J.; Li, K. The effect of resistance training on cognitive function in the older adults: A systematic review of randomized clinical trials. Aging Clin. Exp. Res. 2018, 30, 1259–1273. [Google Scholar] [CrossRef]
- Gomes-Osman, J.; Cabral, D.F.; Morris, T.P.; McInerney, K.; Cahalin, L.P.; Rundek, T.; Oliveira, A.; Pascual-Leone, A. Exercise for cognitive brain health in aging: A systematic review for an evaluation of dose. Neurol. Clin. Pract. 2018, 8, 257–265. [Google Scholar] [CrossRef]
- Frost, N.J.; Weinborn, M.; Gignac, G.E.; Rainey-Smith, S.R.; Markovic, S.; Gordon, N.; Sohrabi, H.R.; Laws, S.M.; Martins, R.N.; Peiffer, J.J.; et al. A Randomized Controlled Trial of High-Intensity Exercise and Executive Functioning in Cognitively Normal Older Adults. Am. J. Geriatr. Psychiatry 2021, 29, 129–140. [Google Scholar] [CrossRef]
- Smiley-Oyen, A.L.; Lowry, K.A.; Francois, S.J.; Kohut, M.L.; Ekkekakis, P. Exercise, fitness, and neurocognitive function in older adults: The “selective improvement” and “cardiovascular fitness” hypotheses. Ann. Behav. Med. 2008, 36, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Coetsee, C.; Terblanche, E. The effect of three different exercise training modalities on cognitive and physical function in a healthy older population. Eur. Rev. Aging Phys. Act. 2017, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.F.; Hahn, S.; Cohen, N.J.; Banich, M.T.; McAuley, E.; Harrison, C.R.; Chason, J.; Vakil, E.; Bardell, L.; Boileau, R.A.; et al. Ageing, fitness and neurocognitive function. Nature 1999, 400, 418–419. [Google Scholar] [CrossRef] [PubMed]
- Fabre, C.; Chamari, K.; Mucci, P.; Massé-Biron, J.; Préfaut, C. Improvement of cognitive function by mental and/or individualized aerobic training in healthy elderly subjects. Int. J. Sports Med. 2002, 23, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.; Merchant, R.A.; Morley, J.E.; Anker, S.D.; Aprahamian, I.; Arai, H.; Aubertin-Leheudre, M.; Bernabei, R.; Cadore, E.L.; Cesari, M.; et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J. Nutr. Health Aging 2021, 25, 824–853. [Google Scholar] [CrossRef] [PubMed]
- Gronwald, T.; Törpel, A.; Herold, F.; Budde, H. Perspective of Dose and Response for Individualized Physical Exercise and Training Prescription. J. Funct. Morphol. Kinesiol. 2020, 5, 48. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, H.B. The effects of whole-body vibration exercise intervention on electroencephalogram activation and cognitive function in women with senile dementia. J. Exerc. Rehabil. 2018, 14, 586–591. [Google Scholar] [CrossRef]
- Heesterbeek, M.; Van der Zee, E.A.; van Heuvelen, M.J.G. Passive exercise to improve quality of life, activities of daily living, care burden and cognitive functioning in institutionalized older adults with dementia—A randomized controlled trial study protocol. BMC Geriatr. 2018, 18, 182. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).