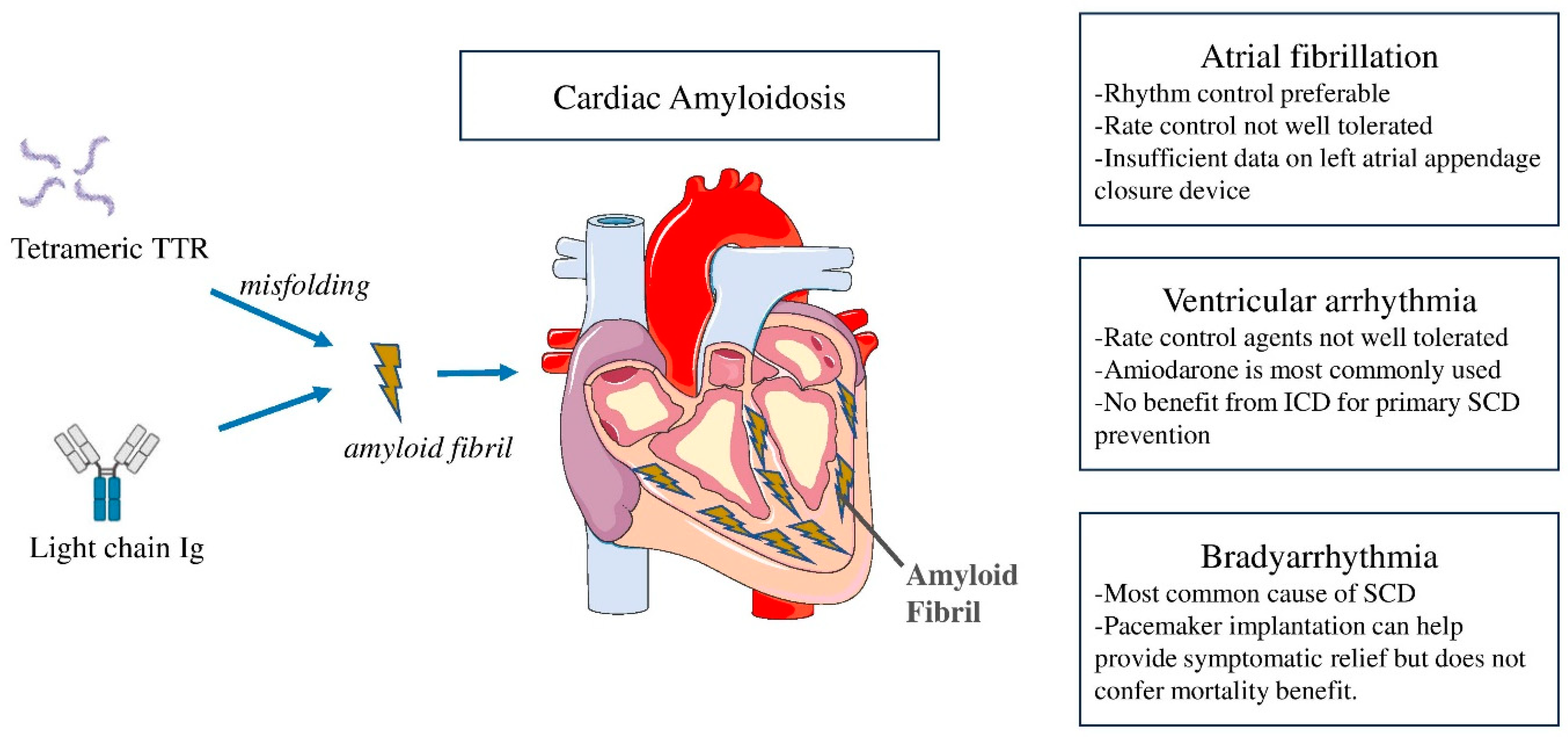
Arrhythmias and Device Therapies in Cardiac Amyloidosis
Abstract
1. Introduction
2. Atrial Arrhythmias in CA
2.1. Prevalence and Mechanisms
2.2. Management of AF and Role of Left Appendage Closure Device
3. Ventricular Tachyarrhythmias
3.1. Prevalence and Mechanisms
3.2. Management of VT and Role of Implantable Cardioverter-Defibrillator
3.3. Epidemiology of Bradyarrhythmias and Role of Pacemaker in CA
3.4. Knowledge Gaps and Future Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maleszewski, J.J. Cardiac amyloidosis: Pathology, nomenclature, and typing. Cardiovasc. Pathol. 2015, 24, 343–350. [Google Scholar] [CrossRef]
- Falk, R.H.; Alexander, K.M.; Liao, R.; Dorbala, S. AL (Light-Chain) Cardiac Amyloidosis: A Review of Diagnosis and Therapy. J. Am. Coll. Cardiol. 2016, 68, 1323–1341. [Google Scholar] [CrossRef]
- Masri, A.; Bukhari, S.; Eisele, Y.S.; Soman, P. Molecular Imaging of Cardiac Amyloidosis. J. Nucl. Med. 2020, 61, 965–970. [Google Scholar] [CrossRef]
- Merlini, G.; Dispenzieri, A.; Sanchorawala, V.; Schönland, S.O.; Palladini, G.; Hawkins, P.N.; Gertz, M.A. Systemic immunoglobulin light chain amyloidosis. Nat. Rev. Dis. Primers 2018, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.; Khan, S.Z.; Bashir, Z. Atrial Fibrillation, Thromboembolic Risk, and Anticoagulation in Cardiac Amyloidosis: A Review. J. Card. Fail. 2023, 29, 76–86. [Google Scholar] [CrossRef]
- Marin-Argany, M.; Lin, Y.; Misra, P.; Williams, A.; Wall, J.S.; Howell, K.G.; Elsbernd, L.R.; McClure, M.; Ramirez-Alvarado, M. Cell Damage in Light Chain Amyloidosis: Fibril Internalization, Toxicity and Cell-Mediated Seeding. J. Biol. Chem. 2016, 291, 19813–19825. [Google Scholar] [CrossRef]
- Brenner, D.A.; Jain, M.; Pimentel, D.R.; Wang, B.; Connors, L.H.; Skinner, M.; Apstein, C.S.; Liao, R. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ. Res. 2004, 94, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- González-López, E.; Gallego-Delgado, M.; Guzzo-Merello, G.; de Haro-Del Moral, F.J.; Cobo-Marcos, M.; Robles, C.; Bornstein, B.; Salas, C.; Lara-Pezzi, E.; Alonso-Pulpon, L.; et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur. Heart J. 2015, 36, 2585–2594. [Google Scholar] [CrossRef]
- Lo Presti, S.; Horvath, S.A.; Mihos, C.G.; Rajadhyaksha, C.; McCloskey, V.; Santana, O. Transthyretin Cardiac Amyloidosis as Diagnosed by 99mTc-PYP Scanning in Patients with Acute Heart Failure and Preserved Ejection Fraction. Crit. Pathw. Cardiol. 2019, 18, 195–199. [Google Scholar] [CrossRef]
- Devesa, A.; Camblor Blasco, A.; Pello Lázaro, A.M.; Askari, E.; Lapeña, G.; Gómez Talavera, S.; Taibo Urquía, M.; Rodríguez Olleros, C.; Tuñón, J.; Ibáñez, B.; et al. Prevalence of transthyretin amyloidosis in patients with heart failure and no left ventricular hypertrophy. ESC Heart Fail. 2021, 8, 2856–2865. [Google Scholar] [CrossRef] [PubMed]
- Quarta, C.C.; Buxbaum, J.N.; Shah, A.M.; Falk, R.H.; Claggett, B.; Kitzman, D.W.; Mosley, T.H.; Butler, K.R.; Boerwinkle, E.; Solomon, S.D. The amyloidogenic V122I transthyretin variant in elderly black Americans. N. Engl. J. Med. 2015, 372, 21–29. [Google Scholar] [CrossRef]
- Parker, M.M.; Damrauer, S.M.; Tcheandjieu, C.; Erbe, D.; Aldinc, E.; Hawkins, P.N.; Gillmore, J.D.; Hull, L.E.; Lynch, J.A.; Joseph, J.; et al. Association of the transthyretin variant V122I with polyneuropathy among individuals of African ancestry. Sci. Rep. 2021, 11, 11645. [Google Scholar] [CrossRef]
- 13. Porcari, A.; Razvi, Y.; Masi, A.; Patel, R.; Ioannou, A.; Rauf, M.U.; Hutt, D.F.; Rowczenio, D.; Gilbertson, J.; Martinez-Naharro, A.; et al. Prevalence, characteristics and outcomes of older patients with hereditary versus wild-type transthyretin amyloid cardiomyopathy. Eur. J. Heart Fail. 2023, 25, 515–524. [Google Scholar] [CrossRef]
- Arvidsson, S.; Pilebro, B.; Westermark, P.; Lindqvist, P.; Suhr, O.B. Amyloid Cardiomyopathy in Hereditary Transthyretin V30M Amyloidosis—Impact of Sex and Amyloid Fibril Composition. PLoS ONE 2015, 10, e0143456. [Google Scholar] [CrossRef]
- Ihse, E.; Ybo, A.; Suhr, O.; Lindqvist, P.; Backman, C.; Westermark, P. Amyloid fibril composition is related to the phenotype of hereditary transthyretin V30M amyloidosis. J. Pathol. 2008, 216, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.; Brownell, A.; Nieves, R.; Eisele, Y.S.; Follansbee, W.P.; Soman, P. Clinical Predictors of positive Tc-99m pyrophosphate scan in patients hospitalized for decompensated heart failure. J. Nucl. Med. 2020, 61 (Suppl. S1), 659, Abstract. [Google Scholar]
- Mints, Y.Y.; Doros, G.; Berk, J.L.; Connors, L.H.; Ruberg, F.L. Features of atrial fibrillation in wild-type transthyretin cardiac amyloidosis: A systematic review and clinical experience. ESC Heart Fail. 2018, 5, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.; Barakat, A.F.; Eisele, Y.S.; Nieves, R.; Jain, S.; Saba, S.; Follansbee, W.P.; Brownell, A.; Soman, P. Prevalence of Atrial Fibrillation and Thromboembolic Risk in Wild-Type Transthyretin Amyloid Cardiomyopathy. Circulation 2021, 143, 1335–1337. [Google Scholar] [CrossRef]
- Staerk, L.; Sherer, J.A.; Ko, D.; Benjamin, E.J.; Helm, R.H. Atrial Fibrillation: Epidemiology, Pathophysiology, and Clinical Outcomes. Circ. Res. 2017, 120, 1501–1517. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, M.; Jakstaite, A.M.; Oubari, S.; Siebermair, J.; Wakili, R.; Hoffmann, J.; Carpinteiro, A.; Hagenacker, T.; Thimm, A.; Rischpler, C.; et al. Clinical features and predictors of atrial fibrillation in patients with light-chain or transthyretin cardiac amyloidosis. ESC Heart Fail. 2022, 9, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Hylek, E.M.; Phillips, K.A.; Chang, Y.; Henault, L.E.; Selby, J.V.; Singer, D.E. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001, 285, 2370–2375. [Google Scholar] [CrossRef]
- Rosenberg, M.A.; Manning, W.J. Diastolic dysfunction and risk of atrial fibrillation: A mechanistic appraisal. Circulation 2012, 126, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Röcken, C.; Peters, B.; Juenemann, G.; Saeger, W.; Klein, H.U.; Huth, C.; Roessner, A.; Goette, A. Atrial amyloidosis: An arrhythmogenic substrate for persistent atrial fibrillation. Circulation 2002, 106, 2091–2097. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bhaskaran, A. Atrial Fibrillation in Transthyretin Cardiac Amyloidosis: The Growing Need to Look Forward. JACC Clin. Electrophysiol. 2020, 6, 1128–1130. [Google Scholar] [CrossRef] [PubMed]
- Henein, M.Y.; Suhr, O.B.; Arvidsson, S.; Pilebro, B.; Westermark, P.; Hörnsten, R.; Lindqvist, P. Reduced left atrial myocardial deformation irrespective of cavity size: A potential cause for atrial arrhythmia in hereditary transthyretin amyloidosis. Amyloid 2018, 25, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.; Oliveros, E.; Parekh, H.; Farmakis, D. Epidemiology, Mechanisms, and Management of Atrial Fibrillation in Cardiac Amyloidosis. Curr. Probl. Cardiol. 2023, 48, 101571. [Google Scholar] [CrossRef] [PubMed]
- Pollak, A.; Falk, R.H. Left ventricular systolic dysfunction precipitated by verapamil in cardiac amyloidosis. Chest 1993, 104, 618–620. [Google Scholar] [CrossRef]
- Gertz, M.A.; Skinner, M.; Connors, L.H.; Falk, R.H.; Cohen, A.S.; Kyle, R.A. Selective binding of nifedipine to amyloid fibrils. Am. J. Cardiol. 1985, 55, 1646. [Google Scholar] [CrossRef]
- Ioannou, A.; Massa, P.; Patel, R.K.; Razvi, Y.; Porcari, A.; Rauf, M.U.; Jiang, A.; Cabras, G.; Filisetti, S.; Bolhuis, R.E.; et al. Conventional heart failure therapy in cardiac ATTR amyloidosis. Eur. Heart J. 2023, 44, 2893–2907. [Google Scholar] [CrossRef]
- Rubinow, A.; Skinner, M.; Cohen, A.S. Digoxin sensitivity in amyloid cardiomyopathy. Circulation 1981, 63, 1285–1288. [Google Scholar] [CrossRef]
- Cassidy, J.T. Cardiac amyloidosis. Two cases with digitalis sensitivity. Ann. Intern Med. 1961, 55, 989–994. [Google Scholar] [CrossRef]
- Muchtar, E.; Gertz, M.A.; Kumar, S.K.; Lin, G.; Boilson, B.; Clavell, A.; Lacy, M.Q.; Buadi, F.K.; Hayman, S.R.; Kapoor, P.; et al. Digoxin use in systemic light-chain (AL) amyloidosis: Contra-indicated or cautious use? Amyloid 2018, 25, 86–92. [Google Scholar] [CrossRef]
- Dale, Z.; Chandrashekar, P.; Al-Rashdan, L.; Kim, M.; Masri, A.; Nazer, B. Management Strategies for Atrial Fibrillation and Flutter in Patients with Transthyretin Cardiac Amyloidosis. Am. J. Cardiol. 2021, 157, 107–114. [Google Scholar] [CrossRef]
- Donnellan, E.; Wazni, O.M.; Hanna, M.; Elshazly, M.B.; Puri, R.; Saliba, W.; Kanj, M.; Vakamudi, S.; Patel, D.R.; Baranowski, B.; et al. Atrial Fibrillation in Transthyretin Cardiac Amyloidosis: Predictors, Prevalence, and Efficacy of Rhythm Control Strategies. JACC Clin. Electrophysiol. 2020, 6, 1118–1127. [Google Scholar] [CrossRef]
- El-Am, E.A.; Dispenzieri, A.; Melduni, R.M.; Ammash, N.M.; White, R.D.; Hodge, D.O.; Noseworthy, P.A.; Lin, G.; Pislaru, S.V.; Egbe, A.C.; et al. Direct Current Cardioversion of Atrial Arrhythmias in Adults With Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2019, 73, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.Y.; Mohsin, Y.; Hodge, D.O.; Lacy, M.Q.; Packer, D.L.; Dispenzieri, A.; Grogan, M.; Asirvatham, S.J.; Madhavan, M.; McLeod, C.J. Catheter Ablation for Atrial Arrhythmias in Patients With Cardiac Amyloidosis. J. Cardiovasc. Electrophysiol. 2016, 27, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Donnellan, E.; Wazni, O.; Kanj, M.; Elshazly, M.B.; Hussein, A.; Baranowski, B.; Hanna, M.; Patel, D.; Trulock, K.; Martyn, M.; et al. Atrial fibrillation ablation in patients with transthyretin cardiac amyloidosis. Europace 2020, 22, 259–264. [Google Scholar] [CrossRef]
- Feng, D.; Edwards, W.D.; Oh, J.K.; Chandrasekaran, K.; Grogan, M.; Martinez, M.W.; Syed, I.S.; Hughes, D.A.; Lust, J.A.; Jaffe, A.S.; et al. Intracardiac thrombosis and embolism in patients with cardiac amyloidosis. Circulation 2007, 116, 2420–2426. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Syed, I.S.; Martinez, M.; Oh, J.K.; Jaffe, A.S.; Grogan, M.; Edwards, W.D.; Gertz, M.A.; Klarich, K.W. Intracardiac thrombosis and anticoagulation therapy in cardiac amyloidosis. Circulation 2009, 119, 2490–2497. [Google Scholar] [CrossRef]
- Bukhari, S. Cardiac amyloidosis: State-of-the-art review. J. Geriatr. Cardiol. 2023, 20, 361–375. [Google Scholar] [CrossRef]
- Mitrani, L.R.; De Los Santos, J.; Driggin, E.; Kogan, R.; Helmke, S.; Goldsmith, J.; Biviano, A.B.; Maurer, M.S. Anticoagulation with warfarin compared to novel oral anticoagulants for atrial fibrillation in adults with transthyretin cardiac amyloidosis: Comparison of thromboembolic events and major bleeding. Amyloid 2021, 28, 30–34. [Google Scholar] [CrossRef]
- Vilches, S.; Fontana, M.; Gonzalez-Lopez, E.; Mitrani, L.; Saturi, G.; Renju, M.; Griffin, J.M.; Caponetti, A.; Gnanasampanthan, S.; De Los Santos, J.; et al. Systemic embolism in amyloid transthyretin cardiomyopathy. Eur. J. Heart Fail. 2022, 24, 1387–1396. [Google Scholar] [CrossRef]
- Bukhari, S.; Fatima, S.; Nieves, R.; Ibrahim, J.; Brownell, A.; Soman, P. Bleeding risk associated with transthyretin cardiac amyloidosis. J. Am. Coll. Cardiol. 2021, 77 (Suppl. S1), 530. [Google Scholar] [CrossRef]
- Nicol, M.; Siguret, V.; Vergaro, G.; Aimo, A.; Emdin, M.; Dillinger, J.G.; Baudet, M.; Cohen-Solal, A.; Villesuzanne, C.; Harel, S.; et al. Thromboembolism and bleeding in systemic amyloidosis: A review. ESC Heart Fail. 2022, 9, 11–20. [Google Scholar] [CrossRef]
- El-Am, E.A.; Grogan, M.; Ahmad, A.; Patlolla, S.H.; Klarich, K.W.; AbouEzzeddine, O.F.; Melduni, R.M.; Maleszewski, J.J.; Dis-penzieri, A.; Nkomo, V.T. Persistence of Left Atrial Appendage Thrombus in Patients With Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2021, 77, 342–343. [Google Scholar] [CrossRef] [PubMed]
- Amat-Santos, I.J.; Delgado-Arana, J.R.; Cruz-González, I.; Gutiérrez, H.; García-Bolao, I.; Millán, X.; Tirado-Conte, G.; Ruiz-Nodar, J.M.; Mohandes, M.; Palazuelos, J.; et al. Cardiac amyloidosis and left atrial appendage closure. The CAMYLAAC study. Rev. Esp. Cardiol. Engl. Ed. 2023, 76, 503–510. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Kuo, M.J.; Chung, F.P.; Lin, Y.J.; Chien, K.L.; Hsieh, Y.C.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Chao, T.F.; et al. Risks of Ventricular Tachyarrhythmia and Mortality in Patients with Amyloidosis—A Long-Term Cohort Study. Acta Cardiol. Sin. 2022, 38, 464–474. [Google Scholar] [PubMed]
- Dubrey, S.W.; Cha, K.; Anderson, J.; Chamarthi, B.; Reisinger, J.; Skinner, M.; Falk, R.H. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM Int. J. Med. 1998, 91, 141–157. [Google Scholar] [CrossRef]
- Goldsmith, Y.B.; Liu, J.; Chou, J.; Hoffman, J.; Comenzo, R.L.; Steingart, R.M. Frequencies and types of arrhythmias in patients with systemic light-chain amyloidosis with cardiac involvement undergoing stem cell transplantation on telemetry monitoring. Am. J. Cardiol. 2009, 104, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Hörnsten, R.; Wiklund, U.; Olofsson, B.O.; Jensen, S.M.; Suhr, O.B. Liver transplantation does not prevent the development of life-threatening arrhythmia in familial amyloidotic polyneuropathy, Portuguese-type (ATTR Val30Met) patients. Transplantation 2004, 78, 112–116. [Google Scholar] [CrossRef]
- Bukhari, S.; Khan, B. Prevalence of ventricular arrhythmias and role of implantable cardioverter-defibrillator in cardiac amyloidosis. J. Cardiol. 2023, 81, 429–433. [Google Scholar] [CrossRef]
- Zampieri, M.; Allinovi, M.; Olivotto, I.; Antonioli, E.; Gabriele, M.; Argirò, A.; Fumagalli, C.; Nardi, G.; Di Mario, C.; Vannucchi, A.M.; et al. Ventricular tachyarrhythmias and sudden cardiac death in light-chain amyloidosis: A clash of cardio-toxicities? Br. J. Haematol. 2021, 193, e27–e31. [Google Scholar] [CrossRef]
- Hashimura, H.; Ishibashi-Ueda, H.; Yonemoto, Y.; Ohta-Ogo, K.; Matsuyama, T.A.; Ikeda, Y.; Morita, Y.; Yamada, N.; Yasui, H.; Naito, H. Late gadolinium enhancement in cardiac amyloidosis: Attributable both to interstitial amyloid deposition and subendocardial fibrosis caused by ischemia. Heart Vessel. 2016, 31, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Sławiński, G.; Dorniak, K.; Lewicka, E. Implantable cardioverter-defibrillators in cardiac amyloidosis: A grey zone requiring an individual approach. Kardiol. Pol. 2020, 78, 934–936. [Google Scholar] [CrossRef] [PubMed]
- Liżewska-Springer, A.; Sławiński, G.; Lewicka, E. Arrhythmic Sudden Cardiac Death and the Role of Implantable Cardioverter-Defibrillator in Patients with Cardiac Amyloidosis-A Narrative Literature Review. J. Clin. Med. 2021, 10, 1858. [Google Scholar] [CrossRef] [PubMed]
- Kristen, A.V.; Dengler, T.J.; Hegenbart, U.; Schonland, S.O.; Goldschmidt, H.; Sack, F.U.; Voss, F.; Becker, R.; Katus, H.A.; Bauer, A. Prophylactic implantation of cardioverter-defibrillator in patients with severe cardiac amyloidosis and high risk for sudden cardiac death. Heart Rhythm 2008, 5, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Varr, B.C.; Zarafshar, S.; Coakley, T.; Liedtke, M.; Lafayette, R.A.; Arai, S.; Schrier, S.L.; Witteles, R.M. Implantable cardioverter-defibrillator placement in patients with cardiac amyloidosis. Heart Rhythm 2014, 11, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Hamon, D.; Algalarrondo, V.; Gandjbakhch, E.; Extramiana, F.; Marijon, E.; Elbaz, N.; Selhane, D.; Dubois-Rande, J.L.; Teiger, E.; Plante-Bordeneuve, V.; et al. Outcome and incidence of appropriate implantable cardioverter-defibrillator therapy in patients with cardiac amyloidosis. Int. J. Cardiol. 2016, 222, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Dispenzieri, A.; Kyle, R.; Grogan, M.; Brady, P.A. Implantable cardioverter defibrillators in patients with cardiac amyloidosis. J. Cardiovasc. Electrophysiol. 2013, 24, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Higgins, A.Y.; Annapureddy, A.R.; Wang, Y.; Minges, K.E.; Lampert, R.; Rosenfeld, L.E.; Jacoby, D.L.; Curtis, J.P.; Miller, E.J.; Freeman, J.V. Survival Following Implantable Cardioverter-Defibrillator Implantation in Patients with Amyloid Cardiomyopathy. J. Am. Heart Assoc. 2020, 9, e016038. [Google Scholar] [CrossRef]
- Donnellan, E.; Wazni, O.M.; Hanna, M.; Saliba, W.; Jaber, W.; Kanj, M. Primary prevention implantable cardioverter-defibrillators in transthyretin cardiac amyloidosis. Pacing Clin. Electrophysiol. 2020, 43, 1401–1403. [Google Scholar]
- Brown, M.T.; Yalamanchili, S.; Evans, S.T.; Ram, P.; Blank, E.A.; Lyle, M.A.; Merchant, F.M.; Bhatt, K.N. Ventricular arrhythmia burden and implantable cardioverter-defibrillator outcomes in transthyretin cardiac amyloidosis. Pacing Clin. Electrophysiol. 2022, 45, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Olausson, E.; Wertz, J.; Fridman, Y.; Bering, P.; Maanja, M.; Niklasson, L.; Wong, T.C.; Fukui, M.; Cavalcante, J.L.; Cater, G.; et al. Diffuse myocardial fibrosis associates with incident ventricular arrhythmia in implantable cardioverter defibrillator recipients. medRxiv 2023. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. ESC Scientific Document Group. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef] [PubMed]
- Barbhaiya, C.R.; Kumar, S.; Baldinger, S.H.; Michaud, G.F.; Stevenson, W.G.; Falk, R.; John, R.M. Electrophysiologic assessment of conduction abnormalities and atrial arrhythmias associated with amyloid cardiomyopathy. Heart Rhythm 2016, 13, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.; Karp, K.; Bjerle, P.; Olofsson, B.O. Disturbances of cardiac rhythm and conduction in familial amyloidosis with polyneuropathy. Br. Heart J. 1984, 51, 658–662. [Google Scholar] [CrossRef]
- Porcari, A.; Rossi, M.; Cappelli, F.; Canepa, M.; Musumeci, B.; Cipriani, A.; Tini, G.; Barbati, G.; Varrà, G.G.; Morelli, C.; et al. Incidence and risk factors for pacemaker implantation in light-chain and transthyretin cardiac amyloidosis. Eur. J. Heart Fail. 2022, 24, 1227–1236. [Google Scholar] [CrossRef]
- Bukhari, S.; Kasi, A.; Khan, B. Bradyarrhythmias in Cardiac Amyloidosis and Role of Pacemaker. Curr. Probl. Cardiol. 2023, 48, 101912. [Google Scholar] [CrossRef]
- Elgendy, I.Y.; Bukhari, S.; Barakat, A.F.; Pepine, C.J.; Lindley, K.J.; Miller, E.C.; American College of Cardiology Cardiovascular Disease in Women Committee. Maternal Stroke: A Call for Action. Circulation 2021, 143, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Ladefoged, B.T.; Dybro, A.; Dahl Pedersen, A.L.; Rasmussen, T.B.; Vase, H.Ø.; Clemmensen, T.S.; Gillmore, J.; Poulsen, S.H. Incidence and predictors of worsening heart failure in patients with wild-type transthyretin cardiac amyloidosis. ESC Heart Fail. 2022, 9, 2978–2987. [Google Scholar] [CrossRef] [PubMed]
- Rehorn, M.R.; Loungani, R.S.; Black-Maier, E.; Coniglio, A.C.; Karra, R.; Pokorney, S.D.; Khouri, M.G. Cardiac Implantable Electronic Devices: A Window Into the Evolution of Conduction Disease in Cardiac Amyloidosis. JACC Clin. Electrophysiol. 2020, 6, 1144–1154. [Google Scholar] [CrossRef]
- Donnellan, E.; Wazni, O.M.; Saliba, W.I.; Baranowski, B.; Hanna, M.; Martyn, M.; Patel, D.; Trulock, K.; Menon, V.; Hussein, A.; et al. Cardiac devices in patients with transthyretin amyloidosis: Impact on functional class, left ventricular function, mitral regurgitation, and mortality. J. Cardiovasc. Electrophysiol. 2019, 30, 2427–2432. [Google Scholar] [CrossRef]
- Donnellan, E.; Wazni, O.M.; Hanna, M.; Kanj, M.; Saliba, W.I.; Jaber, W.A. Cardiac Resynchronization Therapy for Transthyretin Cardiac Amyloidosis. J. Am. Heart Assoc. 2020, 9, e017335. [Google Scholar] [CrossRef]
- Sayed, R.H.; Rogers, D.; Khan, F.; Wechalekar, A.D.; Lachmann, H.J.; Fontana, M.; Mahmood, S.; Sachchithanantham, S.; Patel, K.; Hawkins, P.N.; et al. A study of implanted cardiac rhythm recorders in advanced cardiac AL amyloidosis. Eur. Heart J. 2015, 36, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Slart, R.H.J.A.; Glaudemans, A.W.J.M.; Hazenberg, B.P.C.; Noordzij, W. Imaging cardiac innervation in amyloidosis. J. Nucl. Cardiol. 2019, 26, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Algalarrondo, V.; Dinanian, S.; Juin, C.; Chemla, D.; Bennani, S.L.; Sebag, C.; Planté, V.; Le Guludec, D.; Samuel, D.; Adams, D.; et al. Prophylactic pacemaker implantation in familial amyloid polyneuropathy. Heart Rhythm 2012, 9, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, S.; Estner, H.L.; Näbauer, M.; Hausleiter, J. Percutaneous extraction of a leadless Micra pacemaker after dislocation: A case report. Eur. Heart J. Case Rep. 2019, 3, ytz113. [Google Scholar] [CrossRef] [PubMed]
- Masri, A.; Bukhari, S.; Ahmad, S.; Nieves, R.; Eisele, Y.S.; Follansbee, W.; Brownell, A.; Wong, T.C.; Schelbert, E.; Soman, P. Efficient 1-Hour Technetium-99 m Pyrophosphate Imaging Protocol for the Diagnosis of Transthyretin Cardiac Amyloidosis. Circ. Cardiovasc. Imaging 2020, 13, e010249. [Google Scholar] [CrossRef]
- Bashir, Z.; Chen, E.W.; Tori, K.; Ghosalkar, D.; Aurigemma, G.P.; Dickey, J.B.; Haines, P. Insight into different phenotypic presentations of heart failure with preserved ejection fraction. Prog. Cardiovasc. Dis. 2023, 79, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Emdin, M.; Morfino, P.; Crosta, L.; Aimo, A.; Vergaro, G.; Castiglione, V. Monoclonal antibodies and amyloid removal as a therapeutic strategy for cardiac amyloidosis. Eur. Heart J. Suppl. 2023, 25, B79–B84. [Google Scholar] [CrossRef]
| Study | Population Demographics with ICD | ICD for Primary Prevention, N (%) | Successful ICD Therapy, % | Mean Age, Years | Mean Follow Up Duration, Months | 1-Year Survival, % |
|---|---|---|---|---|---|---|
| Kristen et al. [56] | N = 19 | 19 (100) | 11 | 58 | 27 ± 5.0 | 63 |
| Varr et al. [57] | N = 19 AL-15 ATTR-4 | 15 (79) | 26 | 68 | NA | 50 |
| Hamon et al. [58] | N = 45 AL-12 ATTR-33 | 38 (84) | 27 | 66 | 17 ± 14 | 73 |
| Lin et al. [59] | N = 53 AL-33 ATTR-19 | 41 (77) | 32 | 64 | 23 ± 21 | 22 |
| Hiigins et al. [60] | N = 472 | 359 (76) | NA | 68 | NA | 73 |
| Donnellan et al. [61] | N = 19 ATTR-19 | 19 (100) | NA | 73 | 23 ± 19 | 16 |
| Brown et al. [62] | N = 32 ATTR-32 | 32 (100) | 25 | 74 | 38 ± 3.6 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukhari, S.; Khan, S.Z.; Ghoweba, M.; Khan, B.; Bashir, Z. Arrhythmias and Device Therapies in Cardiac Amyloidosis. J. Clin. Med. 2024, 13, 1300. https://doi.org/10.3390/jcm13051300
Bukhari S, Khan SZ, Ghoweba M, Khan B, Bashir Z. Arrhythmias and Device Therapies in Cardiac Amyloidosis. Journal of Clinical Medicine. 2024; 13(5):1300. https://doi.org/10.3390/jcm13051300
Chicago/Turabian StyleBukhari, Syed, Syed Zamrak Khan, Mohamed Ghoweba, Bilal Khan, and Zubair Bashir. 2024. "Arrhythmias and Device Therapies in Cardiac Amyloidosis" Journal of Clinical Medicine 13, no. 5: 1300. https://doi.org/10.3390/jcm13051300
APA StyleBukhari, S., Khan, S. Z., Ghoweba, M., Khan, B., & Bashir, Z. (2024). Arrhythmias and Device Therapies in Cardiac Amyloidosis. Journal of Clinical Medicine, 13(5), 1300. https://doi.org/10.3390/jcm13051300







