Abstract
A certain proportion of patients undergoing cardiac surgery may experience bleeding complications that worsen outcomes. Numerous studies have investigated bleeding in cardiac surgery and some evaluate the role of hemostatic point-of-care tests in cardiac surgery patients. The prevalence of excessive bleeding varies in the literature, and such variability stems from the lack of a standardized definition of excessive bleeding. Herein, we report numerous definitions of excessive bleeding and methodological considerations for studies evaluating bleeding using hemostatic point-of-care tests in cardiac surgery patients. We evaluated the role of hemostatic point-of-care devices in contemporary research on bleeding complications and hemostatic management in cardiac surgery. The type of studies (prospective vs. retrospective, interventional vs. observational), patient selection (less complex vs. complex cases), as well as data analysis with comprehensive statistical considerations have also been provided. This article provides a comprehensive insight into the research field of bleeding complications in cardiac surgery and may help readers to better understand methodological flaws and how they influence current evidence.
1. Introduction
1.1. The Relevance of Bleeding Complications in Cardiac Surgery
A negligible proportion of patients undergoing cardiac surgery may experience significant chest tube drainage (CTD) requiring intervention ranging from transfusion of blood components to re-exploration for excessive bleeding [1,2,3,4]. Dixon et al. [5] showed that CTD was an independent predictor of mortality in patients undergoing cardiac surgery [5]. Furthermore, an independent association between excessive bleeding and adverse clinical outcomes has been reported by Chrisensen et al. [6]. Also, Rannuci et al. described a significant association between postoperative bleeding and operative mortality [7]. This association was further enhanced by the administration of red blood cells (RBC) and to a lesser extent with preoperative anemia [7]. Furthermore, Petrou et al. reported a dose-dependent effect of RBC, plasma, and platelet transfusion on mortality in cardiac surgery [8].
Excessive postoperative bleeding is a relatively common complication in cardiac surgery occurring in about 20% of patients [9] and remains to be a persistent complication of cardiac surgery procedures over a long period.
Re-exploration for excessive bleeding occurs in 5–9% of all unselected cardiac surgical patients [3] and remains a very important complication associated with patient mortality rates up to 14% [3,10,11]. It seems that the risk of an adverse outcome parallels the time for re-exploration [12]. Furthermore, the multicausality of excessive postoperative bleeding makes it somehow challenging to precisely understand the underlying mechanisms. The mechanisms of hemostatic disorders in patients undergoing cardiac surgery with cardiopulmonary bypass (CPB) have recently been described [11]. Hemostatic disorder includes different mechanisms such as hyperfibrinolysis, heparin anticoagulation, protamine overdose, dilution and consumption of fibrinogen, and other coagulation factors, as well as thrombocytopenia and platelet dysfunction [11,13,14,15,16]. Numerous clinical factors contributing to post-CPB bleeding are well recognized [1]; however, not all patients with risk factors will bleed (low positive predictive value), nor all patients without known clinical risk factors will not bleed.
Additional preoperative factors should inevitably be considered, such as platelet dysfunction due to preoperatively administered antiplatelet therapy (APT) or impaired thrombin generation due to oral anticoagulation with vitamin K-antagonists (VKAs) or direct oral anticoagulants (DOACs). Furthermore, an acquired von Willebrand syndrome must be considered in patients with structural heart disease (e.g., aortic stenosis) resulting in high shear stress, which increases ADAMTS13 mediated cleavage of von Willebrand factor multimers. All these factors, either alone or in combination, cause hemostatic disbalance, which in turn may contribute to bleeding complications.
1.2. The Relevance of Transfusion Requirements in Cardiac Surgery
Adult cardiac surgery accounts for up to 20% of all RBC transfusion in the Unites States [17,18,19,20,21].
It is obvious that cardiac surgery patients receiving blood transfusion are at risk for increased mortality and morbidity, and that the transfusion practice expresses huge variability with a widespread range of transfusion triggers. Shaw et al. attempted to elucidate whether this increased risk occurs across all hematocrite (HCT) levels [22]. The authors concluded that patients who received blood at all HCT levels may be placed at an increased risk of operative mortality and/or other surgical complications [22]. Unexpectedly, transfusion was still associated with a higher complication and mortality rate even in patients with low HCT [22]. A lack of benefit of transfusing patients at all HCT levels was noted [22].
In a study by Ranucci et al. [7], they showed the deleterious effect of major bleeding had a multiplying effect if coexisting with RBC transfusion and to a lesser extent, preoperative anemia [7]. The importance of the three aforementioned factors may be easily understood by the information that patients without preoperative anemia or major bleeding and not being transfused have a very low operative mortality rate from 0.6% to 0.7% [7]. This inevitably leads to the obvious consideration that controlling these risk factors may be of paramount importance in cardiac surgery.
Considering the impact of bleeding and transfusion on clinical outcomes, determining which of these factors is the predominant driver of adverse outcomes is paramount to advancing our knowledge regarding perioperative bleeding management.
1.3. The Role of Hemostatic Point-of-Care Devices in Contemporary Research of Bleeding Complications and Hemostatic Management in Cardiac Surgery
Despite the increased understanding of the hemostatic disorder mechanism, the question “How to predict and prevent excessive bleeding?” remains challenging. Conventional coagulation tests failed to predict postoperative bleeding [23]. Furthermore, long turnaround times from blood sampling to obtaining results make conventional laboratory tests to be of limited value in the clinical decision-making process. The complexity of bleeding mechanisms hampers the possibility of simple detection and treatment, and rather requires a comprehensive approach.
It sounds reasonable that hemostatic point of care testing (HPOCT) may help to better understand the underlying mechanisms of hemostatic disorders, and therefore, improve diagnostic performance and accuracy in predicting bleeding complications in cardiac surgery.
Since platelets may play a pivotal role in hemostatic disorders in cardiac surgery and the importance of short turnaround times, several HPOCT devices have been developed.
These point-of-care hemostatic tests may be divided into the following: (1) Viscoelastic tests such as ROTEM (TEM Innovations, Munich, Germany) and TEG (Haemonetics Corporation, Braintree, MA, USA); and (2) Platelet function analyzers based on either whole blood impedance aggregometry [Multiplate—(Roche Diagnostics, Mannheim, Germany), ROTEM platelet—(TEM Innovations, Munich, Germany)], whole blood light transmission (VerifyNow, Werfen, San Diego, CA, USA), viscoelastic testing (TEG Platelet Mapping, Haemonetics Corporation, Braintree, Massachusetts, United States), or shear stress aggregometry (PFA—200, Siemens GmbH, Munich, Germany).
Both platelet function testing and viscoelastic testing, have been used in numerous trials to assess the predictability of bleeding complications; however, contradictory data exists which hampers the pooling of the evidence and drawing clear conclusions on the role of these devices in everyday clinical hemostatic management. The heterogeneity of reported data is further corroborated by different study settings with different non-standardized endpoints assessed, along with different devices used.
1.4. Contemporary Management of Hemostasis in Cardiac Surgery: What Do Guidelines Say?
The American Society of Anesthesiologists Task Force of Perioperative Blood Management came out with updated practice guidelines for perioperative blood management [24]. These guidelines include a greater emphasis on the preoperative assessment of patients and adjuvant therapies such as drugs and techniques to reduce or prevent bleeding complications and transfusion requirements [24]. For intraoperative and postoperative monitoring of hemostasis, these guidelines recommend that point-of-care platelet function testing and/or viscoelastic testing (e.g., TEG or ROTEM) may be included in the monitoring of perioperative hemostasis [24].
The European Society of Anesthesiology published guidelines on the management of severe perioperative bleeding [25]. These guidelines recommend the use of transfusion algorithms based on HPOCT for the evaluation of coagulation status [26]. Evaluation of platelet function is suggested for patients with positive bleeding history, medical conditions that may result in platelet dysfunction, or patients exposed to APT prior to surgery [26]. The guidelines [26] do not support the routine use of platelet function testing despite emerging evidence on the utility of preoperative platelet function testing in tailoring the timing of surgery [27] or predicting bleeding complications based on preoperative platelet function testing [28,29,30].
Platelet dysfunction is a major cause of bleeding following cardiac surgery [31,32].
Apparently, clinical trials are needed to assess the value of preoperative drug-specific platelet function testing in predicting bleeding complications.
Platelet function tests have the potential to predict bleeding complications and transfusion requirements; however, no “gold standard” test or cut-off value has yet been established.
Platelet function testing may be useful for monitoring platelet (dys) function during CPB and early after surgery upon arrival to the intensive care unit (ICU). Here, platelet dysfunction due to CPB and protamine may be of higher importance compared to platelet dysfunction caused by preoperatively administered APT. In different phases of cardiac surgery, platelet function is influenced by different modulating factors such as APT, CPB, and protamine administration for heparin reversal [14,15,33,34].
In fact, the multicausality of bleeding does not allow for strong correlations obtained by the assessment of a single hemostatic mechanism, and therefore, we must address some methodological considerations for studies investigating this issue. In this extremely challenging field, attention should be paid to every single detail and the appropriateness of study designs remains one of the most important issues. Compared to the other methodological issues in this field, the appropriate study design seems an easy reachable aim. Being aware that it is not possible to achieve an ideal experimental laboratory setting in real clinical life, we would like to address some very important methodological considerations for studies evaluating the prediction of excessive bleeding using HPOCT in cardiac surgery. Herein, we discuss methodological issues related to the investigation of the relationship between HPOCT and bleeding outcomes.
2. Methodological Considerations for Studies Evaluating the Prediction of Bleeding Complications Using Hemostatic Point-of-Care Tests in Cardiac Surgery
2.1. Type of Study
There are many different types of trials, and each type has distinct strengths and weaknesses (Figure 1). In general, there are two basic concepts that determine the type of study: (1) In regard to timing, studies may be conducted in a prospective or retrospective way. (2) In regard to patients’ exposure, studies may be either interventional or observational. Apparently, retrospective studies may only be observational, whereas prospective studies may be either interventional or observational in nature (Figure 1).
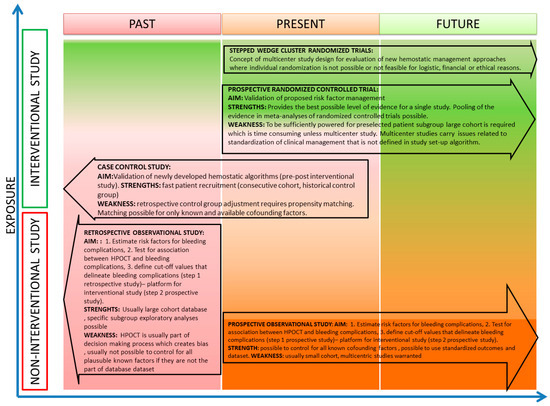
Figure 1.
Methodological considerations for studies with respect to intervention and timeline.
Different study settings were used in investigating the relationship between HPOCT and bleeding amount as well as transfusion outcomes [35].
2.2. Prospective Studies
From a historical viewpoint, the majority of studies pertaining to this issue were small prospective, observational cohort studies [35]. In recent times, there has been a growing proportion of retrospective studies [35]. When establishing research in this field, prospective observational studies are the most appropriate study type. In this way, it is possible to evaluate the association between observed parameters, i.e., platelet function test results and bleeding amount. The measurements may be performed at different time points and a correlation coefficient may be observed at each time point which reflects the different parameters influencing bleeding risk. For instance, performing platelet function testing prior to surgery may help to detect patients that have pronounced response to administered APT who could benefit either from earlier APT discontinuation or postponing surgery. At this time point, it is very important to use drug-specific platelet function tests that may direct further APT management. In cases of pronounced platelet inhibition, early discontinuation of APT should be considered. In contrast, if platelet function tests show high residual platelet reactivity despite administered APT, patients could undergo surgery even without APT discontinuation. In this way, it would be possible to avoid the rebound phenomenon which may occur after APT discontinuation in the subgroup of patients with hyperactive platelets [36].
Platelet function testing after weaning from CPB and heparin reversal by protamine may assess the effect of CPB and protamine on platelet function which may be particularly relevant in patients with long CPB times [33,34]. However, it is still a matter of debate whether platelet function tests or platelet contribution of clot firmness in viscoelastic testing better predicts bleeding complications in major cardiac surgery and other patients with thrombocytopenia [37].
However, prospective observational studies have some drawbacks that should be addressed. Even though consultant anesthesiologists and surgeons are unaware of the platelet function test findings, patients may receive procoagulant blood components according to predefined empirical clinical protocols. Thus, procoagulant blood component administration certainly hampers the observation of relationships between platelet function test results and the amount of postoperative bleeding by reducing the sensitivity of each test. It is not possible to assess the exact relationship between HPOCT results and the amount of bleeding, as the latter is the target of treatment, thus influenced by anesthetic measures during surgery. Obviously, in clinical situations it would not be ethically acceptable to avoid administration of procoagulant blood component therapy in bleeding patients, thereby making this methodology be compromised. Furthermore, prospective studies usually recruit only a relatively small number of patients which makes them frequently underpowered for assessment of some important clinical outcomes (i.e., mortality) which would confirm the relevance of bleeding complications.
If prospective observational studies detect a significant association between platelet function test values and bleeding extent, receiver operating characteristics (ROC) curve analysis should be performed [38] with the aim of delineating the cut-off value with the best sensitivity and specificity in predicting a particular outcome such as excessive bleeding. ROC curve analysis may define cut-offs that have the best positive predictive values (PPV) and negative predictive values (NPV) [38]. ROC curve analysis-derived cut-off values may direct the clinical decision-making process for preoperative APT discontinuation management or intraoperative transfusion management. The definition of cut-off values that delineate bleeding tendency presents the cornerstone according to which interventions may be directed. Interventions based on ROC curve analysis cut-offs may direct clinical practice, and may be used as a subject to further evaluate through interventional studies.
Prospective studies in this field should be designed as a part of a research project encompassing three stages: (1) Monocentric prospective studies evaluating the association between hemostatic blood properties and bleeding complications. In this stage, cut-off values that delineate bleeding complications should be defined. (2) Multicentric validation studies should confirm the association and cut-off values detected in the first step. (3) Prospective interventional studies should confirm the causal relationship and usefulness to guide interventions to prevent or stop bleeding and to develop validated hemostatic algorithms based on pre-defined cut-off values as recommended by the bleeding management guidelines [25].
There is a shortage of prospective interventional studies in this field. Mahla et al. conducted the first prospective interventional study using a platelet function testing-based strategy to reduce the bleeding and waiting time in clopidogrel treated patients undergoing coronary artery bypass grafting (CABG) [27]. In the absence of validated cut-off values that predict excessive bleeding, authors divided patients into three groups according to expert opinion-based cut-off values that might delineate bleeding tendency [27]. Even though this study used surrogate cut-off values, the authors reported positive results with a reduction in surgery waiting time after clopidogrel withdrawal without an increased risk of excessive bleeding [27]. This study shows how the definition of cut-off values can be performed through interventional studies. In cases of preoperatively performed platelet function tests, the intervention may be either the decision to discontinue or continue with APT preoperatively or to guide the timing of surgery based on pre-defined cut-off values [27].
Weber et al. [39] conducted a prospective randomized clinical trial aiming to evaluate the efficacy of hemostatic therapy guided by an HPOCT algorithm vs. an algorithm guided by conventional coagulation tests in patients undergoing major cardiac surgery experiencing clinically relevant bleeding after weaning from CPB and heparin reversal by protamine [39]. Algorithms for hemostatic management were based on either conventional coagulation tests or HPOCT (thromboelastometry and whole blood impedance aggregometry) and covered intraoperative as well as postoperative hemostatic management [39]. The study was terminated early since the planned interim analysis showed a highly significant reduction in RBC transfusion with a p-value < 0.01 according to predefined conditions. Furthermore, according to references [40,41,42,43], the patients in the HPOCT group showed a significant reduction in 6-month mortality (4% vs. 20%; p = 0.013) [39]. To the best of our knowledge, this is the first prospective randomized controlled study that showed clinical benefits when intra- and postoperative hemostatic management was based on HPOCT [39]. However, a significant improvement in patient outcomes and hospital costs could be confirmed in a multicenter trial looking at 5-year mortality, particularly in patients with long CPB times (>115 min) and bleeding after weaning from CPB [44].
2.3. Retrospective Studies
In recent years, there has been a growing number of retrospective cohort studies [40,41,42,43]. Authors may often consider these studies as retrospective analyses of prospectively collected data. This essentially means that authors have created robust databases with all the relevant data including the data on platelet function and viscoelastic testing, bleeding complications, and transfusion requirements. However, retrospective studies are the least appropriate study type for bleeding risk assessment based on HPOCT. In such studies, HPOCT results are usually used in regular clinical practice as a part of clinical decision-making. This results in major bias since the results of platelet function and viscoelastic testing were used as a part of hemostatic management, and therefore, may have influenced both the amount of bleeding and transfusion requirements as a self-full-filling prophecy. However, such a concept of retrospective analysis of prospectively collected data may provide valuable and useful data, but the observed associations should always be interpreted carefully due to the inextricable presence of bias. Ranucci et al. reported a retrospective analysis of prospectively collected data aiming to assess the relationship between platelet function testing and the amount of CTD [40]. Here, it is important to stress that patients with observed microvascular bleeding were treated with desmopressin if the preoperative whole blood impedance aggregometry (Multiplate) ADP test was less than 40 AUC [40]. Apparently, retrospective studies have some obvious shortcomings that actually lead to cut-off values which may be distorted with concomitant use of observed parameters in regular clinical practice [40]. Apart from these shortcomings, these studies may have some advantages [40]. Since they usually rely on huge databases, specific subgroup analyses are feasible which in turn may provide useful and clinically relevant data that would otherwise be time-consuming to obtain throughout prospective observational studies [42]. Furthermore, this type of study is inexpensive and provides results quickly once a database is created. The presence of confounders must be considered, even in cases where the propensity score matching the data analysis is used because such analysis may control only for known cofounders that are available in the database.
2.4. Definition of Bleeding Outcomes
For cardiac surgery patients, abnormal postoperative bleeding remains difficult to define. There is no generally accepted definition of excessive postoperative bleeding in cardiac surgery patients (Table 1). Although some authors offer definitions of abnormal blood loss [23], there is no consensus on the definition of excessive bleeding. Furthermore, the definition of excessive bleeding may be dependent on the kind of cardiac surgery such as routine CABG, valve replacement, complex cardiac surgery, or lung and heart transplantation. Different bleeding classifications exist but are not easily applicable to cardiac surgery patients [45]. Ti et al. discussed this issue [23]. Blood loss, measured as CTD, is a continuous parameter; however, a separation of normal and abnormal bleeding based on a single numerical value is necessary to allow meaningful conclusions [23]. Furthermore, CTD consists of a mixture of fluids, including actual blood loss, serous drainage, and fluid left in the pleural cavity. Furthermore, the actual blood loss through these tubes is the sum of bleeding due to hemostatic disorder and bleeding from wound edges due to surgical issues. When conducting research evaluating the relationship between HPOCT and bleeding outcomes possibility of surgical bleeding should inevitably be considered. Surgical bleeding affects to some degree the extent of postoperative CTD. In 70% of patients undergoing re-exploration for excessive bleeding, a surgical cause of bleeding (bleeding vessels and anastomoses) has been identified [46]. Such patients should be excluded from the analysis. The study’s participants should be well-balanced in regard to surgeons performing hemostasis because there is evidence that individual surgeons’ performance may contribute significantly to bleeding outcomes [46]. Meticulous surgical hemostasis should be the cornerstone of appropriate hemostatic management as it influences bleeding outcomes as much as preoperative APT management and intraoperative transfusion management. For that reason, a formal operative checklist to reduce surgical bleeding should be part of the hemostatic protocol in every study evaluating the relationship between HPOCT and bleeding outcomes that aims to provide accurate and reliable correlations not distorted by factors unrelated to hemostatic alteration. Unfortunately, even then it is impossible to differentiate bleeding volume according to surgical or coagulopathic bleeding. The higher the proportion of “surgical bleeding” in CTD, the lesser the NPV for each HPOCT when predicting bleeding outcomes. Unfortunately, without surgical re-exploration, it is impossible to differentiate surgical bleeding and bleeding due to hemostatic disturbances. We suggest that re-exploration for excessive bleeding with bleeding vessels identified should inevitably be considered as exclusion criteria in this type of study.

Table 1.
Various definitions of excessive bleeding across studies recruiting patients undergoing cardiac surgery procedures.
International Initiative on Haemostasis Management in Cardiac Surgery provided a universal definition of perioperative bleeding (UDPB) in adult cardiac surgery [125]. Dyke et al. addressed the obvious lack of standardization in the definition of excessive bleeding [125]; with the attempt to precisely describe and quantify bleeding and to facilitate future investigations, the authors proposed a UDPB for adult cardiac surgery [125]. UDPB consists of five gradual bleeding categories based on several parameters such as re-exploration/tamponade, delayed sternal closure, the amount of CTD in the first 12 postoperative hours, transfusion of RBCs, FFP, PC, cryoprecipitate, prothrombin complex concentrate, as well as administration of recombinant factor VIIa [125]. Prior to this recent proposal for a standardized definition of excessive bleeding, published evidence lacked standardization in defining bleeding outcomes. Different definitions of bleeding outcomes exist (Table 1), and different definitions lead to different reported prevalence of excessive bleeding. Furthermore, heterogeneity in the definitions of bleeding outcomes certainly affects the ability of HPOCT to predict bleeding outcomes. Different definitions of bleeding outcomes result in different predictabilities of bleeding complications and pooling of the data from different studies is almost impossible in such a setting. We support the idea of a UDPB [125] even though one should be aware that the recently proposed definition by Dyke et al. [125] is based on factors that would be expected to be highly influenced by transfusion policies that are well known to vary widely among institutions and surgical teams and are prone to changes over time [21,102,125,126]. Despite all these possible shortcomings, the application of this definition in prospective studies may allow for the pooling of data from different studies. Pooling of data may provide more reliable data on the role of HPOCT in the prediction of bleeding complications.
The same holds true for transfusion requirements. Thresholds for transfusion of blood components vary widely among different centers and sometimes are based on anesthesiologists/surgeons personal experiences and preferences. The exact thresholds for transfusion of each blood component and other hemostatic therapy should be defined and, if possible, used consistently in studies that aim to provide the pooling of the evidence that would allow for meaningful conclusions. Therefore, the monitoring of adherence to the protocol (algorithm) is of outstanding importance in prospective interventional studies and should always be reported in order to avoid misinterpretation of study results [127,128,129]. Apparently, to extract the best out of the standardized definition, each component that contributes to the definition should be standardized, which is particularly important in transfusion management.
For research centers that, for some reason, consider Dyke’s definition of perioperative bleeding not to be suitable or feasible, we propose to create their definition in order to adjust the CTD volume to anthropometric properties of their cohort, as well as to surgical technique, perfusion, and anesthetic management. As stressed earlier, the separation of normal and abnormal bleeding based on a single numerical value is necessary, and, herein, we propose our way to define excessive bleeding. The CTD amount should inevitably be divided by the patient’s weight and the patients should be characterized as bleeders if their 24-h CTD (mL/kg) exceeds the 75th percentile of distribution within the study cohort. This way of excessive bleeding definition has already been described in the literature [29,30,74]. We believe that such a definition allows for the most reliable correlation, and is not distorted with different perfusion, surgical, and anesthesia techniques attributable to different centers. The amount of postoperative bleeding may be recorded in different timeframes, and measurement of the CTD on an hourly basis allows for precise bleeding dynamic assessment. Assessment of bleeding amounts in an hourly manner may add some information on the predictability of bleeding in early postoperative hours versus bleeding in the late postoperative phase.
Considering the prediction of bleeding outcomes using HPOCT, the majority of studies report poor PPVs, whereas NPVs are quite high [35]. The question of how to improve PPVs remains challenging. One of the parameters that certainly influence PPV is the transfusion of procoagulant blood components in the intra- and postoperative phases. For instance, in our previously published prospective observational studies [29,30], clinicians were not aware of the HPOCT results. However, they managed patients according to the best available hemostatic management and patients were transfused with procoagulant blood components, whenever deemed necessary. Thus, patients with poor HPOCT values may at the same time be treated with procoagulant therapy by attending the physician in line with his clinical judgment or regular clinical hemostatic management. In an observational study, such a transfusion may reduce the volume of CTD and consequently prevent bleeding complications (primary outcome) which in turn diminishes the PPV. Another reason for the low PPV of HPOCT is that microvascular bleeding is most often multifactorial and both, platelet function testing and viscoelastic testing, assess specific hemostasis issues only, and therefore, should be used complementarily.
When assessing bleeding complications, the amount of postoperative bleeding and transfusion requirements are inextricably associated. Rosengart et al. [43] evaluated the ability of preoperative platelet function testing to identify patients at increased risk of bleeding and transfusion outcomes. The authors described a very interesting way of defining bleeding outcomes in cardiac surgery patients [43]. CTD was dichotomized as normal versus significantly higher than normal [43]. Transfusion requirements were also coded dichotomously with regard to whether any amount of given product—allogeneic RBCs, fresh frozen plasma, or platelets concentrate—had been transfused intraoperatively or within 24 h postoperatively [43]. A composite outcome indicative of surgery-related bleeding events was defined as “high” CTD or procoagulant blood component transfusion [43]. In conducting research, such a composite endpoint may provide better positive predictive values as it incorporates two outcomes whose incidence is inversely related [43]. In this way, it is possible to control variables that hamper the evaluated correlations. In prospective observational studies, investigating the relationship between HPOCT and CTD amount, attending physicians are unaware of HPOCT results, thereby, administering procoagulant blood components according to regular clinical practice protocols. Transfusion of procoagulant blood components distorts correlations between the HPOCT results and bleeding outcomes in terms of reducing the sensitivity of HPOCT. Therefore, it is impossible to control transfusion effects on bleeding outcomes, and the use of composite endpoints including both, bleeding amount and transfusion of procoagulant blood components, sounds reasonable.
2.5. Bleeding Risk Stratification Models
Identification of patients who are at increased bleeding risk would be advantageous in optimizing perioperative management.
Because of multicausality, it is a challenge to develop a bleeding risk prediction model that would be reliable and reproducible, worldwide.
Vuylsteke et al. developed a bleeding risk stratification score to identify patients undergoing cardiac surgery with a higher risk of severe postoperative bleeding [130]. Excessive bleeding was defined as blood loss exceeding 2 mL/kg/h during the first three hours following admission to the ICU [130]. A risk stratification score was constructed to define three categories: (a) high, (b) medium, and (c) low risk of severe postoperative bleeding with rates of severe postoperative bleeding being 21%, 8%, and 3%, respectively [130]. Using this bleeding score [130], authors were able to separate preoperatively patients into bleeding risk groups.
Greiff et al. conducted a study to perform external validation of the Papworth Bleeding Risk Score and to compare the usefulness of the UDPB, proposed by Dyke et al., with the Papworth Bleeding Risk Score [102]. In addition, the authors developed their local bleeding prediction score using stricter statistical criteria. When those two prediction models were compared, both showed a similar pattern with a high NPV but very low PPV, indicating that both scores were superior in identifying patients at low, rather than high risk of bleeding. Even though UDPB seems to be very attractive and calculates several parameters including transfusion requirement [125], it is strongly influenced by the transfusion practice of each institution. This is not the case for the definition of excessive bleeding proposed by Vuylsteke et al. [130]. However, in the study published by Greiff et al. [102], the application of either definition of excessive bleeding did not obtain significantly different results. Therefore, it seems that there is room for improvement and refinement in the development of bleeding stratification models. Even though the present bleeding stratification models do account for preoperative APT administration, they do not account for the platelet inhibitory effect that these drugs reveal. Furthermore, several other drugs not considered “antiplatelet drugs” but often used in cardiac and critically ill patients are known to impact platelet function such as protamine, beta-blockers, calcium antagonists, beta-lactam antibiotics, anti-depressants, and analgetics [131]. We hypothesized that the addition of HPOCT results may improve the PPV of bleeding stratification models, which was found to be the major weakness of the present models [102,130]. Our research group recently developed a SHOULD-NOT-BLEED Score [132]. To address the issue of unnecessary transfusions within the CABG population, we developed a model to predict which patients are at low risk of bleeding for whom transfusion treatment might be considered unnecessary [132]. The study aimed to develop a user-friendly application that stratifies patients with respect to bleeding risk. The statistical model we used to develop the SHOULD-NOT-BLEED Score aimed to detect CABG patients at low risk of bleeding [132]. We developed a Windows platform app based on risk modeling which we previously calculated for 1426 patients undergoing elective CABG [133]. The variables that entered the scoring system were as follows: Age; Body Mass Index; Chronic renal failure; Preoperative clopidogrel exposure; Preoperative red blood cell count; Preoperative fibrinogen level; Preoperative multiplate ASPI test area under the curve (AUC) units [132,133]. The score included a platelet function testing variable and predicted patients without a risk of excessive bleeding with strong discriminatory performance (Receiver Operating Curve (ROC) analysis AUC 72.3%, p < 0.001) [132,133].
2.6. Patient Selection
Selected patient cohorts should be homogeneous as much as possible since patients’ heterogeneity in terms of patient comorbidities, complexity of procedures, and preoperative APT management may diminish the predictability of HPOCT in the assessment of bleeding outcomes. When considering patients to be recruited in studies evaluating the relationship between platelet function testing and bleeding outcomes, one should be aware that with increasing bleeding risk, the higher the possible benefit of the bleeding risk assessment using HPOCT. Therefore, research should be focused on patients considered to be at high bleeding risk.
Preoperative APT management should be consistent among study participants. It seems that preoperative platelet function testing is of limited value in predicting bleeding risk in patients not being exposed to APT, preoperatively [35]. Recently, our working group has published a systematic review evaluating the predictive value of POC PFT for postoperative blood loss and transfusion requirements in cardiac surgery [35]. In a negligible proportion of reviewed studies, patients were not exposed to APT, preoperatively [35]. Moreover, almost half of the studies enrolled patients undergoing isolated CABG, even without the use of CPB [35]. Furthermore, it seems that the great majority of patients actually underwent elective surgery procedures [35]. The results from these studies may only be reliably applied to similar patient populations. Based on the reviewed literature, this means that we should draw the conclusions only from patients undergoing isolated CABG with APT not being administered, preoperatively. In the new era of cardiac surgery, with a growing proportion of high-risk patients undergoing complex procedures with recent exposure to APT, it seems reasonable to shift our focus to this subgroup of patients, as they may benefit the most from further refinements in hemostatic management. Despite the current guidelines, many centers resume with APT until the day of surgery, disregarding the recommendations for a drug-free interval before surgery. For example, the data from 2858 acute coronary syndrome patients in the CRUSADE (Can Rapid risk stratification of Unstable angina patients Supress Adverse Outcomes with Early implementation of the ACC/AHA Guidelines) initiative demonstrated that 87% of patients treated with clopidogrel preoperatively underwent CABG surgery ≤ 5 days after treatment cessation with the result of increased blood transfusion requirements [134]. Furthermore, 5–15% of patients with acute coronary syndrome require urgent cardiac surgery with recently administered APT [134]. These real-life data should direct our research efforts to the subgroup of patients that could benefit the most from improved hemostatic management.
Furthermore, patients exposed to more potent APT should be the focus of research. Studies should be conducted to elucidate the pharmacodynamic profile of novel APT. More pharmacodynamic studies of platelet function recovery after discontinuation of prasugrel and ticagrelor should be conducted. A platelet inhibitory response as well as the recovery rate should be calculated using drug-specific platelet function tests. It is very important to compare these characteristics in cardiac surgery patients as this may influence APT administration/discontinuation management. Another important question is whether the uniform cut-off levels of adenosine diphosphate (ADP) platelet reactivity reflect bleeding tendency. If this hypothesis were confirmed, one could assess the platelet inhibitory response to each ADP platelet receptor blocker and create a unique ADP test cut-off value that corresponds to the bleeding risk. This would allow for the detection of patients with a high response to clopidogrel therapy and these patients may have a similar ADP platelet inhibition as patients exposed to more potent ADP receptor blockers. One could assume that a similar level of platelet ADP receptor inhibition may be achieved with clopidogrel, prasugrel, and ticagrelor but with different probability odds to achieve certain levels of platelet reactivity. More potent ADP receptor blockers may have a higher odds to achieve pronounced platelet inhibition whereas less potent ADP receptor blockers (such as clopidogrel) may have a higher odds for high residual platelet reactivity. These pharmacodynamics hypotheses should be tested in upcoming studies. However, one should be aware that not one single type of receptor activity reflects overall platelet reactivity. In patients treated with ADP receptor blockers, a protease-activated receptor (PAR) that reacts with thrombin stimulation may still be active. Recently, Ranucci et al. evaluated the effect of preoperative ADP and thrombin receptor inhibition on bleeding after cardiac surgery [110]. Both ADP and thrombin receptor-activating peptide (TRAP) test, were significantly associated (p < 0.001) with postoperative bleeding. However, weak ADP receptor activity, if coupled with sufficient TRAP test was not associated with severe bleeding [110]. It turns out that overall platelet reactivity may remain within an acceptable range even in the presence of strong drug-induced inhibition of ADP receptors, which suggests a compensatory mechanism [110]. Even though they were obtained in different clinical settings of percutaneous coronary intervention, a very similar phenomenon has been described by Tricoci et al. [135]. In addition, the role of aspirin should not be underestimated as there is evidence that high platelet inhibition measured in patients while on aspirin therapy (aspirin hyperresponse) may contribute to bleeding diathesis [30]. Accordingly, the take-home message would be that not only one drug-specific test but rather a combination of specific tests should be performed at the same time. Based on recently published data, it seems that impaired the TRAP test should be used to justify platelet transfusion due to low ADP test results [110]. In this way, concomitant assessment of different platelet activation pathways (arachidonic acid, ADP, and TRAP) may reliably provide a level of general platelet reactivity. This strategy detects patients who may proceed with surgery even if they are exposed to ADP receptor blockers and have weak ADP receptor activity [110]. It is well known that a platelet inhibitory response to APT varies widely among individuals [136] and such considerable heterogeneity in platelet inhibitory response to APT makes it difficult to reliably use arbitrary intervals for drug discontinuation prior to surgery without incurring excessive thrombotic or bleeding risks with premature versus too late discontinuation. Therefore, platelet function testing seems reasonable in a group of patients preoperatively exposed to APT in order to detect patients with pronounced platelet inhibition who might benefit from earlier drug discontinuation or delaying surgery until platelet recovery, if clinical condition allows. Vice versa, continuing APT up to the day of surgery in patients who have high residual platelet reactivity may help to avoid adverse thrombotic events associated with platelet recovery and possible rebound phenomena. Regarding preoperative APT management and patient selection, research should be focused on and provide priority to patients exposed to dual APT in close proximity to surgery. Furthermore, special attention should be paid to patients exposed to more potent APT such as prasugrel [121,137,138] and ticagrelor [138,139]. Pharmacodynamic evaluation of novel, more potent APT and the clinical implications of switching between them in the preoperative phase should be considered in future trials.
Furthermore, patients should be comparable in regard to the complexity of the surgical procedures performed. First, recruited patients should be equal in regard to the use of CPB during the surgical procedure. Merging patients undergoing off-pump and on-pump cardiosurgical procedures would be detrimental in terms of creating great heterogeneity and bias. More complex procedures are associated with a higher bleeding risk attributable to surgical complexity and duration of CPB. In addition to complexity and length of incision-to-suture times, there are several other factors related to CPB that contribute to the onset of hemostatic disorder such as foreign surface contact, consumption of fibrinogen and enzymatic coagulation factors, platelet dysfunction, fibrinolysis, hypothermia, acidosis, and hypocalcemia [11]. The multicausality of hemostatic disorders related to CPB requires well-balanced homogeneous populations with a minimum risk for confounding factors since heterogeneity of patient population makes it difficult to exclude the effects of complexity of the cardiac pathology condition, such as levels of hypothermia and duration of CPB, on the postoperative blood loss.
Considering patient selection, we would like to provide case-based learning. Agarwal et al. [112] recently conducted a prospective randomized control trial aiming to investigate whether the use of preoperative platelet function testing as a part of a transfusion algorithm may reduce transfusion requirements in cardiac surgery patients [112]. In our opinion, patient selection remains the main shortcoming of the study [112]. This study enrolled both patients undergoing elective and urgent surgery. Furthermore, they enrolled patients who underwent various types of cardiosurgical procedures and cohorts consisted of both, patients undergoing cardiac surgery with and without CPB [112]. Accordingly, we may conclude that the heterogeneity of the study cohorts makes it difficult to control the confounding factors [112]. Even if studies report positive results, as in this study [112], the observed results may be distorted and hampered by the heterogeneity of the study cohorts [112]. With this example, we would like to stress how valuable and robust studies may be improved by addressing some methodological considerations. In our opinion, such a type of study should have a narrow focus on one particular subgroup of cardiac surgery patients. The first discrimination should be with respect to the use of CPB and the second discrimination should address different types of procedures with respect to surgical complexity. Surgical complexity certainly affects bleeding outcomes and transfusion requirements due to the extent of surgical stress with the number of suture lines and the duration of CPB time which has deleterious effects on hemostasis, particularly in patients with a long CPB time.
Accordingly, the utility of HPOCT-guided hemostatic management should be the highest in the group of patients considered to be at the highest bleeding risk, which in turn may be determined with preoperative exposure to APT as well as with complexity of procedure (long CPB time with hypothermia, sometimes deep hypothermic circulatory arrest).
2.7. How to Set Up Appropriate Timing for Point-of-Care Measurements
Because the platelet dysfunction may be caused by either APT being administered preoperatively or by CPB effects, it is necessary to make a clear distinction between these two potential causes of platelet dysfunction. However, in procedures with long CPB times, both causes may contribute to postoperative platelet dysfunction. To make a clear distinction between these two causes, it is necessary to study patients undergoing CPB-assisted cardiac surgery procedures with preoperative exposure to APT. In this type of trial, platelet function assessment should be performed at different time points. Thereby, we would be able to reliably describe the mechanisms by which APT and CPB achieve harmful effects of platelet function. This should be coupled with assessing the clinical relevance of the described phenomenon by correlating the hemostatic properties (i.e., platelet function) with relevant clinical outcomes such as bleeding amount or transfusion requirements.
When considering hemostatic properties in cardiac surgery patients, clinicians are usually focused on the following:
- (1)
- Baseline (preoperative) hemostatic properties, in particular platelet function that may often be under considerable influence of preoperatively administered APT;
- (2)
- Intraoperative changes in hemostatic blood properties that are mainly influenced by the effects of CPB or heparin reversal by protamine;
- (3)
- Hemostatic properties following arrival to the ICU is extremely important and should not be underestimated [1].
Hemostatic alterations following arrival to the ICU are related to hemostatic disorder secondary to blood loss and dilution followed by fibrinolysis. In particular, attention should be paid to fibrinolytic bleeding, especially in centers that perform reinfusion of mediastinal blood (without washing) which in turn can result in systematic fibrinolytic coagulopathy [140] and centers that do not administer antifibrinolytic therapy topically or systemically during surgery [141,142].
Hemostatic alterations in cardiac surgery patients are moving targets, constantly changing over time during and after CPB and following admission to the ICU. Appropriate timing for HPOCT is of utmost importance. In general, the more frequent the timing for measurements, the better the approach to detecting hemostatic alterations that rapidly change over time.
The evaluation of the relationship between CPB duration and hemostatic disturbances is very important in the assessment of bleeding risk. Recently, Mutlak et al. [143] conducted a prospective observational study that aimed to assess the capability of the whole blood impedance aggregometry (Multiplate) to assess the extent of CPB-associated platelet dysfunction [143]. They showed that platelet function, assessed by ADP test reflected the time-dependent extent of CPB-induced platelet dysfunction [143]. The authors provided valuable data on the dynamics of platelet function during CPB; however, failed to correlate these findings with clinical outcomes. In our opinion, such studies should be focused on the gaps in our knowledge regarding this specific issue. The observed phenomena, such as the dynamics of platelet function over time on CPB, should inevitably be analyzed in the context of clinical outcomes such as bleeding complications and transfusion requirements. Accordingly, the evaluation of platelet function should inextricably be coupled with clinical endpoints, in this type of trial. Only in this way, it will be possible to draw conclusions that are of clinical relevance.
HPOCT may help in clarifying the mechanisms of hemostatic disorders before, during, and after CPB. Based on our own experience [28,29,30,144], we may conclude that PFTs may be more useful in managing platelet dysfunction caused by exposure to APT. Using these devices, it is possible to perform bleeding risk stratification by detecting patients with pronounced platelet inhibition (hyperresponders) [30]. Furthermore, PFTs may be useful for the assessment of platelet dysfunction during and after CPB, although it seems that viscoelastic coagulation tests are of greater usefulness in terms of diagnosing mechanisms for hemostatic disorder as well as directing transfusion practice [29,39,145].
Presumably, the assessment of platelet function at different time points may elucidate the relative role of each modulating factor. Later measurement time points may more accurately predict bleeding as they assess platelet function influenced by accumulative effects of different modulating effects such as APT, CPB, and protamine. However, the platelet function assessment in earlier periods (i.e., in the preoperative phase) enables a wider spectrum of hemostatic measures to prevent bleeding complications such as personalized timing of surgery or personalized APT administration/discontinuation management.
2.8. Data Analysis—Statistical Considerations
Sample Size Calculation
A minimum sample size required for a study is a major consideration which all scientists need to address at an early stage of creating a research protocol [146]. Furthermore, for type I errors and power of the study, some estimates for the effect sizes will also need to be determined in the process of calculating or estimating the sample size [146]. The appropriateness of calculating or estimating the sample size will enable the researchers to better plan their study, particularly pertaining to the recruitment of subjects and to avoid underpowering the study [146]. The type of sample size calculation depends on the study design and the type of statistical analyses performed to compare subjects and/or outcomes.
2.9. Which Tests Should Be Used and When?
2.9.1. Correlation Tests
Studies evaluating the association of bleeding outcomes with haemostatic properties or subject properties usually rely on correlation tests. Correlation is a bivariate analysis that measures both the strength and direction of association between two variables. Regarding the strength of the relationship, the value of the correlation coefficient varies between +1 and −1, where a value of ±1 indicates a perfect degree of association between the two variables. As the correlation coefficient value goes towards zero, the relationship between the two variables will be weaker. The direction of the relationship is indicated by the sign of the coefficient; a + sign indicates a positive relationship and a negative sign indicates a negative relationship. In statistics, there are four types of correlations: Pearson correlation; Kendall rank correlation; Spearman correlation; and the Point-Biserial correlation.
Pearson r correlation is the most widely used correlation statistic to measure the degree of the relationship between linearly related variables. For the Pearson r correlation, both variables should be normally distributed (normally distributed variables have a bell-shaped curve). Spearman rank correlation is a non-parametric test that is used to measure the degree of association between two variables. The Spearman rank correlation test does not carry any assumptions about the distribution of the data and is the appropriate correlation analysis when the variables are measured on a scale that is at least ordinal.
2.9.2. ROC Curve Analysis
Using diagnostic testing to determine the presence of a specific outcome (be it the presence or absence of) i.e., excessive bleeding is essential in clinical research [147]. In many cases, test results are obtained as continuous values and require a process of conversion and interpretation into a dichotomous form to determine the presence of a disease or outcome [147]. The primary method used for this process is the receiver operating characteristic (ROC) curve analysis [147]. The ROC curve analysis is used to assess the overall diagnostic performance of a test and to compare the performance of two or more diagnostic tests [147]. It is also used to select an optimal cut-off value for determining the presence or absence of a disease or outcome [147]. Furthermore, for optimum cut-off values, ROC curve analyses provide information about accuracy (ROC AUC), significance (standard error, 95% confidence interval, and p-value), sensitivity, specificity, positive predictive value, and negative predictive value.
2.10. Sensitivity, Specificity, Positive Predictive Value, and Negative Predictive Value
Monaghan et al. recently described foundational statistical principles in medical research such as sensitivity, specificity, positive predictive value, and negative predictive value [148].
Briefly, sensitivity denotes the proportion of subjects correctly given a positive assignment out of all subjects who are actually positive for the outcome. On the other hand, specificity denotes the proportion of subjects correctly given a negative assignment out of all subjects who are actually negative for the outcome [148]. A positive predictive value reflects the proportion of subjects with a positive test result who truly have the outcome of interest whereas a negative predictive value reflects the proportion of subjects with a negative test result who truly do not have the outcome of interest. According to ref. [148], sensitivity and specificity are inversely related, wherein one increases as the other decreases. Positive and negative predictive values do inherently vary with pre-test probability (e.g., changes in population disease prevalence). Positive and negative predictive values are influenced by the prevalence of disease in the population that is being tested. If we test in a high prevalence setting, it is more likely that persons who test positive truly have the disease than if the test is performed in a population with low prevalence [148].
3. Conclusions
The developed models for prediction of excessive bleeding show consistently low PPVs coupled with acceptable to high NPVs indicating that available scores are better in identifying patients at low risk rather than high risk of bleeding [102]. Accordingly, it seems easier to identify patients who should not bleed rather than patients who should bleed. This may have numerous implications for clinical practice [102] in terms of selecting patients for whom HPOCT might not be necessary.
For studies addressing this research field it is of utmost importance to adequately select patients (i.e., patients exposed to APT preoperatively who require complex surgical procedures using CPB). Particular attention should be given to patients with renal failure.
Preoperative APT certainly influences bleeding outcomes. Well-known wide variability in the platelet inhibitory response to APT directs how powerful a correlation will be between APT and bleeding outcomes. Inclusion of PFT should inevitably be considered for bleeding risk stratification as PFT may clearly distinguish patients with pronounced platelet inhibition (hyperresponders) with a high bleeding risk, from those with high residual platelet reactivity (low responders) who may in fact proceed to surgery without risk of bleeding despite APT administered in close proximity to surgery. Appropriate methodology in conducting such studies may be of utmost importance leading to adequate bias control.
Even if positive correlations between preoperative HPOCT and bleeding outcomes are obtained through well-designed prospective studies, it may be too optimistic to expect that only one PFT assay may be sufficient to predict bleeding. The adequate value of a single patient characteristic or a single laboratory biomarker in the prediction of excessive bleeding has not been proven, yet [149,150]. The best bleeding risk prediction score should consider non-surgical risk factors (age, sex, renal disease, administration of antiplatelet/antithrombotic drugs, platelet function test values) and surgical procedure-related risk factors. In order to optimize perioperative management, bleeding risk prediction scores should provide better PPVs. With acceptable PPVs, it would be possible to control and treat factors contributing to the bleeding risk.
It is obvious that there is a need to consider the diagnostic approach and the management of hemorrhage in cardiac surgery. Further refinements in hemostatic management are warranted. Newly designed hemostatic algorithms should inevitably encompass preoperative, intraoperative, and postoperative time points.
The preoperative part should provide a preoperative bleeding risk stratification based on the assessment of platelet function in relation to APT administration/discontinuation management. Exact cut-off values that delineate the bleeding risk should be defined using drug-specific platelet function tests, and preoperative APT administration/discontinuation management should be adjusted, accordingly.
Considering the intraoperative part, again, exact cut-off values that delineate excessive bleeding should be defined and validated. Assessment of viscoelastic blood clot properties provides greater usefulness to diagnose bleeding mechanisms and should be used complementary to PFT in bleeding patients. Platelet function testing appears to be more useful for the preoperative assessment of platelet function in patients being exposed to APT, preoperatively. Notably, some authors reported intraoperative administration of desmopressin or even platelet concentrates based on platelet function testing results performed intraoperatively in cases of microvascular bleeding occurrence [40]. However, here, viscoelastic testing seems to be more useful and reliable.
Postoperative hemostatic measures should provide a differential diagnosis of the underlying hemostatic disorders, such as dilutional coagulopathy, fibrinolysis, platelet dysfunction, or fibrinogen deficiency. On-time detection of surgical bleeding should be given priority. Any assessment of possible hemostatic disturbances starts with two questions and answers:
- Question 1: Do we have wet surgical field?—Answer 1: Yes
- Question 2: Do we have surgical bleeding?—Answer 2: No
The use of HPOCT makes no sense in cases of the dry surgical field. If surgical bleeding persists, HPOCT should generally not be used as an alternative to appropriate surgical haemostasis. In general, if bleeding occurs in patients considered to be at low risk of bleeding, the focus should be directed toward possible surgical causes for excessive bleeding. However, in real clinical scenarios, it is not always “either black or white” but rather “shades of gray” scenarios where we may have “small” surgical sources of bleeding that could be stopped if the patient’s haemostatic properties were optimized. Patients that are primarily considered to be at low risk of bleeding may undergo a procedure with, for example, a long pump run time, which in turn may significantly alter the haemostasis. With such haemostatic disturbances patient may experience “oozing” and a wet surgical field resulting in excessive chest tube output. In such cases, HPOCT-guided optimization of the haemostatic blood properties may be considered as a first step to stop bleeding, and if bleeding persists despite optimized haemostasis, surgical re-exploration may be the next step. The message herein is that there are some clinical scenarios where we may experience surgical bleeding with small sources of bleeding that could efficiently be treated with optimizing blood haemostasis rather than immediate chest re-exploration. Such an approach may optimize clinical outcomes by avoiding unnecessary surgical revisions.
Author Contributions
Conceptualization, M.P. (Mirna Petricevic) and K.G.; methodology, M.P. (Mate Petricevic) and M.M.; resources, M.P. (Mirna Petricevic) and M.M.; writing—original draft preparation, M.P. (Mirna Petricevic); writing—review and editing, K.G. and M.P. (Mate Petricevic); visualization, M.M.; supervision, M.P. (Mate Petricevic) and K.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
Adenosine Di-Phosphate (ADP); Antiplatelet Therapy (APT); Cardiopulmonary Bypass (CPB); Chest Tube Drainage (CTD); Coronary Artery Bypass Grafting (CABG); Direct Oral Anticoagulants (DOACs); Hematocrite (HCT); Hemostatic Point-of-Care Testing (HPOCT); Intensive Care Unit (ICU); Negative Predictive Value (NPV); Positive Predictive Value (PPV); Receiver Operating Characteristics (ROC); Red Blood Cells (RBC); Thrombin Receptor Activating Peptice (TRAP); Universal Definition of Perioperative Bleeding (UDPB); Vitamin K Antagonist (VKA).
References
- Nielsen, V.G. Coagulation crystal ball: Why can’t we predict bleeding after cardiac surgery? Anesth. Analg. 2012, 115, 490–492. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.C.; Chavez, J.J.; Snider, C.C.; Meyer, D.S.; Muenchen, R.A. Correlation of perioperative platelet function and coagulation tests with bleeding after cardiopulmonary bypass surgery. J. Lab. Clin. Med. 2006, 147, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, K.L.; Rauer, L.J.; Mortensen, P.E.; Kjeldsen, B.J. Reoperation for bleeding in cardiac surgery. Interact. Cardiovasc. Thorac. Surg. 2012, 14, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.I.; Solbeck, S.; Genet, G.; Stensballe, J.; Ostrowski, S.R. Coagulopathy and hemostatic monitoring in cardiac surgery: An update. Scand. Cardiovasc. J. 2012, 46, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Dixon, B.; Santamaria, J.D.; Reid, D.; Collins, M.; Rechnitzer, T.; Newcomb, A.E.; Nixon, I.; Yii, M.; Rosalion, A.; Campbell, D.J. The association of blood transfusion with mortality after cardiac surgery: Cause or confounding? (CME). Transfusion 2013, 53, 19–27. [Google Scholar] [CrossRef]
- Christensen, M.C.; Dziewior, F.; Kempel, A.; von Heymann, C. Increased chest tube drainage is independently associated with adverse outcome after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2012, 26, 46–51. [Google Scholar] [CrossRef]
- Ranucci, M.; Baryshnikova, E.; Castelvecchio, S.; Pelissero, G.; Surgical Clinical Outcome Research Group. Major bleeding, transfusions, and anemia: The deadly triad of cardiac surgery. Ann. Thorac. Surg. 2013, 96, 478–485. [Google Scholar] [CrossRef]
- Petrou, A.; Tzoka, T.; Tzimas, P.; Apostolakis, E.; Papadopoulos, G.S.; Zervou, E. Mortality associated with standard prescription transfusions in cardiac surgery. Hippokratia 2018, 22, 68–74. [Google Scholar]
- Paparella, D.; Brister, S.J.; Buchanan, M.R. Coagulation disorders of cardiopulmonary bypass: A review. Intensive Care Med. 2004, 30, 1873–1881. [Google Scholar] [CrossRef]
- Ranucci, M.; Bozzetti, G.; Ditta, A.; Cotza, M.; Carboni, G.; Ballotta, A. Surgical reexploration after cardiac operations: Why a worse outcome? Ann. Thorac. Surg. 2008, 86, 1557–1562. [Google Scholar] [CrossRef]
- Ranucci, M. Hemostatic and thrombotic issues in cardiac surgery. Semin. Thromb. Hemost. 2015, 41, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Choong, C.K.; Gerrard, C.; Goldsmith, K.A.; Dunningham, H.; Vuylsteke, A. Delayed re-exploration for bleeding after coronary artery bypass surgery results in adverse outcomes. Eur. J. Cardio-Thorac. Surg. 2007, 31, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Bolliger, D.; Gorlinger, K.; Tanaka, K.A. Pathophysiology and treatment of coagulopathy in massive hemorrhage and hemodilution. Anesthesiology 2010, 113, 1205–1219. [Google Scholar] [CrossRef] [PubMed]
- Meesters, M.I.; Veerhoek, D.; de Lange, F.; de Vries, J.W.; de Jong, J.R.; Romijn, J.W.; Kelchtermans, H.; Huskens, D.; van der Steeg, R.; Thomas, P.W.; et al. Effect of high or low protamine dosing on postoperative bleeding following heparin anticoagulation in cardiac surgery. A randomised clinical trial. Thromb. Haemost. 2016, 116, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Boer, C.; Meesters, M.I.; Veerhoek, D.; Vonk, A.B.A. Anticoagulant and side-effects of protamine in cardiac surgery: A narrative review. Br. J. Anaesth. 2018, 120, 914–927. [Google Scholar] [CrossRef]
- Gorlinger, K.; Perez-Ferrer, A.; Dirkmann, D.; Saner, F.; Maegele, M.; Calatayud, A.A.P.; Kim, T.Y. The role of evidence-based algorithms for rotational thromboelastometry-guided bleeding management. Korean J. Anesthesiol. 2019, 72, 297–322. [Google Scholar] [CrossRef]
- Crescenzi, G.; Torracca, L.; Capestro, F.; Matteucci, M.L.; Rossi, M. Allogenic blood transfusion in cardiac surgery. J. Card. Surg. 2012, 27, 594–599. [Google Scholar] [CrossRef]
- Sullivan, M.T.; Cotten, R.; Read, E.J.; Wallace, E.L. Blood collection and transfusion in the United States in 2001. Transfusion 2007, 47, 385–394. [Google Scholar] [CrossRef]
- Whitson, B.A.; Huddleston, S.J.; Savik, K.; Shumway, S.J. Bloodless cardiac surgery is associated with decreased morbidity and mortality. J. Card. Surg. 2007, 22, 373–378. [Google Scholar] [CrossRef]
- Ferraris, V.A.; Ferraris, V.A.; Brown, J.R.; Despotis, G.J.; Hammon, J.W.; Reece, T.B.; Saha, S.P.; Song, H.K.; Clough, E.R.; Shore-Lesserson, L.J.; et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann. Thorac. Surg. 2011, 91, 944–982. [Google Scholar] [CrossRef]
- Bennett-Guerrero, E.; Zhao, Y.; O’Brien, S.M.; Ferguson, T.B., Jr.; Peterson, E.D.; Gammie, J.S.; Song, H.K. Variation in use of blood transfusion in coronary artery bypass graft surgery. JAMA 2010, 304, 1568–1575. [Google Scholar] [CrossRef]
- Shaw, R.E.; Johnson, C.K.; Ferrari, G.; Zapolanski, A.; Brizzio, M.; Rioux, N.; Edara, S.; Sperling, J.; Grau, J.B. Balancing the benefits and risks of blood transfusions in patients undergoing cardiac surgery: A propensity-matched analysis. Interact. Cardiovasc. Thorac. Surg. 2013, 17, 96–102. [Google Scholar] [CrossRef]
- Ti, L.K.; Cheong, K.F.; Chen, F.G. Prediction of excessive bleeding after coronary artery bypass graft surgery: The influence of timing and heparinase on thromboelastography. J. Cardiothorac. Vasc. Anesth. 2002, 16, 545–550. [Google Scholar] [CrossRef]
- Practice guidelines for perioperative blood management: An updated report by the american society of anesthesiologists task force on perioperative blood management*. Anesthesiology 2015, 122, 241–275. [CrossRef]
- Kietaibl, S.; Ahmed, A.; Afshari, A.; Albaladejo, P.; Aldecoa, C.; Barauskas, G.; De Robertis, E.; Faraoni, D.; Filipescu, D.C.; Fries, D.; et al. Management of severe peri-operative bleeding: Guidelines from the European Society of Anaesthesiology and Intensive Care: Second update 2022. Eur. J. Anaesthesiol. 2023, 40, 226–304. [Google Scholar] [CrossRef]
- Kozek-Langenecker, S.A.; Afshari, A.; Albaladejo, P.; Santullano, C.A.; De Robertis, E.; Filipescu, D.C.; Fries, D.; Gorlinger, K.; Haas, T.; Imberger, G.; et al. Management of severe perioperative bleeding: Guidelines from the European Society of Anaesthesiology. Eur. J. Anaesthesiol. 2013, 30, 270–382. [Google Scholar] [CrossRef]
- Mahla, E.; Suarez, T.A.; Bliden, K.P.; Rehak, P.; Metzler, H.; Sequeira, A.J.; Cho, P.; Sell, J.; Fan, J.; Antonino, M.J.; et al. Platelet function measurement-based strategy to reduce bleeding and waiting time in clopidogrel-treated patients undergoing coronary artery bypass graft surgery: The timing based on platelet function strategy to reduce clopidogrel-associated bleeding related to CABG (TARGET-CABG) study. Circ. Cardiovasc. Interv. 2012, 5, 261–269. [Google Scholar] [CrossRef]
- Petricevic, M.; Biocina, B.; Boban, M.; Samardzic, J.; Zrno Mihaljevic, M.; Milicic, D. Bleeding risk assessment using point-of-care platelet function testing in patients undergoing coronary artery surgery: How to improve predictability. J. Card. Surg. 2014, 29, 806–807. [Google Scholar] [CrossRef]
- Petricevic, M.; Biocina, B.; Milicic, D.; Konosic, S.; Svetina, L.; Lekic, A.; Zdilar, B.; Burcar, I.; Milosevic, M.; Brahimaj, R.; et al. Bleeding risk assessment using whole blood impedance aggregometry and rotational thromboelastometry in patients following cardiac surgery. J. Thromb. Thrombolysis 2013, 36, 514–526. [Google Scholar] [CrossRef]
- Petricevic, M.; Biocina, B.; Milicic, D.; Konosic, S.; Ivancan, V.; Milosevic, M.; Burcar, I.; Gasparovic, H. Bleeding risk assessment using multiple electrode aggregometry in patients following coronary artery bypass surgery. J. Thromb. Thrombolysis 2013, 35, 31–40. [Google Scholar] [CrossRef]
- Straub, A.; Schiebold, D.; Wendel, H.P.; Hamilton, C.; Wagner, T.; Schmid, E.; Dietz, K.; Ziemer, G. Using reagent-supported thromboelastometry (ROTEM) to monitor haemostatic changes in congenital heart surgery employing deep hypothermic circulatory arrest. Eur. J. Cardio-Thorac. Surg. 2008, 34, 641–647. [Google Scholar] [CrossRef]
- Hartmann, M.; Sucker, C.; Boehm, O.; Koch, A.; Loer, S.; Zacharowski, K. Effects of cardiac surgery on hemostasis. Transfus. Med. Rev. 2006, 20, 230–241. [Google Scholar] [CrossRef]
- Petricevic, M.; Konosic, S.; Biocina, B.; Dirkmann, D.; White, A.; Mihaljevic, M.Z.; Ivancan, V.; Konosic, L.; Svetina, L.; Gorlinger, K. Bleeding risk assessment in patients undergoing elective cardiac surgery using ROTEM((R)) platelet and Multiplate((R)) impedance aggregometry. Anaesthesia 2016, 71, 636–647. [Google Scholar] [CrossRef]
- Ortmann, E.; Klein, A.A.; Sharples, L.D.; Walsh, R.; Jenkins, D.P.; Luddington, R.J.; Besser, M.W. Point-of-care assessment of hypothermia and protamine-induced platelet dysfunction with multiple electrode aggregometry (Multiplate(R)) in patients undergoing cardiopulmonary bypass. Anesth. Analg. 2013, 116, 533–540. [Google Scholar] [CrossRef]
- Petricevic, M.; Kopjar, T.; Biocina, B.; Milicic, D.; Kolic, K.; Boban, M.; Skoric, B.; Lekic, A.; Gasparovic, H. The predictive value of platelet function point-of-care tests for postoperative blood loss and transfusion in routine cardiac surgery: A systematic review. Thorac. Cardiovasc. Surg. 2015, 63, 2–20. [Google Scholar] [CrossRef]
- Sambu, N.; Warner, T.; Curzen, N. Clopidogrel withdrawal: Is there a “rebound” phenomenon? Thromb. Haemost. 2011, 105, 211–220. [Google Scholar] [CrossRef]
- Ranucci, M.; Baryshnikova, E. Sensitivity of Viscoelastic Tests to Platelet Function. J. Clin. Med. 2020, 9, 189. [Google Scholar] [CrossRef]
- Metz, C.E. Basic principles of ROC analysis. Semin. Nucl. Med. 1978, 8, 283–298. [Google Scholar] [CrossRef]
- Weber, C.F.; Gorlinger, K.; Meininger, D.; Herrmann, E.; Bingold, T.; Moritz, A.; Cohn, L.H.; Zacharowski, K. Point-of-care testing: A prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology 2012, 117, 531–547. [Google Scholar] [CrossRef]
- Ranucci, M.; Baryshnikova, E.; Soro, G.; Ballotta, A.; De Benedetti, D.; Conti, D.; Surgical Clinical Outcome Research Group. Multiple electrode whole-blood aggregometry and bleeding in cardiac surgery patients receiving thienopyridines. Ann. Thorac. Surg. 2011, 91, 123–129. [Google Scholar] [CrossRef]
- Schimmer, C.; Hamouda, K.; Sommer, S.P.; Ozkur, M.; Hain, J.; Leyh, R. The predictive value of multiple electrode platelet aggregometry (multiplate) in adult cardiac surgery. Thorac. Cardiovasc. Surg. 2013, 61, 733–743. [Google Scholar] [CrossRef]
- Di Dedda, U.; Ranucci, M.; Baryshnikova, E.; Castelvecchio, S. Thienopyridines resistance and recovery of platelet function after discontinuation of thienopyridines in cardiac surgery patients. Eur. J. Cardio-Thorac. Surg. 2014, 45, 165–170. [Google Scholar] [CrossRef]
- Rosengart, T.K.; Romeiser, J.L.; White, L.J.; Fratello, A.; Fallon, E.; Senzel, L.; Shroyer, A.L. Platelet activity measured by a rapid turnaround assay identifies coronary artery bypass grafting patients at increased risk for bleeding and transfusion complications after clopidogrel administration. J. Thorac. Cardiovasc. Surg. 2013, 146, 1259–1266; discussion 1266. [Google Scholar] [CrossRef]
- Haensig, M.; Kempfert, J.; Kempfert, P.M.; Girdauskas, E.; Borger, M.A.; Lehmann, S. Thrombelastometry guided blood-component therapy after cardiac surgery: A randomized study. BMC Anesth. 2019, 19, 201. [Google Scholar] [CrossRef]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef]
- Biancari, F.; Mikkola, R.; Heikkinen, J.; Lahtinen, J.; Kettunen, U.; Juvonen, T. Individual surgeon’s impact on the risk of re-exploration for excessive bleeding after coronary artery bypass surgery. J. Cardiothorac. Vasc. Anesth. 2012, 26, 550–556. [Google Scholar] [CrossRef]
- Marengo-Rowe, A.J.; Lambert, C.J.; Leveson, J.E.; Thiele, J.P.; Geisler, G.F.; Adam, M.; Mitchel, B.F., Jr. The evaluation of hemorrhage in cardiac patients who have undergone extracorporeal circulation. Transfusion 1979, 19, 426–433. [Google Scholar] [CrossRef]
- Michelson, E.L.; Torosian, M.; Morganroth, J.; MacVaugh, H., 3rd. Early recognition of surgically correctable causes of excessive mediastinal bleeding after coronary artery bypass graft surgery. Am. J. Surg. 1980, 139, 313–317. [Google Scholar] [CrossRef]
- Bagge, L.; Lilienberg, G.; Nystrom, S.O.; Tyden, H. Coagulation, fibrinolysis and bleeding after open-heart surgery. Scand. J. Thorac. Cardiovasc. Surg. 1986, 20, 151–160. [Google Scholar] [CrossRef]
- Hirayama, T.; Roberts, D.G.; Allers, M.; Belboul, A.; al-Khaja, N.; Olsson, G.W. Association between bleeding and reduced red cell deformability following cardiopulmonary bypass. Scand. J. Thorac. Cardiovasc. Surg. 1988, 22, 171–174. [Google Scholar] [CrossRef]
- Gram, J.; Janetzko, T.; Jespersen, J.; Bruhn, H.D. Enhanced effective fibrinolysis following the neutralization of heparin in open heart surgery increases the risk of post-surgical bleeding. Thromb. Haemost. 1990, 63, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Ratnatunga, C.P.; Rees, G.M.; Kovacs, I.B. Preoperative hemostatic activity and excessive bleeding after cardiopulmonary bypass. Ann. Thorac. Surg. 1991, 52, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Villarino, M.E.; Gordon, S.M.; Valdon, C.; Potts, D.; Fish, K.; Uyeda, C.; McCarthy, P.M.; Bland, L.A.; Anderson, R.L.; Jarvis, W.R. A cluster of severe postoperative bleeding following open heart surgery. Infect. Control. Hosp. Epidemiol. 1992, 13, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Lin, C.Y.; Hung, W.T.; O’Connor, M.F.; Thisted, R.A.; Lee, B.K.; Karp, R.B.; Yang, M.W. Thromboelastogram fails to predict postoperative hemorrhage in cardiac patients. Ann. Thorac. Surg. 1992, 53, 435–439. [Google Scholar] [CrossRef]
- Hartstein; Janssens, M. Treatment of excessive mediastinal bleeding after cardiopulmonary bypass. Ann. Thorac. Surg. 1996, 62, 1951–1954. [Google Scholar] [CrossRef]
- Despotis, G.J.; Filos, K.S.; Zoys, T.N.; Hogue, C.W., Jr.; Spitznagel, E.; Lappas, D.G. Factors associated with excessive postoperative blood loss and hemostatic transfusion requirements: A multivariate analysis in cardiac surgical patients. Anesth. Analg. 1996, 82, 13–21. [Google Scholar]
- Despotis, G.J.; Levine, V.; Filos, K.S.; Santoro, S.A.; Joist, J.H.; Spitznagel, E.; Goodnough, L.T. Evaluation of a new point-of-care test that measures PAF-mediated acceleration of coagulation in cardiac surgical patients. Anesthesiology 1996, 85, 1311–1323. [Google Scholar] [CrossRef]
- Nuttall, G.A.; Oliver, W.C.; Ereth, M.H.; Santrach, P.J. Coagulation tests predict bleeding after cardiopulmonary bypass. J. Cardiothorac. Vasc. Anesth. 1997, 11, 815–823. [Google Scholar] [CrossRef]
- Brown, R.S.; Thwaites, B.K.; Mongan, P.D. Tranexamic acid is effective in decreasing postoperative bleeding and transfusions in primary coronary artery bypass operations: A double-blind, randomized, placebo-controlled trial. Anesth. Analg. 1997, 85, 963–970. [Google Scholar]
- Ereth, M.H.; Nuttall, G.A.; Klindworth, J.T.; MacVeigh, I.; Santrach, P.J.; Orszulak, T.A.; Harmsen, W.S.; Oliver, W.C., Jr. Does the platelet-activated clotting test (HemoSTATUS) predict blood loss and platelet dysfunction associated with cardiopulmonary bypass? Anesth. Analg. 1997, 85, 259–264. [Google Scholar]
- Dacey, L.J.; Munoz, J.J.; Baribeau, Y.R.; Johnson, E.R.; Lahey, S.J.; Leavitt, B.J.; Quinn, R.D.; Nugent, W.C.; Birkmeyer, J.D.; O’Connor, G.T. Reexploration for hemorrhage following coronary artery bypass grafting: Incidence and risk factors. Arch. Surg. 1998, 133, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Ereth, M.H.; Nuttall, G.A.; Santrach, P.J.; Klindworth, J.T.; Oliver, W.C., Jr.; Schaff, H.V. The relation between the platelet-activated clotting test (HemoSTATUS) and blood loss after cardiopulmonary bypass. Anesthesiology 1998, 88, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Herwaldt, L.A.; Swartzendruber, S.K.; Edmond, M.B.; Embrey, R.P.; Wilkerson, K.R.; Wenzel, R.P.; Perl, T.M. The epidemiology of hemorrhage related to cardiothoracic operations. Infect. Control. Hosp. Epidemiol. 1998, 19, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Despotis, G.J.; Levine, V.; Saleem, R.; Spitznagel, E.; Joist, J.H. Use of point-of-care test in identification of patients who can benefit from desmopressin during cardiac surgery: A randomised controlled trial. Lancet 1999, 354, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Mongan, P.D.; Brown, R.S.; Thwaites, B.K. Tranexamic acid and aprotinin reduce postoperative bleeding and transfusions during primary coronary revascularization. Anesth. Analg. 1998, 87, 258–265. [Google Scholar] [CrossRef]
- Lasne, D.; Fiemeyer, A.; Chatellier, G.; Chammas, C.; Baron, J.F.; Aiach, M. A study of platelet functions with a new analyzer using high shear stress (PFA 100) in patients undergoing coronary artery bypass graft. Thromb. Haemost. 2000, 84, 794–799. [Google Scholar]
- Casati, V.; Guzzon, D.; Oppizzi, M.; Bellotti, F.; Franco, A.; Gerli, C.; Cossolini, M.; Torri, G.; Calori, G.; Benussi, S.; et al. Tranexamic acid compared with high-dose aprotinin in primary elective heart operations: Effects on perioperative bleeding and allogeneic transfusions. J. Thorac. Cardiovasc. Surg. 2000, 120, 520–527. [Google Scholar] [CrossRef]
- Yende, S.; Wunderink, R.G. Effect of clopidogrel on bleeding after coronary artery bypass surgery. Crit. Care Med. 2001, 29, 2271–2275. [Google Scholar] [CrossRef]
- Slaughter, T.F.; Sreeram, G.; Sharma, A.D.; El-Moalem, H.; East, C.J.; Greenberg, C.S. Reversible shear-mediated platelet dysfunction during cardiac surgery as assessed by the PFA-100 platelet function analyzer. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2001, 12, 85–93. [Google Scholar] [CrossRef]
- Ascione, R.; Williams, S.; Lloyd, C.T.; Sundaramoorthi, T.; Pitsis, A.A.; Angelini, G.D. Reduced postoperative blood loss and transfusion requirement after beating-heart coronary operations: A prospective randomized study. J. Thorac. Cardiovasc. Surg. 2001, 121, 689–696. [Google Scholar] [CrossRef]
- Ereth, M.H.; Nuttall, G.A.; Ericson, D.G.; Cooney, W.P.; Fisher, B.R.; Oliver, W.C., Jr.; Schaff, H.V.; Fass, D.N. Platelet glass bead retention predicts bleeding after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2001, 15, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Forestier, F.; Coiffic, A.; Mouton, C.; Ekouevi, D.; Chene, G.; Janvier, G. Platelet function point-of-care tests in post-bypass cardiac surgery: Are they relevant? Br. J. Anaesth. 2002, 89, 715–721. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fattorutto, M.; Pradier, O.; Schmartz, D.; Ickx, B.; Barvais, L. Does the platelet function analyser (PFA-100) predict blood loss after cardiopulmonary bypass? Br. J. Anaesth. 2003, 90, 692–693. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cammerer, U.; Dietrich, W.; Rampf, T.; Braun, S.L.; Richter, J.A. The predictive value of modified computerized thromboelastography and platelet function analysis for postoperative blood loss in routine cardiac surgery. Anesth. Analg. 2003, 96, 51–57. [Google Scholar] [CrossRef]
- Casati, V.; Della Valle, P.; Benussi, S.; Franco, A.; Gerli, C.; Baili, P.; Alfieri, O.; D’Angelo, A. Effects of tranexamic acid on postoperative bleeding and related hematochemical variables in coronary surgery: Comparison between on-pump and off-pump techniques. J. Thorac. Cardiovasc. Surg. 2004, 128, 83–91. [Google Scholar] [CrossRef]
- Pleym, H.; Stenseth, R.; Wahba, A.; Bjella, L.; Tromsdal, A.; Karevold, A.; Dale, O. Prophylactic treatment with desmopressin does not reduce postoperative bleeding after coronary surgery in patients treated with aspirin before surgery. Anesth. Analg. 2004, 98, 578–584. [Google Scholar] [CrossRef]
- Poston, R.; Gu, J.; Manchio, J.; Lee, A.; Brown, J.; Gammie, J.; White, C.; Griffith, B.P. Platelet function tests predict bleeding and thrombotic events after off-pump coronary bypass grafting. Eur. J. Cardio-Thorac. Surg. 2005, 27, 584–591. [Google Scholar] [CrossRef][Green Version]
- Nuttall, G.A.; Henderson, N.; Quinn, M.; Blair, C.; Summers, L.; Williams, B.A.; Oliver, W.C.; Santrach, P.J. Excessive bleeding and transfusion in a prior cardiac surgery is associated with excessive bleeding and transfusion in the next surgery. Anesth. Analg. 2006, 102, 1012–1017. [Google Scholar] [CrossRef]
- Marietta, M.; Facchini, L.; Pedrazzi, P.; Busani, S.; Torelli, G. Pathophysiology of bleeding in surgery. Transplant. Proc. 2006, 38, 812–814. [Google Scholar] [CrossRef]
- Gerrah, R.; Brill, A.; Tshori, S.; Lubetsky, A.; Merin, G.; Varon, D. Using cone and plate(let) analyzer to predict bleeding in cardiac surgery. Asian Cardiovasc. Thorac. Ann. 2006, 14, 310–315. [Google Scholar] [CrossRef]
- Jimenez Rivera, J.J.; Iribarren, J.L.; Raya, J.M.; Nassar, I.; Lorente, L.; Perez, R.; Brouard, M.; Lorenzo, J.M.; Garrido, P.; Barrios, Y. Factors associated with excessive bleeding in cardiopulmonary bypass patients: A nested case-control study. J. Cardiothorac. Surg. 2007, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Katori, N.; Tanaka, K.A.; Takeda, J. Impact of Sonoclot hemostasis analysis after cardiopulmonary bypass on postoperative hemorrhage in cardiac surgery. J. Anesth. 2007, 21, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Ouattara, A.; Bouzguenda, H.; Le Manach, Y.; Leger, P.; Mercadier, A.; Leprince, P.; Bonnet, N.; Montalescot, G.; Riou, B.; Coriat, P. Impact of aspirin with or without clopidogrel on postoperative bleeding and blood transfusion in coronary surgical patients treated prophylactically with a low-dose of aprotinin. Eur. Heart J. 2007, 28, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Newby, L.K.; Clare, R.M.; Shaw, L.K.; Lodge, A.J.; Smith, P.K.; Jolicoeur, E.M.; Rao, S.V.; Becker, R.C.; Mark, D.B.; et al. Clopidogrel use and bleeding after coronary artery bypass graft surgery. Am. Heart J. 2008, 156, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.S.; Frye, C.B.; Harshaw, Q.; Edwards, F.H.; Steinhubl, S.R.; Becker, R.C. Impact of clopidogrel in patients with acute coronary syndromes requiring coronary artery bypass surgery: A multicenter analysis. J. Am. Coll. Cardiol. 2008, 52, 1693–1701. [Google Scholar] [CrossRef]
- Davidson, S.J.; McGrowder, D.; Roughton, M.; Kelleher, A.A. Can ROTEM thromboelastometry predict postoperative bleeding after cardiac surgery? J. Cardiothorac. Vasc. Anesth. 2008, 22, 655–661. [Google Scholar] [CrossRef]
- Reinhofer, M.; Brauer, M.; Franke, U.; Barz, D.; Marx, G.; Losche, W. The value of rotation thromboelastometry to monitor disturbed perioperative haemostasis and bleeding risk in patients with cardiopulmonary bypass. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2008, 19, 212–219. [Google Scholar] [CrossRef]
- Rahe-Meyer, N.; Solomon, C.; Winterhalter, M.; Piepenbrock, S.; Tanaka, K.; Haverich, A.; Pichlmaier, M. Thromboelastometry-guided administration of fibrinogen concentrate for the treatment of excessive intraoperative bleeding in thoracoabdominal aortic aneurysm surgery. J. Thorac. Cardiovasc. Surg. 2009, 138, 694–702. [Google Scholar] [CrossRef]
- Gill, R.; Herbertson, M.; Vuylsteke, A.; Olsen, P.S.; von Heymann, C.; Mythen, M.; Sellke, F.; Booth, F.; Schmidt, T.A. Safety and efficacy of recombinant activated factor VII: A randomized placebo-controlled trial in the setting of bleeding after cardiac surgery. Circulation 2009, 120, 21–27. [Google Scholar] [CrossRef]
- Preisman, S.; Kogan, A.; Itzkovsky, K.; Leikin, G.; Raanani, E. Modified thromboelastography evaluation of platelet dysfunction in patients undergoing coronary artery surgery. Eur. J. Cardio-Thorac. Surg. 2010, 37, 1367–1374. [Google Scholar] [CrossRef]
- Wasowicz, M.; McCluskey, S.A.; Wijeysundera, D.N.; Yau, T.M.; Meinri, M.; Beattie, W.S.; Karkouti, K. The incremental value of thrombelastography for prediction of excessive blood loss after cardiac surgery: An observational study. Anesth. Analg. 2010, 111, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Herman, C.R.; Buth, K.J.; Kent, B.A.; Hirsch, G.M. Clopidogrel increases blood transfusion and hemorrhagic complications in patients undergoing cardiac surgery. Ann. Thorac. Surg. 2010, 89, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Nesher, N.; Singh, S.K.; Fawzy, H.F.; Sever, J.Y.; Goldman, B.E.; Cohen, G.N.; Laflamme, C.; Fremes, S.E. Impact of clopidogrel use on mortality and major bleeding in patients undergoing coronary artery bypass surgery. Interact. Cardiovasc. Thorac. Surg. 2010, 10, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Kwak, Y.L.; Kim, J.C.; Choi, Y.S.; Yoo, K.J.; Song, Y.; Shim, J.K. Clopidogrel responsiveness regardless of the discontinuation date predicts increased blood loss and transfusion requirement after off-pump coronary artery bypass graft surgery. J. Am. Coll. Cardiol. 2010, 56, 1994–2002. [Google Scholar] [CrossRef] [PubMed]
- Coakley, M.; Hall, J.E.; Evans, C.; Duff, E.; Billing, V.; Yang, L.; McPherson, D.; Stephens, E.; Macartney, N.; Wilkes, A.R. Assessment of thrombin generation measured before and after cardiopulmonary bypass surgery and its association with postoperative bleeding. J. Thromb. Haemost. 2011, 9, 282–292. [Google Scholar] [CrossRef]
- Weitzel, N.S.; Weitzel, L.B.; Epperson, L.E.; Karimpour-Ford, A.; Tran, Z.V.; Seres, T. Platelet mapping as part of modified thromboelastography (TEG(R)) in patients undergoing cardiac surgery and cardiopulmonary bypass. Anaesthesia 2012, 67, 1158–1165. [Google Scholar] [CrossRef]
- Lee, G.C.; Kicza, A.M.; Liu, K.Y.; Nyman, C.B.; Kaufman, R.M.; Body, S.C. Does rotational thromboelastometry (ROTEM) improve prediction of bleeding after cardiac surgery? Anesth. Analg. 2012, 115, 499–506. [Google Scholar] [CrossRef]
- Deja, M.A.; Kargul, T.; Domaradzki, W.; Stacel, T.; Mazur, W.; Wojakowski, W.; Gocol, R.; Gaszewska-Zurek, E.; Zurek, P.; Pytel, A.; et al. Effects of preoperative aspirin in coronary artery bypass grafting: A double-blind, placebo-controlled, randomized trial. J. Thorac. Cardiovasc. Surg. 2012, 144, 204–209. [Google Scholar] [CrossRef]
- Wang, G.; Xie, G.; Jiang, T.; Wang, Y.; Wang, W.; Ji, H.; Liu, M.; Chen, L.; Li, L. Tranexamic acid reduces blood loss after off-pump coronary surgery: A prospective, randomized, double-blind, placebo-controlled study. Anesth. Analg. 2012, 115, 239–243. [Google Scholar] [CrossRef]
- Yang, L.; Vuylsteke, A.; Gerrard, C.; Besser, M.; Baglin, T. Postoperative fibrinogen level is associated with postoperative bleeding following cardiothoracic surgery and the effect of fibrinogen replacement therapy remains uncertain. J. Thromb. Haemost. 2013, 11, 1519–1526. [Google Scholar] [CrossRef]
- Emeklibas, N.; Kammerer, I.; Bach, J.; Sack, F.U.; Hellstern, P. Preoperative hemostasis and its association with bleeding and blood component transfusion requirements in cardiopulmonary bypass surgery. Transfusion 2013, 53, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Greiff, G.; Pleym, H.; Stenseth, R.; Berg, K.S.; Wahba, A.; Videm, V. Prediction of Bleeding after Cardiac Surgery: Comparison of Model Performances: A Prospective Observational Study. J. Cardiothorac. Vasc. Anesth. 2014, 29, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Devenraj, V.; Tewarson, V.; Kumar, S.; Kaushal, D.; Chandra, T. Thromboelastography in off-pump coronary artery bypass grafting. Asian Cardiovasc. Thorac. Ann. 2014, 23, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Welsh, K.J.; Padilla, A.; Dasgupta, A.; Nguyen, A.N.; Wahed, A. Thromboelastography is a suboptimal test for determination of the underlying cause of bleeding associated with cardiopulmonary bypass and may not predict a hypercoagulable state. Am. J. Clin. Pathol. 2014, 142, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Walden, K.; Jeppsson, A.; Nasic, S.; Backlund, E.; Karlsson, M. Low preoperative fibrinogen plasma concentration is associated with excessive bleeding after cardiac operations. Ann. Thorac. Surg. 2014, 97, 1199–1206. [Google Scholar] [CrossRef]
- Orlov, D.; McCluskey, S.A.; Selby, R.; Yip, P.; Pendergrast, J.; Karkouti, K. Platelet dysfunction as measured by a point-of-care monitor is an independent predictor of high blood loss in cardiac surgery. Anesth. Analg. 2014, 118, 257–263. [Google Scholar] [CrossRef]
- Doussau, A.; Perez, P.; Puntous, M.; Calderon, J.; Jeanne, M.; Germain, C.; Rozec, B.; Rondeau, V.; Chene, G.; Ouattara, A.; et al. Fresh-frozen plasma transfusion did not reduce 30-day mortality in patients undergoing cardiopulmonary bypass cardiac surgery with excessive bleeding: The PLASMACARD multicenter cohort study. Transfusion 2014, 54, 1114–1124. [Google Scholar] [CrossRef]
- Chowdhury, M.; Shore-Lesserson, L.; Mais, A.M.; Leyvi, G. Thromboelastograph with Platelet Mapping(TM) predicts postoperative chest tube drainage in patients undergoing coronary artery bypass grafting. J. Cardiothorac. Vasc. Anesth. 2014, 28, 217–223. [Google Scholar] [CrossRef]
- Dalen, M.; van der Linden, J.; Holm, M.; Lindvall, G.; Ivert, T. Adenosine diphosphate-induced single-platelet count aggregation and bleeding in clopidogrel-treated patients undergoing coronary artery bypass grafting. J. Cardiothorac. Vasc. Anesth. 2014, 28, 230–234. [Google Scholar] [CrossRef]
- Ranucci, M.; Colella, D.; Baryshnikova, E.; Di Dedda, U.; Surgical Clinical Outcome Research Group. Effect of preoperative P2Y12 and thrombin platelet receptor inhibition on bleeding after cardiac surgery. Br. J. Anaesth. 2014, 113, 970–976. [Google Scholar] [CrossRef]
- Negargar, S.; Anvari, S.; Abbasi, K.; Enamzadeh, E. Immediate Postoperative Complications in Patients Undergoing CABG.; Investigating the Role of Prior Coronary Stenting. J. Cardiovasc. Thorac. Res. 2014, 6, 229–234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Agarwal, S.; Johnson, R.I.; Shaw, M. Preoperative Point-of-Care Platelet Function Testing in Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2014, 29, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Totonchi, Z.; Baazm, F.; Chitsazan, M.; Seifi, S. Predictors of prolonged mechanical ventilation after open heart surgery. J. Cardiovasc. Thorac. Res. 2014, 6, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Fassl, J.; Lurati Buse, G.; Filipovic, M.; Reuthebuch, O.; Hampl, K.; Seeberger, M.D.; Bolliger, D. Perioperative administration of fibrinogen does not increase adverse cardiac and thromboembolic events after cardiac surgery. Br. J. Anaesth. 2015, 114, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.; Stenseth, R.; Videm, V.; Pleym, H. Comparison of three point-of-care testing devices to detect hemostatic changes in adult elective cardiac surgery: A prospective observational study. BMC Anesthesiol. 2014, 14, 80. [Google Scholar] [CrossRef]
- Kindo, M.; Hoang Minh, T.; Gerelli, S.; Perrier, S.; Meyer, N.; Schaeffer, M.; Bentz, J.; Announe, T.; Mommerot, A.; Collange, O.; et al. Plasma fibrinogen level on admission to the intensive care unit is a powerful predictor of postoperative bleeding after cardiac surgery with cardiopulmonary bypass. Thromb. Res. 2014, 134, 360–368. [Google Scholar] [CrossRef]
- Kim, N.Y.; Shim, J.K.; Song, J.W.; Kim, E.K.; Kwak, Y.L. Impact of preoperative fibrinogen concentration on postoperative outcome in patients who received dual antiplatelet therapy in proximity to off-pump coronary bypass surgery. Circ. J. 2014, 78, 1661–1666. [Google Scholar] [CrossRef]
- Sharma, A.D.; Al-Achi, A.; Seccombe, J.F.; Hummel, R.; Preston, M.; Behrend, D. Does incorporation of thromboelastography improve bleeding prediction following adult cardiac surgery? Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2014, 25, 561–570. [Google Scholar] [CrossRef]
- Ghavidel, A.A.; Toutounchi, Z.; Shahandashti, F.J.; Mirmesdagh, Y. Rotational thromboelastometry in prediction of bleeding after cardiac surgery. Asian Cardiovasc. Thorac. Ann. 2015, 23, 525–529. [Google Scholar] [CrossRef]
- Mishra, P.K.; Thekkudan, J.; Sahajanandan, R.; Gravenor, M.; Lakshmanan, S.; Fayaz, K.M.; Luckraz, H. The role of point-of-care assessment of platelet function in predicting postoperative bleeding and transfusion requirements after coronary artery bypass grafting. Ann. Card. Anaesth. 2015, 18, 45–51. [Google Scholar] [CrossRef]
- Drews, S.; Bolliger, D.; Kaiser, C.; Grapow, M.; Reuthebuch, O.; Eckstein, F.; Matt, P. Prasugrel increases the need for platelet transfusions and surgical reexploration rates compared with clopidogrel in coronary artery bypass surgery. Thorac. Cardiovasc. Surg. 2015, 63, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Besser, M.W.; Ortmann, E.; Klein, A.A. Haemostatic management of cardiac surgical haemorrhage. Anaesthesia 2015, 70 (Suppl. 1), 87–95, e29–e31. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, P.; Pitkanen, O.; Musialowicz, T. Levosimendan increases bleeding risk after heart valve surgery: A retrospective analysis of a randomized trial. J. Cardiothorac. Vasc. Anesth. 2014, 28, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.W.; Kumar, A.; Guo, J.; Aranki, S.; Shekar, P.; Agnihotri, A.; Maree, A.O.; McLean, D.S.; Rosenfield, K.; Cannon, C.P. Point-of-Care Platelet Function Testing Predicts Bleeding in Patients Exposed to Clopidogrel Undergoing Coronary Artery Bypass Grafting: Verify Pre-Op TIMI 45-A Pilot Study. Clin. Cardiol. 2015, 38, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Dyke, C.; Aronson, S.; Dietrich, W.; Hofmann, A.; Karkouti, K.; Levi, M.; Murphy, G.J.; Sellke, F.W.; Shore-Lesserson, L.; von Heymann, C.; et al. Universal definition of perioperative bleeding in adult cardiac surgery. J. Thorac. Cardiovasc. Surg. 2014, 147, 1458–1463 e1. [Google Scholar] [CrossRef]
- Goodnough, L.T.; Johnston, M.F.; Toy, P.T. The variability of transfusion practice in coronary artery bypass surgery. JAMA 1991, 265, 86–90. [Google Scholar] [CrossRef]
- Gratz, J.; Guting, H.; Thorn, S.; Brazinova, A.; Gorlinger, K.; Schafer, N.; Schochl, H.; Stanworth, S.; Maegele, M. Protocolised thromboelastometric-guided haemostatic management in patients with traumatic brain injury: A pilot study. Anaesthesia 2019, 74, 883–890. [Google Scholar] [CrossRef]
- Rimaitis, M.; Bilskiene, D.; Tamosuitis, T.; Vilcinis, R.; Rimaitis, K.; Macas, A. Implementation of Thromboelastometry for Coagulation Management in Isolated Traumatic Brain Injury Patients Undergoing Craniotomy. Med. Sci. Monit. 2020, 26, e922879. [Google Scholar] [CrossRef]
- Lumbreras-Marquez, M.I.; Singh, S.; King, C.H.; Nelson, C.I.; Jespersen, K.N.; Fields, K.G.; Wang, P.; Carusi, D.A.; Farber, M.K. Rotational thromboelastometry for the transfusion management of postpartum hemorrhage after cesarean or vaginal delivery: A single-center randomized controlled trial. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102470. [Google Scholar] [CrossRef]
- Vuylsteke, A.; Pagel, C.; Gerrard, C.; Reddy, B.; Nashef, S.; Aldam, P.; Utley, M. The Papworth Bleeding Risk Score: A stratification scheme for identifying cardiac surgery patients at risk of excessive early postoperative bleeding. Eur. J. Cardio-Thorac. Surg. 2011, 39, 924–930. [Google Scholar] [CrossRef]
- Scharf, R.E. Drugs that affect platelet function. Semin. Thromb. Hemost. 2012, 38, 865–883. [Google Scholar] [CrossRef] [PubMed]
- Petricevic, M.; Petricevic, M.; Pasalic, M.; Golubic Cepulic, B.; Raos, M.; Vasicek, V.; Goerlinger, K.; Rotim, K.; Gasparovic, H.; Biocina, B. Bleeding risk stratification in coronary artery surgery: The should-not-bleed score. J. Cardiothorac. Surg. 2021, 16, 103. [Google Scholar] [CrossRef] [PubMed]
- Petricevic, M.; Petricevic, M.; Pasalic, M.; Cepulic, B.G.; Raos, M.; Dujmic, D.; Kalamar, V.; Mestrovic, V.; Gasparovic, H.; Vasicek, V.; et al. Cost Analysis of Transfusion Therapy in Coronary Artery Surgery. Thorac. Cardiovasc. Surg. 2019, 69, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.H.; Roe, M.T.; Mulgund, J.; Ohman, E.M.; Cannon, C.P.; Gibler, W.B.; Pollack, C.V., Jr.; Smith, S.C., Jr.; Ferguson, T.B.; Peterson, E.D. Acute clopidogrel use and outcomes in patients with non-ST-segment elevation acute coronary syndromes undergoing coronary artery bypass surgery. J. Am. Coll. Cardiol. 2006, 48, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Tricoci, P.; Huang, Z.; Held, C.; Moliterno, D.J.; Armstrong, P.W.; Van de Werf, F.; White, H.D.; Aylward, P.E.; Wallentin, L.; Chen, E.; et al. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N. Engl. J. Med. 2012, 366, 20–33. [Google Scholar] [CrossRef]
- Ben-Dor, I.; Kleiman, N.S.; Lev, E. Assessment, mechanisms, and clinical implication of variability in platelet response to aspirin and clopidogrel therapy. Am. J. Cardiol. 2009, 104, 227–233. [Google Scholar] [CrossRef]
- Petricevic, M.; Biocina, B.; Mihaljevic, M.Z.; Boban, M.; Samardzic, J.; Konosic, S.; Ivancan, V. Preoperative prasugrel discontinuation management in coronary artery surgery. Thorac. Cardiovasc. Surg. 2015, 63, 36–37. [Google Scholar] [CrossRef]
- Dalen, M.; Ivert, T.; Lindvall, G.; van der Linden, J. Prasugrel-related late cardiac tamponade after coronary artery bypass surgery in a patient with a recently implanted bare metal stent. J. Cardiothorac. Vasc. Anesth. 2013, 27, e57–e58. [Google Scholar] [CrossRef]
- Schotola, H.; Brauer, A.; Meyer, K.; Hinz, J.; Schondube, F.A.; Bauer, M.; Mohite, P.N.; Danner, B.C.; Sossalla, S.; Popov, A.F. Perioperative outcomes of cardiac surgery patients with ongoing ticagrelor therapy: Boon and bane of a new drug. Eur. J. Cardio-Thorac. Surg. 2014, 46, 198–205. [Google Scholar] [CrossRef][Green Version]
- Vertrees, R.A.; Conti, V.R.; Lick, S.D.; Zwischenberger, J.B.; McDaniel, L.B.; Shulman, G. Adverse effects of postoperative infusion of shed mediastinal blood. Ann. Thorac. Surg. 1996, 62, 717–723. [Google Scholar] [CrossRef]
- Baric, D.; Biocina, B.; Unic, D.; Sutlic, Z.; Rudez, I.; Vrca, V.B.; Brkic, K.; Ivkovic, M. Topical use of antifibrinolytic agents reduces postoperative bleeding: A double-blind, prospective, randomized study. Eur. J. Cardio-Thorac. Surg. 2007, 31, 366–371; discussion 371. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, H.; Elmistekawy, E.; Bonneau, D.; Latter, D.; Errett, L. Can local application of Tranexamic acid reduce post-coronary bypass surgery blood loss? A randomized controlled trial. J. Cardiothorac. Surg. 2009, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Mutlak, H.; Reyher, C.; Meybohm, P.; Papadopoulos, N.; Hanke, A.A.; Zacharowski, K.; Weber, C.F. Multiple electrode aggregometry for the assessment of acquired platelet dysfunctions during extracorporeal circulation. Thorac. Cardiovasc. Surg. 2015, 63, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Petricevic, M.; Biocina, B.; Milicic, D.; Svetina, L.; Boban, M.; Lekic, A.; Konosic, S.; Milosevic, M.; Gasparovic, H. Activated coagulation time vs. intrinsically activated modified rotational thromboelastometry in assessment of hemostatic disturbances and blood loss after protamine administration in elective cardiac surgery: Analysis from the clinical trial (NCT01281397). J. Cardiothorac. Surg. 2014, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Baryshnikova, E.; Di Dedda, U.; Ranucci, M. Are Viscoelastic Tests Clinically Useful to Identify Platelet-Dependent Bleeding in High-Risk Cardiac Surgery Patients? Anesth. Analg. 2022, 135, 1198–1206. [Google Scholar] [CrossRef]
- Bujang, M.A. A Step-by-Step Process on Sample Size Determination for Medical Research. Malays. J. Med. Sci. 2021, 28, 15–27. [Google Scholar] [CrossRef]
- Nahm, F.S. Receiver operating characteristic curve: Overview and practical use for clinicians. Korean J. Anesthesiol. 2022, 75, 25–36. [Google Scholar] [CrossRef]
- Monaghan, T.F.; Rahman, S.N.; Agudelo, C.W.; Wein, A.J.; Lazar, J.M.; Everaert, K.; Dmochowski, R.R. Foundational Statistical Principles in Medical Research: Sensitivity, Specificity, Positive Predictive Value, and Negative Predictive Value. Medicina 2021, 57, 503. [Google Scholar] [CrossRef]
- Gombotz, H.; Knotzer, H. Preoperative identification of patients with increased risk for perioperative bleeding. Curr. Opin. Anaesthesiol. 2013, 26, 82–90. [Google Scholar] [CrossRef]
- Pandit, T.N.; Sarode, R. Blood component support in acquired coagulopathic conditions: Is there a method to the madness? Am. J. Hematol. 2012, 87 (Suppl. 1), S56–S62. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).