Abstract
Background: Individuals undergoing cardiac surgery face an increased risk of bleeding, as well as alterations in biochemical and coagulation patterns. Therefore, assessing the effectiveness of systems such as Cell Salvage is necessary to prevent potential surgical complications. Objective: To evaluate the efficacy of Cell Salvage in relation to the biochemical parameters of the red blood series and coagulation, as well as the risk of hemorrhage. Methods: A systematic review, accompanied by a meta-analysis, was executed via an extensive literature exploration encompassing Medline, CINAHL, Scopus, Web of Science, and the Cochrane Library. The inclusion criteria comprised studies in English or Spanish, without year restrictions, conducted in adults and with a randomized controlled trial design. Results: Twenty-six studies were included in the systematic review, involving a total of 2850 patients (experimental group = 1415; control group = 1435). Cell Salvage did not demonstrate superior outcomes compared to allogeneic transfusions in the management of post-surgical hemorrhage, as well as in total blood loss, platelet count, fresh frozen plasma, and fibrinogen. However, Cell Salvage showed a greater effectiveness for hemoglobin (moderate evidence), hematocrit (low evidence), post intervention D-dimer (low evidence), and some coagulation-related parameters (low evidence) compared to allogeneic transfusions. Finally, better results were found in the control group for INR parameters. Conclusions: The use of the Cell Salvage system holds high potential to improve the postoperative levels of biochemical and coagulation parameters. However, the results do not provide definitive evidence regarding its effectiveness for hemorrhage control, platelet count, fresh frozen plasma, and fibrinogen. Therefore, it is recommended to increase the number of studies to assess the impact of the Cell Salvage system on improvements in the red blood cell count and patient coagulation patterns. In addition, protocols should be homogenized, and variables such as the sex of the participants should be taken into account.
1. Introduction
Throughout history, cardiac surgery has been a major consumer of blood products worldwide, and specifically, it is the surgery that consumes the most blood resources [1,2,3]. Patients undergoing cardiac surgery face a high risk of bleeding [4,5]. Consequently, these patients receive a significant amount of allogeneic blood transfusions, accounting for between 15% and 20% of perioperative transfusions [6,7]. Similarly, in recent years, there has been a decline in blood donations, a trend exacerbated by the COVID-19 pandemic [8,9,10].
On the other hand, there is concern about the side effects of allogeneic blood transfusion. This has driven the development of methods aimed at minimizing these risks in the perioperative period. These methods include preoperative autologous donation (PAD) [11,12], isovolemic hemodilution [13], and the use of medications such as aprotinin [14], tranexamic acid [15], and erythropoietin [16].
In addition to the mentioned methods, there is the Cell Salvage approach, which can reduce the need for allogeneic blood transfusions during the perioperative period. Cell Salvage is well-established in cardiac surgery [17]. It allows for the reinfusion of blood extracted from the surgical field, either directly or after a centrifugation process to remove non-cellular components [18]. This potentially reduces the risk of blood transfusions while maintaining acceptable hemoglobin (Hb) levels during the intraoperative and early postoperative periods [19,20]. In addition to its primary role in managing patient blood [21], there is evidence that Cell Salvage is associated with a decrease in systemic inflammation and a reduced incidence of postoperative atrial fibrillation, a common arrhythmia after cardiac surgery [22]. The first autotransfusion of blood extracted from a patient was described in 1860 [23]. The devices used at that time were often associated with serious complications, such as gas embolism, although nowadays, these complications are rare [24].
While numerous articles have been published on Cell Salvage use, there is considerable controversy regarding its effectiveness, with studies supporting its use [25,26,27] and others opposing it due to a lack of conclusive results [28,29].
Due to the variability in methodologies, procedures, and results obtained, there is a need for a synthesis of the existing literature to analyze the effectiveness of Cell Salvage and update the latest findings. Furthermore, exploring alternative approaches to allogeneic transfusions becomes more necessary when facing a lack of blood resources in blood banks, along with the side effects and the high prevalence of bleeding in cardiac surgery. Therefore, the objective of this meta-analysis of randomized clinical trials was to evaluate the effectiveness of Cell Salvage regarding biochemical parameters of the red blood cell series in the complete blood count and coagulation, as well as the risk of bleeding.
2. Materials and Methods
2.1. Search Strategy and Inclusion Criteria
An exhaustive search was conducted in databases such as Medline (PubMed), CINAHL, Scopus, Web of Science, and The Cochrane Library, following the recommended guidelines in Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Protocols (PRISMA) [30]. This search was assessed using A Measurement Tool to Assess Systematic Reviews (AMSTAR-2) [31,32]. Additionally, a search was carried out on ClinicalTrials.gov, confirming the absence of similar documents in the Prospero registry. Google Scholar was also explored to identify unconventional literature and minimize potential publication biases. The protocol for this study was registered on the Prospero website in July 2023 (CRD42023446583).
A PICO question was formulated as follows: Patient/Problem: Individuals undergoing cardiovascular, cardiac, and/or thoracic surgery; Intervention: Use of a cell saver; Comparison: Conventional blood transfusion or alternative controls; Outcomes: Evaluation of the reduction in bleeding risk, along with key biochemical parameters, including hemoglobin levels, platelet count, prothrombin time, and activated partial thromboplastin time.
The search terms used were (“blood retrievers” OR “intraoperative cell salvage” OR “autotransfusion system” OR “cell saver” OR “Operative Blood Salvage” OR “Blood Transfusion, Autologous”) AND (“cardiac surgery” OR “cardiopulmonary bypass”) AND (“Hemoglobins” OR “Hemorrhage” OR “Hematocrit” OR “Erythrocyte Count” OR Erythrocytes OR “Blood Component Transfusion” OR “International Normalized Ratio” OR “Partial Thromboplastin Time” OR “Prothrombin Time” OR “Thrombin Time” OR “fresh frozen plasma” OR “Platelet Count” OR “Pyrimidine Dimers” OR “Fibrinogen”) and their equivalent in Spanish or French. The search equation descriptors were chosen from the Medical Subject Headings (MeSH) thesaurus.
These search terms were obtained from the Medical Subject Headings (MeSH) and were used during the search conducted from January to June 2024 by two researchers (M.P.-C. and R.C.-M.). The inclusion criteria comprised articles published without year restriction, in English or Spanish, related to the objectives of this study, and randomized clinical trials (RCTs) conducted in adult humans. The exclusive selection of RCTs was performed to enhance the methodological quality of the review and mitigate biases in an intrinsically complex subject due to the diversity of factors influencing its success, such as the type of surgery, cardiopulmonary bypass time, professional experience, patients’ body mass index, and patients’ comorbidities, among others.
2.2. Data Extraction
The search and article selection were independently conducted by two researchers (M.P.-C. and R.C.-M.), and in the case of disagreement, the opinion of an expert in cardiac surgery was sought for resolution. Initially, the titles and abstracts of articles were reviewed, followed by a full article assessment. Additionally, a bibliographic search was performed both forward and backward in the references cited in the selected studies. The agreement between the two researchers in assessing the suitability of the studies was quantified using the Kappa statistical test.
A data coding manual was followed to gather information from each study, including (1) author’s name; (2) year of publication; (3) country of origin; (4) study design; (5) sample size; (6) type of intervention (use of Cell Salvage versus control group); (7) participants’ age; (8) objectives of each study; and (9) outcomes obtained. The primary dichotomous outcomes analyzed included continuous outcomes focused on hemorrhage, FFP, hemoglobin, fibrinogen, aPTT and aPTT ratio, PT, TT, D-dimer, and INR.
2.3. Quality and Bias Risk Assessment
The Cochrane Risk of Bias Tool [33] was utilized, categorizing each type of risk into three levels: low, high, or unclear. The evaluated risk types encompassed aspects such as random sequence generation, allocation concealment, blinding of participants and personnel, blinding in outcome assessment, integrity of outcome data, selective reporting, and other potential sources of bias. Studies without a high risk of bias in any category were considered high quality (1++), while those with a high risk or two unclear risks were rated as medium quality (1+). Other studies were considered low quality (1−).
Additionally, the modified Jadad scale was applied to assess the internal validity of each study, where a score of ≥4 indicated high quality.
For the risk of bias assessment, the Cochrane Handbook for Intervention Reviews (Revman Version 5.4) was used. Two independent reviewers subjectively assessed all articles and assigned ratings of “high”, “low”, or “unclear” based on selection, performance, detection, attrition biases, and other potential biases. Disagreements were resolved through discussions to reach a consensus. If a consensus was not reached, the opinion of a third investigator (M.-L.O.) was sought.
Statistical analysis and bias assessment were conducted using the Review Manager software, version 5.4 (Cochrane Library, London, UK). Additionally, the data were imported into the Grade Pro application, allowing for the assessment of the recommendation grade for the obtained data [34].
2.4. Data Synthesis and Statistical Analysis
The relative risk (RR) was used to compare dichotomous variables, and 95% confidence intervals (CIs) were provided. Continuous variables were evaluated using mean differences (MDs) along with a 95% CI. Data for dichotomous outcomes were pooled using a random-effects model [6] to provide a more cautious estimate of the effects of Cell Salvage. When standard deviation data were not available in the study, the method recommended by Hozo et al. was applied [35]. Both binary and continuous data were calculated using fixed or random-effects models. The fixed-effects model was chosen initially if there was no significant heterogeneity between studies (I2 ≤ 50%). Otherwise, the random-effects model was used [36].
Heterogeneity among studies was assessed through chi-square tests and the I2 test, with a statistical significance level of p-value < 0.05. I2 values between 0% and 25% indicated low heterogeneity, between 25% and 75% moderate heterogeneity, and over 75% high heterogeneity [37].
A forest plot was used to visualize the results of the meta-analysis, and a funnel plot was employed to assess possible publication bias among studies. The asymmetry of the funnel plot was analyzed using the funnel plot representation and evaluated with the Egger’s test, considering a statistical significance level of p-value < 0.05 as indicative of publication bias evidence.
Subgroup analysis based on biochemical patterns was conducted. A sensitivity analysis was also performed to assess the robustness of the results by sequentially omitting each study. p-values < 0.05 were considered statistically significant.
The comparison of the impact of Cell Salvage in relation to allogeneic transfusions was expressed as the RR of reintervention or subsequent bleeding, along with 95% confidence intervals. The units of measurement in which the variables were expressed were as follows: for bleeding and the volume of FFP in milliliters (mL); hemoglobin and fibrinogen in grams per deciliter (g/dL); aPTT, PT, and TT in seconds; and D-dimer in nanograms per milliliter (ng/mL).
3. Results
3.1. Results Obtained in the Selection of Articles
In the initial literature search, a total of 2838 articles were identified, with no additional documents excluded from specific clinical trial registries (ClinicalTrials.gov and Prospero). After removing 135 duplicate articles using the Zotero® reference manager, applying the inclusion criteria, and evaluating the titles and abstracts of the articles, 856 were excluded for not meeting the inclusion criteria. Finally, 26 studies were selected for the systematic review analysis, of which 25 provided data for the meta-analysis, encompassing a sample of 2850 participants (74.4% men vs. 25.6% women) who underwent cardiac surgery (experimental group with Cell Salvage, n = 1415; control group, n = 1435). The flow diagram (Figure 1) illustrates the review process. There was excellent agreement between the researchers regarding the eligibility assessment of the trials (Kappa statistic = 0.95).
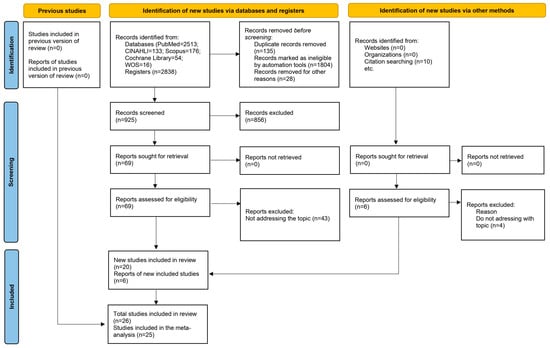
Figure 1.
Flow diagram that illustrates the review process.
3.2. Descriptive Analysis of the Results Found
The years with the highest scientific production were 2015, with three articles, and 2006, 2007, and 2012, each with two publications. The levels of evidence assessed based on the quality of the selected articles received a score of 1++ in 13.63% (n = 3) of cases, 27.27% received a score of 1+ (n = 6), and 59.09% received a score of 1− (n = 13).
The included studies addressed hemorrhage (n = 11; 42.31%), hemoglobin (n = 18; 69.23%), hematocrit (n = 7; 26.92%), fibrinogen (n = 4; 15.38%), INR (n = 2; 7.69%), aPTT (n = 5; 19.23%), PT (n = 3; 11.54%), TT (n = 1; 3.85%), platelet count (n = 11; 42.31%), and aPTT ratio (n = 2; 7.69%). The details of each item included are provided in Table 1.

Table 1.
Characteristics of the included studies.
A moderate grade of recommendation was observed for overall hemoglobin (MD 0.48, 95 CI 0.28–0.69), immediate postoperative hemoglobin (MD 0.65, 95 CI 0.27–1.04), and hemoglobin at 24 h (MD 0.56, 95 CI 0.23–0.90). All other variables had a low or very low grade of recommendation. All analyses were performed using Grade PRO® (Table S1).
3.3. Bias Risk Assessment of the Selected Studies and Publication Bias
The risk of bias was assessed using RevMan 5®, represented in Supplementary Figures S1 and S2 by the bias assessment plots of all included studies and by a one-to-one summary plot. Allocation concealment was evident in about 35% of included studies, with approximately 15% blinding of participants and staff and 20% blinding of outcome assessment. In relation to publication bias, a funnel plot for each study objective assessed shows an inverted funnel, with the strongest studies concentrated in the center (Figure S3).
3.4. Results of the Meta-Analysis
3.4.1. Efficacy of Cell Salvage in Hemorrhage
In eleven clinical trials involving 2056 participants, with 1015 in the intervention group and 1041 in the control group, the efficacy of utilizing Cell Salvage in hemorrhage was assessed [38,43,45,54,55,56,57,59,60,61,63]. Seven studies exhibited a high risk of bias [38,43,57,59,60,61,63].
At six hours post-surgical intervention, blood losses were higher in the control group than in the Cell Salvage group in three studies. However, no significant differences were found between the two groups. An MD of 11.92 was obtained, with a 95% confidence interval of −80.21 to 104.04 (p = 0.80), and significant heterogeneity was noted among the studies (I2 = 84%, p < 0.001).
Twelve hours post-surgical intervention, both studies hit the no-effect line with no difference found between the two groups. An MD of 25.71 was obtained, with a 95% confidence interval of −64.16 to 115.58 (p = 0.57), and heterogeneity was found among the studies (I2 = 0%, p = 0.45).
Regarding the amount of blood lost at 24 h, four studies tended to have higher losses in the control group, two in the intervention group, and another two had similar losses between the two groups. We obtained an MD of 0.79, with a 95% confidence interval of −67.07 to 68.65 (p = 0.98), and heterogeneity between studies (I2 = 59%, p = 0.02).
Concerning the total amount of blood lost, one study found greater losses in the control group and another in the intervention group. An MD of 217.92 was obtained, with a 95% confidence interval of −489.53 to 925.37 (p = 0.55), and significant heterogeneity among the studies (I2 = 98%, p < 0.001).
Ultimately, no statistically significant differences were observed between the experimental and control group (p = 0.56). This is evident in the confidence intervals as well as in the forest plot (no-effect line) (Figure 2).
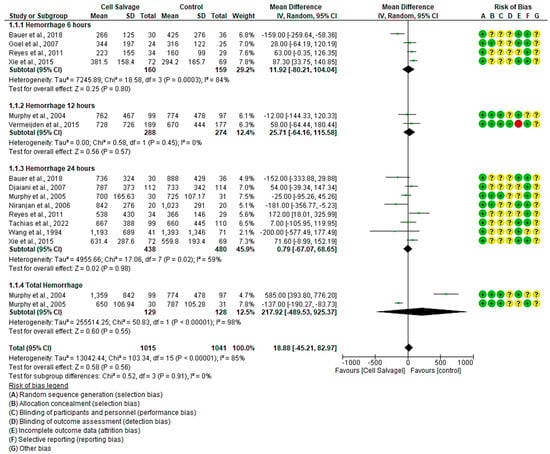
Figure 2.
Forest plot depicting hemorrhage at different time slices.
3.4.2. Efficacy of Cell Salvage on Hemoglobin Levels
In seventeen clinical trials involving 3671 participants, with 1806 in the intervention group and 1865 in the control group, the efficacy of utilizing Cell Salvage on hemoglobin was examined. Eleven studies exhibited a high risk of bias [38,41,43,46,49,52,57,58,59,60,61,62,63].
In the immediate postoperative period, hemoglobin levels were higher in eight studies, while one favored Cell Salvage. In three studies, the results were not statistically significant, approaching the no-effect line. An MD of 0.65 was obtained, with a 95% confidence interval of 0.27 to 1.04 (p < 0.001), and there was significant heterogeneity among the studies (I2 = 91%, p < 0.001).
At six hours, more ambiguous results were shown: two studies tended to have better hemoglobin results in the Cell Salvage group, while one study favored the control group. An MD of 0.39, with a 95% confidence interval of −0.21 to 1.00 (p = 0.20), and heterogeneity between studies (I2 = 80%, p < 0.05) were obtained, so that at 6 h, no significant differences between groups were obtained.
After twenty-four hours of surgery, a total of ten clinical trials were found. The results show an improvement in Cell Salvage compared to the control group. The MD was 0.46, with a 95% confidence interval of 0.23 to 0.90 (p = 0.0001), and significant heterogeneity was noted between studies (I2 = 92%, p < 0.001).
Regarding the amount of hemoglobin at discharge, four studies did not show statistically significant differences, approaching the no-effect line. An MD of −0.05 was obtained, with a 95% confidence interval of −0.23 to 0.13 (p = 0.57), and no heterogeneity among the studies was observed (I2 = 0%, p = 0.77).
Finally, statistically significant differences were observed (p < 0.001), with the Cell Salvage being more efficient (Figure 3).
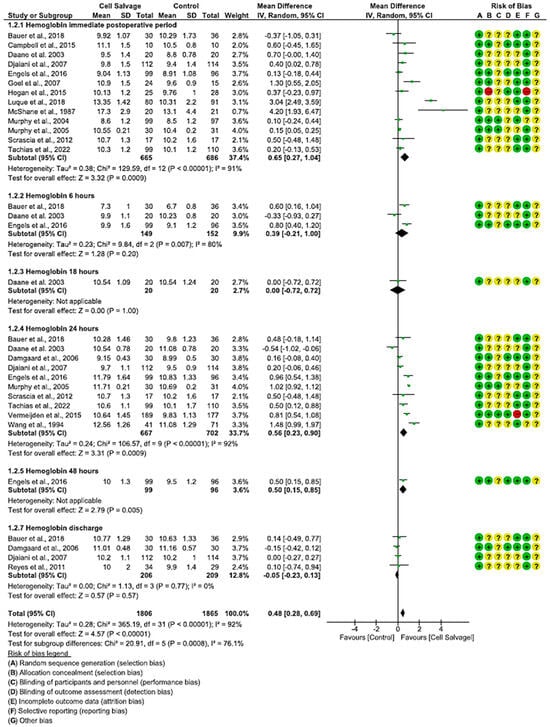
Figure 3.
Forest plot presenting hemoglobin at different time slices.
3.4.3. Efficacy of Cell Salvage on Hematocrit
In six clinical trials involving 666 participants, with 311 in the intervention group and 355 in the control group, the efficacy of using Cell Salvage to improve hematocrit was compared with traditional methods based on transfusions. Five studies showed a high risk of bias [38,41,42,43,45], while one exhibited an adequate quality level [42].
In the immediate postoperative period, four studies indicated better scores for the Cell Salvage group, while only one did not show a statistically significant association, approaching the no-effect line. The results showed an MD of 3.91, with a 95% confidence interval of 0.44 to 7.37 (p = 0.03), and significant heterogeneity between studies (I2 = 99%, p < 0.001). Therefore, an improvement in hematocrit was observed in the Cell Salvage group.
There was only one study that assessed hematocrit both at 6 h and at 18 h. The results tended to favor the Cell Salvage (p = 0.29).
At 24 h, while two studies showed better levels for the Cell Salvage group, one favored the control group. An MD of 2.33 was obtained, with a 95% confidence interval of −0.07 to 4.74 (p = 0.06), and there was significant heterogeneity between studies (I2 = 94%, p < 0.001).
Finally, statistically significant differences were observed in favor of the Cell Salvage (p < 0.001) (Figure 4).
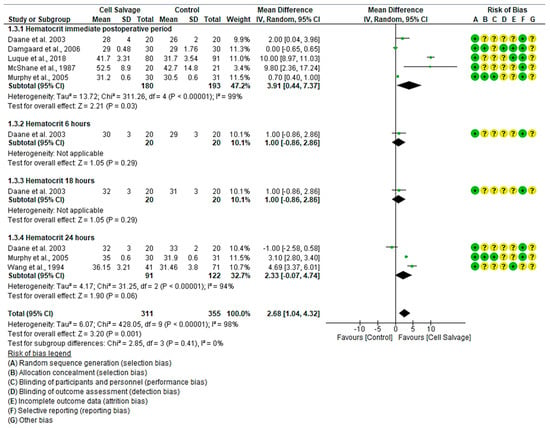
Figure 4.
Forest plot presenting hematocrit at different time slices.
3.4.4. Efficacy of Cell Salvage on Coagulation Parameters
For aPTT, five clinical trials with 1363 participants were identified, with 665 in the intervention group and 698 in the control group, comparing the efficacy of Cell Salvage use. All five studies showed a high risk of bias [39,41,43,46,59]. In the immediate postoperative period, two studies indicated better aPTT times for the control group, while one clearly favored the use of Cell Salvage. The rest have disparate results based on their confidence intervals. An MD of 0.87 was obtained, with a 95% confidence interval of −5.01 to 6.76 (p = 0.77), and significant heterogeneity was observed among the studies (I2 = 97%, p < 0.001).
At 6 h (p = 0.21) and 18 h (p = 0.43) post-surgical intervention, and upon hospital discharge (p = 0.93), aPTT was only assessed in one study, showing a trend toward better times for Cell Salvage.
At 24 h, three studies showed no difference between the groups. It can be seen that the confidence intervals touch the no-effect line. An MD of 0.77, with a 95% confidence interval of −0.46 to 2.00 (p = 0.22) was obtained, and heterogeneity was noted between studies (I2 = 48%, p < 0.001).
Finally, no statistically significant differences were found between groups (95% CI = −2.50 to 3.49, p = 0.74).
For the aPTT ratio, two clinical trials with 575 participants, 288 in the intervention group and 287 in the control group, were identified, comparing the efficacy of Cell Salvage use. Both studies showed a high risk of bias [55,56]. In the immediate postoperative period, both studies indicated better aPTT ratio values for the intervention group with Cell Salvage, with an MD of −0.05, a 95% confidence interval of −0.06 to −0.04 (p < 0.001), and no heterogeneity among the studies (I2 = 0%, p = 0.33). At 24 h post-surgical intervention, only one study evaluated the aPTT ratio, finding no statistically significant differences between groups, approaching the no-effect line. Lastly, statistically significant differences were found between groups (p-value < 0.001).
Regarding the PT ratio, two clinical trials with a total of 257 participants, 129 in the intervention group and 128 in the control group, were identified, both showing a medium risk of bias [54,55]. One of the two clinical trials showed better values for Cell Salvage, while the second did not show a statistically significant association, approaching the no-effect line. An MD of −0.05 was obtained, with a 95% confidence interval of −0.14 to 0.05 (p = 0.35), and significant heterogeneity among the studies was observed (I2 = 92%, p < 0.001). Results favoring Cell Salvage are evident (Figure 5).
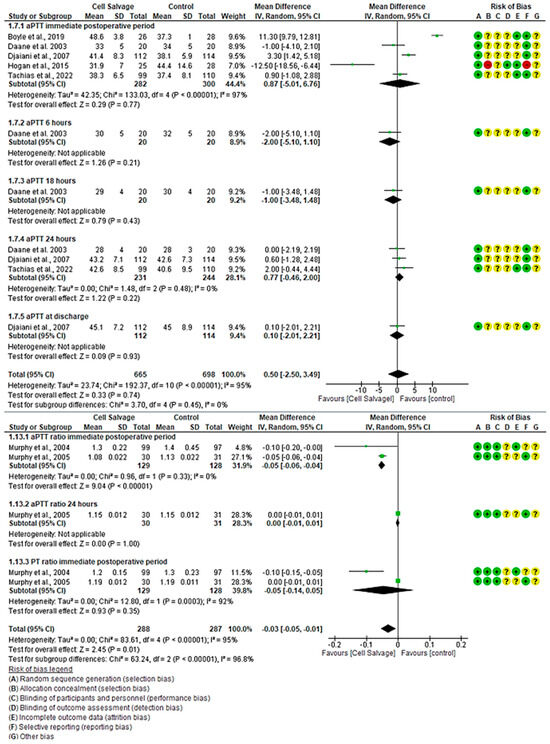
Figure 5.
Forest plot presenting the aPPT and aPPT ratio over time.
For PT, three clinical trials with 267 participants were identified, with 131 in the intervention group and 136 in the control group, comparing the efficacy of Cell Salvage use with conventional techniques based on traditional transfusions. All three studies showed a high risk of bias [39,43,46]. Regarding PT, two studies demonstrated better results for the Cell Salvage, while one did not show a statistically significant relationship, touching the no-effect line. An MD of −0.88 was obtained, with a 95% confidence interval of −1.48 to −0.29 (p < 0.001), with heterogeneity among the studies (I2 = 66%, p = 0.05). At 6, 18, and 24 h, only one study evaluated PT, finding no statistically significant association, touching the no-effect line (p = 0.999). Finally, there was also no association between groups when assessing combined outcomes, touching the no-effect line (p = 0.16).
For TT, one clinical trial with 160 participants was identified, with 80 in the intervention group and 80 in the control group, comparing the efficacy of Cell Salvage use with traditional transfusions. The study showed a high risk of bias [43]. In the immediate postoperative period and at 18 h, the study demonstrated better results for the control group, while it was better in the Cell Salvage group at 6 and 24 h. However, all confidence intervals run along the no-effect line (Figure 6).
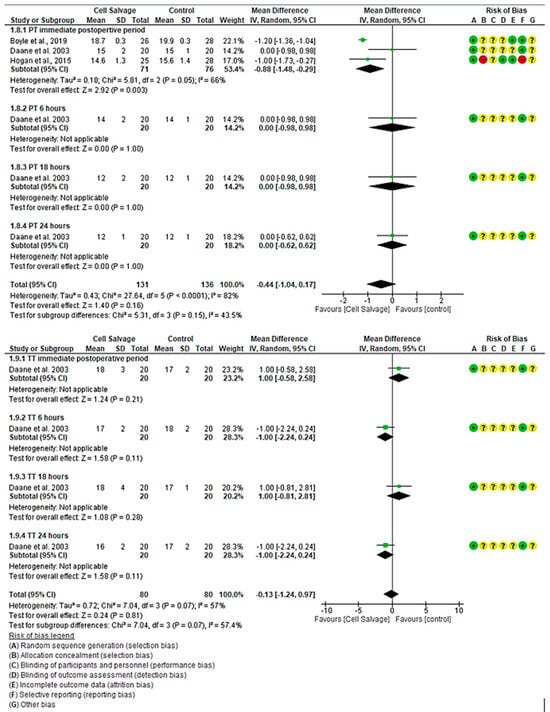
Figure 6.
Forest plot presenting PT and TT over time.
3.4.5. The Efficacy of Cell Salvage in Fresh Frozen Plasma Transfusion
In eleven clinical trials, comprising a total of 1153 participants in both the intervention group (n = 602) and the control group (n = 551), the effectiveness of using Cell Salvage was compared with the control group, where traditional methods based on allogeneic transfusion were employed. Seven studies exhibited a high risk of bias [43,45,50,53,57,58,63]. Regarding the number of individuals transfused with FFP, a total of 100 transfusion cases (16.61%) were observed in the intervention group, while this figure rose to 72 (13.07%) in the control group. One study indicated a higher number of individuals transfused in the control group, while another study showed more cases for the intervention group. The remaining studies yielded inconclusive results, with confidence intervals bordering on the no-effect line. No statistically significant differences were found between the two groups (OR 1.28, 95% CI = 0.92 to 1.78), with moderate heterogeneity between studies (I2 = 44%, p = 0.06).
For platelet count, a total of 2186 participants were involved in eleven clinical trials, with 1062 in the intervention group and 1126 in the control group, comparing the efficacy of Cell Salvage use. Seven studies exhibited a high risk of bias [41,43,46,49,57,59,61], while the remaining studies showed low levels of bias [40,44,54,55]. In the immediate postoperative period, one study indicated a higher number of individuals transfused in the control group, while another study showed more cases for the intervention group. The remaining studies yielded inconclusive results, with confidence intervals bordering on the no-effect line to a greater or lesser extent. An MD of −0.24 was obtained, with a 95% confidence interval of −4.71 to 4.23 (p = 0.92), and there was significant heterogeneity among the studies (I2 = 54%, p < 0.02).
At 24 h, among the four studies that evaluated platelet count, only one study showed better results for the group that used Cell Salvage. An MD of 0.11 was obtained, with a 95% confidence interval of −8.76 to 8.98 (p = 0.98), and there was significant heterogeneity between studies (I2 = 71%, p < 0.001).
No group differences were found at both 6 h (p = 0.10) and 18 h (p = 0.73). Likewise, there was no group difference at patient discharge (p = 0.24). In these cases, only one study was found.
Finally, no statistically significant differences were found between groups (p-value = 0.29) (Figure 7).
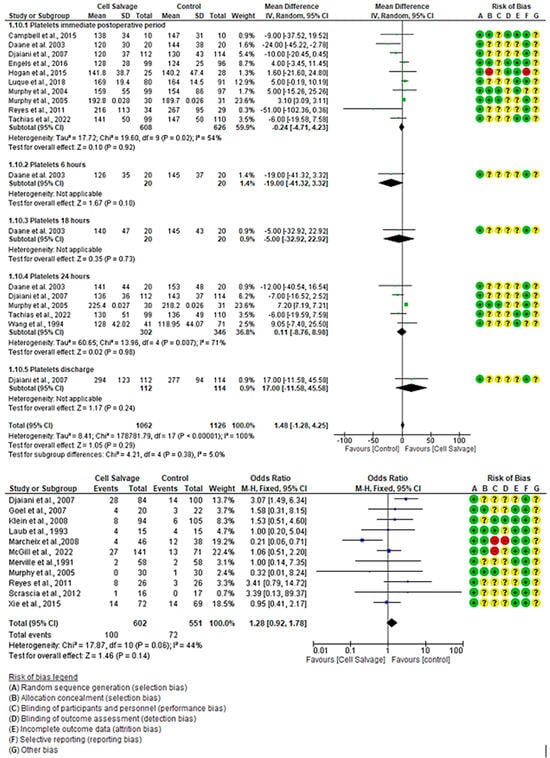
Figure 7.
Forest plot presenting platelet count over time and number of fresh frozen plasma transfusions.
3.4.6. The Efficacy of Cell Salvage on Other Coagulation Factors
Four clinical trials assessed postoperative fibrinogen levels with a total of 362 participants, including 172 in the intervention group and 189 in the control group, comparing the efficacy of Cell Salvage use. Four studies demonstrated a high risk of bias [39,41,59], while the remaining study showed a low risk of bias [55]. In the immediate postoperative period, one study showed better levels in the group that employed Cell Salvage and another in the control group. The remaining two studies did not show a statistically significant association, with the diamond touching the no-effect line. An MD of 0.11 was obtained, with a 95% confidence interval of −0.07 to 0.29 (p = 0.23), and there was high heterogeneity among the studies (I2 = 96%, p < 0.001).
At 24 h, among the three studies assessing fibrinogen levels, one study showed better levels for the control group, while the other two invaded the no-effect line. An MD of −0.01 was obtained, with a 95% confidence interval of −0.20 to 0.19 (p = 0.96), and significant heterogeneity among the studies was observed (I2 = 63%, p = 0.07).
At 6 and 18 h, only one study assessed fibrinogen, showing no better values between groups. Lastly, no statistically significant differences were found between groups (p-value = 0.67) (Figure S4).
Regarding D-dimer, it was evaluated in a total of three studies, involving 410 participants (205 in the Cell Salvage group and 205 in the control group). Two studies showed a high risk of bias [39,41], while the third study demonstrated a low risk of bias [54]. In the immediate postoperative period, two studies showed better results for Cell Salvage, and the third did not show a statistically significant association, with the diamond touching the no-effect line. An MD of −0.38 was obtained, with a 95% confidence interval of −0.73 to −0.02 (p = 0.04), and there was significant heterogeneity among the studies (I2 = 52%, p = 0.12). At 6 h, 18 h, and 24 h, only one study assessed D-dimer, showing a trend of better values for the control group. Lastly, no statistically significant differences were found between groups (p-value = 0.60) (Figure S5).
Finally, for the INR, the two identified studies included a total of 1096 participants, with 534 in the Cell Salvage group and 562 in the control group, showing a high risk of bias [43,59]. In the immediate postoperative period, both studies showed better INR levels for the control group. An MD of 0.06 was obtained, with a 95% confidence interval of 0.03 to 0.10 (p < 0.001), with high heterogeneity among the studies (I2 = 75%, p = 0.05). At 24 h, the same pattern as in the immediate postoperative period was observed, showing better results in the control group. The MD was 0.04, with a 95% confidence interval of 0.02 to 0.07 (p < 0.001), with no heterogeneity among the studies (I2 = 0%, p = 0.73). At discharge, only one study assessed the INR, obtaining better results for Cell Salvage. Ultimately, no statistically significant differences were observed (p < 0.35) (Figure S6).
4. Discussion
The results show that Cell Salvage does not present statistically significant differences in relation to blood loss in the postoperative period. In fact, during the first 6 h, 12 h, and 24 h after surgery, they present similar losses. However, in the red series, a greater effectiveness of Cell Salvage was observed in terms of the increase in hemoglobin and hematocrit in the patient, above the normal range. This is significant because, given the dynamics of fluids, with blood being a non-Newtonian fluid, the increase in hematocrit figures allows the viscosity to increase in turn. Taking this increase as a reference, it has a proportional effect on a greater adherence in the circulatory stream, with the negative consequences that derive from it.
Furthermore, the results show that, between the groups (control/experimental), as well as their divergence in analytical values, not only did the hematocrit and hemoglobin increase, but there were significant differences in the PT, D-dimer, total PT, and aPTT values post-surgery. This suggests that, although Cell Salvage does not directly affect hemorrhage, it does modify other blood parameters, especially those affecting coagulation. Similar results were obtained for platelets, FFP, and fibrinogen, where there was also a significant statistical difference between the two groups, although it is true that the results were better for the control group in the INR measurement.
These facts present us with a turning point on the operability of the electronic device, especially knowing when it should be used and what consequences are associated with its use. For this reason, it is necessary to discuss in detail each of the most striking and necessary results for the knowledge of the scientific community.
4.1. Cell Salvage-Associated Hemorrhage and Red Series Effectiveness
Bleeding, with its different locations and ranges of severity, is a relatively frequent cause in this type of extracorporeal surgery; however, it should also be noted, from the point of view of the use of the cell saver, that the results obtained did not reveal statistically significant differences, nor was there even a firm and clear tendency to obtain benefits with the use of this device. In contrast, a previous meta-analysis found that people who had undergone cardiac surgery with this device had a 31% decrease in the risk of requiring a CBR blood transfusion [29]. However, the authors report a large heterogeneity in the clinical trials included in their study, as is the case in the present meta-analysis. This situation undermines the possibility to extrapolate the results and to determine the exact effectiveness that the use of Cell Salvage may have in the control or prevention of bleeding.
In contrast, other cohort studies by Vonk et al. found that the mean number of transfusions in control patients was higher when compared to Cell Salvage patients (control: mean 2, 95% CI: 1–5 vs. Cell Salvage: mean 1, 95% CI: 0–3; p-value < 0.001). Similarly, they assessed postoperative blood loss. The authors concluded that these losses were lower in the Cell Salvage group compared to control patients at 6, 12, and 24 h postoperatively [64]. These results are in line with Bauer et al. at 6 h and Niranjan et al. at 24 h but do not agree with the other authors included in this review [38,43,45,54,55,57,59,60,63]. These differences may stem from a lack of consensus in the protocols, variations in the perfusionist’s expertise, or differences in the comorbidities of the patients included.
Another associated alteration is the irreversible modification of the structure of the blood components due to their interaction with the biomaterials that make up the shunt circuit, which may alter their function [65]. This does not suggest that technological advances will be able to remedy these alterations; however, if they are not published in the scientific evidence, it will be difficult to contribute to the development of new devices to alleviate this deficit.
4.2. Hemoglobin and Hematocrit Levels after Use of Cell Salvage
The American Heart Association (AHA) [66] states that hemoglobin levels of 12 g/dL and hematocrit of 28% are considered adequate in cardiac surgery, with other studies [67] supporting this position. In our meta-analysis, only two studies met the hemoglobin criteria, one being in the immediate postoperative period [49] and the other trial at 24 h [61].
Regarding hematocrit, all the patients in the Cell Salvage group had percentages above 28%. However, if we take into account the criteria where hemoglobin less than 9 g/dL and hematocrit less than 27% are considered strong candidates for transfusion of blood or blood products [68], we would only have one study in the immediate postoperative period in the Cell Salvage group that would require an additional allogeneic transfusion [54] compared to the three studies requiring such transfusion in the control group [41,44,54]. This is in agreement with the results obtained by Vonk et al., (2015), where the Cell Salvage group had a slightly higher hemoglobin level at discharge (6.9 ± 0.7 mmol/L vs. 6.7 ± 0.7 mmol/L; p < 0.05) than those in the control group [64]. This is refuted by the more recent trial conducted by Tachias et al. in 2022, where it was observed that the mean postoperative hemoglobin concentrations at 24 h and their postoperative values were in favor of patients in the Cell Salvage group [59]. The authors explain the results by noting the relatively low concentration, with a hematocrit level close to 42%, compared to the hematocrit > 50% indicated in the manufacturer’s specifications. Specifically, the type of device used at the time of the surgery in question (as there are different electronic devices on the market), as well as the operability according to the practitioner using it, produced a hemoglobin concentration effect of 150% [69]. This fact, combined with a shorter lifespan of the recovered red blood cells [47,70], could have contributed reciprocally to the comparable red blood cell transfusions between the different groups.
This observation is not novel, as several studies in the last decade [58,71,72], as well as one of the most recent meta-analyses, have shown that cell recovery did not influence the number of red blood cells transfused. They also suggested a trend, although not statistically significant, towards greater use of fresh frozen plasma (FFP) and platelets [29,72]. In contrast, another meta-analysis from 2018 found that cell salvage reduces both the percentage of patients transfused during the perioperative period and the volume of allogeneic blood products administered [20].
4.3. Effectiveness of Cell Salvage on Clotting Times
One of the most controversial points is the coagulation parameters and the use of Cell Salvage, given the variability of results across studies. In 2022, Tachias et al. reported low platelet and fibrinogen concentrations and an undetectable INR > 10 and aPTT (>180 s). A decrease in the platelet count at ICU admission was observed in both groups, in contrast to other techniques as found by Boyle et al. with ultrafiltration using the HemoSep© device [39] or by authors who found an increased platelet content and preserved platelet functionality as assessed by thromboelastography [73].
Similarly, Rubens et al. performed a cardiotomy trial that revealed prolongation of the INR and thrombin time and reductions in fibrinogen levels for at least 12 h postoperatively [74], while other authors described a consumption coagulopathy in the Cell Salvage group [58] and a significant decrease in several coagulation factors (I, II, VII, XI, XIII) in the Cell Salvage concentrate [75].
Therefore, due to activated coagulation combined with accelerated fibrinolysis and postoperative bleeding in their Cell Salvage group, some investigators called for a more cautious use of Cell Salvage techniques in patients at high risk of perioperative bleeding [58], while others indicated higher costs in Cell Salvage [60] and recommended avoiding re-transfusion volumes higher than 1 L [76], despite the recommendation of widespread use by several scientific societies [76,77,78,79,80]. In fact, scientific evidence already exists, such as the study by Luque, who specified the red blood cell reinfusion threshold with the Cell Salvage device based on a specific volume of blood in the reservoir to be processed, which has greater or lesser repercussions for the patient, as well as the relationship between the processing time and obtaining the reinfusion volume [49].
From a critical perspective, the impact of Cell Salvage on coagulation times highlights key clinical challenges. The variability in study outcomes underscores the need for standardized protocols, as some research indicates significant alterations in coagulation parameters, such as a prolonged INR and reduced fibrinogen levels, while others do not. This inconsistency may be influenced by differences in devices and patient characteristics, particularly in those at high risk of perioperative bleeding.
Although Cell Salvage can reduce the need for allogeneic transfusions, the potential for coagulation disturbances poses a risk, especially for vulnerable patients. A cautious, individualized approach is essential, balancing the hematological risks with potential benefits. Technological improvements should focus not only on efficiency but also on minimizing adverse effects on coagulation. Studies such as Luque’s offer a path toward more evidence-based, tailored thresholds for reinfusion, which could enhance clinical outcomes.
On the other hand, our results are in line with the meta-analysis by Al Khabori M et al., where no increase in the rate of platelet transfusion or FFP was found [29]. However, the same authors in a previous cohort study observed an increased risk of FFP transfusion and increased platelet transfusion rate. The latter would be in line with the hypothesis of hemostasis triggered either by profuse bleeding or by extracorporeal reinfusion of large amounts of blood [72]. One of the factors that may influence this lack of consensus in the studies carried out seems to be caused by the process of massive heparinization with subsequent administration of protamine to which cardiac surgery patients are subjected. Both drugs are associated with significant alterations in platelet function and fibrinolysis. It should be noted that, in this type of surgery under an extracorporeal circuit, the pulmonary or minor circulation is no longer active, as the circuit itself acts as an exchanger of the blood–gas barrier. As a result, the inactivity of the lungs during the time of connection to the extracorporeal machine means that the lungs act as a reservoir, allowing part of the heparin to lodge, and once it has left the extracorporeal circuit, and after the administration of protamine (heparin antagonist), the heparin returns to the bloodstream, which may cause alterations in platelet function [81,82].
Another factor to consider is the type of electronic device that each hospital has and, more specifically, the professional who uses it. These devices can operate continuously or discontinuously, with a high margin of variability in terms of the revolutions per minute in the centrifugation chamber, the crystalloid used with its corresponding heparin to prevent clotting of the aspirated blood, the vacuum pressure activated, and even the moment when the recovered and processed red blood cells are reinfused. These differences between the devices are so significant that there are already studies that address them in part, such as the study by Wang et al. (2012), where they evaluated three types of Cell Salvage (Cell Saver 5+, Haemonetics; autolog, Medtronic; and CATS Fresenius HemoCare). The authors concluded that the function of washed red cells and the efficacy in removing waste products differed widely from one device to another (p-value = 0.021 for hematocrit and p-value = 0.008 for hemoglobin). In our meta-analysis, six different types of brand names were identified, with C.A.T.S., Fresenius Hemocare GmbH©, Bad Homberg, Germany (n = 7), and Haemonetics, Braintree, MA, USA (n = 5) being the most represented [62]. In addition, each of their own characteristics must be taken into account, which has a direct impact on the quality of the red blood cells obtained.
4.4. Gender Differences in Cell Salvage Effectiveness
Despite the heterogeneity of the product, it is important to note that no studies have been identified that address gender differences in the effectiveness of cell salvage, although several studies have found that women are at greater risk of bleeding and transfusion. These notable differences may be due to the disparity in the size of blood vessels, cardiovascular system organs, or anatomical disposition, as their smaller size (given their femininity) has a direct impact on the choice of tubing for the extracorporeal circuit and thus directly affects fluid dynamics. It must be taken into account that the larger the diameter of the tubing chosen to connect the patient to the extracorporeal circuit is, the higher the fluid pressure, the higher the viscosity of the blood, the lower the adherence, and the lower the velocity. In addition, the smaller the diameter is, the lower the pressure and the greater the resistance, speed, and adherence. All this makes it necessary to calculate the theoretical priming volume of the machine, as well as the hemodilutional volume. For this reason, the patient’s cardiac output and body surface area index are always taken into account.
To this must be added the fact that women have a different blood volume due to their different body size, as well as the losses that occur monthly with menstruation, which leads to unequal hemoglobin levels, increasing the risk of anemia in women. These aspects have not been taken into consideration in any of the studies included, so we believe that they should have analyzed the results separately, setting specific reference values for women and men. In addition, there are other factors such as hormonal fluctuations in the different stages of the menstrual cycle or other reproductive stages such as pregnancy or menopause that have also not been considered [83,84,85,86].
4.5. Limitations and Strengths
The results of this meta-analysis should be interpreted with some caution. The sample size of most of the studies included in the analyses was small. The mean number of patients was 120 subjects ± 84 (95% CI 145 to 76; Max: 352 Min: 20). Studies have shown that the results of meta-analyses involving trials with small numbers of patients may lead to confounding bias compared to trials with a large sample size [87,88].
In addition, the heterogeneity found in some of the analyses makes it difficult to interpret the results. There was no obvious cause for the heterogeneity, but it is possible that it reflects the lack of working protocols on the use of cellular rescue, especially with regard to the type of device chosen and how it is used. In addition, because it is difficult to blind the intervention, practitioners’ behavior could have been driven by knowledge of group allocation, especially when transfusion guidelines were not established. The decrease in the proportion of patients receiving allogeneic transfusion may be related to the intrinsic efficacy of Cell Salvage or to clinician transfusion behavior, especially when transfusion guidelines have not been established. Because most studies do not state their transfusion protocols, nor do they indicate how rigorously these protocols were followed, it is possible that there was a difference in the indications for the use of Cell Salvage, which could have favored the Cell Salvage group.
Another very important limitation concerns the use of the Cell Salvage. As the authors do not state how the device is used (continuous or discontinuous), their results have serious interpretation biases. The variability in the way the cell saver is used leads to different results. There are already studies that deal with this, as well as the different retriever devices, which have different characteristics and thus bias the results of the studies. Therefore, it is crucial to develop and adhere to standardized protocols for the use of Cell Salvage and transfusion practices. This includes clear guidelines on when and how to apply Cell Salvage techniques, which can minimize variability in the results due to different practices among clinicians.
Regarding recommendations for future cellular retriever research, we recommend that implementing strategies to blind intervention assignments whenever possible could help mitigate bias introduced by the practitioners’ knowledge of group assignments. Improved randomization techniques could also enhance the validity of the results. In addition, future studies should require the inclusion of detailed transfusion protocols to facilitate the comparison and synthesis of results across studies. This would help identify whether variations in protocols contribute to differences in outcomes.
Similarly, conducting longitudinal studies could provide information on the long-term effects of Cell Salvage and transfusion practices, thereby enriching the understanding of their efficacy over time. Therefore, it is recommended that patients be monitored when they are transferred to the hospital ward or post-surgical recovery unit.
On the other hand, including diverse patient populations in studies may help generalize the findings and assess the effects of biological sex and other demographic factors more accurately.
The strengths of this meta-analysis include the clear inclusion and exclusion criteria, a comprehensive literature search, and large subgroup analyses. In addition, all studies identified in languages other than English (German, French, Spanish, Italian, Danish, and Norwegian) were included because the exclusion of articles for linguistic reasons was found to affect the results of meta-analyses [89,90]. In addition, the biological sex perspective was assessed according to the new international recommendations, and a large gap was observed between men and women. Furthermore, above all, this research contributes to a growing body of knowledge with a disparity of results, which makes this meta-analysis even more important for the scientific community.
4.6. Clinical Prospective
The need to unify protocols to determine the effectiveness of Cell Salvage to improve biochemical patterns is evident, especially to elucidate those points where there is great controversy such as platelet consumption and coagulation times. These devices present different options for use (continuous/discontinuous; with higher/lower processing revolution; with higher/lower volume of crystalloid mixing, etc.) given their broad portfolio of operational characteristics. Therefore, the volume of blood processed and the volume obtained from the flow recuperator depend on the perfusionist’s knowledge and experience. From this, their performance is derived, and thus, the re-infusion of high-quality red blood cells in turn allows for a higher consumption of the patient’s reserve of platelets and clotting factors. This leads to significant differences in analytical values and, hence, the possible consumption of clotting factors, plasma, or platelets. The use of blood spilled during surgery or from CPB machines can activate hemostatic pathways and lead to low-grade disseminated intravascular coagulopathy in patients receiving this blood, especially if it is received in large volumes [29].
It would be interesting to have the option of manufacturing a device that obtains other components such as platelets, plasma, or coagulation factors from the patient themself, thus saving indirect and direct bench costs. On the other hand, most studies do not indicate the type of priming machine used by perfusionists, which directly influences the hemodynamics of the patient and indirectly influences the use of Cell Salvage in terms of hemoglobin and hematocrit figures.
On the other hand, it would be of interest for studies to homogeneously determine erythrocytes, strain rate, hematocrit viscosity, 2,3 diphosphoglycerate, hematocrit, hemoglobin, free hemoglobin clearance, glucose, lactate, and urea nitrogen, among others. In addition, international measures should be used to express the results. Similarly, patient comorbidities should be identified in order to make more accurate comparisons. Additionally, a protocol for common use by perfusionists should be established, which includes the choice of Cell Salvage.
5. Conclusions
The use of the Cell Salvage system has great uncertainty in the improvement of biochemical parameters, primarily clotting times. The results do not provide definitive evidence regarding its efficacy in the control of bleeding, platelet count, fresh frozen plasma, and fibrinogen. Therefore, it is recommended to increase the number of studies to assess the impact of the Cell Salvage system on improvements in patients’ red blood cell counts and coagulation patterns. Furthermore, protocols should be standardized, and variables such as the sex of the participants, type of device used, and time of reinfusion of the red blood cells obtained should be taken into account, as well as standardizing the protocols of action by those in charge of these devices, in particular perfusionists. For all these reasons, we can affirm that caution should be exercised today in its indiscriminate use until its benefits (with all the aforementioned items) with respect to allogeneic transfusion can be confirmed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13206073/s1, Table S1: Grades of recommendations; Figure S1: Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies. Red = high risk; Green = low risk; Yellow/? = unclear risk; +/− = risk percentage. Figure S2: Risk of bias summary: review authors’ judgements about each risk of bias item for each included study. Red = high risk; Green = low risk; Yellow/? = unclear risk; +/− = risk percentage. Figure S3: Analysis of publication bias by means of a funnel plot; Figure S4: Forest plot presenting fibrinogen over time; Figure S5: Forest plot presenting D-dimer over time; Figure S6: Forest plot presenting INR over time.
Author Contributions
Conceptualization, R.C.-M., M.L.-O. and M.P.-C.; methodology, R.C.-M. and M.P.-C.; software R.C.-M. and M.P.-C.; formal analysis, R.C.-M. and M.P.-C.; investigation, R.C.-M. and M.P.-C.; data curation, R.C.-M. and M.P.-C.; writing—original draft preparation, R.C.-M., M.L.-O. and M.P.-C.; writing—review and editing, R.C.-M., M.L.-O. and M.P.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on requirement.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- NICE. NICE Recommends New Tests for Bleeding Problems during and after Cardiac Surgery. 2014. Available online: https://www.nice.org.uk/guidance/dg13/chapter/1-recommendations (accessed on 26 July 2024).
- Geissler, R.G.; Rotering, H.; Buddendick, H.; Franz, D.; Bunzemeier, H.; Roeder, N.; Kwiecien, R.; Sibrowski, W.; Scheld, H.H.; Martens, S.; et al. Utilisation of blood components in cardiac surgery: A single-centre retrospective analysis with regard to diagnosis-related procedures. Transfus. Med. Hemother. 2015, 42, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Stoicea, N.; Bergese, S.D.; Ackermann, W.; Moran, K.R.; Hamilton, C.; Joseph, N.; Steiner, N.; Barnett, C.J.; Smith, S.; Ellis, T.J. Current status of blood transfusion and antifibrinolytic therapy in orthopedic surgeries. Front. Surg. 2015, 12, 3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McKay, C. Perfusion approaches to blood conservation. Semin. Cardiothorac. Vasc. Anesth. 2007, 11, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Bainbridge, D.; Martin, J.; Cheng, D. The efficacy of an intraoperative cell saver during cardiac surgery: A meta-analysis of randomized trials. Anesth. Analg. 2009, 109, 320–330. [Google Scholar] [CrossRef]
- Stover, P.E.; Siegel, L.C.; Parks, R.; Levin, J.; Body, S.C.; Maddi, R.; D’Ambra, M.N.; Mangano, D.T.; Spiess, B.D. Variability in transfusion practice for coronary artery bypass surgery persists despite national consensus guidelines: A 24-institution study. Institutions of the Multicenter Study of Perioperative Ischemia Research Group. Anesthesiology 1998, 88, 327–333. [Google Scholar] [CrossRef]
- Wells, A.W.; Mounter, P.J.; Chapman, C.E.; Stainsby, D.; Wallis, J.P. Where does blood go? Prospective observational study of red cell transfusion in north England. BMJ 2002, 325, 803. [Google Scholar] [CrossRef]
- Pati, I.; Velati, C.; Mengoli, C.; Franchini, M.; Masiello, F.; Marano, G.; Veropalumbo, E.; Vaglio, S.; Piccinini, V.; Pupella, S.; et al. A forecasting model to estimate the drop in blood supplies during the SARS-CoV-2 pandemic in Italy. Transfus. Med. 2021, 31, 200–205. [Google Scholar] [CrossRef]
- Robich, M.P.; Koch, C.G.; Johnston, D.R.; Schiltz, N.; Pillai, A.C.; Hussain, S.T.; Soltesz, E.G. Trends in blood utilization in United States cardiac surgical patients. Transfusion 2015, 55, 805–814. [Google Scholar] [CrossRef]
- Goodnough, L.T.; Shafron, D.; Marcus, R.E. The impact of preoperative autologous blood donation on orthopaedic surgical practice. Vox Sang. 1990, 59, 65–69. [Google Scholar] [CrossRef]
- Toy, P.T.; Kaplan, E.B.; McVay, P.A.; Lee, S.J.; Strauss, R.G.; Stehling, L.C. Blood loss and replacement in total hip arthroplasty: A multicenter study. The Preoperative Autologous Blood Donation Study Group. Transfusion 1992, 32, 63–67. [Google Scholar] [CrossRef]
- Stehling, L.; Zauder, H.L. Acute normovolemic hemodilution. Transfusion 1991, 31, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Scott, W.J.; Kessler, R.; Wernly, J.A. Blood conservation in cardiac surgery. Ann. Thorac. Surg. 1990, 50, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Rubens, F.; Wells, P. Tranexamic acid use during coronary artery bypass grafting. Ann. Thorac. Surg. 1996, 61, 774–775. [Google Scholar] [CrossRef] [PubMed]
- Goodnough, L.T. Clinical application of recombinant erythropoietin in the perioperative period. Hematol. Oncol. Clin. N. Am. 1994, 8, 1011–1020. [Google Scholar] [CrossRef]
- Brainard, D.M.D. Amputation of the thigh for disease of the knee joint: Transfusion of blood. Chic. Med. J. 1860, 18, 116–117. [Google Scholar]
- Klein, A.; Agarwal, S.; Cholley, B.; Fassl, J.; Griffin, M.; Kaakinen, T.; Mzallassi, Z.; Paulus, P.; Rex, S.; Siegemund, M.; et al. A survey of patient blood management for patients undergoing cardiac surgery in nine European countries. J. Clin. Anesth. 2021, 72, 110311. [Google Scholar] [CrossRef]
- Schulz, K.F.; Chalmers, I.; Hayes, R.J.; Altman, D.G. Empirical evidence of bias: Dimensions of methodological quality associated with estimates of treatment effect in controlled trials. JAMA 1995, 273, 408–412. [Google Scholar] [CrossRef]
- Van Klarenbosch, J.; Van den Heuvel, E.R.; Van Oeveren, W.; De Vries, A.J. Does Intraoperative Cell Salvage Reduce Postoperative Infection Rates in Cardiac Surgery? J. Cardiothorac. Vasc. Anesth. 2020, 34, 1457–1463. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, W.; Fang, W.; Meng, G.; Zhang, L.; Zhou, Y.; Gu, E.; Liu, X. Safety, efficacy, and cost-effectiveness of intraoperative blood salvage in OPCABG with different amount of bleeding: A single-center, retrospective study. J. Cardiothorac. Surg. 2018, 13, 109. [Google Scholar] [CrossRef]
- Yao, Y.; Yuan, X.; He, L.; Yu, Y.; Du, Y.; Liu, G.; Tian, L.; Ma, Z.; Zhang, Y.; Ma, J. Patient Blood Management: Single Center Evidence and Practice at Fuwai Hospital. Chin. Med. Sci. J. 2022, 37, 246–260. [Google Scholar] [CrossRef]
- Koçyiğit, M.; Koçyiğit, Ö.I.; Güllü, A.Ü.; Şenay, Ş.; Alhan, C. Postoperative Atrial Fibrillation Reduced by Intraoperative and Postoperative Cell Saver System in Coronary Artery Bypass Graft Surgery. Turk. J. Anaesthesiol. Reanim. 2022, 50, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Spence, R.K.; Erhard, J. History of patient blood management. Best. Pract. Res. Clin. Anaesthesiol. 2013, 27, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Faught, C.; Wells, P.; Fergusson, D.; Laupacis, A. Adverse effects of methods for minimizing perioperative allogeneic transfusion: A critical review of the literature. Transfus. Med. Rev. 1998, 12, 206–225. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.A.; Silva, J.P.; Silva Lda, F.; Sousa, A.G.; Piotto, R.F.; Baumgratz, J.F. Therapeutic options to minimize allogeneic blood transfusions and their adverse effects in cardiac surgery: A systematic review. Rev. Bras. Cir. Cardiovasc. 2014, 29, 606–621. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gupta, S.; McEwen, C.; Basha, A.; Panchal, P.; Eqbal, A.; Wu, N.; Belley-Cote, E.P.; Whitlock, R. Retrograde autologous priming in cardiac surgery: A systematic review and meta-analysis. Eur. J. Cardiothorac. Surg. 2021, 60, 1245–1256. [Google Scholar] [CrossRef]
- Garg, P.; Malhotra, A.; Desai, M.; Sharma, P.; Bishnoi, A.K.; Tripathi, P.; Rodricks, D.; Pandya, H. Pretransfusion Comparison of Dialyser-Based Hemoconcentrator With Cell Saver System for Perioperative Cell Salvage. Innovations 2015, 10, 334–341. [Google Scholar] [CrossRef]
- Stoneham, M.D.; Barbosa, A.; Maher, K.; Douglass, P.; Desborough, M.J.R.; Von Kier, S. Intraoperative cell salvage using swab wash and serial thromboelastography in elective abdominal aortic aneurysm surgery involving massive blood loss. Br. J. Haematol. 2023, 200, 652–659. [Google Scholar] [CrossRef]
- Al Khabori, M.; Al Riyami, A.; Siddiqi, M.S.; Sarfaraz, Z.K.; Ziadinov, E.; Al Sabti, H. Impact of cell saver during cardiac surgery on blood transfusion requirements: A systematic review and meta-analysis. Vox Sang. 2019, 114, 553–565. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Estarli, M.; Martínez-Rodríguez, R.; Baladia, E.; Camacho, S.; Buhring, K.; Herrero-López, A. Reference items for publishing protocols of systematic reviews and meta-analyses: PRISMA-P 2015 statement. Rev. Esp. Nutr. Hum. Diet. 2016, 20, 148. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; The Cochrane Collaboration: London, UK, 2011; Available online: https://training.cochrane.org/handbook/current (accessed on 25 July 2024).
- GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime. 2022. Available online: https://Gradepro.org (accessed on 1 July 2024).
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Hausmann, H.; Schaarschmidt, J.; Scharpenberg, M.; Troitzsch, D.; Johansen, P.; Nygaard, H.; Eberle, T.; Hasenkam, J.M. Shed-blood-separation and cell-saver: An integral Part of MiECC? Shed-blood-separation and its influence on the perioperative inflammatory response during coronary revascularization with minimal invasive extracorporeal circulation systems - a randomized controlled trial. Perfusion 2018, 33, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Boyle, G.; Kuffel, A.; Parmar, K.; Gibson, K.; Smith, M.; Grehan, A.; Hunt, B.J.; Chambers, D.J. A comparison of haemostatic biomarkers during low-risk patients undergoing cardiopulmonary bypass using either conventional centrifugal cell salvage or the HemoSep device. Perfusion 2019, 34, 76–83. [Google Scholar] [CrossRef]
- Campbell, J.; Holland, C.; Richens, D.; Skinner, H. Impact of cell salvage during cardiac surgery on the thrombelastomeric coagulation profile: A pilot study. Perfusion 2012, 27, 221–224. [Google Scholar] [CrossRef]
- Daane, C.R.; Golab, H.D.; Meeder, J.H.; Wijers, M.J.; Bogers, A.J. Processing and transfusion of residual cardiopulmonary bypass volume: Effects on haemostasis, complement activation, postoperative blood loss and transfusion volume. Perfusion 2003, 18, 115–121. [Google Scholar] [CrossRef]
- Damgaard, S.; Steinbrüchel, D.A. Autotransfusion with cell saver for off-pump coronary artery bypass surgery: A randomized trial. Scand. Cardiovasc. J. 2006, 40, 194–198. [Google Scholar] [CrossRef]
- Djaiani, G.; Fedorko, L.; Borger, M.A.; Green, R.; Carroll, J.; Marcon, M.; Karski, J. Continuous-flow cell saver reduces cognitive decline in elderly patients after coronary bypass surgery. Circulation 2007, 116, 1888–1895. [Google Scholar] [CrossRef]
- Engels, G.E.; Van Klarenbosch, J.; Gu, Y.J.; Van Oeveren, W.; de Vries, A.J. Intraoperative cell salvage during cardiac surgery is associated with reduced postoperative lung injury. Interact. Cardiovasc. Thorac. Surg. 2016, 22, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Goel, P.; Pannu, H.; Mohan, D.; Arora, R. Efficacy of cell saver in reducing homologous blood transfusions during OPCAB surgery: A prospective randomized trial. Transfus. Med. 2007, 17, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.; Needham, A.; Ortmann, E.; Bottrill, F.; Collier, T.J.; Besser, M.W.; Klein, A.A. Haemoconcentration of residual cardiopulmonary bypass blood using Hemosep®: A randomised controlled trial. Anaesthesia 2015, 70, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.A.; Nashef, S.A.M.; Sharples, L.; Bottrill, F.; Dyer, M.; Armstrong, J.; Vuylsteke, A. A randomized controlled trial of cell salvage in routine cardiac surgery. Anesth. Analg. 2008, 107, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Laub, G.W.; Dharan, M.; Riebman, J.B.; Chen, C.; Moore, R.; Bailey, B.M.; Fernandez, J.; Adkins, M.S.; Anderson, W.; McGrath, L.B. The impact of intraoperative autotransfusion on cardiac surgery. A prospective randomized double-blind study. Chest 1993, 104, 686–689. [Google Scholar] [CrossRef]
- Luque Oliveros, M.; Domínguez Baños, M.A.; Gutiérrez Plata, M. Autologous blood for reinfusion using a cell saver in cardiac patients in response to blood transfusions. Cardiocore 2018, 53, 122–127. [Google Scholar] [CrossRef]
- Marcheix, B.; Carrier, M.; Martel, C.; Cossette, M.; Pellerin, M.; Bouchard, D.; Perrault, L.P. Effect of pericardial blood processing on postoperative inflammation and the complement pathways. Ann. Thorac. Surg. 2008, 85, 530–535. [Google Scholar] [CrossRef]
- McGill, N.; O’Shaughnessy, D.; Pickering, R.; Herbertson, M.; Gill, R. Mechanical methods of reducing blood transfusion in cardiac surgery: Randomised controlled trial. BMJ 2002, 324, 1299. [Google Scholar] [CrossRef]
- Mcshane, A.J.; Power, C.; Jackson, J.F.; Murphy, D.F.; Macdonald, A.; Moriarty, D.C.; Otridge, B.W. Autotransfusion: Quality of blood prepared with a red cell processing device. Br. J. Anaesth. 1987, 59, 1035–1039. [Google Scholar] [CrossRef]
- Merville, C.; Charlet, P.; Zerr, C.; Bricard, H. Efficacité respective du Cell Saver et de la récupération du circuit de CEC ultrafiltré en chirurgie cardiaque. Ann. Fr. Anesth. Reanim. 1991, 10, 548–553. [Google Scholar] [CrossRef]
- Murphy, G.J.; Allen, S.M.; Unsworth-White, J.; Lewis, C.T.; Dalrymple-Hay, M.J. Safety and efficacy of perioperative cell salvage and autotransfusion after coronary artery bypass grafting: A randomized trial. Ann. Thorac. Surg. 2004, 77, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Rogers, C.; Lansdowne, W.; Channon, I.; Alwair, H.; Cohen, A.; Caputo, M.; Angelini, G. Safety, efficacy, and cost of intraoperative cell salvage and autotransfusion after off-pump coronary artery bypass surgery: A randomized trial. J. Thorac. Cardiovasc. Surg. 2005, 130, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, G.; Asimakopoulos, G.; Karagounis, A.; Cockerill, G.; Thompson, M.; Chandrasekaran, V. Effects of cell saver autologous blood transfusion on blood loss and homologous blood transfusion requirements in patients undergoing cardiac surgery on- versus off-cardiopulmonary bypass: A randomised trial. Eur. J. Cardiothorac. Surg. 2006, 30, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Reyes, G.; Prieto, M.; Alvarez, P.; Orts, M.; Bustamante, J.; Santos, G.; Sarraj, A.; Planas, A. Cell saving systems do not reduce the need of transfusion in low-risk patients undergoing cardiac surgery. Interact. Cardiovasc. Thorac. Surg. 2011, 12, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Scrascia, G.; Rotunno, C.; Nanna, D.; Rociola, R.; Guida, P.; Rubino, G.; Schinosa, L.d.L.T.; Paparella, D. Pump blood processing, salvage and re-transfusion improves hemoglobin levels after coronary artery bypass grafting, but affects coagulative and fibrinolytic systems. Perfusion 2012, 27, 270–277. [Google Scholar] [CrossRef]
- Tachias, F.; Samara, E.; Petrou, A.; Karakosta, A.; Siminelakis, S.; Apostolakis, E.; Tzimas, P. The Effect of Cell Salvage on Bleeding and Transfusion Needs in Cardiac Surgery. Anesth. Res. Pract. 2022, 2022, 3993452. [Google Scholar] [CrossRef]
- Vermeijden, W.J.; van Klarenbosch, J.; Gu, Y.J.; Mariani, M.A.; Buhre, W.F.; Scheeren, T.W.; Hagenaars, J.A.; Tan, M.E.S.; Haenen, J.S.; Bras, L.; et al. Effects of cell-saving devices and filters on transfusion in cardiac surgery: A multicenter randomized study. Ann. Thorac. Surg. 2015, 99, 26–32. [Google Scholar] [CrossRef]
- Wang, M.J.; Chen, T.L.; Huang, C.H.; Chao, A.; Chu, S.H. Difference in efficacy of the Cell Saver in coronary bypass grafting surgery and cardiac valvular reoperations. J. Formos. Med. Assoc. 1994, 93, 117–121. [Google Scholar]
- Wang, X.; Ji, B.; Zhang, Y.; Zhu, X.; Liu, J.; Long, C.; Zheng, Z. Comparison of the effects of three cell saver devices on erythrocyte function during cardiopulmonary bypass procedure--a pilot study. Artif. Organs 2012, 36, 931–935. [Google Scholar] [CrossRef]
- Xie, Y.; Shen, S.; Zhang, J.; Wang, W.; Zheng, J. The efficacy, safety and cost-effectiveness of intra-operative cell salvage in high-bleeding-risk cardiac surgery with cardiopulmonary bypass: A prospective randomized and controlled trial. Int. J. Med. Sci. 2015, 12, 322–328. [Google Scholar] [CrossRef]
- Vonk, A.B.; Meesters, M.I.; Garnier, R.P.; Romijn, J.W.; van Barneveld, L.J.; Heymans, M.W.; Jansen, E.K.; Boer, C. Intraoperative cell salvage is associated with reduced postoperative blood loss and transfusion requirements in cardiac surgery: A cohort study. Transfusion 2013, 53, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- Huët, C.; Salmi, L.R.; Fergusson, D.; Koopman-van Gemert, A.W.; Rubens, F.; Laupacis, A. A meta-analysis of the effectiveness of cell salvage to minimize perioperative allogeneic blood transfusion in cardiac and orthopedic surgery. International Study of Perioperative Transfusion (ISPOT) Investigators. Anesth. Analg. 1999, 89, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.-P.; Fullerton, H.J.; et al. Heart Disease and Strole Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, 38–360. [Google Scholar] [CrossRef]
- Drouet, N. European transfusion practices:the SANGUIS survey. Cah. Anesthesiol. 1994, 42, 425–428. [Google Scholar] [PubMed]
- Murphy, G.J.; Pike, K.; Rogers, C.A.; Wordsworth, S.; Stokes, E.A.; Angelini, G.D.; Reeves, B.C.; TITRe2 Investigators. Liberal or restrictive transfusion after cardiac surgery. N. Engl. J. Med. 2015, 372, 997–1008, Erratum in N. Engl. J. Med. 2015, 372, 2274. [Google Scholar] [CrossRef]
- Working with the Haemonetics® Cell Saver® 5. Available online: https://manualzz.com/doc/27212870/working-with-the-haemonetics®-cell-saver®-52022 (accessed on 1 July 2024).
- Liao, X.-Y.; Zuo, S.-S.; Meng, W.-T.; Zhang, J.; Huang, Q.; Gou, D.-M. Intraoperative blood salvage may shorten the lifespan of red blood cells within 3 days postoperatively. Medicine 2017, 96, e8143. [Google Scholar] [CrossRef]
- Álvarez Gallesio, J.M.; Bertolino, T.; Méndez, M.M.; David, M.; Tenorio Núñez, O.M.; Borracci, R.A. Recuperación rutinaria de sangre con cell saver durante la cirugía cardíaca electiva. Rev. Argent. Cardiol. 2020, 88, 276–278. [Google Scholar] [CrossRef]
- Al-Riyami, A.Z.; Al-Khabori, M.; Baskaran, B.; Siddiqi, M.; Al-Sabti, H. Intra-operative cell salvage in cardiac surgery may increase platelet transfusion requirements: A cohort study. Vox Sang. 2015, 109, 280–286. [Google Scholar] [CrossRef]
- Gunaydin, S.; Robertson, C.; Budak, A.B.; Gourlay, T. Comparative evaluation of blood salvage techniques in patients undergoing cardiac surgery with cardiopulmonary bypass. Perfusion 2018, 33, 105–109. [Google Scholar] [CrossRef]
- Rubens, F.D.; Boodhwani, M.; Mesana, T.; Wozny, D.; Wells, G.; Nathan, H.J. Cardiotomy Investigators The cardiotomy trial: A randomized, double-blind study to assess the effect of processing of shed blood during cardiopulmonary bypass on transfusion and neurocognitive function. Circulation 2007, 116, I89–I97. [Google Scholar]
- Adam, E.H.; Funke, M.; Zacharowski, K.; Meybohm, P.; Keller, H.; Weber, C.F. Impact of intraoperative cell salvage on blood coagulation factor concentrations in patients undergoing cardiac surgery. Anesth. Analg. 2020, 130, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Terwindt, L.; Karlas, A.; Eberl, S.; Wijnberge, M.; Driessen, A.; Veelo, D.; Geerts, B.; Hollmann, M.; Vlaar, A. Patient blood management in the cardiac surgical setting: An updated overview. Transfus. Apher. Sci. 2019, 58, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Boer, C.; Meesters, M.I.; Milojevic, M.; Benedetto, U.; Bolliger, D.; von Heymann, C.; Jeppsson, A.; Koster, A.; Osnabrugge, R.L.; Ranucci, M.; et al. 2017 EACTS/EACTA guidelines on patient blood management for adult cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2018, 32, 88–120. [Google Scholar] [CrossRef] [PubMed]
- Raphael, J.; Mazer, C.D.; Subramani, S.; Schroeder, A.; Abdalla, M.; Ferreira, R.; Roman, P.E.; Patel, N.; Welsby, I.; Greilich, P.E.; et al. Society of cardiovascular anesthesiologists clinical practice improvement advisory for management of perioperative bleeding and hemostasis in cardiac surgery patients. Anesth. Analg. 2019, 129, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Bolliger, D.; Buser, A.; Erb, J.M. Patient blood management in cardiac surgery. Curr. Anesthesiol. Rep. 2019, 9, 215–222. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, J.; Wang, W.; Zheng, J.; Xie, Y. Impact of intra-operative cell salvage on blood coagulation in high-bleeding-risk patients undergoing cardiac surgery with cardiopulmonary bypass: A prospective randomized and controlled trial. J. Transl. Med. 2016, 14, 228. [Google Scholar] [CrossRef]
- Jobes, D.R.; Aitken, G.L.; Shaffer, G.W. Increased accuracy and precision of heparin and protamine dosing reduces blood loss and transfusion in patients undergoing primary cardiac operations. J. Thorac. Cardiovasc. Surg. 1995, 110, 36–45. [Google Scholar] [CrossRef][Green Version]
- Khuri, S.F.; Valeri, C.R.; Loscalzo, J.; Weinstein, M.J.; Birjiniuk, V.; Healey, N.A.; MacGregor, H.; Doursounian, M.; Zolkewitz, M.A. Heparin causes platelet dysfunction and induces fibrinolysis before cardiopulmonary bypass. Ann. Thorac. Surg. 1995, 60, 1008–1014. [Google Scholar] [CrossRef]
- Koch, C.G.; Weng, Y.-S.; Zhou, S.X.; Savino, J.S.; Mathew, J.P.; Hsu, P.H.; Saidman, L.J.; Mangano, D.T. Prevalence of risk factors, and not gender per se, determines short- and long-term survival after coronary artery bypass surgery. J. Cardiothorac. Vasc. Anesth. 2003, 17, 585–593. [Google Scholar] [CrossRef]
- Ried, M.; Lunz, D.; Kobuch, R.; Rupprecht, L.; Keyser, A.; Hilker, M.; Schmid, C.; Diez, C. Gender’s impact on outcome in coronary surgery with minimized extracorporeal circulation. Clin. Res. Cardiol. 2012, 101, 437–444. [Google Scholar] [CrossRef]
- Othman, H.; Khambatta, S.; Seth, M.; Lalonde, T.A.; Rosman, H.S.; Gurm, H.S.; Mehta, R.H. Differences in sex-related bleeding and outcomes after percutaneous coronary intervention: Insights from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) registry. Am. Heart J. 2014, 168, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Mehran, R.; Grinfeld, L.; Xu, K.; Nikolsky, E.; Brodie, B.R.; Witzenbichler, B.; Kornowski, R.; Dangas, G.D.; Lansky, A.J.; et al. Sex-based differences in bleeding and long term adverse events after percutaneous coronary intervention for acute myocardial infarction: Three year results from the HORIZONS-AMI trial. Catheter. Cardiovasc. Interv. 2015, 85, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Egger, M. Misleading meta-analysis: Lessons from “an effective, safe, simple” intervention that wasn’t. BMJ 1995, 310, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Cappelleri, J.C.; Ioannidis, J.P.; Schmid, C.H.; de Ferranti, S.D.; Aubert, M.; Chalmers, T.C.; Lau, J. Large trials vs meta-analysis of smaller trials: How do their results compare? JAMA 1996, 276, 1332–1338. [Google Scholar] [CrossRef]
- Grégoire, G.; Derderian, F.; Le Lorier, J. Selecting the language of the publications included in a meta-analysis: Is there a tower of Babel bias? J. Clin. Epidemiol. 1995, 48, 159–163. [Google Scholar] [CrossRef]
- Egger, M.; Zellweger-Zähner, T.; Schneider, M.; Junker, C.; Lengeler, C.; Antes, G. Language bias in randomised controlled trials published in English and German. Lancet 1997, 350, 326–329. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).