Intravenous Infusion of Autologous Mesenchymal Stem Cells Expanded in Auto Serum for Chronic Spinal Cord Injury Patients: A Case Series
Abstract
1. Introduction
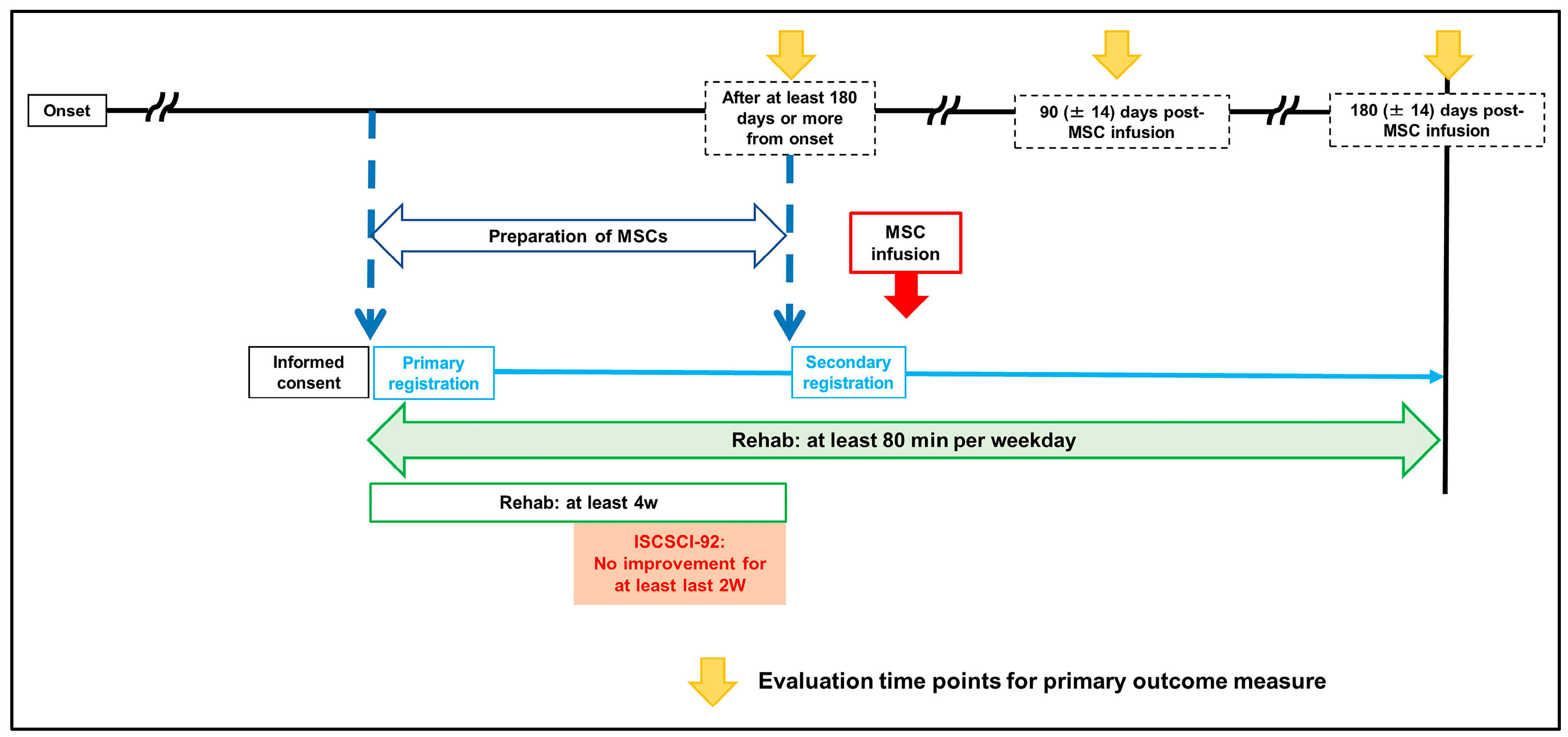
2. Methods
2.1. Patients and Study Design
2.2. Preparation of Autologous Human Mesenchymal Stem Cells (MSCs): STR01
2.3. Study Procedures
2.4. Assessments
2.5. Statistical Analysis
2.6. Ethics
3. Results
3.1. Cell Preparation
3.2. Patient Characteristics
3.3. Case Presentations
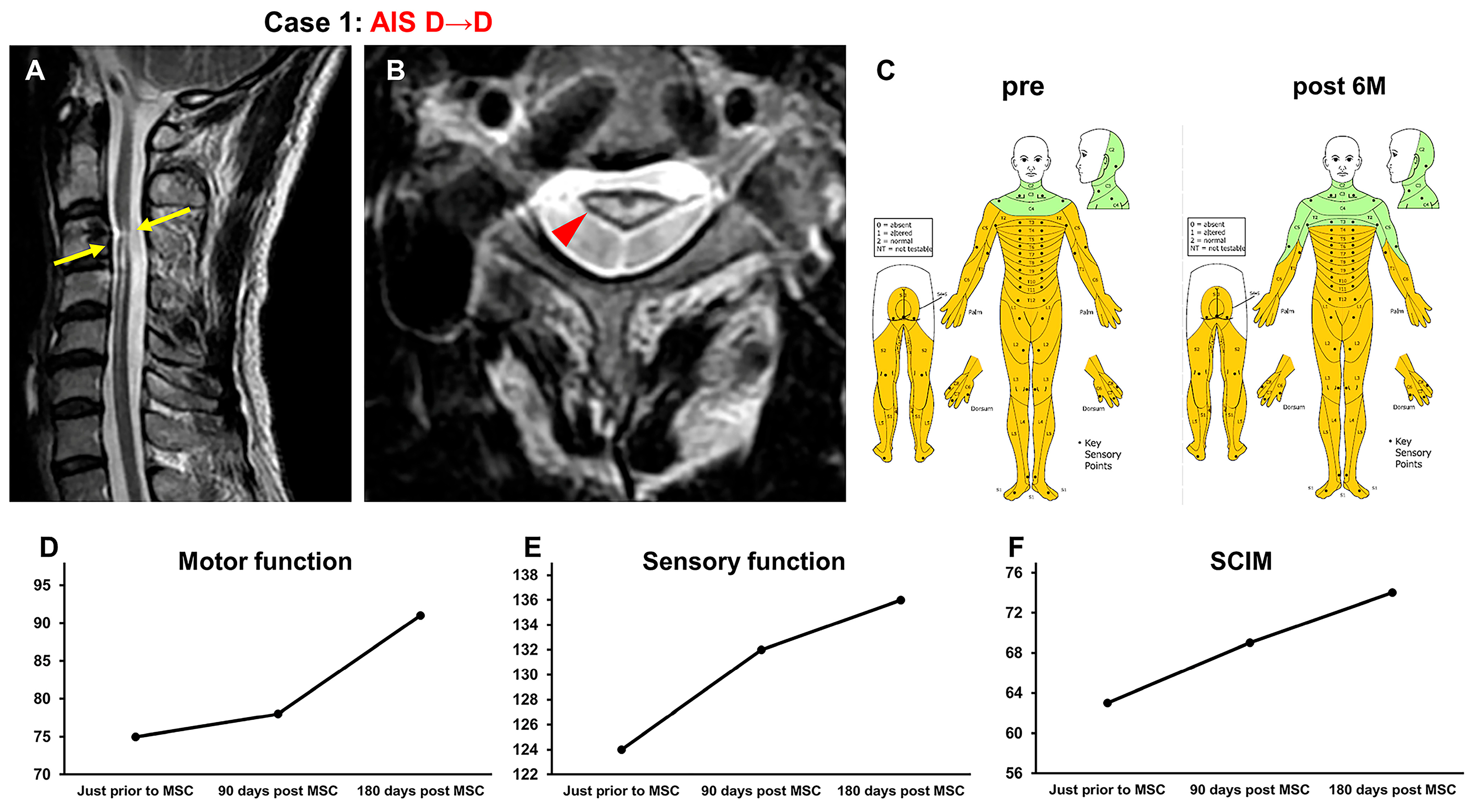
3.3.1. Case 1 (Figure 2)
3.3.2. Case 2 (Figure 3)
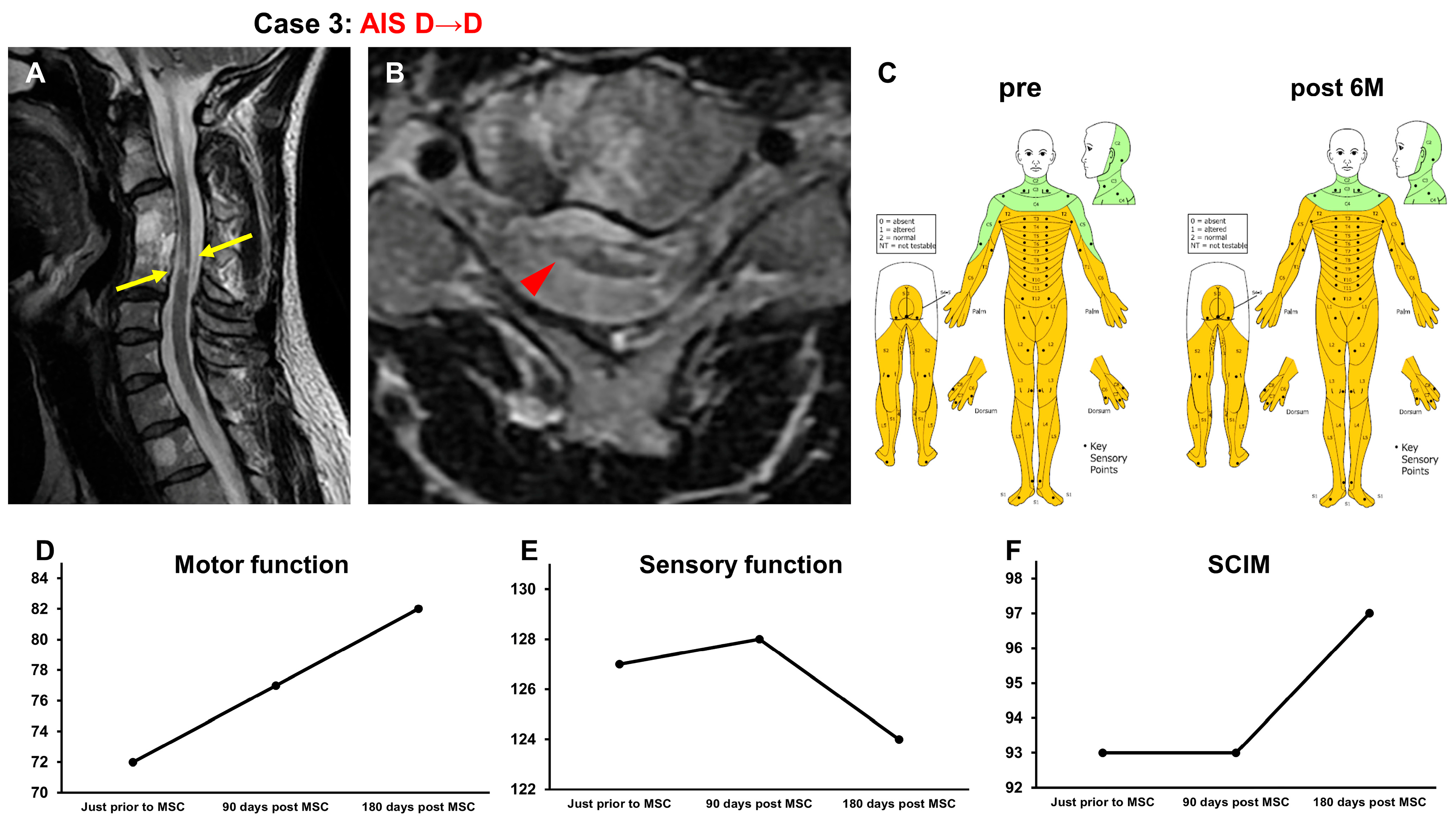
3.3.3. Case 3 (Figure 4)
3.3.4. Case 4 (Figure 5)
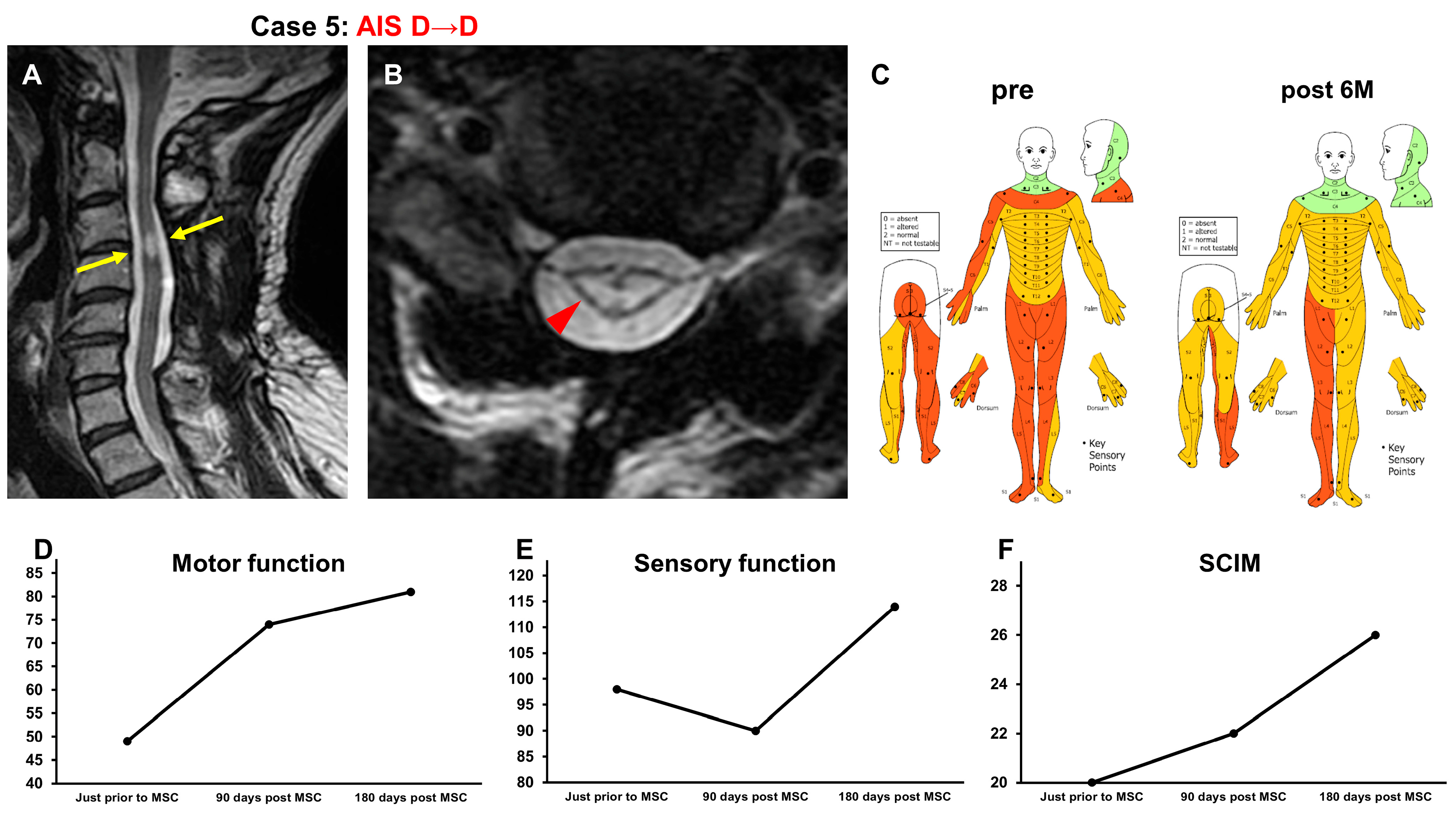
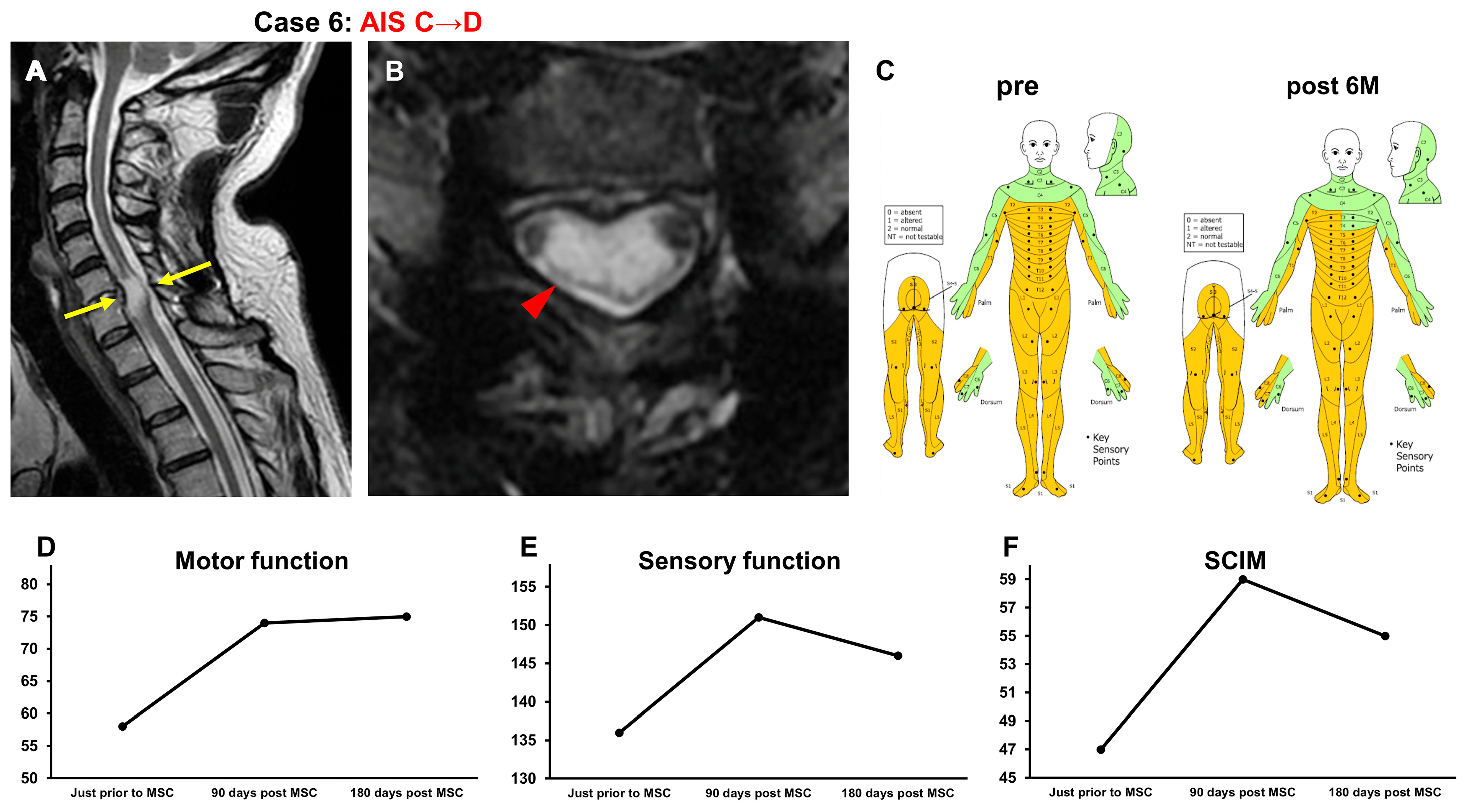
3.3.5. Case 5 (Figure 6)
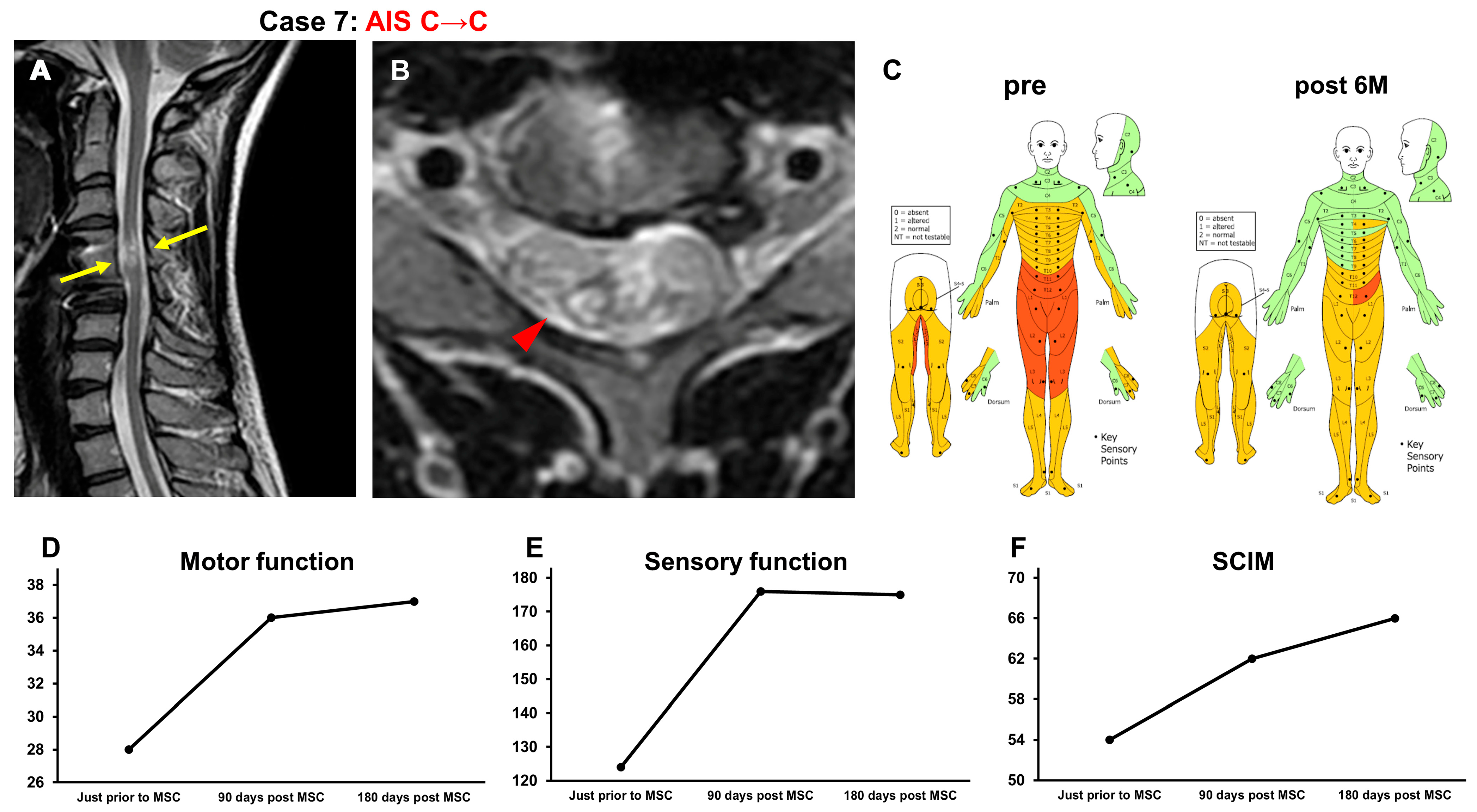
3.3.6. Case 6 (Figure 7)
3.3.7. Case 7 (Figure 8)
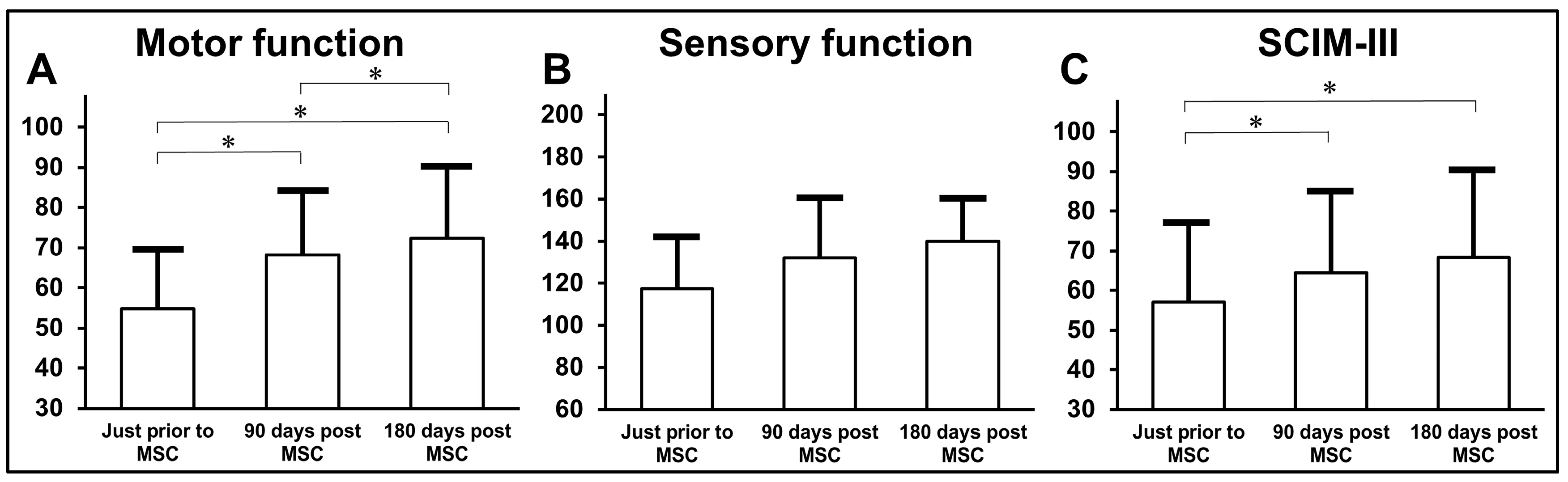
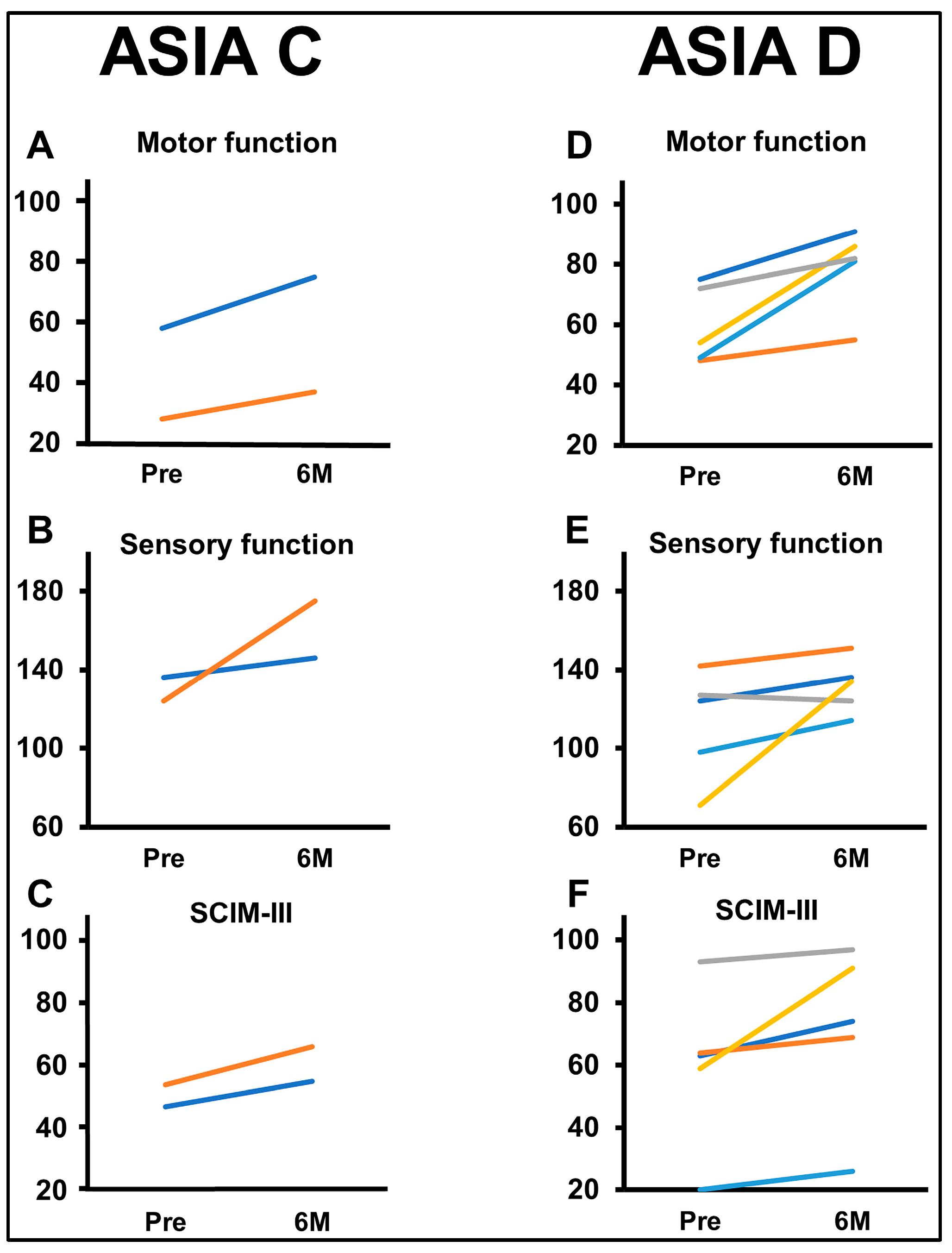
3.4. Clinical Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse events |
| AIS | American Spinal Injury Association Impairment Scale |
| BSCB | Blood–spinal cord barrier |
| ISCSCI | International Standards for Neurological Classification of Spinal Cord Injury |
| MSC | Mesenchymal stem cells |
| PMDA | Pharmaceuticals and Medical Devices Agency |
| QOL | Quality of life |
| SCI | Spinal cord injury |
| SCIM | Spinal Cord Independence Measure |
References
- Kumar, R.; Lim, J.; Mekary, R.A.; Rattani, A.; Dewan, M.C.; Sharif, S.Y.; Osorio-Fonseca, E.; Park, K.B. Traumatic Spinal Injury: Global Epidemiology and Worldwide Volume. World Neurosurg. 2018, 113, e345–e363. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Theadom, A.; Ellenbogen, R.G.; Bannick, M.S.; Montjoy-Venning, W.; Lucchesi, L.R.; Abbasi, N.; Abdulkader, R.; Abraha, H.N.; Adsuar, J.C.; et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef] [PubMed]
- Center NSCIS. Spinal Cord Injury Facts and Figures at a Glance. J. Spinal Cord Med. 2013, 36, 1–2, Updated (2023). [Google Scholar] [CrossRef] [PubMed]
- Levi, A.D.; Anderson, K.D.; Okonkwo, D.O.; Park, P.; Bryce, T.N.; Kurpad, S.N.; Aarabi, B.; Hsieh, J.; Gant, K. Clinical Outcomes from a Multi-Center Study of Human Neural Stem Cell Transplantation in Chronic Cervical Spinal Cord Injury. J. Neurotrauma 2019, 36, 891–902. [Google Scholar] [CrossRef]
- Abolghasemi, R.; Davoudi-Monfared, E.; Allahyari, F.; Farzanegan, G. Systematic Review of Cell Therapy Efficacy in Human Chronic Spinal Cord Injury. Tissue Eng. Part B Rev. 2024, 30, 254–269. [Google Scholar] [CrossRef]
- Morita, T.; Sasaki, M.; Kataoka-Sasaki, Y.; Nakazaki, M.; Nagahama, H.; Oka, S.; Oshigiri, T.; Takebayashi, T.; Yamashita, T.; Kocsis, J.D.; et al. Intravenous infusion of mesenchymal stem cells promotes functional recovery in a model of chronic spinal cord injury. Neuroscience 2016, 335, 221–231. [Google Scholar] [CrossRef]
- Hirota, R.; Sasaki, M.; Kataoka-Sasaki, Y.; Oshigiri, T.; Kurihara, K.; Fukushi, R.; Oka, S.; Ukai, R.; Yoshimoto, M.; Kocsis, J.D.; et al. Enhanced Network in Corticospinal Tracts after Infused Mesenchymal Stem Cells in Spinal Cord Injury. J. Neurotrauma 2022, 39, 1665–1677. [Google Scholar] [CrossRef]
- Oshigiri, T.; Sasaki, T.; Sasaki, M.; Kataoka-Sasaki, Y.; Nakazaki, M.; Oka, S.; Morita, T.; Hirota, R.; Yoshimoto, M.; Yamashita, T.; et al. Intravenous Infusion of Mesenchymal Stem Cells Alters Motor Cortex Gene Expression in a Rat Model of Acute Spinal Cord Injury. J. Neurotrauma 2019, 36, 411–420. [Google Scholar] [CrossRef]
- Honmou, O.; Yamashita, T.; Morita, T.; Oshigiri, T.; Hirota, R.; Iyama, S.; Kato, J.; Sasaki, Y.; Ishiai, S.; Ito, Y.M.; et al. Intravenous infusion of auto serum-expanded autologous mesenchymal stem cells in spinal cord injury patients: 13 case series. Clin. Neurol. Neurosurg. 2021, 203, 106565. [Google Scholar] [CrossRef]
- Piltti, K.M.; Salazar, D.L.; Uchida, N.; Cummings, B.J.; Anderson, A.J. Safety of human neural stem cell transplantation in chronic spinal cord injury. Stem Cells Transl. Med. 2013, 2, 961–974. [Google Scholar] [CrossRef]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Yamaki, T.; Oka, S.; Iyama, S.; Sasaki, M.; Onodera, R.; Kataoka-Sasaki, Y.; Namioka, T.; Namioka, A.; Nakazaki, M.; Takemura, M.; et al. Intravenous infusion of auto-serum-expanded autologous mesenchymal stem cells into chronic severe brain injury patients. Interdiscip. Neurosurg. 2024, 36, 101927. [Google Scholar] [CrossRef]
- Ditunno, J.F., Jr.; Young, W.; Donovan, W.H.; Creasey, G. The international standards booklet for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Paraplegia 1994, 32, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Catz, A.; Itzkovich, M.; Tesio, L.; Biering-Sorensen, F.; Weeks, C.; Laramee, M.T.; Craven, B.C.; Tonack, M.; Hitzig, S.L.; Glaser, E.; et al. A multicenter international study on the Spinal Cord Independence Measure, version III: Rasch psychometric validation. Spinal Cord 2007, 45, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Jeong, S.; Lee, B.S.; Lim, J.C.; Kim, O. Association between functional outcomes and psychological variables in persons with spinal cord injury. Sci. Rep. 2023, 13, 23092. [Google Scholar] [CrossRef]
- Kramer, J.L.; Minhas, N.K.; Jutzeler, C.R.; Erskine, E.L.; Liu, L.J.; Ramer, M.S. Neuropathic pain following traumatic spinal cord injury: Models, measurement, and mechanisms. J. Neurosci. Res. 2017, 95, 1295–1306. [Google Scholar] [CrossRef]
- Beck, K.D.; Nguyen, H.X.; Galvan, M.D.; Salazar, D.L.; Woodruff, T.M.; Anderson, A.J. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: Evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 2010, 133, 433–447. [Google Scholar] [CrossRef]
- Bartanusz, V.; Jezova, D.; Alajajian, B.; Digicaylioglu, M. The blood-spinal cord barrier: Morphology and clinical implications. Ann. Neurol. 2011, 70, 194–206. [Google Scholar] [CrossRef]
- Noble, L.J.; Wrathall, J.R. Distribution and time course of protein extravasation in the rat spinal cord after contusive injury. Brain Res. 1989, 482, 57–66. [Google Scholar] [CrossRef]
- Popovich, P.G.; Horner, P.J.; Mullin, B.B.; Stokes, B.T. A quantitative spatial analysis of the blood-spinal cord barrier. I. Permeability changes after experimental spinal contusion injury. Exp. Neurol. 1996, 142, 258–275. [Google Scholar] [CrossRef]
- Kaptanoglu, E.; Okutan, O.; Akbiyik, F.; Solaroglu, I.; Kilinc, A.; Beskonakli, E. Correlation of injury severity and tissue Evans blue content, lipid peroxidation and clinical evaluation in acute spinal cord injury in rats. J. Clin. Neurosci. 2004, 11, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Figley, S.A.; Khosravi, R.; Legasto, J.M.; Tseng, Y.F.; Fehlings, M.G. Characterization of vascular disruption and blood-spinal cord barrier permeability following traumatic spinal cord injury. J. Neurotrauma 2014, 31, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Keirstead, H.S. Stem cells for the treatment of myelin loss. Trends Neurosci. 2005, 28, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Telegin, G.B.; Chernov, A.S.; Malyavina, E.V.; Minakov, A.N.; Kazakov, V.A.; Rodionov, M.V.; Belogurov, A.A.; Spallone, A. CSF—injected contrast medium enhances post-traumatic spinal cord cysts. An experimental study in rats. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 6132–6139. [Google Scholar] [CrossRef] [PubMed]
- Soderblom, C.; Lee, D.H.; Dawood, A.; Carballosa, M.; Jimena Santamaria, A.; Benavides, F.D.; Jergova, S.; Grumbles, R.M.; Thomas, C.K.; Park, K.K.; et al. 3D Imaging of Axons in Transparent Spinal Cords from Rodents and Nonhuman Primates. eNeuro 2015, 2, 102074. [Google Scholar] [CrossRef]
- Matsumoto, K.; Mitani, T.T.; Horiguchi, S.A.; Kaneshiro, J.; Murakami, T.C.; Mano, T.; Fujishima, H.; Konno, A.; Watanabe, T.M.; Hirai, H.; et al. Advanced CUBIC tissue clearing for whole-organ cell profiling. Nat. Protoc. 2019, 14, 3506–3537. [Google Scholar] [CrossRef]
- De Simone, M.; De Feo, R.; Choucha, A.; Ciaglia, E.; Fezeu, F. Enhancing Sleep Quality: Assessing the Efficacy of a Fixed Combination of Linden, Hawthorn, Vitamin B1, and Melatonin. Med. Sci. 2023, 12, 2. [Google Scholar] [CrossRef]


| Case Number | Total Cell Numbers (Cells) | Concentration of Injected Cells (Cells/mL) | Volume (mL) | CD105 (%) | CD34 (%) | CD45 (%) | Cell Viability (%) | Number of Culture Days (Day) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.36 × 108 | 3.40 × 106 | 40 | 100.0 | 0.2 | 0.7 | 94.7 | 15 |
| 99.9 | 0.1 | 0.7 | 97.8 | |||||
| 2 | 1.74 × 108 | 4.35 × 106 | 40 | 99.7 | 0.0 | 0.2 | 95.7 | 15 |
| 99.9 | 0.0 | 0.0 | 96.8 | |||||
| 3 | 1.00 × 108 | 2.50 × 106 | 40 | 99.6 | 0.1 | 0.0 | 96.4 | 22 |
| 99.8 | 0.0 | 0.2 | 97.1 | |||||
| 4 | 1.22 × 108 | 3.05 × 106 | 40 | 100.0 | 0.0 | 1.2 | 97.9 | 15 |
| 99.9 | 0.0 | 1.4 | 96.7 | |||||
| 5 | 1.88 × 108 | 4.70 × 106 | 40 | 100.0 | 0.0 | 1.2 | 97.9 | 15 |
| 100.0 | 0.3 | 0.9 | 98.5 | |||||
| 6 | 1.90 × 108 | 4.75 × 106 | 40 | 99.9 | 0.1 | 0.4 | 98.4 | 15 |
| 100.0 | 0.1 | 0.4 | 97.7 | |||||
| 7 | 1.30 × 108 | 3.25 × 106 | 40 | 98.0 | 0.0 | 0.0 | 97.0 | 32 |
| 98.0 | 0.0 | 0.0 | 97.0 |
| Case Number | Age (y.o) | Sex | Level of Injury | Time of Injury until MSC Infusion | AIS Grade just before MSC Infusion | AIS Grade 6 Months Post-MSC Infusion | Difference in Motor Function | Difference in SCIM-III | Major Adverse Effects |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 50 | male | C4 | 4.7 y | D | D | 16 | 11 | None |
| 2 | 20 | male | C4 | 1.3 y | D | D | 7 | 5 | None |
| 3 | 48 | male | C5 | 27 y | D | D | 10 | 4 | None |
| 4 | 28 | female | C3 | 2.1 y | D | D | 32 | 32 | None |
| 5 | 49 | male | C4 | 2.9 y | D | D | 32 | 6 | None |
| 6 | 52 | male | C7 | 4.0 y | C | D | 17 | 8 | None |
| 7 | 30 | female | C6 | 18.0 y | C | C | 9 | 12 | None |
| AIS at 180 Days Post-MSC Infusion | |||
|---|---|---|---|
| AIS prior to MSC infusion | AIS C | AIS D | |
| AIS C | 50% (1/2) | 50% (1/2) | |
| AIS D | 0% | 100% (5/5) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirota, R.; Sasaki, M.; Iyama, S.; Kurihara, K.; Fukushi, R.; Obara, H.; Oshigiri, T.; Morita, T.; Nakazaki, M.; Namioka, T.; et al. Intravenous Infusion of Autologous Mesenchymal Stem Cells Expanded in Auto Serum for Chronic Spinal Cord Injury Patients: A Case Series. J. Clin. Med. 2024, 13, 6072. https://doi.org/10.3390/jcm13206072
Hirota R, Sasaki M, Iyama S, Kurihara K, Fukushi R, Obara H, Oshigiri T, Morita T, Nakazaki M, Namioka T, et al. Intravenous Infusion of Autologous Mesenchymal Stem Cells Expanded in Auto Serum for Chronic Spinal Cord Injury Patients: A Case Series. Journal of Clinical Medicine. 2024; 13(20):6072. https://doi.org/10.3390/jcm13206072
Chicago/Turabian StyleHirota, Ryosuke, Masanori Sasaki, Satoshi Iyama, Kota Kurihara, Ryunosuke Fukushi, Hisashi Obara, Tsutomu Oshigiri, Tomonori Morita, Masahito Nakazaki, Takahiro Namioka, and et al. 2024. "Intravenous Infusion of Autologous Mesenchymal Stem Cells Expanded in Auto Serum for Chronic Spinal Cord Injury Patients: A Case Series" Journal of Clinical Medicine 13, no. 20: 6072. https://doi.org/10.3390/jcm13206072
APA StyleHirota, R., Sasaki, M., Iyama, S., Kurihara, K., Fukushi, R., Obara, H., Oshigiri, T., Morita, T., Nakazaki, M., Namioka, T., Namioka, A., Onodera, R., Kataoka-Sasaki, Y., Oka, S., Takemura, M., Ukai, R., Yokoyama, T., Sasaki, Y., Yamashita, T., ... Honmou, O. (2024). Intravenous Infusion of Autologous Mesenchymal Stem Cells Expanded in Auto Serum for Chronic Spinal Cord Injury Patients: A Case Series. Journal of Clinical Medicine, 13(20), 6072. https://doi.org/10.3390/jcm13206072







