Abstract
The migration or translocation of an intrauterine device (IUD) in the urinary tract is a rare event. Here, we present the case of a 55-year-old woman who accidentally discovered the ectopic presence of an IUD following a radiological examination for pelvic pain caused by a lumbar discopathy. Over the years, the patient had several IUDs inserted without being able to specify which one had migrated. The removal of the IUD was performed laparoscopically with the minimum resection of the bladder wall and the subsequent cystorrhaphy. The evolution of the patient was favorable. To better analyze these events, we conducted an all-time extensive electronic search of the PubMed database and identified 94 eligible articles, with a total of 115 cases. The literature analysis on the IUD migrations shows either the simultaneous existence of the second IUD or of a maximum number of up to two IUD insertions during the life of patients. Thus, in the presented case, we identified five IUD insertions over time, which explained the chronic inflammatory process by forming an important mass of adherents that included the urinary bladder, uterus, omentum, sigmoid colon, and abdominal wall. Therapeutic management must be adapted to each case depending on the intra/extravesical location of the migrated IUD evaluated by imaging.
Keywords:
intrauterine device; translocated; migration; intraperitoneally; embedment; bladder; ureter; urinary tract 1. Introduction
The intrauterine device (IUD) is one of the most widely used long-term contraceptive methods in the world. This device is effective, long-acting, and reversible and can be used by many women [1]. It is known that 23% of those who use contraceptive methods have IUDs. The IUD’s popularity is probably due to its similar effectiveness to surgical sterilization and its safety and reversible effect. There are many mechanisms of action for the IUD, which vary by type of IUD (inert, copper, or hormonal) [2]. The most popular types of IUDs are copper T-shaped ones and those that release levonorgestrel, a progestin with similar contraceptive effectiveness to combined oral contraceptives when used correctly.
In contrast, IUDs are safer than improperly used oral contraceptives. Once inserted into the uterus, the IUD leads to a sterile inflammatory response that is unfavorable to sperm and eggs. Progestin-based IUDs have the added benefit of causing a thickening of the cervical mucus, making it more difficult for the sperm to reach the egg [3]. Progestins can cause endometrial decidualization and glandular atrophy, preventing implantation [2]. Almost 35% of patients may experience amenorrhea after 2 years of use. For patients with a history of deep vein thrombosis, pulmonary embolism, or myocardial infarction, the copper IUD is the first-line contraceptive method, thus avoiding hormonal treatment and its prothrombotic effects [3,4]. Among those using IUDs, there is an average rate of about six pregnancies per 1000 women, the failure rate being approximately 0.8% for the copper-containing IUD and 0.2% for the levonorgestrel-releasing IUD [5].
The most common side effects of the IUD are pain and irregular bleeding, especially in the first several months after insertion. Rarely, the IUD can be complicated by pelvic inflammatory disease, contraceptive failure, expulsion, perforation, or migration [6].
Uterine perforation during insertion is rare, occurring in 0.6 to 16 cases per 1000 procedures. The risk of perforation is higher when it is inserted between 4 and 6 weeks after delivery or elective abortion. There are two types of uterine perforations, and both are prone to severe complications. Perforation usually occurs at the time of IUD insertion but rarely can occur later [7]. Uterine perforation after IUD insertion may be observed during device insertion, immediately after the procedure, or as a delayed event.
Primary perforation may occur during insertion and is usually associated with severe abdominal pain. Risk factors for perforation include inexperience of the person inserting the IUD, retroverted uterus, immobile uterus, and myometrial defect from a previous cesarean section or myomectomy [8,9]. Delayed perforation may be due to uterine spasms, and it can be due to gradual pressure necrosis of the uterine wall [7]. Approximately 80% of IUDs migrate into the peritoneal cavity after perforation. Migration into surrounding organs is a rare but severe complication [10,11]. After perforation, IUDs can migrate to any location, including the omentum, pouch of Douglas, or adherent to the sigmoid colon. Less commonly, IUDs can migrate into the bladder, small intestine, appendix, or colon [1].
About 30% of those with uterine perforation are asymptomatic, while about 70% have abdominal pain or uterine bleeding [12]. The most common finding associated with IUD migration is “missing strings” [13]. The effectiveness of the IUD is maintained only if the device is correctly placed in the uterine cavity; therefore, it is important to teach the patient how to check the presence of the IUD strings at the first visit [14]. Long-term complications include the formation of abscesses and fistulas. The copper IUD causes a greater inflammatory process than the progesterone-based ones [13].
If the IUD is not found, different methods can be used to recover the device. The World Health Organization recommends removing the migrated device as soon as possible [15]. Surgical removal is recommended even in asymptomatic patients once the IUD has migrated outside the uterus. The recommendation is to use minimally invasive methods as far as possible. These include hysteroscopy, cystoscopy, gastrointestinal tract endoscopy, or laparoscopy, depending on where the IUD has migrated [16].
If the strings are not seen or felt on examination, it is important to proceed with transvaginal ultrasound or other imaging techniques before assuming that the IUD has been expelled through the cervix and vagina. If migration is identified, the next step would be to obtain a cross-sectional image with CT or MRI to evaluate the involvement of other organs. If the device is embedded in an organ, for example, the bladder or bowel, it is not recommended to remove it using minimally invasive methods; instead, exploratory laparoscopy or even laparotomy should be performed. If the IUD is suspected to be embedded in a vessel or cannot be accurately visualized, a multidisciplinary approach is necessary, and collaboration between experienced surgeons is mandatory [15,16,17].
We aimed to present a case of IUD migration in the bladder wall and an all-time extensive literature review regarding these cases’ diagnosis and therapeutic management.
2. Case Presentation
A 55-year-old woman presented to our hospital, having been referred by a radiology service with the diagnosis of an IUD migrated outside the uterine cavity. The discovery was accidental following a radiological evaluation to investigate pelvic pain caused by a lumbar discopathy. During radiography, a radiopaque image was visualized in the hypogastrium, suggesting an extrauterine migrated IUD intraperitoneally (lateral deviated to the left). The patient is currently in menopause and has had two natural births and two miscarriages. She also has a history of appendectomy and glaucoma. The patient stated that the IUD was removed by another gynecologist 2 years ago, being surprised by the result of the X-ray and the fact that she was asymptomatic. The patient specified that she was not instructed to self-check her IUD strings.
Later, she attended a vaginal examination, during which the IUD strings were not visualized. The 2D/3D transvaginal and abdominal ultrasound scan did not show any evidence of the presence of an IUD inside the uterus. Also, the uterus shows two intramural uterine leiomyomas, located posteriorly, measuring 3.4 × 3.2 cm and 3.3 × 3.7 cm, respectively. Subsequent CT of the abdomen and pelvis revealed a T-shaped IUD-like structure that migrated anterior and superior to the uterine fundus region, positioned slightly obliquely to the right. Urinalysis and urine culture were normal.
A hysterolaparoscopy was proposed to specify the patient’s diagnosis. Diagnostic hysteroscopy revealed a regular cervical canal, a normal-looking uterine cavity, and a visible tubal ostia bilaterally. The surgical intervention was continued by performing a laparoscopy, which highlighted a pelvic mass with adhesions between the omentum, sigmoid colon, uterine fundus, and bladder. During the adhesiolysis, using the laparoscopic graspers, the limbs of the IUD intimately adherent to the serosa of the sigmoid colon and the long arm at the level of the submucosa of the urinary bladder could be identified. The dissection continued by traction of the IUD in its axis, with the IUD strings highlighted, without being able to detach the urinary bladder after sectioning the peritoneum over the bladder. The IUD was extracted en bloc to detach the strings with a limited resection of the bladder wall due to the technical difficulties created by the adhesions. A double-layer cystorrhaphy with 2-0 wires was performed, the associated adhesions were lysed, and the migrated IUD was successfully removed. The patient had a favorable evolution postoperatively and was discharged 48 h later. The evolution after one year was good, with the recommendation regarding the follow-up being regular medical check-ups (Figure 1 and Figure 2).
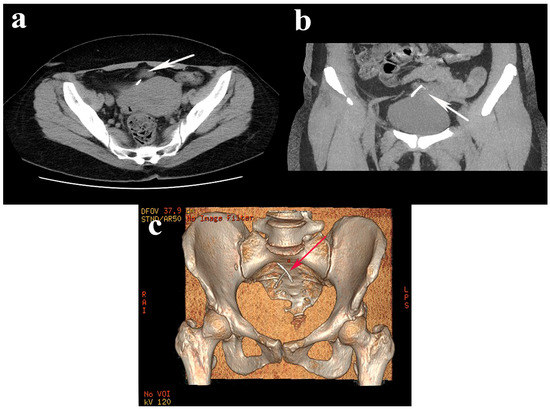
Figure 1.
Axial (a) and sagittal (b) abdominopelvic computed tomography images revealed a translocated IUD above the bladder and uterus (white arrows), oriented slightly obliquely to the right; (c) 3D reconstruction of the IUD topography (red arrow).
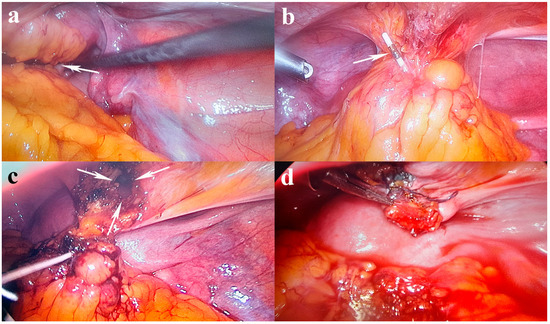
Figure 2.
Laparoscopic findings: (a) pelvic mass (white arrow) with adhesions between the omentum, sigmoid colon, uterine fundus, and bladder; (b) embedded IUD in the pelvic mass (white arrow); (c) IUD removal from the mass with long arm penetrated in the bladder wall (white arrows show the bladder limited resection); (d) a double-layer cystorrhaphy with 2-0 wires.
3. Discussion
The IUD is a globally accepted contraceptive method. IUDs are a popular method of reversible contraception because they have high efficacy, low risks, and relatively low cost. Post-insertion follow-up and awareness of complications are important to assess when to return to the gynecologist [18]. Expulsion, intrauterine displacement, translocation, and migration of the IUD lead to decreased contraceptive effectiveness and require immediate removal of the defective IUD with possible subsequent replacement to achieve contraception [1]. The first report on intravesical migration was made in 1972 by Rubin, who identified a device like the Lippes loop IUD [18].
Without a standardization of the management of these cases, we considered it necessary to conduct an all-time extensive literature search, accessing the PubMed database. Selection criteria were articles published in English and involving human subjects using the following methodological approaches: case series and case reports. The criteria followed were age, clinical manifestations, type of IUD, time of insertion, localization of IUD migration at the level of the urinary tract, size of the bladder stone, used imaging, therapeutic procedures, monitoring, and prognosis of these cases.
The key MeSH terms used in the electronic search technique were “intrauterine device”, “bladder”, “ureter”, “urinary tract”, “migration”, “translocation”, “embedment”, “diagnosis”, “treatment”, and “prognosis”. Finally, 105 articles were found, of which 94 met the eligibility criteria and were included in this review (Figure 3, Table 1).
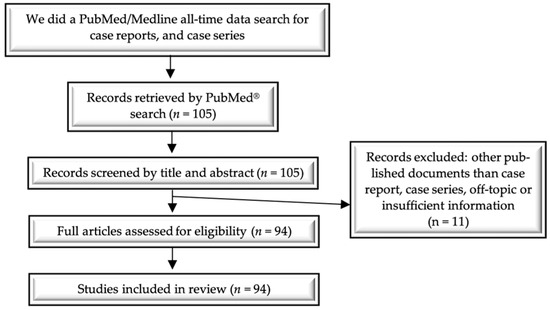
Figure 3.
Flow diagram of the literature search and study selection process.

Table 1.
Synopsis of all-time literature studies regarding translocation/migration of IUD in the urinary tract.
Thus, since 1980, 115 cases have been reported in which intrauterine contraceptive devices underwent a migration or translocation process with the interest of the urinary tract (Figure 4). More than 87 cases had bladder interest and 7 cases ureters. The average age of the patients in the review was 37.2 ± 10.71 years (range 19–74), with 11 cases >50 years. The average duration of IUD remaining in place after insertion reported in 96 cases was 8.4 ± 7.46 years (with an interval between 1 week and 35 years).
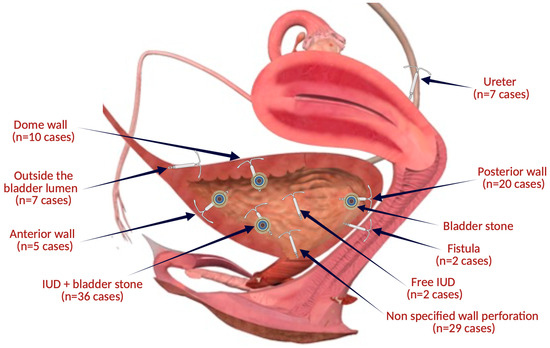
Figure 4.
The topographical sites of IUD migrations related to the articles included in the review (the figure was made with the help of Biorender.com).
The main clinical findings encountered were the following: asymptomatic in 4.3% (n = 5), dysuria in 33.04% (n = 38), hematuria in 26.1% (n = 30), pelvic pain in 20% (n = 23), urinary tract infections (UTI) in 15.6% (n = 18), urinary tract symptoms (UTS) in 14.8% (n = 17), suprapubic pain in 11.3% (n = 13), abdominal pain in 6.1% (n = 7), and loin pain in 2.6% (n = 3). The imaging evaluation highlighted the use of US in 76 cases (66.1%), X-ray in 71 cases (61.7%), CT in 41 cases (35.6%), and IV urography in 13 cases (11.3%).
Regarding the therapeutic modalities, cystoscopy was recorded in 76 cases (66.1%), cystolithotomy in 11 cases (9.5%), cystotomy in 9 cases (7.8%), laparoscopy in 20 cases (17.4%) (1 case with Da Vinci), laparotomy in 9 cases (7.8%), cystolitholapaxy in 10 cases (8.7%), partial cystectomy in 1 case (0.87%), lithotripsy in 16 cases (13.8%) (including 2 cases with laser). The prognosis was favorable in 110 cases (95.6%), and complications (fistulas) were identified in 5 cases (4.34%).
To increase the quality of the study methodology and reduce bias in the case reports we analyzed in this review, we assessed articles using the criteria outlined by Murad et al. [111] (Table S1).
The incidence of uterine perforation secondary to the migration/translocation of an IUD is very low, ranging from 0.1 to 0.9% [35], but its real rate is unknown because many patients are asymptomatic, and discovery may be accidental during investigation for another possible pathology. The uterine perforation has an estimated incidence of 1.6 to 1000 inserts [4].
You should be especially concerned about perforation if the IUD has been inserted by an inexperienced healthcare provider or is placed in an inappropriate position [112]. Secondary perforation is a delayed event proposed to be due to gradual pressure necrosis of the uterine wall [7]. Another situation in which we might suspect a perforation is when the patient has a weakened uterine wall near the insertion site, which frequently occurs as a result of multiparity, cesarean section, or abortion [15]. Regarding risk factors, the patient presented in the above case gave birth twice vaginally, and she had two abortions.
Additional risk factors for perforation regarding the indication for IUD insertion can be found immediately postabortion or postpartum, and uterine malpositions (retroversion, post-cesarean scars). The transmigration of the inserted IUD is favored by uterine contractions that further push the IUD out of the intraperitoneal uterine wall or into adjacent areas such as the bladder, ureter, bowel, peritoneum, appendix, adnexa, and abdominal wall.
Spontaneous migration was not highlighted, but the mechanism was favored by the degree of penetration of the uterine wall at the time of insertion. The migration of the IUD into the urinary bladder can be associated with the formation of stones at the moment of penetration into the lumen of the urinary bladder, a process found in 65.2% (n = 75) of the studied cases. The longer the interval, the more the incidence of these bladder stones can increase.
The IUD’s postpartum insertion increases the risk of perforation due to the reduced consistency of the uterus, the reduced dimensions of the uterine wall, and the uterine contractions of increased intensity. Other factors, such as intestinal peristalsis and bladder muscle contractions, may promote progressive migration of the IUD. The moment when the IUD exceeds the mucosa and enters the bladder can be accompanied by certain clinical symptoms. Bjornerem et al. showed that about 2% of the IUD migrates involve the bladder [26].
Knowing the type and form of IUD is important because it is known that the copper IUD determines an important inflammatory process and must be removed. The prevention of IUD migration can be achieved by identifying risk factors and increasing doctors’ learning curves.
Many patients with uterine perforation and IUD migration may present with symptoms, but as many as 30% are asymptomatic [12], as in the presented case. The clinical findings of IUD migration can occur with wide variability (from one week to 35 years, according to Table 1) because some patients are asymptomatic, and others are not recognized as belonging to this situation. Studies show that even in patients who have been asymptomatic for a long time, the therapeutic removal of the IUD is necessary due to the possible complications that can occur and that increase the morbidity of these cases (abscess, fistulas, hydronephrosis, obstructions).
Patients at the time of presentation may have a series of clinical symptoms (recurrent UTIs, UTSs, urinary irritation, hematuria, incontinence, and abdominal pain) until the time of diagnosis and may be treated for long periods with antibiotics. The diagnosis of faulty placement of the IUD at the time of insertion can be indicated by bleeding, pain, or the lack of visualization of the strings in the vast majority of cases, but some occult perforations can be diagnosed after a variable period.
Transmigration of the IUD leads to the lack of contraceptive effect, which is why some patients became pregnant, and some gave birth at term. Extraction of the IUD is mandatory in the event of pregnancy or when the patient wishes to conceive due to the risk that may occur when it migrates. IUD left in situ during pregnancy predisposes to spontaneous abortions, placenta previa or abruption, preterm premature rupture of the membranes (PPROM), chorioamnionitis, or cesarean delivery [113].
IUDs are effective if they are used correctly, removed, and replaced at the right time. Most frequently, intraperitoneally migration occurs in patients with scarred uterus, but as in our presented case, it can also occur in those who have not undergone cesarean sections.
If a patient has a lost IUD and the strings are not visible during the pelvic exam, the correct diagnosis must be obtained before surgery, including transvaginal or abdominal ultrasound or even radiography to confirm the position of the IUD. If IUD migration is still suspected, cross-sectional imaging such as CT or MRI is recommended to rule out the involvement of adjacent organs before surgical removal [114,115].
We encounter diagnostic and therapeutic challenges in devices partially migrated into the bladder wall. The role of ultrasound is important when we suspect the existence of IUD migration due to the lack of intrauterine evidence of it or its presence in the adjacent structures. The imaging diagnosis of translocated IUDs can sometimes be performed by a single technique or by combinations thereof (US, X-ray, CT).
Cystoscopy in these cases is important for the intraluminal identification of the IUD at the level of the urinary bladder and therapeutic resolution where possible, either by pulling it or by using a forceps to crush the stones in case the IUD is embedded in the bladder calculus. Currently, minimally invasive endoscopic techniques prevail over open surgical interventions. The more extensive the involved area of the bladder wall, the higher the use rate of open surgical procedures that will repair the defect after IUD extraction.
In selected cases, in elderly patients with comorbidities who accidentally discovered the presence of a migrated IUD, we can either try a conservative treatment or postpone an intervention until we can perform it due to the increased risk of morbidity secondary to possible complications. Fortunately, few cases in the literature have been reported in menopausal women.
The World Health Organization recommends removing the migrated device immediately after it is detected. It is suggested that surgical removal should be considered even in asymptomatic patients once it has migrated outside the uterus, as it was in the case presented [116]. In most cases, the therapeutic solution was customized through minimally invasive combined techniques (cystoscopy ± hysteroscopy ± laparoscopy) depending on the intravesical or extravesical position of the IUD. Furthermore, these patients require a multidisciplinary approach (gynecologist, urologist) and a general surgeon’s presence in complicated cases. In the case of the presence of fistulas, they are solved exclusively by the urologist. Minimally invasive procedures, including hysteroscopy and laparoscopy, should be attempted first, as in our case. Since our patient had a history of deliveries and post-appendectomy adhesions, extra caution should be taken when exploring the abdomen for the migrated device. Locating the strings in the submucosa of the urinary bladder is a rare and potentially serious complication, but with careful adhesiolysis, the IUD removal was completed successfully.
In our case, an X-ray was performed first for another medical reason. After that, the asymptomatic patient underwent a vaginal examination, and the IUD strings were not visualized. CT was performed to identify the exact position of the migrated IUD. The migration of the IUD with the highlighting of the strings in the submucosal region of the urinary bladder and the adhesions of this pelvic mass with the omentum and the sigmoid colon represents a particularity of this case. Removing the IUD required adhesiolysis and limited resection of the bladder wall. A limitation of this study is the use of a single database search.
4. Conclusions
IUDs are effective and are frequently used as a contraceptive method, but patients must be instructed to self-check their IUD strings and return for periodic control to check the correct position of the IUD for contraceptive effectiveness and to avoid possible complications due to its migration. Early identification of IUD migration and its removal significantly lowers the complication rate. The removal of an IUD that has migrated to the level of the urinary tract is a major concern both in the case of patients with recurrent symptoms and in asymptomatic patients with an uncertain history regarding the inserted IUD. Therapeutic management must be adapted to each case depending on the intra/ extravesical location of the migrated IUD evaluated by imaging.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jcm13144233/s1: Table S1. Representation of the risk of bias of the case reports we analyzed in this review.
Author Contributions
Conceptualization, V.N.V.; methodology, R.G.V. and A.I.M.; software, R.G.V.; validation, R.G.V. and I.B.; formal analysis, V.N.V.; investigation, R.G.V. and I.B.; resources, R.G.V.; data curation, A.I.M. and A.I.R.; writing—original draft preparation, V.N.V.; writing—review and editing, V.N.V., and N.B.; visualization, V.N.V. and N.B.; supervision, V.N.V. and N.B.; project administration, V.N.V.; funding acquisition, V.N.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Filantropia Clinical Hospital (no. 807/ 31 January 2024).
Informed Consent Statement
Informed consent was obtained from the subject involved in the study.
Data Availability Statement
The data presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gunbey, H.P.; Tanrivermis Sayit, A.; Sedakat Idilman, I.; Aksoy, O. Migration of Intrauterine Devices with Radiological Findings: Report on Two Cases. BMJ Case Rep. 2014, 2014, bcr2013202522. [Google Scholar] [CrossRef] [PubMed]
- Jb, S.; Rt, M. Mechanisms of Action of Intrauterine Devices: Update and Estimation of Postfertilization Effects. Am. J. Obstet. Gynecol. 2002, 187, 1699–1708. [Google Scholar] [CrossRef]
- Lewis, R.A.; Taylor, D.; Natavio, M.F.; Melamed, A.; Felix, J.; Mishell, D., Jr. Effects of the Levonorgestrel-Releasing Intrauterine System on Cervical Mucus Quality and Sperm Penetrability. Contraception 2010, 82, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Intrauterine Devices: An Effective Alternative to Oral Hormonal Contraception. Prescrire Int. 2009, 18, 125–130.
- Cheung, M.-L.; Rezai, S.; Jackman, J.M.; Patel, N.D.; Bernaba, B.Z.; Hakimian, O.; Nuritdinova, D.; Turley, C.L.; Mercado, R.; Takeshige, T.; et al. Retained Intrauterine Device (IUD): Triple Case Report and Review of the Literature. Case Rep. Obstet. Gynecol. 2018, 2018, 9362962. [Google Scholar] [CrossRef] [PubMed]
- Aoun, J.; Dines, V.A.; Stovall, D.W.; Mete, M.; Nelson, C.B.; Gomez-Lobo, V. Effects of Age, Parity, and Device Type on Complications and Discontinuation of Intrauterine Devices. Obstet. Gynecol. 2014, 123, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Jindal, R.P.; Deep, A. Intravesical Migration of Intrauterine Contraceptive Devices with Stone Formation. J. Fam. Med. Prim. Care 2014, 3, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Puttler, K.M.; Hong, C.; Ayzengart, A.L. Sigmoid Colon Penetration by an Intrauterine Device: A Case Report and Literature Review. Mil. Med. 2014, 179, e127–e129. [Google Scholar] [CrossRef] [PubMed]
- Aydogdu, O.; Pulat, H. Asymptomatic Far-Migration of an Intrauterine Device into the Abdominal Cavity: A Rare Entity. Can. Urol. Assoc. J. J. Assoc. Urol. Can. 2012, 6, E134–E136. [Google Scholar] [CrossRef]
- Rajaie Esfahani, M.; Abdar, A. Unusual Migration of Intrauterine Device into Bladder and Calculus Formation. Urol. J. 2007, 4, 49–51. [Google Scholar]
- Mülayim, B.; Mülayim, S.; Celik, N.Y. A Lost Intrauterine Device. Guess Where We Found It and How It Happened? Eur. J. Contracept. Reprod. Health Care Off. J. Eur. Soc. Contracept. 2006, 11, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Sharifiaghdas, F.; Mohammad Ali Beigi, F.; Abdi, H. Laparoscopic Removal of a Migrated Intrauterine Device. Urol. J. 2007, 4, 177–179. [Google Scholar]
- Kho, K.A.; Chamsy, D.J. Perforated Intraperitoneal Intrauterine Contraceptive Devices: Diagnosis, Management, and Clinical Outcomes. J. Minim. Invasive Gynecol. 2014, 21, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Gavin, L.; Pazol, K. Update: Providing Quality Family Planning Services—Recommendations from CDC and the U.S. Office of Population Affairs, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 231–234. [Google Scholar] [CrossRef]
- WHO Scientific Group on Mechanism of Action, Efficacy of Intrauterine Devices. Mechanism of Action, Safety and Efficacy of Intrauterine Devices. Report of a WHO Scientific Group; World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 1987; Volume 753, pp. 1–91. [Google Scholar]
- Sun, C.-C.; Chang, C.-C.; Yu, M.-H. Far-Migrated Intra-Abdominal Intrauterine Device with Abdominal Pain. Taiwan J. Obstet. Gynecol. 2008, 47, 244–246. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cetinkaya, K.; Kumtepe, Y.; Ingec, M. Minimally Invasive Approach to Cases of Lost Intra-Uterine Device: A 7-Year Experience. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 159, 119–121. [Google Scholar] [CrossRef]
- Rubin, A. Complications Due to Lippe’s Loop: Report of a Death and Other Complications Seen over an 18-Month Period at Baragwanath Hospital. S. Afr. J. Obstet. Gynaecol. 1972, 10, 45–48. [Google Scholar]
- Woods, M.; Wise, H.M. An Unusual Cause of Cystolithiasis: A Migrant Intrauterine Device. J. Urol. 1980, 124, 720–721. [Google Scholar] [CrossRef]
- Sasidharan, K.; Chally, R. Intravesical Migration of Lippes Loop with Stone Formation. Br. J. Urol. 1988, 61, 363–364. [Google Scholar] [CrossRef]
- Khan, S.R.; Wilkinson, E.J. Bladder Stone in a Human Female: The Case of an Abnormally Located Intrauterine Contraceptive Device. Scanning Microsc. 1990, 4, 395–398. [Google Scholar]
- Dietrick, D.D.; Issa, M.M.; Kabalin, J.N.; Bassett, J.B. Intravesical Migration of Intrauterine Device. J. Urol. 1992, 147, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Shratter, L.A. Ultrasonographic Identification of an Ectopic Intrauterine Contraceptive Device within the Bladder: A Case Report. Am. J. Obstet. Gynecol. 1993, 169, 1608–1609. [Google Scholar] [CrossRef] [PubMed]
- el-Diasty, T.A.; Shokeir, A.A.; el-Gharib, M.S.; Sherif, L.S.; Shamaa, M.A. Bladder Stone: A Complication of Intravesical Migration of Lippes Loop. Scand. J. Urol. Nephrol. 1993, 27, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Caspi, B.; Rabinerson, D.; Appelman, Z.; Kaplan, B. Penetration of the Bladder by a Perforating Intrauterine Contraceptive Device: A Sonographic Diagnosis. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 1996, 7, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Bjørnerem, A.; Tollan, A. Intrauterine Device--Primary and Secondary Perforation of the Urinary Bladder. Acta Obstet. Gynecol. Scand. 1997, 76, 383–385. [Google Scholar] [CrossRef]
- Szabó, Z.; Ficsór, E.; Nyirádi, J.; Nyirádi, T.; Pásztor, I.; Papp, F.; Danka, R. Rare Case of the Utero-Vesical Fistula Caused by Intrauterine Contraceptive Device. Acta Chir. Hung. 1997, 36, 337–339. [Google Scholar]
- Maskey, C.P.; Rahman, M.; Sigdar, T.K.; Johnsen, R. Vesical Calculus around an Intra-Uterine Contraceptive Device. Br. J. Urol. 1997, 79, 654–655. [Google Scholar] [CrossRef] [PubMed]
- Cumming, G.P.; Bramwell, S.P.; Lees, D.A. An Unusual Case of Cystolithiasis: A Urological Lesson for Gynaecologists. Br. J. Obstet. Gynaecol. 1997, 104, 117–118. [Google Scholar] [CrossRef]
- Yalçin, V.; Demirkesen, O.; Alici, B.; Onol, B.; Solok, V. An Unusual Presentation of a Foreign Body in the Urinary Bladder: A Migrant Intrauterine Device. Urol. Int. 1998, 61, 240–242. [Google Scholar] [CrossRef]
- Olaore, J.A.; Shittu, O.B.; Adewole, I.F. Intravesical Lippes Loop Following Insertion for the Treatment of Asherman’s Syndrome: A Case Report. Afr. J. Med. Med. Sci. 1999, 28, 207–208. [Google Scholar]
- Sehgal, A.; Gupta, B.; Malhotra, S. Intravesical Migration of Copper-T. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2000, 68, 265–266. [Google Scholar] [CrossRef]
- Güvel, S.; Tekin, M.I.; Kilinç, F.; Peskircioglu, L.; Ozkardeş, H. Bladder Stones around a Migrated and Missed Intrauterine Contraceptive Device. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2001, 8, 78–79. [Google Scholar] [CrossRef] [PubMed]
- Atakan, r.H.; Kaplan, M.; Ertrk, E. Intravesical Migration of Intrauterine Device Resulting in Stone Formation. Urology 2002, 60, 911. [Google Scholar] [CrossRef] [PubMed]
- Mahmutyazicioğlu, K.; Ozdemir, H.; Ozkan, P. Migration of an Intrauterine Contraceptive Device to the Urinary Bladder: Sonographic Findings. J. Clin. Ultrasound JCU 2002, 30, 496–498. [Google Scholar] [CrossRef]
- Senanayake, H.M.; Fernando, H. An Intrauterine Device in the Bladder Mimicking Urinary Tract Infection. Ceylon Med. J. 2002, 47, 28. [Google Scholar] [CrossRef]
- Dabbas, M.; Maaita, M. Ureteric Calculus around an Intrauterine Contraceptive Device. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2002, 22, 101–102. [Google Scholar] [CrossRef] [PubMed]
- Demirci, D.; Ekmekçioğlu, O.; Demirtaş, A.; Gülmez, I. Big Bladder Stones around an Intravesical Migrated Intrauterine Device. Int. Urol. Nephrol. 2003, 35, 495–496. [Google Scholar] [CrossRef]
- Wei, T.-Y.; Hsieh, C.-C.; Lo, T.-S. Intravesical Migration of an Intrauterine Device with Stone Formation. Aust. N. Z. J. Obstet. Gynaecol. 2003, 43, 391–392. [Google Scholar] [CrossRef] [PubMed]
- Rafique, M.; Rauf, A.; Khan, N.A.; Haque, T.U. An Unusual Cause of Vesical Stone: A Migrant Intrauterine Device. Eur. J. Contracept. Reprod. Health Care Off. J. Eur. Soc. Contracept. 2003, 8, 170–172. [Google Scholar] [CrossRef]
- Eke, N.; Okpani, A.O. Extrauterine Translocated Contraceptive Device: A Presentation of Five Cases and Revisit of the Enigmatic Issues of Iatrogenic Perforation and Migration. Afr. J. Reprod. Health 2003, 7, 117–123. [Google Scholar] [CrossRef][Green Version]
- Eskandar, O.S.; Eckford, S.D. Intravesical Migration of a GyneFix Intrauterine Device. J. Fam. Plann. Reprod. Health Care 2003, 29, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Nwofor, A.M.E.; Ikechebelu, J.I. Uterovesical Fistula and Bladder Stones Following Bladder Penetration by a Perforating Intrauterine Contraceptive Device. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2003, 23, 683–684. [Google Scholar] [CrossRef]
- Ozçelik, B.; Serin, I.S.; Basbug, M.; Aygen, E.; Ekmekçioglu, O. Differential Diagnosis of Intra-Uterine Device Migrating to Bladder Using Radiographic Image of Calculus Formation and Review of Literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 108, 94–96. [Google Scholar] [CrossRef]
- Ozgür, A.; Sişmanoğlu, A.; Yazici, C.; Coşar, E.; Tezen, D.; Ilker, Y. Intravesical Stone Formation on Intrauterine Contraceptive Device. Int. Urol. Nephrol. 2004, 36, 345–348. [Google Scholar] [CrossRef]
- Hick, E.J.; Hernández, J.; Yordán, R.; Morey, A.F.; Avilés, R.; García, C.R. Bladder Calculus Resulting from the Migration of an Intrauterine Contraceptive Device. J. Urol. 2004, 172 Pt 1, 1903. [Google Scholar] [CrossRef] [PubMed]
- Tunçay, Y.A.; Tunçay, E.; Güzin, K.; Oztürk, D.; Omurcan, C.; Yücel, N. Transuterine Migration as a Complication of Intrauterine Contraceptive Devices: Six Case Reports. Eur. J. Contracept. Reprod. Health Care Off. J. Eur. Soc. Contracept. 2004, 9, 194–200. [Google Scholar] [CrossRef]
- Zafar, M.; Murtaza, B.; Saeed, S. Two Displaced Intrauterine Contraceptive Devices (Copper-T). J. Coll. Physicians Surg.—Pak. JCPSP 2004, 14, 427–429. [Google Scholar]
- Dede, F.S.; Dilbaz, B.; Sahin, D.; Dilbaz, S. Vesical Calculus Formation around a Migrated Copper-T 380-A. Eur. J. Contracept. Reprod. Health Care Off. J. Eur. Soc. Contracept. 2006, 11, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Hoşcan, M.B.; Koşar, A.; Gümüştaş, U.; Güney, M. Intravesical Migration of Intrauterine Device Resulting in Pregnancy. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2006, 13, 301–302. [Google Scholar] [CrossRef]
- Gillis, E.; Chhiv, N.; Kang, S.; Sayegh, R.; Lotfipour, S. Case of Urethral Foreign Body: IUD Perforation of the Bladder with Calculus Formation. Calif. J. Emerg. Med. 2006, 7, 47–53. [Google Scholar]
- Khan, Z.A.; Khan, S.A.; Williams, A.; Mobb, G.E. Intravesical Migration of Levonorgestrel-Releasing Intrauterine System (LNG-IUS) with Calculus Formation. Eur. J. Contracept. Reprod. Health Care Off. J. Eur. Soc. Contracept. 2006, 11, 243–245. [Google Scholar] [CrossRef]
- Singh, I. Intravesical Cu-T Emigration: An Atypical and Infrequent Cause of Vesical Calculus. Int. Urol. Nephrol. 2007, 39, 457–459. [Google Scholar] [CrossRef]
- Nouira, Y.; Rakrouki, S.; Gargouri, M.; Fitouri, Z.; Horchani, A. Intravesical Migration of an Intrauterine Contraceptive Device Complicated by Bladder Stone: A Report of Six Cases. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2007, 18, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Istanbulluoglu, M.O.; Ozcimen, E.E.; Ozturk, B.; Uckuyu, A.; Cicek, T.; Gonen, M. Bladder Perforation Related to Intrauterine Device. J. Chin. Med. Assoc. JCMA 2008, 71, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Rafique, M. Intravesical Foreign Bodies: Review and Current Management Strategies. Urol. J. 2008, 5, 223–231. [Google Scholar] [PubMed]
- El-Hefnawy, A.S.; El-Nahas, A.R.; Osman, Y.; Bazeed, M.A. Urinary Complications of Migrated Intrauterine Contraceptive Device. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2008, 19, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Yensel, U.; Bezircioglu, I.; Yavuzcan, A.; Baloglu, A.; Cetinkaya, B. Migration of an Intrauterine Device into the Bladder: A Rare Case. Arch. Gynecol. Obstet. 2009, 279, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M. Erosion of an Intrauterine Contraceptive Device through the Bladder Wall Causing Calculus: Management and Review of the Literature. Urol. Int. 2009, 82, 370–371. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-T.; Yang, W.-J.; Lee, R.K.-K.; Hwu, Y.-M. Laparoscopic Removal of a Migrated Intrauterine Contraceptive Device with Bladder Penetration. Taiwan J. Obstet. Gynecol. 2010, 49, 518–520. [Google Scholar] [CrossRef]
- Vural, M.; Toy, H.; Camuzcuoglu, H.; Sezgin, B. Lost IUD Penetrating Bladder Wall. J. Fam. Plann. Reprod. Health Care 2010, 36, 182. [Google Scholar] [CrossRef][Green Version]
- Karsmakers, R.; Weis-Potters, A.E.; Buijs, G.; Joustra, E.B. Chronic Kidney Disease after Vesico-Vaginal Stone Formation around a Migrated Intrauterine Device. BMJ Case Rep. 2010, 2010, bcr1220092547. [Google Scholar] [CrossRef] [PubMed]
- Al-Awadi, K.A.; Zaghloul, A.S.; Kehinde, E.O. Symptomatic Secondary Vesical Calculus Formed on an Intrauterine Contraceptive Device Inserted 25 Years Previously. Urol. Int. 2011, 86, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Dar, L.A.; Khan, P.S.; Mushtaque, M. Giant Calculus Formation after Migration of an Intrauterine Device into the Urinary Bladder. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2011, 113, 243. [Google Scholar] [CrossRef] [PubMed]
- Ko, P.-C.; Lin, Y.-H.; Lo, T.-S. Intrauterine Contraceptive Device Migration to the Lower Urinary Tract: Report of 2 Cases. J. Minim. Invasive Gynecol. 2011, 18, 668–670. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.Y.; Kim, J.W.; Yoon, C.Y.; Park, H.S.; Moon, D.G.; Oh, M.M. Bladder Stone Due to Accidentally Intravesically Inserted Intrauterine Device. Urol. Res. 2012, 40, 429–430. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.G.; Kim, T.N.; Lee, W. Intrauterine Device Embedded into the Bladder Wall with Stone Formation: Laparoscopic Removal Is a Minimally Invasive Alternative to Open Surgery. Int. Urogynecol. J. 2012, 23, 1129–1131. [Google Scholar] [CrossRef]
- Jeje, E.A.; Ojewola, R.W.; Atoyebi, O.A. Intravesical Migration of a Failed and Forgotten Intrauterine Contraceptive Device after 20 Years of Insertion—A Case Report. Niger. Q. J. Hosp. Med. 2012, 22, 91–93. [Google Scholar]
- Guner, B.; Arikan, O.; Atis, G.; Canat, L.; Çaskurlu, T. Intravesical Migration of an Intrauterine Device. Urol. J. 2013, 10, 818–820. [Google Scholar] [PubMed]
- Ebner, F.; Petirsch, O.; Rempen, A. A Lost LNG-IUP in the Bladder. Arch. Gynecol. Obstet. 2013, 287, 1055–1056. [Google Scholar] [CrossRef]
- Campobasso, D.; Ciuffreda, M.; Maestroni, U.; Dinale, F.; Frattini, A.; Ferretti, S. Laparoscopic Removal of an Intrauterine Contraceptive Device Migrated into the Bladder: A Case Report. Urol. J. 2014, 11, 1847–1848. [Google Scholar]
- Scovell, J.M.; Chan, R.C.; Smith, C.P. Transurethral Use of a Nephroscope Significantly AIDS in the Surgical Management of an Intrauterine Device Eroding into the Bladder. Female Pelvic Med. Reconstr. Surg. 2014, 20, e8–e11. [Google Scholar] [CrossRef] [PubMed]
- Simsek, A.; Ozgor, F.; Akbulut, M.F.; Sönmezay, E.; Yuksel, B.; Sarılar, O.; Berberoglu, A.Y.; Gurbuz, Z.G. Management of Bladder Stones Associated with Foreign Bodies Following Incontinence and Contraception Surgery. Arch. Ital. Urol. Androl. Organo Uff. Soc. Ital. Ecogr. Urol. E Nefrol. 2014, 86, 108–111. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Voulgaris, A.M.; Lee, T.; Habib, A.; Kavia, R. The Case of the Migrating IUD. Practitioner 2015, 259, 3, 25–27. [Google Scholar] [PubMed]
- Kart, M.; Gülecen, T.; Üstüner, M.; Çiftçi, S.; Yavuz, U.; Özkürkçügil, C. Intravesical Migration of Missed Intrauterine Device Associated with Stone Formation: A Case Report and Review of the Literature. Case Rep. Urol. 2015, 2015, 581697. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, H.; Zhang, X. Intravesical Migration of a Chinese Intrauterine Device and Secondary Stone Formation: Diagnostic Investigation and Laparoscopic Management. Int. Urogynecol. J. 2015, 26, 1715–1716. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Qu, Q.; Yang, X.; Zhang, L. Partial Perforation of the Bladder by an Intrauterine Device in a Pregnant Woman: A Case Report. J. Reprod. Med. 2015, 60, 543–546. [Google Scholar] [PubMed]
- Bashir, B.M.; Atobatele, M.O.; Illo, H.G. Bladder Calculus Resulting from an Intravesical Translocation of Intrauterine Contraceptive Device in a Postmenopausal Woman. Niger. J. Med. J. Natl. Assoc. Resid. Dr. Niger. 2016, 25, 90–92. [Google Scholar] [CrossRef]
- Shen, J.K.; Ko, E.Y.; Staack, A. Early Pregnancy Likely Caused by an Intravesical Intrauterine Device. Can. J. Urol. 2016, 23, 8487–8490. [Google Scholar] [PubMed]
- Gyasi-Sarpong, C.K.; Maison, P.O.M.; Morhe, E.; Aboah, K.; Appiah, K.A.-A.; Azorliade, R.; Baah-Nyamekye, K.; Otu-Boateng, K.; Amoah, G.; Antwi, I.; et al. Intravesical Migration of an Intrauterine Device. BMC Res. Notes 2016, 9, 4. [Google Scholar] [CrossRef][Green Version]
- Jin, C.; Fan, Y.; Zhang, Q.; Wang, Y.; Wu, S.; Jin, J. Removal of Foreign Bodies Embedded in the Urinary Bladder Wall by a Combination of Laparoscopy and Carbon Dioxide Cystoscopic Assistance: Case Report and Literature Review. Investig. Clin. Urol. 2016, 57, 449–452. [Google Scholar] [CrossRef]
- Chai, W.; Zhang, W.; Jia, G.; Cui, M.; Cui, L. Vesical Transmigration of an Intrauterine Contraceptive Device: A Rare Case Report and Literature Review. Medicine 2017, 96, e8236. [Google Scholar] [CrossRef] [PubMed]
- De Silva, W.S.L.; Kodithuwakku, K.A.S.U.A.; Aponsu, G.U.E.; Rathnayake, R.M.M.; Rajasegaram, E. A Large Bladder Stone Caused by the Intravesical Migration of an Intrauterine Contraceptive Device: A Case Report. J. Med. Case Rep. 2017, 11, 293. [Google Scholar] [CrossRef]
- Clancy, A.A.; Gerridzen, R.; Pascali, D. Intrauterine Device Visualized as Extrinsic Bladder Mass on Cystoscopy. Int. Urogynecol. J. 2017, 28, 1429–1430. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Zhao, X.P.; Zhang, W.H.; Bai, W.; He, Y.G. Hydronephrosis Caused by Intrauterine Contraceptive Device Migration: Three Case Reports with Literature Review. Clin. Exp. Obstet. Gynecol. 2017, 44, 301–304. [Google Scholar] [CrossRef]
- Vahdat, M.; Gorginzadeh, M.; Mousavi, A.S.; Afshari, E.; Ghaed, M.A. Cystoscopic Removal of a Migrated Intrauterine Device to the Bladder; a Case Report. Contracept. Reprod. Med. 2019, 4, 7. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Li, C.; Luo, X.; Song, Y.; Li, S.; Luo, S.; Wang, Y. Migration of an Intrauterine Device Causing Severe Hydronephrosis Progressing to Renal Failure: A Case Report. Medicine 2019, 98, e13872. [Google Scholar] [CrossRef]
- Niu, H.; Zhang, L.; Yao, S.; Qu, Q. Successful Removal of an Intrauterine Device Perforating the Uterus and the Bladder with the Aid of a Transurethral Nephroscope. Int. Urogynecol. J. 2019, 30, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Jievaltienė, G.; Surgontaitė, D.; Aniulienė, R.; Venskutonis, D. Intrauterine Device Penetrating the Anterior Urinary Bladder Wall Discovered during Caesarean Section: A Case Report. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2020, 40, 718–720. [Google Scholar] [CrossRef]
- Basiri, A.; Shakiba, B.; Rostaminejad, N. Removal of Intramural Trapped Intrauterine Device by Cystoscopic Incision of Bladder Wall. Int. Braz J Urol Off. J. Braz. Soc. Urol. 2019, 45, 408–409. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, T.; Yang, L. Extensive Intravesical Benign Hyperplasia Induced by an Extravesical Migrated Intrauterine Device: A Case Report. Medicine 2019, 98, e15671. [Google Scholar] [CrossRef]
- Zhang, N.-N.; Zuo, N.; Sun, T.-S.; Yang, Q. An Effective Method Combining Various Endoscopes in the Treatment of Intravesical Migrated Intrauterine Device. J. Minim. Invasive Gynecol. 2020, 27, 582. [Google Scholar] [CrossRef] [PubMed]
- Dappa, E.; Ramos, R.; Cunha, T.M.; Ramos, N.; Silva, E. Intrauterine Device Migration into the Bladder with Stone Formation after Radiochemotherapy for Cervical Cancer: A Case Report. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2020, 40, 1166–1168. [Google Scholar] [CrossRef] [PubMed]
- Badu-Peprah, A.; Adu-Bredu, T.K.; Adu-Takyi, C. The Role of Multimodality Radiological Imaging in Extrauterine Misplaced IUCD: A Case Report. Afr. J. Reprod. Health 2020, 24, 213–217. [Google Scholar] [PubMed]
- Christodoulides, A.P.; Karaolides, T. Intravesical Migration of an Intrauterine Device (IUD)-Case Report. Urology 2020, 139, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Benaguida, H.; Kiram, H.; Telmoudi, E.C.; Ouafidi, B.; Benhessou, M.; Ennachit, M.; Elkarroumi, M. Intraperitoneal Migration of an Intrauterine Device (IUD): A Case Report. Ann. Med. Surg. 2021, 68, 102547. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-L.; Wang, Y.-L.; Weng, S.-S.; Huang, W.-C. Laparoscopic Repair of the Bladder: A Case of Intrauterine Device Migration to the Urinary Bladder. J. Minim. Invasive Gynecol. 2021, 28, 1433–1435. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, M.; Zi, D.; Duan, K.; Fu, K.; Guan, X. Single-Incision Laparoscopic Surgery for Removal of Ectopic Intrauterine Devices with Bladder Repair. Asian J. Surg. 2021, 44, 401. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, F.; Ao, M.; Huang, G. Intrauterine Devices Migrated into the Bladder: Two Case Reports and Literature Review. BMC Womens Health 2021, 21, 301. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, O.S.; Rasool, S.; Nazir, S.S. Migrated Intravesical Intrauterine Contraceptive Devices: A Case Series and a Suggested Algorithm for Management. Cureus 2021, 13, e12987. [Google Scholar] [CrossRef]
- Han, X.; Yang, H. Successful Endoscopic Management of 3 Cases of Translocated Intrauterine Devices: A Case Report. Ann. Palliat. Med. 2021, 10, 2371–2378. [Google Scholar] [CrossRef]
- Qu, R.; Yang, L.; Dai, Y. Cystoscopy to Remove an Intrauterine Contraceptive Device Embedded in the Urinary Bladder Wall: A Case Report and Literature Review. J. Int. Med. Res. 2021, 49, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Salih, M.A.; Tefera, A.T.; Gebrehiwot, F.G.; Mideksa, A.G.; Halala, N.S.; Gebreselassie, K.H. Gross Hematuria Caused by Intravesical Migration of a Forgotten Intrauterine Device: A Case Report and Literature Review. Res. Rep. Urol. 2022, 14, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Ago, B.U.; Enakirerhi, G. Bladder Stone Caused by Misplaced Intrauterine Contraceptive Device: A Case Report. Pan Afr. Med. J. 2022, 42, 143. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, I.; Agarwal, H. IUCD as Bladder Stone. Trop. Doct. 2022, 52, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Moy, B.M.; Andino, J.J.; Sadeghi, Z.; Gupta, P. Da Vinci-Assisted Laparoscopic Removal of an Intrauterine Device in the Bladder. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2022, 159, 601–603. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatlan, H.S.; Al-Tuhoo, A.M.; Abu-Faza, M.; Obaid, M.; Abdelazim, I.A.; Al-Kandari, I.M. Intraperitoneally Retained Contraceptive Device After Uterine Perforation: A Case Report. J. Mother Child 2023, 27, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Adeyanju, A.S.; Ogunsola, J.A.; Obajimi, G.O. Uterovesical Migration of Copper-Containing Intrauterine Device Complicated by Bladder Stone Formation. J.-Life Health 2023, 14, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Saputra, A.N.D.; Permatasari, N.U.I. Intrauterine Device Migration into the Bladder Leading to Stones Formation. BMJ Case Rep. 2023, 16, e256547. [Google Scholar] [CrossRef] [PubMed]
- Agil, A.; Tjahjodjati, T.; Atik, N.; Rachmadi, D.; Zahrina, T.T. Case Report: Iatrogenic Trauma of the Bladder Due to Long-Term Unidentified Intrauterine Device Malposition inside the Bladder with Rectovesical Fistula. F1000Research 2023, 12, 1390. [Google Scholar] [CrossRef]
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological Quality and Synthesis of Case Series and Case Reports. BMJ Evid.-Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice; Long-Acting Reversible Contraceptive Expert Work Group. Committee Opinion No 672: Clinical Challenges of Long-Acting Reversible Contraceptive Methods. Obstet. Gynecol. 2016, 128, e69–e77. [Google Scholar] [CrossRef] [PubMed]
- Eka R Gunardi, Raymond Surya. Outcome of Pregnancy with Intrauterine Device In Situ: A Meta-Analysis. J. South Asian Fed. Obstet. Gynaecol. 2019, 11, 212–216. [Google Scholar] [CrossRef]
- Baker, C.C.; Creinin, M.D. Long-Acting Reversible Contraception. Obstet. Gynecol. 2022, 140, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Berzenji, L.; Hosten, L.; Van Schil, P. The Lost Intrauterine Device. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2021, 60, 1477. [Google Scholar] [CrossRef]
- Pont, M.; Lantheaume, S. [Abdominal migration of a levonorgestrel-releasing intrauterine device. Case report and review of the literature]. J. Gynecol. Obstet. Biol. Reprod. 2009, 38, 179–181. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).