Gut Microbiota in Chronic Kidney Disease: From Composition to Modulation towards Better Outcomes—A Systematic Review
Abstract
1. Introduction
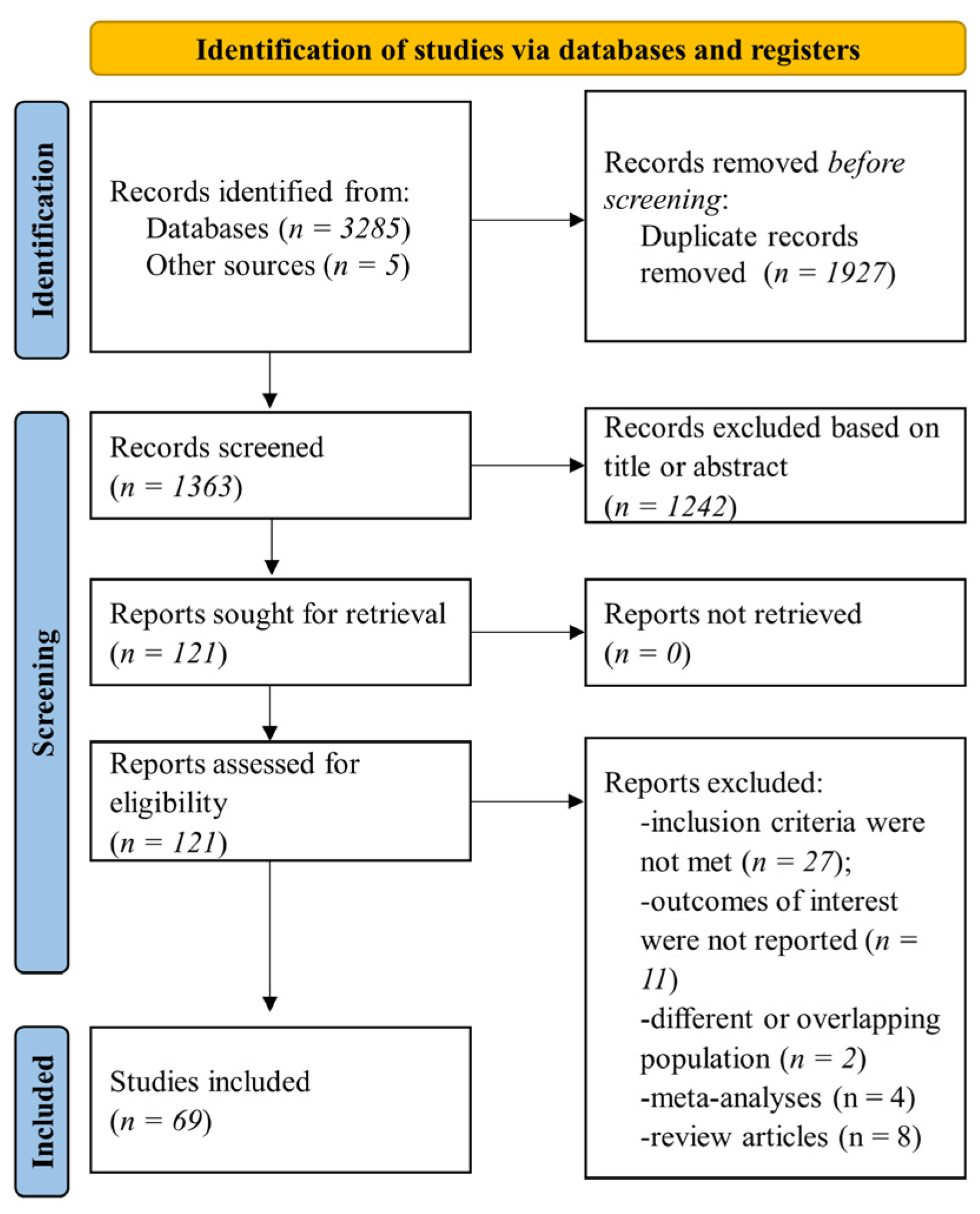
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Eligibility Criteria and Outcomes
2.3. Data Collection and Synthesis
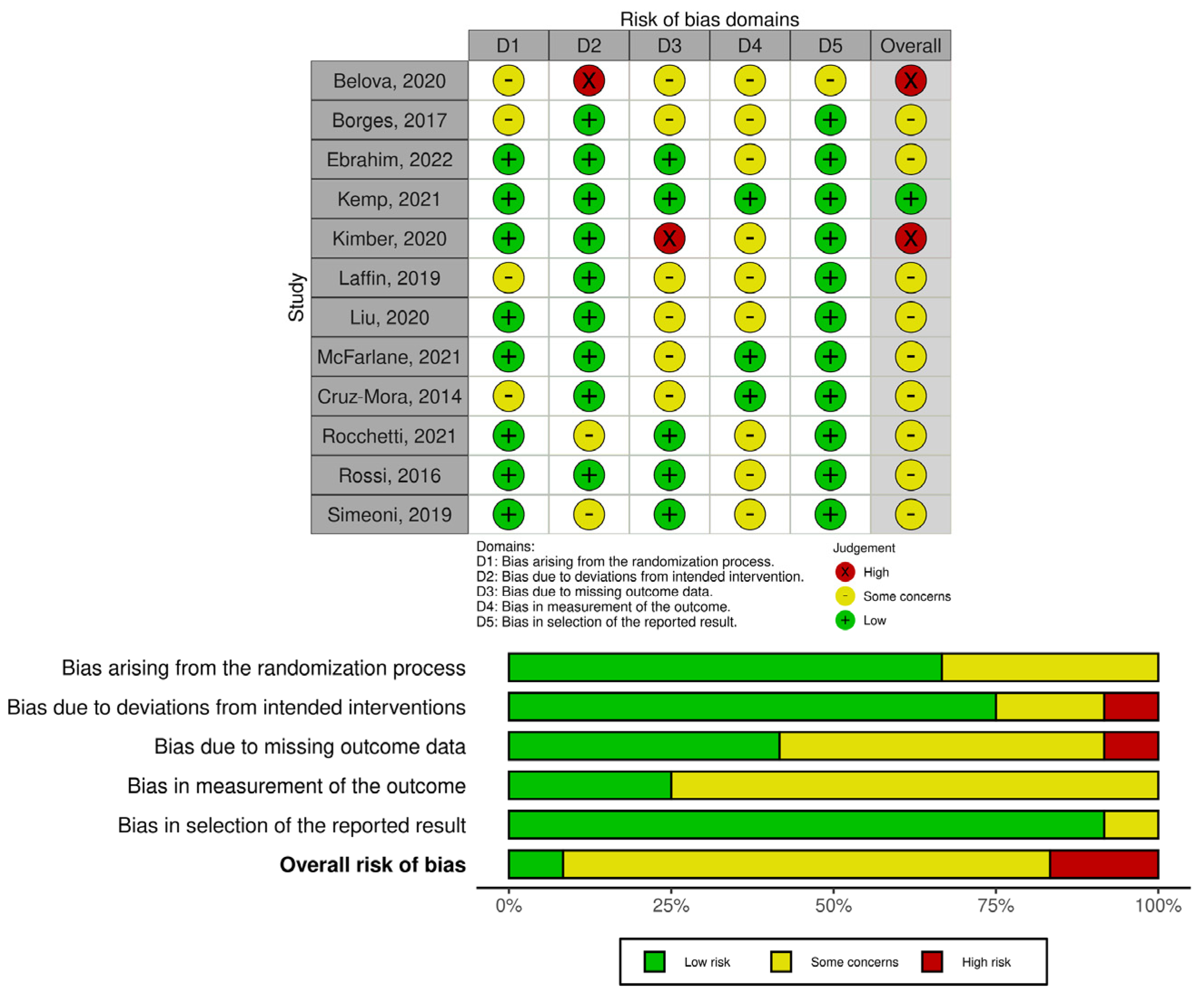
2.4. Quality and Risk of Bias Assessment
3. Results
3.1. Early-Stage CKD
3.2. ESKD
3.3. Diabetic Nephropathy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Baquero, F.; Nombela, C. The microbiome as a human organ. Clin. Microbiol. Infect. 2012, 18 (Suppl. 4), 2–4. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut microbiota: The neglected endocrine organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Al Khodor, S.; Shatat, I.F. Gut microbiome and kidney disease: A bidirectional relationship. Pediatr. Nephrol. 2017, 32, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Hamooya, B.; Hangoma, J.; Hayumbu, V.; Ertuglu, L.A.; Ishimwe, J.; Rahman, S.; Saleem, M.; Laffer, C.L.; Elijovich, F.; et al. Recent advances in modulation of cardiovascular diseases by the gut microbiota. J. Hum. Hypertens. 2022, 36, 952–959. [Google Scholar] [CrossRef]
- Twardowska, A.; Makaro, A.; Binienda, A.; Fichna, J.; Salaga, M. Preventing Bacterial Translocation in Patients with Leaky Gut Syndrome: Nutrition and Pharmacological Treatment Options. Int. J. Mol. Sci. 2022, 23, 3204. [Google Scholar] [CrossRef]
- de Waal, G.M.; de Villiers, W.J.S.; Pretorius, E. The Link Between Bacterial Inflammagens, Leaky Gut Syndrome and Colorectal Cancer. Curr. Med. Chem. 2021, 28, 8534–8548. [Google Scholar] [CrossRef]
- Schwabkey, Z.I.; Jenq, R.R. Microbiome Anomalies in Allogeneic Hematopoietic Cell Transplantation. Annu. Rev. Med. 2020, 71, 137–148. [Google Scholar] [CrossRef]
- Ananya, F.N.; Ahammed, M.R.; Fahem, M.M.; Kafle, S.; Viswanathan, M.; Desai, D.; Akku, R.; Khan, F.; Hernandez, T.E.; Bala, S.K.; et al. Association of Intestinal Microbial Dysbiosis With Chronic Obstructive Pulmonary Disease. Cureus 2021, 13, e19343. [Google Scholar] [CrossRef] [PubMed]
- Forkosh, E.; Ilan, Y. The heart-gut axis: New target for atherosclerosis and congestive heart failure therapy. Open Heart 2019, 6, e000993. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, S.D.; Schold, J.D.; Arrigain, S.; Jolly, S.E.; Nally, J.V., Jr. Cause-Specific Deaths in Non-Dialysis-Dependent CKD. J. Am. Soc. Nephrol. 2015, 26, 2512–2520. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Barros, A.F.; Borges, N.A.; Ferreira, D.C.; Carmo, F.L.; Rosado, A.S.; Fouque, D.; Mafra, D. Is there interaction between gut microbial profile and cardiovascular risk in chronic kidney disease patients? Future Microbiol. 2015, 10, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-H.; Liu, C.-W.; Ho, Y.-H.; Huang, C.-K.; Hung, C.-S.; Smith, B.; Lin, J.-C. Gut Microbiota Composition and Its Metabolites in Different Stages of Chronic Kidney Disease. J. Clin. Med. 2021, 10, 3881. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Liu, J.; Xue, Y.; Kong, X.; Lv, C.; Li, Z.; Huang, Y.; Wang, B. Alteration of gut microbial profile in patients with diabetic nephropathy. Endocrine 2021, 73, 71–84. [Google Scholar] [CrossRef]
- Gao, B.; Jose, A.; Alonzo-Palma, N.; Malik, T.; Shankaranarayanan, D.; Regunathan-Shenk, R.; Raj, D.S. Butyrate producing microbiota are reduced in chronic kidney diseases. Sci. Rep. 2021, 11, 23530. [Google Scholar] [CrossRef] [PubMed]
- Gryp, T.; Faust, K.; Van Biesen, W.; Huys, G.R.B.; Verbeke, F.; Speeckaert, M.; Raes, J.; Vaneechoutte, M.; Joossens, M.; Glorieux, G. Gut Microbiome Profiling Uncovers a Lower Abundance of Butyricicoccus in Advanced Stages of Chronic Kidney Disease. J. Pers. Med. 2021, 11, 1118. [Google Scholar] [CrossRef] [PubMed]
- Gryp, T.; Huys, G.R.B.; Joossens, M.; Van Biesen, W.; Glorieux, G.; Vaneechoutte, M. Isolation and Quantification of Uremic Toxin Precursor-Generating Gut Bacteria in Chronic Kidney Disease Patients. Int. J. Mol. Sci. 2020, 21, 1986. [Google Scholar] [CrossRef]
- Guirong, Y.E.; Minjie, Z.; Lixin, Y.U.; Junsheng, Y.E.; Lin, Y.; Lisha, S. [Gut microbiota in renal transplant recipients, patients with chronic kidney disease and healthy subjects]. Nan Fang Yi Ke Xue Xue Bao 2018, 38, 1401–1408. [Google Scholar] [CrossRef]
- Hanifi, G.R.; Samadi Kafil, H.; Tayebi Khosroshahi, H.; Shapouri, R.; Asgharzadeh, M. Bifidobacteriaceae Family Diversity in Gut Microbiota of Patients with Renal Failure. Arch. Razi Inst. 2021, 76, 521–528. [Google Scholar] [CrossRef]
- He, H.; Xie, Y. Alteration of Intestinal Microflora in Uremia Patients With or Without Blood Purification. Niger. J. Clin. Pract. 2021, 24, 1133–1137. [Google Scholar] [CrossRef]
- Hu, J.; Zhong, X.; Yan, J.; Zhou, D.; Qin, D.; Xiao, X.; Zheng, Y.; Liu, Y. High-throughput sequencing analysis of intestinal flora changes in ESRD and CKD patients. BMC Nephrol. 2020, 21, 12. [Google Scholar] [CrossRef]
- Iguchi, A.; Yamamoto, S.; Oda, A.; Tanaka, K.; Kazama, J.J.; Saeki, T.; Yamazaki, H.; Ishioka, K.; Suzutani, T.; Narita, I. Effect of sucroferric oxyhydroxide on gastrointestinal microbiome and uremic toxins in patients with chronic kidney disease undergoing hemodialysis. Clin. Exp. Nephrol. 2020, 24, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xie, S.; Lv, D.; Zhang, Y.; Deng, J.; Zeng, L.; Chen, Y. A reduction in the butyrate producing species Roseburia spp. and Faecalibacterium prausnitzii is associated with chronic kidney disease progression. Antonie Leeuwenhoek 2016, 109, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xie, S.; Lv, D.; Wang, P.; He, H.; Zhang, T.; Zhou, Y.; Lin, Q.; Zhou, H.; Jiang, J.; et al. Alteration of the gut microbiota in Chinese population with chronic kidney disease. Sci. Rep. 2017, 7, 2870. [Google Scholar] [CrossRef] [PubMed]
- Khiabani, S.A.; Haghighat, S.; Khosroshahi, H.T.; Asgharzadeh, M.; Kafil, H.S. Clostridium species diversity in gut microbiota of patients with renal failure. Microb. Pathog. 2022, 169, 105667. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, H.E.; Park, J.I.; Cho, H.; Kwak, M.J.; Kim, B.Y.; Yang, S.H.; Lee, J.P.; Kim, D.K.; Joo, K.W.; et al. The Association between Gut Microbiota and Uremia of Chronic Kidney Disease. Microorganisms 2020, 8, 907. [Google Scholar] [CrossRef]
- Sugurmar, A.N.K.; Mohd, R.; Shah, S.A.; Neoh, H.M.; Cader, R.A. Gut microbiota in Immunoglobulin A Nephropathy: A Malaysian Perspective. BMC Nephrol. 2021, 22, 145. [Google Scholar] [CrossRef]
- Lai, S.; Molfino, A.; Testorio, M.; Perrotta, A.M.; Currado, A.; Pintus, G.; Pietrucci, D.; Unida, V.; La Rocca, D.; Biocca, S.; et al. Effect of Low-Protein Diet and Inulin on Microbiota and Clinical Parameters in Patients with Chronic Kidney Disease. Nutrients 2019, 11, 3006. [Google Scholar] [CrossRef]
- Lecamwasam, A.; Nelson, T.M.; Rivera, L.; Ekinci, E.I.; Saffery, R.; Dwyer, K.M. Gut Microbiome Composition Remains Stable in Individuals with Diabetes-Related Early to Late Stage Chronic Kidney Disease. Biomedicines 2020, 9, 19. [Google Scholar] [CrossRef]
- Li, F.; Wang, M.; Wang, J.; Li, R.; Zhang, Y. Alterations to the Gut Microbiota and Their Correlation With Inflammatory Factors in Chronic Kidney Disease. Front. Cell. Infect. Microbiol. 2019, 9, 206. [Google Scholar] [CrossRef]
- Lin, T.Y.; Hung, S.C. Association of subjective global assessment of nutritional status with gut microbiota in hemodialysis patients: A case-control study. Nephrol. Dial. Transplant. 2021, 36, 1104–1111. [Google Scholar] [CrossRef]
- Lin, T.Y.; Wu, P.H.; Lin, Y.T.; Hung, S.C. Characterization of Gut Microbiota Composition in Hemodialysis Patients With Normal Weight Obesity. J. Clin. Endocrinol. Metab. 2020, 105, 2006–2014. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liang, W.; Li, L.; Xiong, Q.; He, S.; Zhao, J.; Guo, X.; Xiang, S.; Zhang, P.; Wang, H.; et al. The Accumulation of Gut Microbiome-derived Indoxyl Sulfate and P-Cresyl Sulfate in Patients With End-stage Renal Disease. J. Ren. Nutr. 2022, 32, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xu, X.; Chao, L.; Chen, K.; Shao, A.; Sun, D.; Hong, Y.; Hu, R.; Jiang, P.; Zhang, N.; et al. Alteration of the Gut Microbiome in Chronic Kidney Disease Patients and Its Association With Serum Free Immunoglobulin Light Chains. Front. Immunol. 2021, 12, 609700. [Google Scholar] [CrossRef] [PubMed]
- Lun, H.; Yang, W.; Zhao, S.; Jiang, M.; Xu, M.; Liu, F.; Wang, Y. Altered gut microbiota and microbial biomarkers associated with chronic kidney disease. MicrobiologyOpen 2019, 8, e00678. [Google Scholar] [CrossRef]
- Luo, D.; Zhao, W.; Lin, Z.; Wu, J.; Lin, H.; Li, Y.; Song, J.; Zhang, J.; Peng, H. The Effects of Hemodialysis and Peritoneal Dialysis on the Gut Microbiota of End-Stage Renal Disease Patients, and the Relationship Between Gut Microbiota and Patient Prognoses. Front. Cell. Infect. Microbiol. 2021, 11, 579386. [Google Scholar] [CrossRef]
- Margiotta, E.; Miragoli, F.; Callegari, M.L.; Vettoretti, S.; Caldiroli, L.; Meneghini, M.; Zanoni, F.; Messa, P. Gut microbiota composition and frailty in elderly patients with Chronic Kidney Disease. PLoS ONE 2020, 15, e0228530. [Google Scholar] [CrossRef]
- Al-Obaide, M.A.I.; Singh, R.; Datta, P.; Rewers-Felkins, K.A.; Salguero, M.V.; Al-Obaidi, I.; Kottapalli, K.R.; Vasylyeva, T.L. Gut Microbiota-Dependent Trimethylamine-N-oxide and Serum Biomarkers in Patients with T2DM and Advanced CKD. J. Clin. Med. 2017, 6, 86. [Google Scholar] [CrossRef]
- Pivari, F.; Mingione, A.; Piazzini, G.; Ceccarani, C.; Ottaviano, E.; Brasacchio, C.; Dei Cas, M.; Vischi, M.; Cozzolino, M.G.; Fogagnolo, P.; et al. Curcumin Supplementation (Meriva(®)) Modulates Inflammation, Lipid Peroxidation and Gut Microbiota Composition in Chronic Kidney Disease. Nutrients 2022, 14, 231. [Google Scholar] [CrossRef]
- Ren, Z.; Fan, Y.; Li, A.; Shen, Q.; Wu, J.; Ren, L.; Lu, H.; Ding, S.; Ren, H.; Liu, C.; et al. Alterations of the Human Gut Microbiome in Chronic Kidney Disease. Adv. Sci. 2020, 7, 2001936. [Google Scholar] [CrossRef]
- Salguero, M.V.; Al-Obaide, M.A.I.; Singh, R.; Siepmann, T.; Vasylyeva, T.L. Dysbiosis of Gram-negative gut microbiota and the associated serum lipopolysaccharide exacerbates inflammation in type 2 diabetic patients with chronic kidney disease. Exp. Ther. Med. 2019, 18, 3461–3469. [Google Scholar] [CrossRef]
- Sato, N.; Kakuta, M.; Hasegawa, T.; Yamaguchi, R.; Uchino, E.; Murashita, K.; Nakaji, S.; Imoto, S.; Yanagita, M.; Okuno, Y. Metagenomic profiling of gut microbiome in early chronic kidney disease. Nephrol. Dial. Transplant. 2021, 36, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, V.; Horvath, A.; Ribitsch, W.; Schmerböck, B.; Schilcher, G.; Lemesch, S.; Stiegler, P.; Rosenkranz, A.R.; Fickert, P.; Leber, B. Structural and functional differences in gut microbiome composition in patients undergoing haemodialysis or peritoneal dialysis. Sci. Rep. 2017, 7, 15601. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lv, D.; Jiang, S.; Jiang, J.; Liang, M.; Hou, F.; Chen, Y. Quantitative reduction in short-chain fatty acids, especially butyrate, contributes to the progression of chronic kidney disease. Clin. Sci. 2019, 133, 1857–1870. [Google Scholar] [CrossRef]
- Wang, Y.F.; Zheng, L.J.; Liu, Y.; Ye, Y.B.; Luo, S.; Lu, G.M.; Gong, D.; Zhang, L.J. The gut microbiota-inflammation-brain axis in end-stage renal disease: Perspectives from default mode network. Theranostics 2019, 9, 8171–8181. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, S.; Li, S.; Zhao, L.; Hao, Y.; Qin, J.; Zhang, L.; Zhang, C.; Bian, W.; Zuo, L.; et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut 2020, 69, 2131–2142. [Google Scholar] [CrossRef]
- Wu, I.W.; Gao, S.S.; Chou, H.C.; Yang, H.Y.; Chang, L.C.; Kuo, Y.L.; Dinh, M.C.V.; Chung, W.H.; Yang, C.W.; Lai, H.C.; et al. Integrative metagenomic and metabolomic analyses reveal severity-specific signatures of gut microbiota in chronic kidney disease. Theranostics 2020, 10, 5398–5411. [Google Scholar] [CrossRef]
- Wu, I.W.; Lin, C.Y.; Chang, L.C.; Lee, C.C.; Chiu, C.Y.; Hsu, H.J.; Sun, C.Y.; Chen, Y.C.; Kuo, Y.L.; Yang, C.W.; et al. Gut Microbiota as Diagnostic Tools for Mirroring Disease Progression and Circulating Nephrotoxin Levels in Chronic Kidney Disease: Discovery and Validation Study. Int. J. Biol. Sci. 2020, 16, 420–434. [Google Scholar] [CrossRef]
- Wu, R.; Ruan, X.L.; Ruan, D.D.; Zhang, J.H.; Wang, H.L.; Zeng, Q.Z.; Lu, T.; Gan, Y.M.; Luo, J.W.; Wu, J.B. Differences in gut microbiota structure in patients with stages 4-5 chronic kidney disease. Am. J. Transl. Res. 2021, 13, 10056–10074. [Google Scholar]
- Xu, K.Y.; Xia, G.H.; Lu, J.Q.; Chen, M.X.; Zhen, X.; Wang, S.; You, C.; Nie, J.; Zhou, H.W.; Yin, J. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci. Rep. 2017, 7, 1445. [Google Scholar] [CrossRef]
- Zhang, P.; Fang, J.; Li, G.; Zhang, L.; Lai, X.; Xu, L.; Liu, L.; Xiong, Y.; Li, L.; Zhang, T.; et al. Sex Differences in Fecal Microbiota Correlation With Physiological and Biochemical Indices Associated With End-Stage Renal Disease Caused by Immunoglobulin a Nephropathy or Diabetes. Front. Microbiol. 2021, 12, 752393. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, D.; Lin, Z.; Zhou, W.; Rao, J.; Li, Y.; Wu, J.; Peng, H.; Lou, T. Dysbiosis of gut microbiota in adult idiopathic membranous nephropathy with nephrotic syndrome. Microb. Pathog. 2020, 147, 104359. [Google Scholar] [CrossRef]
- Zheng, L.J.; Lin, L.; Zhong, J.; Zhang, Z.; Ye, Y.B.; Zhang, X.Y.; Wang, Y.F.; Zhang, H.; Liu, Y.; Lu, G.M.; et al. Gut dysbiosis-influence on amygdala-based functional activity in patients with end stage renal disease: A preliminary study. Brain Imaging Behav. 2020, 14, 2731–2744. [Google Scholar] [CrossRef]
- Miao, Y.Y.; Xu, C.M.; Xia, M.; Zhu, H.Q.; Chen, Y.Q. Relationship between Gut Microbiota and Phosphorus Metabolism in Hemodialysis Patients: A Preliminary Exploration. Chin. Med. J. 2018, 131, 2792–2799. [Google Scholar] [CrossRef]
- Wu, I.W.; Lee, C.C.; Hsu, H.J.; Sun, C.Y.; Chen, Y.C.; Yang, K.J.; Yang, C.W.; Chung, W.H.; Lai, H.C.; Chang, L.C.; et al. Compositional and Functional Adaptations of Intestinal Microbiota and Related Metabolites in CKD Patients Receiving Dietary Protein Restriction. Nutrients 2020, 12, 2799. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.H.; Liu, P.Y.; Chiu, Y.W.; Hung, W.C.; Lin, Y.T.; Lin, T.Y.; Hung, S.C.; Delicano, R.A.; Kuo, M.C.; Wu, C.Y. Comparative Gut Microbiome Differences between Ferric Citrate and Calcium Carbonate Phosphate Binders in Patients with End-Stage Kidney Disease. Microorganisms 2020, 8, 2040. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhong, X.; Liu, Y.; Yan, J.; Zhou, D.; Qin, D.; Xiao, X.; Zheng, Y.; Wen, L.; Tan, R.; et al. Correlation between intestinal flora disruption and protein–energy wasting in patients with end-stage renal disease. BMC Nephrol. 2022, 23, 130. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, B.; Sha, T.; Li, X. Changes in the Intestinal Microbiota in Patients with Stage 5 Chronic Kidney Disease on a Low-Protein Diet and the Effects of Human to Rat Fecal Microbiota Transplantation. Med. Sci. Monit. 2020, 26, e921557. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, R.; Nugent, M.; Cai, W.; Nadkarni, G.N.; Chaves, L.D.; Abyad, S.; Honan, A.M.; Thomas, S.A.; Zheng, W.; Valiyaparambil, S.A.; et al. Advanced glycation end products dietary restriction effects on bacterial gut microbiota in peritoneal dialysis patients; a randomized open label controlled trial. PLoS ONE 2017, 12, e0184789. [Google Scholar] [CrossRef]
- Zhu, Y.; Tang, Y.; He, H.; Hu, P.; Sun, W.; Jin, M.; Wang, L.; Xu, X. Gut Microbiota Correlates With Clinical Responsiveness to Erythropoietin in Hemodialysis Patients With Anemia. Front. Cell. Infect. Microbiol. 2022, 12, 919352. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, C.; Lei, W.; Yang, Z.; Chen, J.; Lin, H.; Li, Q.; Yuan, W. Exploration of the correlation between intestinal flora and Escherichia coli peritoneal dialysis-related peritonitis. BMC Nephrol. 2022, 23, 76. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ouyang, S.; Xie, Y.; Gong, Z.; Du, J. Characterizing the gut microbiota in patients with chronic kidney disease. Postgrad. Med. 2020, 132, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-Y.; Wu, P.-H.; Lin, Y.-T.; Hung, S.-C. Gut dysbiosis and mortality in hemodialysis patients. NPJ Biofilms Microbiomes 2021, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Xie, Y. Effect of Different Hemodialysis Methods on Microbiota in Uremic Patients. BioMed Res. Int. 2020, 2020, 6739762. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wu, K.; Pan, W.; Zeng, Y.; Hu, K.; Chen, D.; Huang, X.; Zhang, Q. Intestinal flora alterations in patients with early chronic kidney disease: A case-control study among the Han population in southwestern China. J. Int. Med. Res. 2020, 48, 300060520926033. [Google Scholar] [CrossRef]
- Abdelbary, M.M.H.; Kuppe, C.; Michael, S.S.-Y.; Krüger, T.; Floege, J.; Conrads, G. Impact of sucroferric oxyhydroxide on the oral and intestinal microbiome in hemodialysis patients. Sci. Rep. 2022, 12, 9614. [Google Scholar] [CrossRef]
- Liu, H.; Wu, W.; Luo, Y. Oral and intravenous iron treatment alter the gut microbiome differentially in dialysis patients. Int. Urol. Nephrol. 2022, 55, 759–767. [Google Scholar] [CrossRef]
- Nazzal, L.; Roberts, J.; Singh, P.; Jhawar, S.; Matalon, A.; Gao, Z.; Holzman, R.; Liebes, L.; Blaser, M.J.; Lowenstein, J. Microbiome perturbation by oral vancomycin reduces plasma concentration of two gut-derived uremic solutes, indoxyl sulfate and p-cresyl sulfate, in end-stage renal disease. Nephrol. Dial. Transplant. 2017, 32, 1809–1817. [Google Scholar] [CrossRef]
- Belova, I.V.; Khrulev, A.E.; Tochilina, A.G.; Khruleva, N.S.; Lobanova, N.A.; Zhirnov, V.A.; Molodtsova, S.B.; Lobanov, V.N.; Solovieva, I.V. Colon Microbiocenosis and Its Correction in Patients Receiving Programmed Hemodialysis. Sovrem. Tekhnologii V Meditsine 2021, 12, 62–68. [Google Scholar] [CrossRef]
- Borges, N.A.; Carmo, F.L.; Stockler-Pinto, M.B.; de Brito, J.S.; Dolenga, C.J.; Ferreira, D.C.; Nakao, L.S.; Rosado, A.; Fouque, D.; Mafra, D. Probiotic Supplementation in Chronic Kidney Disease: A Double-blind, Randomized, Placebo-controlled Trial. J. Ren. Nutr. 2018, 28, 28–36. [Google Scholar] [CrossRef]
- Ebrahim, Z.; Proost, S.; Tito, R.Y.; Raes, J.; Glorieux, G.; Moosa, M.R.; Blaauw, R. The Effect of ß-Glucan Prebiotic on Kidney Function, Uremic Toxins and Gut Microbiome in Stage 3 to 5 Chronic Kidney Disease (CKD) Predialysis Participants: A Randomized Controlled Trial. Nutrients 2022, 14, 805. [Google Scholar] [CrossRef]
- Kemp, J.A.; Regis de Paiva, B.; Fragoso Dos Santos, H.; Emiliano de Jesus, H.; Craven, H.; U, Z.I.; Alvarenga Borges, N.; P, G.S.; Mafra, D. The Impact of Enriched Resistant Starch Type-2 Cookies on the Gut Microbiome in Hemodialysis Patients: A Randomized Controlled Trial. Mol. Nutr. Food Res. 2021, 65, e2100374. [Google Scholar] [CrossRef] [PubMed]
- Kimber, C.; Zhang, S.; Johnson, C.; West, R.E., 3rd; Prokopienko, A.J.; Mahnken, J.D.; Yu, A.S.; Hoofnagle, A.N.; Ir, D.; Robertson, C.E.; et al. Randomized, Placebo-Controlled Trial of Rifaximin Therapy for Lowering Gut-Derived Cardiovascular Toxins and Inflammation in CKD. Kidney360 2020, 1, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, H.; Chen, L.; Liang, S.S.; Shi, K.; Meng, W.; Xue, J.; He, Q.; Jiang, H. Effect of probiotics on the intestinal microbiota of hemodialysis patients: A randomized trial. Eur. J. Nutr. 2020, 59, 3755–3766. [Google Scholar] [CrossRef]
- McFarlane, C.; Krishnasamy, R.; Stanton, T.; Savill, E.; Snelson, M.; Mihala, G.; Kelly, J.T.; Morrison, M.; Johnson, D.W.; Campbell, K.L. Synbiotics Easing Renal Failure by Improving Gut Microbiology II (SYNERGY II): A Feasibility Randomized Controlled Trial. Nutrients 2021, 13, 4481. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Mora, J.; Martínez-Hernández, N.E.; Martín del Campo-López, F.; Viramontes-Hörner, D.; Vizmanos-Lamotte, B.; Muñoz-Valle, J.F.; García-García, G.; Parra-Rojas, I.; Castro-Alarcón, N. Effects of a symbiotic on gut microbiota in Mexican patients with end-stage renal disease. J. Ren. Nutr. 2014, 24, 330–335. [Google Scholar] [CrossRef]
- Rocchetti, M.T.; Di Iorio, B.R.; Vacca, M.; Cosola, C.; Marzocco, S.; di Bari, I.; Calabrese, F.M.; Ciarcia, R.; De Angelis, M.; Gesualdo, L. Ketoanalogs’ Effects on Intestinal Microbiota Modulation and Uremic Toxins Serum Levels in Chronic Kidney Disease (Medika2 Study). J. Clin. Med. 2021, 10, 840. [Google Scholar] [CrossRef]
- Rossi, M.; Johnson, D.W.; Morrison, M.; Pascoe, E.M.; Coombes, J.S.; Forbes, J.M.; Szeto, C.C.; McWhinney, B.C.; Ungerer, J.P.; Campbell, K.L. Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): A Randomized Trial. Clin. J. Am. Soc. Nephrol. 2016, 11, 223–231. [Google Scholar] [CrossRef]
- Simeoni, M.; Citraro, M.L.; Cerantonio, A.; Deodato, F.; Provenzano, M.; Cianfrone, P.; Capria, M.; Corrado, S.; Libri, E.; Comi, A.; et al. An open-label, randomized, placebo-controlled study on the effectiveness of a novel probiotics administration protocol (ProbiotiCKD) in patients with mild renal insufficiency (stage 3a of CKD). Eur. J. Nutr. 2019, 58, 2145–2156. [Google Scholar] [CrossRef]
- Laffin, M.R.; Tayebi Khosroshahi, H.; Park, H.; Laffin, L.J.; Madsen, K.; Kafil, H.S.; Abedi, B.; Shiralizadeh, S.; Vaziri, N.D. Amylose resistant starch (HAM-RS2) supplementation increases the proportion of Faecalibacterium bacteria in end-stage renal disease patients: Microbial analysis from a randomized placebo-controlled trial. Hemodial. International. Int. Symp. Home Hemodial. 2019, 23, 343–347. [Google Scholar] [CrossRef]
- Mosca, A.; Leclerc, M.; Hugot, J.P. Gut Microbiota Diversity and Human Diseases: Should We Reintroduce Key Predators in Our Ecosystem? Front. Microbiol. 2016, 7, 455. [Google Scholar] [CrossRef]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia intestinalis: A Beneficial Gut Organism From the Discoveries in Genus and Species. Front. Cell. Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, G.; Ossiprandi, M.C.; MacSharry, J.; van Sinderen, D.; Ventura, M. Bifidobacterial Dialogue With Its Human Host and Consequent Modulation of the Immune System. Front. Immunol. 2019, 10, 2348. [Google Scholar] [CrossRef]
- Zhao, J.; Ning, X.; Liu, B.; Dong, R.; Bai, M.; Sun, S. Specific alterations in gut microbiota in patients with chronic kidney disease: An updated systematic review. Ren. Fail. 2021, 43, 102–112. [Google Scholar] [CrossRef]
- Onal, E.M.; Afsar, B.; Covic, A.; Vaziri, N.D.; Kanbay, M. Gut microbiota and inflammation in chronic kidney disease and their roles in the development of cardiovascular disease. Hypertens. Res. 2019, 42, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, A.N.; West, N.R.; Stubbington, M.J.T.; Wendt, E.; Suijker, K.I.M.; Datsi, A.; This, S.; Danne, C.; Campion, S.; Duncan, S.H.; et al. Circulating and Tissue-Resident CD4(+) T Cells With Reactivity to Intestinal Microbiota Are Abundant in Healthy Individuals and Function Is Altered During Inflammation. Gastroenterology 2017, 153, 1320–1337.e16. [Google Scholar] [CrossRef]
- Hänninen, A.; Toivonen, R.; Pöysti, S.; Belzer, C.; Plovier, H.; Ouwerkerk, J.P.; Emani, R.; Cani, P.D.; De Vos, W.M. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut 2018, 67, 1445–1453. [Google Scholar] [CrossRef]
- Han, S.; Chen, M.; Cheng, P.; Zhang, Z.; Lu, Y.; Xu, Y.; Wang, Y. A systematic review and meta-analysis of gut microbiota in diabetic kidney disease: Comparisons with diabetes mellitus, non-diabetic kidney disease, and healthy individuals. Front. Endocrinol. 2022, 13, 1018093. [Google Scholar] [CrossRef]
- Deaver, J.A.; Eum, S.Y.; Toborek, M. Circadian Disruption Changes Gut Microbiome Taxa and Functional Gene Composition. Front. Microbiol. 2018, 9, 737. [Google Scholar] [CrossRef]
- Ramezani, A.; Massy, Z.A.; Meijers, B.; Evenepoel, P.; Vanholder, R.; Raj, D.S. Role of the Gut Microbiome in Uremia: A Potential Therapeutic Target. Am. J. Kidney Dis. 2016, 67, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.J.; Guo, J.; Wang, Q.; Wang, L.; Wang, Y.; Zhang, F.; Huang, W.J.; Zhang, W.; Liu, W.J.; Wang, Y. Probiotics, prebiotics, and synbiotics for the improvement of metabolic profiles in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 577–598. [Google Scholar] [CrossRef]
| Author, Year | Design | Patients, No | Age, Median/Mean ± SD | Gut Microbiota Composition |
|---|---|---|---|---|
| Barros et al., 2015 [21] | Cross-sectional study | 20 (CKD stage 3–4) | 64.4 ± 9.1 | Similar number of bands in patients with CKD vs. healthy controls. CKD: Listeria monocytogenes, Flavobacteriaceae bacterium. Healthy controls: Uncultured Lachnospiraceae bacterium, Butyrivibrio crossotus. |
| 19 (healthy participants) | 51.6 ± 6.6 | |||
| Belova et al., 2020 [78] | Randomized, parallel-group, controlled trial | 32 (HD patients who received basic therapy + symbiotic) | 57.1 ± 7.9 | HD patients displayed an increased number and diversity of Bacteroides spp., Clostridium spp., Collinsella spp., Eggerthella spp., and other bacteria. Grade I dysbiosis was observed in 37.5% of patients, grade II dysbiosis in 50.0% of patients, and grade III dysbiosis in 12.5% of patients. |
| 30 (HD patients who received basic therapy + placebo) | 54.7 ± 8.4 | |||
| Chen et al., 2021 [22] | Cross-sectional study | 96 (CKD stage 1–5) | 71 (CKD stage 3–5) | No significant difference was observed in α-diversity across different groups. Relative levels of Streptococcus, Klebsiella pneumonia, and Haemophilus Parainfluenzae increased progressively in advanced CKD. Increased levels of Fusobacterium varium and Fusobacterium mortiferum were observed in CKD stages 3–5. |
| 60 (healthy participants) | 66 | |||
| Du et al., 2021 [23] | Cross-sectional study | 43 (diabetic nephropathy stage 3–4) | 60.86 ± 5.69 | In patients with diabetic nephropathy, several genera were more abundant as compared to healthy individuals: Acidaminococcus, Lactobacillus, Megasphaera, Mitsuokella, Olsenella, Prevotella_7, and Sutterella. A model based on 25 genera discrepancies had a good prediction power for diabetic nephropathy (AUC = 0.972). |
| 37 (healthy individuals) | 61.78 ± 6.40 | |||
| Ebrahim et al., 2022 [80] | Randomized controlled trial | 59 (CKD stage 3–5) | 41.0 ± 11.6 | In CKD patients, several genera were abundant: Faecalibacterium, Prevotella, Bacteroides, Blautia, and Roseburia. |
| Gao et al., 2021 [24] | Cross-sectional study | 52 (CKD stage 3–5, including 10 patients with ESKD) | – | Eubacterium rectale and Collinsealla genera correlated with kidney disease severity. Bifidobacterium increased with kidney disease severity, while Lactobacillus decreased. Methanobacteria was abundant in advanced CKD stages but was not present in ESKD patients. |
| Gryp et al., 2021 [25] | Cross-sectional study | 111 (CKD stage 1–5) | – | Faecalibacterium, Bacteroides, and Roseburia were the most abundant genera in all CKD groups. Butyricicoccus (butyrate-generating properties) decreased in CKD stage 4–5 as compared to CKD stage 1–2 (p = 0.043). |
| Gryp et al., 2020 [26] | Cross-sectional study | 138 (CKD stage 1–5) and 14 controls | – | A. muciniphila, C. dicile, Enterobacteriaceae, Lactobacillus spp., and Streptococcus spp. were increased in HD patients compared to other CKD stages. Bifidobacterium spp. and Streptococcus spp. decreased with kidney function decline, while Enterobacteriaceae and E. coli increased. |
| Guirong et al., 2018 [27] | Cross-sectional study | 16 (kidney transplant) | 42.8 ± 11.5 | Microbial richness was lowest in kidney transplant patients (Chao1 index 249.6 ± 118.7) as compared to CKD group (Chao1 index 286.4 ± 89.3) and healthy controls (Chao1 index 394.5 ± 86.8). Bacteroides and Enterobacteriaceae abundance were increased in CKD and kidney transplant patients, while Lachnospira, Ruminococcaceae, and Faecalibacterium levels were decreased. Gut microbiota profile had a good power to discriminate between CKD and healthy controls (AUC 0.921). |
| 84 (CKD) | 55.9 ± 18.2 | |||
| 53 (healthy controls) | 54.7 ± 12.8 | |||
| Hanifi et al., 2021 [28] | Cross-sectional study | 20 (CKD or ESKD) | 53.20 ± 12.03 | CKD and ESKD patients had similar abundance of different Bifidobacteriaceae species as compared to those without CKD or ESKD. |
| 20 (non-CKD/ESKD) | 59.3 ± 7.89 | |||
| He et al., 2020 [73] | Cross-sectional study | 109 (ESKD patients) | 56.8 ± 15.5 (HD patients) | Bifidobacterium and Lactobacillus acidophilus decreased in ESKD patients as compared to healthy controls, while Escherichia coli and Enterococcus faecalis increased. Bifidobacterium and Lactobacillus acidophilus were higher in HD patients as compared to ESKD patients without RRT, while Escherichia coli and Enterococcus faecalis were decreased. |
| He et al., 2021 [29] | Cross-sectional study | 30 (HD) | 56.3 ± 13.6 | Patients with CKD (including HD) had a higher abundance of Escherichia coli and Enterococcus faecalis compared to healthy controls (p < 0.05), while Bifidobacterium and Lactobacillus acidophilus were decreased (p < 0.05). |
| 24 (non-HD) | 57.2 ± 15.1 | |||
| 30 (healthy controls) | 57.4 ± 14.9 | |||
| Hu et al., 2020 [71] | Cross-sectional study | 95 (CKD stages 1–5, non-HD) | 57.45 ± 11.68 (CKD stage 5) | Several genera (Escherichia-Shigella, Parabacteroides, Roseburia, Pyramidobacter rectale_group, Ruminococcaceae_NK4A214_group, Prevotellaceae_UCG.001, Hungatella, Intestinimonas, Pyramidobacter) discriminated between CKD stage 5 and healthy controls (AUC = 0.938, 95% CI, 0.853–1.000). Patients with CKD exhibited increased levels of Proteobacteria and decreased levels of Synergistetes as compared to healthy controls. |
| Hu et al., 2020 [74] | Case-control study | 47 (early CKD) | 43.2 ± 12.6 | α-diversity was lower in CKD versus healthy control participants. Proteobacteria and Actinobacteria were increased in CKD group vs. control group. Thirty-one species were different in CKD patients compared to healthy control (highest diagnostic power for Ruminococcus and Roseburia). Ruminococcus displayed the highest AUC for CKD prediction (0.771, 95% CI, 0.771–0.852). |
| 150 (healthy controls) | 38.5 ± 15.4 | |||
| Hu et al., 2020 [30] | Case-control study | 166 (47 non-dialysis CKD, 49 HD group, 53 PD group, and 17 healthy controls) | 57.80 ± 10.03 | α-diversity and β-diversity were lower in PD patients as compared to HD patients and control participants. Enterobacteriaceae and Enterococcaceae were highly expressed in PD patients, while Bifidobacteriaceae and Prevotellaceae were increased in the rest of the patients. |
| Iguchi et al., 2020 [31] | Cohort study | 38 (HD patients) | 66.17 ± 12.38 (sucroferric oxyhydroxide group) | Baseline phyla in HD patients: Firmicutes 67.5%; Proteobacteria 11.0%; Actinobacteria 12.2%; Bacteroides 9.2%. PD patients had lower levels of Bifidobacteriaceae and Prevotellaceae compared to other groups, while Enterobacteriaceae and Enterococcaceae were increased. |
| Jiang et al., 2016 [32] | Case-control study | 65 (CKD) | 43.45 ± 16.90 | Patients with advanced CKD and those with ESKD had significantly decreased abundance of Roseburia spp. and F. prausnitzii (respectively, p = 0.000 and p = 0.003). |
| 20 (healthy controls) | 43.05 ± 9.88 | |||
| Jiang et al., 2017 [33] | Cross-sectional study | 52 (ESKD patients) | 51.58 ± 18.33 | E. coli, Bifidobacterium, Bacteroides fragilis group, Enterococcus spp., Clostridium coccoides group, Faecalibacterium prausnitzii, Roseburia spp., and Prevotella were decreased in ESKD patients as compared to healthy controls. Lactobacillus group levels were similar in both groups. |
| 60 (healthy controls) | 52.53 ± 13.98 | |||
| Kemp et al., 2021 [81] | Randomized, double-blind, controlled clinical trial | 10 (Resistant starch type-2 group) | 53.2 ± 12.3 | Firmicutes phylum prevailed in HD patients. Subdoligranum, Fusicatenibacter, Prevotella, and Blautia were observed in HD patients. |
| 10 (placebo group) | 55.1 ± 11.1 | |||
| Khiabani et al., 2022 [34] | Cross-sectional | 20 (CKD/ESKD) | 53.20 ± 12.03 | Clostridium spp. abundance was similar in patients with CKD (including ESKD patients) and in healthy controls (p < 0.05). |
| 20 (healthy controls) | 59.3 ± 7.89 | |||
| Kim et al., 2020 [35] | Cross-sectional study | 103 (CKD stage 1–5) | 48.9 ± 12.2 (ESKD) | Alistipes, Oscillibacter, Lachnospira, Veillonella, and Dialister were higher in control group as compared to patients with moderate to severe CKD. Alistipes, Oscillibacter, Lachnospira, and Veillonella were increased in mild CKD patients as compared to the moderate to severe CKD group. |
| 46 (healthy controls) | 47.0 ± 10.8 | |||
| Kumar et al., 2021 [36] | Cross-sectional study | 36 (IgAN) | 45.5 ± 13.4 | α-diversity was similar between IgAN patients and healthy controls but increased in advanced CKD stages (p = 0.025). Fusobacteria phylum was increased, while Euryarchaoeota phylum was decreased in patients with IgAN, as compared to healthy controls. |
| 12 (healthy controls) | 46.5 ± 13.5 | |||
| Lai et al., 2019 [37] | Observational, prospective study | 16 (CKD stages 3–4) | – | Patients with CKD had increased levels of Bacteroidaceae, Enterobacteriaceae, and Rickenellaceae as compared to healthy controls. |
| 16 (healthy controls) | ||||
| Lecamwasam et al., 2021 [38] | Observational, prospective study | 95 (diabetes-associated CKD stages 1–5) | 66.24 ± 10.22 (early CKD) | β-diversity and α-diversity were similar across all CKD stages. Firmicutes (the most abundant) and Bacteroidetes phyla abundance were similar in early and late CKD stages. Prevotellaceae was decreased across all CKD stages. |
| 72.68 ± 10.21 (late CKD) | ||||
| Li et al., 2019 [39] | Observational, prospective study | 50 (CKD) | 52.40 ± 13.49 | The most prevalent bacteria in CKD patients were Firmicutes (42.27%), Bacteroidetes (37.85%), Proteobacteria (16.70%), Actinobacteria (1.48%), and Verrucomicrobia (0.67%). As compared to healthy controls, patients with CKD had decreased Akkermansia (p = 0.001) and Parasutterella (p = 0.007) levels, while Lactobacillus (p < 0.001), Clostridium IV (p = 0.015), and Alloprevotella (p < 0.001) levels were higher in CKD patients. Akkermansia associated with Lactobacillus had a good predictive value for CKD (AUC 0.830). |
| 22 (healthy controls) | 50.27 ± 7.77 | |||
| Lin et al., 2021 [72] | Prospective, cohort study | 109 (ESKD) | 68.4 ± 10.4 | In the high diversity group of patients, as well as in those with lower microbiota diversity, Bacteroidetes, Firmicutes, and Proteobacteria were the most prevalent phyla. |
| Lin et al., 2020 [41] | Case-control study | 96 (HD) | 68.1 ± 1.0 | α-diversity was decreased in patients with normal-weight obesity as compared to those with normal weight or obesity (p = 0.001). Firmicutes/Bacteroidetes ratio was similar in all weight groups. Faecalibacterium prausnitzii, Faecalibacterium, and Coprococcus were decreased in the normal-weight obesity group. |
| Lin et al., 2020 [40] | Case-control study | 88 (HD) | 68.6 ± 11.0 (protein-energy wasting) | α-diversity was decreased in HD patients with moderate protein-energy wasting as compared to those with a normal nutritional status (p = 0.018). Faecalibacterium prausnitzii was significantly lower in protein-energy wasting patients, while Akkermansia muciniphila levels were higher. |
| 68.6 ± 9.7 (normal nutritional status) | ||||
| Lin et al., 2022 [42] | Case-control study | 11 (ESKD) | 30.93 ± 4.85 | ESKD patients had a higher abundance of Escherichia coli (p < 0.001), Bacteroides fragilis (p = 0.010), Bacteroides fragilis (p = 0.010), and Bacteroides caccae (p = 0.047), as compared to healthy controls. |
| 11 (healthy controls) | 27.99 ± 2.31 | |||
| Liu et al., 2021 [43] | Case-control study | 100 (CKD) | 56.64 ± 17.25 | The Shannon index decrease was associated with CKD (p < 0.05). Actinobacteria levels were higher in CKD patients and predicted CKD prevalence (OR 1.037, 95% CI 1.007–1.068). Bacteroidetes was decreased in CKD patients and predicted CKD prevalence (OR 0.971, 95% CI, 0.951–0.991). Bifidobacterium, Enterococcus, and Streptococcus were more abundant in CKD patients. |
| 100 (healthy controls) | 60.64 ± 16.51 | |||
| Liu et al., 2020 [83] | Randomized controlled trial | 22 (HD + probiotic) | – | Desulfovibrionaceae, Veillonellaceae, and Lactobacillaceae abundance was increased in HD patients with diabetes mellitus, while Halomonadaceae and Bradyrhizobiaceae were decreased as compared to non-diabetic HD patients. |
| 23 (HD + placebo) | ||||
| Lun et al., 2018 [44] | Cross-sectional study | 49 (CKD) | 54 ± 14 | Patients with CKD had increased abundance of Bacteroidetes and Proteobacteria, while Firmicutes was lower compared to healthy controls. Ruminococcus gnavus had the best discrimination power for CKD (AUC 0.764, 95% CI, 0.656–0.873, p = 0.000). |
| 24 (healthy controls) | 56 ± 9 | |||
| Luo et al., 2021 [45] | Observational, cohort study | 73 (ESKD) | 49.71 ± 14.81 (HD) | HD and PD patients had an increased abundance of Blautia and Dorea, while Prevotella was decreased. Akkermansia, Coprococcus, Acinetobacter, Proteus, and Pseudomonas were increased in HD patients. |
| 48.95 ± 10.23 (healthy controls) | ||||
| Margiotta et al., 2020 [46] | Cross-sectional study | 64 (CKD stages 3b-4) | 80.7 ± 6.2 | α-diversity was similar in CKD patients as compared to controls. Patients with CKD had a higher abundance of Lactobacillus, Coprobacillus, Anaerotruncus, Citrobacter, and Ruminococcus torques. Patients with CKD had lower levels of saccharolytic and butyrate-producing bacteria (Prevotella spp., F. prausnitzii, and Roseburia spp.). |
| 15 (healthy controls) | 73.7 ± 7.6 | |||
| Al-Obaide et al., 2017 [47] | Cross-sectional study | 20 (T2DM and advanced CKD) | 64.4 ± 2.3 | In patients with advanced CKD and T2DM, Bifidobacterium abundance was decreased, while Clostridium, Escherichia, Enterobacter, Acinetobacter, Proteus, and Lactobacillus levels were increased as compared to healthy controls. |
| 20 (healthy controls) | 54.3 ± 3.2 | |||
| Pivari et al., 2022 [48] | Observational, cohort study | 24 (CKD) | 72 (67.5–78.8) | CKD patients had higher α-diversity as compared to healthy controls. CKD patients had decreased abundance of Bacteroides (p = 0.037), Lachnoclostridium spp. (p = 0.018), and Escherichia-Shigella (p = 0.048) compared to controls. |
| 20 (healthy controls) | 74 (68.5–78.7) | |||
| Ren et al., 2020 [49] | Observational, cohort study | 110 (CKD) | 51.75 ± 14.60 | In CKD patients, 36 genera were increased (including Klebsiella, Veillonella, and Desulfovibrio), while 16 genera were decreased (including Blautia, Roseburia, and Lachnospira). Clostridia, Verrucomicrobia, and Cyanobacteria were decreased in CKD patients compared to controls. |
| 210 (healthy controls) | 50.02 ± 4.56 | |||
| Salguero et al., 2019 [50] | Cross-sectional study | 20 (T2DM and CKD) | 62.8 ± 3.6 | Proteobacteria, Verrucomicrobia, and Fusobacteria abundance were increased in CKD patients with T2DM as compared to healthy controls (p < 0.05 for all). |
| 20 (healthy controls) | 58.5 ± 4.1 | |||
| Sato et al., 2021 [51] | Cross-sectional study | 30 (early CKD) | 68.83 ± 10.14 | CKD patients had increased abundance of Bacteroides coprocora and Bacteroides caccae, while Roseburia inulinivorans, Ruminococcus torques, and Ruminococcus lactaris were more abundant in the non-CKD group. |
| 60 (non-CKD) | 67.80 ± 11.48 | |||
| Simeoni et al., 2019 [88] | Randomized, placebo-controlled study | 14 (CKD stage 3a + probiotics) | 61.3 ± 5.2 | Lactobacillales and Bifidobacteria had decreased levels in patients with CKD stage 3a (respectively, 2.3 × 103 CFU/gr and 1.7 × 104 CFU/gr). |
| 14 (CKD stage 3a, placebo) | 58.2 ± 6.2 | |||
| Stadlbauer et al., 2017 [52] | Cross-sectional study | 30 (dialysis patients) | 61 (HD patients) | HD and PD patients had lower α-diversity index as compared to the control group (p < 0.05), but it was similar between HD and PD patients. Blautia obeum, Clostridium citroniae, and Clostridium bolteae levels were higher in HD patients compared to the control group. Clostridium citroniae and Clostridium bolteae were increased in PD patients compared to the control group. Faecalibacterium prausnizii, Roseburia intestinalis, and Clostridium nexile were decreased in HD patients compared to the control group. |
| 21 (healthy controls) | 58 | |||
| Wang et al., 2019 [53] | Cross-sectional study | 56 (CKD stages 1–4) | 47.45 ± 15.47 | Patients with CKD stage 5 had lower Enterobacter, Enterococcus, Bifidobacterium, Bacteroides, and Clostridium levels as compared to controls and patients with CKD stages 1–4 (p < 0.01 for all). Faecalibacterium and Roseburia were reduced in CKD stage 5 patients compared to CKD stages 1–4 and healthy controls (respectively, p = 0.018 and p < 0.001). |
| 72 (CKD stage 5) | 51.69 ± 14.05 | |||
| 61 (healthy controls) | 46.80 ± 10.47 | |||
| Wang et al., 2019 [54] | Observational, cohort study | 28 (ESKD group) | 43.9 ± 13.8 | α-diversity was similar between ESKD and healthy controls group. Patients with ESKD had increased levels of Prevotella, Faecalibacterium, and Fusobacterium, while Roseburia, Lachnospira, Dialister, and Bifidobacterium abundance were decreased. |
| 19 (healthy controls) | 44.1 ± 10.0 | |||
| Wang et al., 2020 [55] | Cross-sectional study | 223 (ESKD group) | – | ESKD patients displayed a higher abundance of Eggerthella lenta, Flavonifractor spp., Alistipes spp., Ruminococcus spp., and Fusobacterium spp., while Prevotella spp., Clostridium spp., Roseburia spp., Faecalibacterium prausnitzii, and Eubacterium rectale were decreased. |
| 69 (healthy controls) | ||||
| Wu et al., 2020 [56] | Cross-sectional study | 72 (CKD group) | 65.00 ± 5.94 (advanced CKD) | Bacteroides eggerthii had a good discriminatory power between early-stage CKD and healthy controls (AUC = 0.80, 95% CI, 0.67–0.93), which was higher than in the case of protein/creatinine ratio (AUC = 0.64) and serum urea (AUC = 0.72). |
| 20 (non-CKD group) | 64.00 ± 7.06 | |||
| Wu et al., 2020 [57] | Cross-sectional study | 92 (CKD group) | 66.2 ± 7.4 (advanced CKD) | CKD patients had increased abundance of Bacteroides, Blautia, Escherichia-Shigella, Collinsella, Lachnoclostridium, and Lactobacillus. Paraprevotella displayed a good discriminatory power between CKD patients and the control group (AUC = 0.78, 95% CI, 0.70–0.87). |
| 30 (control group) | 61.6 ± 8.7 | |||
| Wu et al., 2021 [58] | Cross-sectional study | 39 (CKD stages 4–5) | 56.52 ± 15.72 | CKD patients had increased abundance of Proteobacteria, Enterobacteriaceae, Enterobacteriales, Gammaproteobacteria, Lactobacillales, Escherichia_Shigella, Enterococcus, Enterococcaceae, and Lactobacillaceae. |
| 40 (healthy controls) | 56.35 ± 10.96 | |||
| Xu et al., 2017 [59] | Cross-sectional study | 32 (CKD group) | 53.34 ± 14.47 | Enterobacteriaceae and Corynebacteriaceae were more abundant in CKD patients as compared to controls, while Ruminococcaceae levels were decreased. Enterococcus and Clostridium were increased in CKD patients, whereas Roseburia and Coprococcus were decreased. |
| 32 (healthy controls) | 55.03 ± 10.38 | |||
| Zhang et al., 2021 [60] | Cross-sectional study | 46 (ESKD group) | Stratified in groups | Ruminococcus gnavus, Ruminococcus spp., Eubacterium dolichum, Bacteroides ovatus, and Phascolarctobacterium were more abundant in ESKD patients (including immunoglobulin A nephropathy), compared to healthy controls, while Megamonas spp., Roseburia spp., and Eubacterium biforme were decreased. |
| 15 (healthy controls) | ||||
| Zhang et al., 2020 [61] | Cross-sectional study | 80 (CKD stages 3–5) | 49.50 ± 24.80 | Megamonas, Megasphaera, Akkermansia, Lachnospira, Roseburia, and Fusobacterium were increased in healthy controls as compared to patients with CKD and nephrotic syndrome. Patients with CKD and nephrotic syndrome had increased levels of Parabacteroides. Oscillospira and Ruminococcus were more abundant in the CKD group. |
| 48 (nephrotic syndrome) | 48.47 ± 20.47 | |||
| 30 (healthy controls) | 46.50 ± 22.67 | |||
| Zheng et al., 2020 [62] | Observational, cohort study | 28 (ESKD) | 43.9 ± 13.8 | Patients with ESKD had increased levels of Holdemania, Eggerthella, and Phascolarctobacterium, while Roseburia, Bifidobacterium, and Lachnospira were decreased as compared to healthy controls. |
| 19 (healthy controls) | 44.1 ± 10.0 |
| Study, Year | Outcomes | Results |
|---|---|---|
| Barros et al., 2015 [21] | Inflammation | VCAM-1 levels were negatively correlated with number of bands in CKD patients (r = −0.50, p = 0.03) |
| Ebrahim et al., 2022 [80] | Renal function decline | Creatinine levels were similar between intervention (β-glucan prebiotic) and control group during follow-up (14 weeks). |
| Jiang et al., 2017 [33] | Inflammation | Roseburia spp., Faecalibacterium prausnitzii, and Prevotella were negatively correlated with CRP (respectively, r = −0.452, p = 0.001; r = −0.431, p = 0.002 and r = −0.480, p = 0.000) |
| Renal function | Roseburia spp., Faecalibacterium prausnitzii, Clostridium coccoides group, Prevotella were negatively correlated with Cystatin C levels (respectively, r = −0.414, p = 0.003; r = −0.395, p = 0.005; r = −0.400, p = 0.001 and r = −0.441, p = 0.001) Bifidobacterium was correlated with creatinine and blood urea nitrogen (r = −0.538, p = 0.000 and r = −0.495, p = 0.000, respectively) | |
| Jiang et al., 2016 [32] | Inflammation | In CKD patients, Roseburia spp. and F. prausnitzii were negatively correlated with CRP (respectively, (r = −0.493, p = 0.00; r = -0.528, p = 0.000). |
| Disease progression | In CKD patients, Roseburia spp. and F. prausnitzii were negatively correlated with Cystatin C (r = −0.321, p = 0.006; r = −0.445, p = 0.000) and positively corelated with eGFR (respectively, r = 0.347, p = 0.002 and r = 0.416, p = 0.000). | |
| Lin et al., 2021 [72] | Mortality | The Simpson index and the Shannon index were lower in non-survivors as compared to patients who survived (respectively, p = 0.007 and p = 0.028). Non-survivors had higher levels of Oscillospira, Achromobacter, Agrobacterium, PSB_M_3, Lactobacillus, vadinCA02, Alloscardovia, Anoxybacillus, Devosia, and Yersinia. |
| Lin et al., 2020 [41] | Inflammation | The Shannon diversity index was negatively corelated with IL-6 (r = −0.253, p = 0.015) and TNFα (r = −0.260, p = 0.011). Faecalibacterium prausnitzii was negatively correlated with TNFα (r = −0.204, p = 0.047). |
| Lin et al., 2020 [40] | Inflammation | The Shannon diversity index was negatively corelated with IL-6 (r = −0.339, p = 0.001) and TNFα (r = −0.331, p = 0.002). |
| Luo et al., 2021 [45] | Mortality | ESKD patients with cardiovascular mortality had a lower proportion of Bacteroides and Phascolarctobacterium compared to survivors (p < 0.05). |
| Peritonitis | PD patients with peritonitis had decreased Dorea, Clostridium, and SMB53 proportions as compared to those without peritonitis (p < 0.05). | |
| Margiotta et al., 2020 [46] | Inflammation | Mogibacteriaceae and Oscillospira were correlated with CRP levels. Akkermansia, Ruminococcus, and Eubacterium were negatively correlated with the neutrophil-to-lymphocyte ratio. |
| Zhou et al., 2022 [70] | Peritonitis | PD patients with Escherichia coli peritonitis had higher abundance of Bacteroidetes and Synergistetes compared to the non-peritonitis group, while Bacilli and Lactobacillus were decreased. |
| Zhu et al., 2022 [69] | Responsiveness to erythropoietin | Neisseria, Streptococcus, Porphyromonas, Fusobacterium, Prevotella_7, Rothia, Leptotrichia, Prevotella, and Actinomyces could predict a poor response to erythropoietin in ESKD patients. Neisseria had an excellent power to discriminate between good and poor response to erythropoietin in ESKD patients (AUC 0.9535, 95% CI, 0.902–1.0, p < 0.0001). |
| Study, Year | Type of Therapy | Results |
|---|---|---|
| Abdelbary et al., 2022 [75] | Sucroferric oxyhydroxide | In hemodialysis patients, Veillonella spp. and Ruminococcus torques levels increased (p = 0.0351 for both), while Subdoligranulum decreased (p = 0.0496). |
| Belova et al., 2020 [78] | Immobilized synbiotic LB-complex L vs. placebo | In 56% of patients in the treatment group, gut microbiota recovered as compared to placebo (grade III dysbiosis was absent after therapy). CRP decreased from 6.8 ± 3.1 g/L to 5.3 g/L in the treatment group. |
| Borges et al., 2017 [79] | Probiotics | Gut microbiota profile was similar in the probiotic group (Streptococcus thermophilus, Lactobacillus acidophilus, and Bifidobacteria longum strains) and placebo group after 3 months of therapy (similar number of bands). |
| Ebrahim et al., 2022 [80] | β-glucan prebiotic | Prevotella tended to increase in the intervention group (β-glucan) as compared to the control group, while Bacteroides and Blautia tended to decrease. |
| Hu et al., 2022 [66] | Dietary intervention | HD patients from the protein-energy wasting group had lower abundance of Roseburia as compared to HD patients in the non-protein energy wasting group (p = 0.022). Escherichia abundance was increased in PD patients from the protein-energy wasting group compared to PD patients from the non-protein-energy wasting group (p = 0.022). |
| Iguchi et al., 2020 [31] | Sucroferric oxyhydroxide | In HD patients, sucroferric oxyhydroxide did not affect major phyla (p = 0.849 for Firmicutes, p = 0.776 for Proteobacteria, p = 0.517 for Actinobacteria, p = 0.728 for Bacteroides). |
| Jiang et al., 2020 [67] | Dietary intervention | Patients with CKD stage 5 who received a very low protein diet had higher levels of Escherichia, Shigella, and Klebsiella, while Blautia was decreased. |
| Kemp et al., 2021 [81] | Resistant starch type-2 | Resistant starch type-2 increased Oscillosperaceae, Roseburia, and Ruminococcus gauvreauii levels. Resistant starch type-2 decreased Ruminococcus champanellens, Dialister, and Coprococcus. |
| Kimber et al., 2020 [82] | Rifaximin | Rifaximin was linked to reduced diversity and richness of microbiota as compared to placebo. Rifaximin reduced 10 bacterial taxa from Firmicutes and Actinobacteria phyla (including Clostridium, Turicibacter, and Anaerotruncus). |
| Laffin et al., 2019 [89] | Amylose-resistant starch | Amylose-resistant starch increased levels of Faecalibacterium in ESKD patients as compared to placebo (from 0.40 ± 0.50% to 3.21 ± 4.97%, p = 0.03), while Parabacteroides, Bifidobacteria, Ruminococcus, and Prevotella levels did not change. |
| Lai et al., 2019 [37] | Low-protein diet | Low-protein diet increased Akkermansiaceae and Bacteroidaceae and decreased Christensenellaceae, Clostridiaceae, Lactobacillaceae, and Pasteurellaceae levels. |
| Low-protein diet + inulin | Low-protein diet associated with inulin therapy increased Bifidobacteriaceae levels. | |
| Inulin | Inulin decreased Enterobacteriaceae family. | |
| Liu et al., 2020 [83] | Probiotics | Probiotics increased Bacteroidaceae and Enterococcaceae abundance compared to placebo. Probiotics decreased Ruminococcaceae, Halomonadaceae, Peptostreptococcaceae, and Clostridiales Family XIII levels compared to placebo. |
| Liu et al., 2022 [76] | Iron supplementation | After oral iron supplementation, α-diversity and Firmicutes levels decreased, while Bacteroides increased. Moreover, Blautia and Coprococcus levels decreased, while Bacteroidetes increased. |
| McFarlane et al., 2021 [84] | Synbiotics vs. placebo | Synbiotic therapy increased Bifidobacterium animalis (p < 0.001) and Blautia spp. levels (p = 0.004). Synbiotics decreased Bacteroides cellulosilyticus and Ruminiclostridium spp. (p < 0.05 for both). Synbiotic therapy was linked to eGFR decrease with 3.14 mL/min/1.73 m2 (p < 0.01). |
| Miao et al., 2018 [63] | Lanthanum carbonate | In HD patients, lanthanum carbonate decreased Bacteroides and Proteobacteria but increased Actinobacteria levels. Shannon index decreased following lanthanum carbonate therapy. |
| Cruz-Mora et al., 2014 [85] | Synbiotics | In HD patients, synbiotic therapy increased Bifidobacterium abundance (p = 0.0344) but decreased Lactobacillus levels. |
| Nazzal et al., 2017 [77] | Oral vancomycin | Following vancomycin therapy, Clostridia, Roseburia, Enterococcaceae, and Bacteroidales decreased, while Veillonellaceae increased. |
| Pivari et al., 2022 [48] | Curcumin supplementation | After 6 months of dietary intervention, Escherichia-Shigella levels significantly decreased, while Lachnoclostridium and Lactobacillaceae spp. increased. |
| Rocchetti et al., 2021 [86] | Dietary intervention | The keto analogs-supplemented Mediterranean diet reduced Clostridiaceae, Methanobacteriaceae, Prevotellaceae, and Lactobacillaceae abundance, while Bacteroidaceae and Lachnospiraceae levels increased. |
| Rossi et al., 2016 [87] | Synbiotics | Compared to placebo, synbiotics were linked to a 5-fold increase in Bifidobacterium spp. (p = 0.003), while Lactobacillus spp. abundance was similar. |
| Simeoni et al., 2019 [88] | Probiotics | Compared to the placebo group, probiotics increased Lactobacillales and Bifidobacteria levels from 2.1 × 103 CFU/gr and 1.9 × 104 CFU/gr to 2.2 × 106 CFU/gr and 2.5 × 107 CFU/gr, respectively (p < 0.001 for both). Iron and ferritin levels were significantly increased after probiotic therapy (p < 0.001 for both), while CRP, total cholesterol, and triglycerides levels were decreased in patients who received probiotics (respectively, p < 0.001, p < 0.01, and p < 0.01). |
| Wu et al., 2020 [64] | Dietary intervention | CKD patients who received a low protein diet had lower levels of Lachnospiraceae and Bacteroidaceae as compared to those receiving a normal protein diet. |
| Wu et al., 2020 [65] | Phosphate binders | α-diversity and Simpson index were decreased in HD patients receiving calcium carbonate compared to the ferric citrate group (respectively, p = 0.049 and p = 0.001). Patients receiving ferric citrate had increased levels of Bacteroidetes phylum levels, while Firmicutes phylum was decreased. |
| Yacoub et al., 2017 [68] | Advanced glycation end products | PD patients who received a one-month advanced glycation end-product restriction had a lower abundance of Prevotella copri compared to those with a normal diet. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voroneanu, L.; Burlacu, A.; Brinza, C.; Covic, A.; Balan, G.G.; Nistor, I.; Popa, C.; Hogas, S.; Covic, A. Gut Microbiota in Chronic Kidney Disease: From Composition to Modulation towards Better Outcomes—A Systematic Review. J. Clin. Med. 2023, 12, 1948. https://doi.org/10.3390/jcm12051948
Voroneanu L, Burlacu A, Brinza C, Covic A, Balan GG, Nistor I, Popa C, Hogas S, Covic A. Gut Microbiota in Chronic Kidney Disease: From Composition to Modulation towards Better Outcomes—A Systematic Review. Journal of Clinical Medicine. 2023; 12(5):1948. https://doi.org/10.3390/jcm12051948
Chicago/Turabian StyleVoroneanu, Luminita, Alexandru Burlacu, Crischentian Brinza, Andreea Covic, Gheorghe G. Balan, Ionut Nistor, Cristina Popa, Simona Hogas, and Adrian Covic. 2023. "Gut Microbiota in Chronic Kidney Disease: From Composition to Modulation towards Better Outcomes—A Systematic Review" Journal of Clinical Medicine 12, no. 5: 1948. https://doi.org/10.3390/jcm12051948
APA StyleVoroneanu, L., Burlacu, A., Brinza, C., Covic, A., Balan, G. G., Nistor, I., Popa, C., Hogas, S., & Covic, A. (2023). Gut Microbiota in Chronic Kidney Disease: From Composition to Modulation towards Better Outcomes—A Systematic Review. Journal of Clinical Medicine, 12(5), 1948. https://doi.org/10.3390/jcm12051948









