Upadacitinib for Patients with Rheumatoid Arthritis: A Comprehensive Review
Abstract
1. Introduction
2. Pharmacodynamics
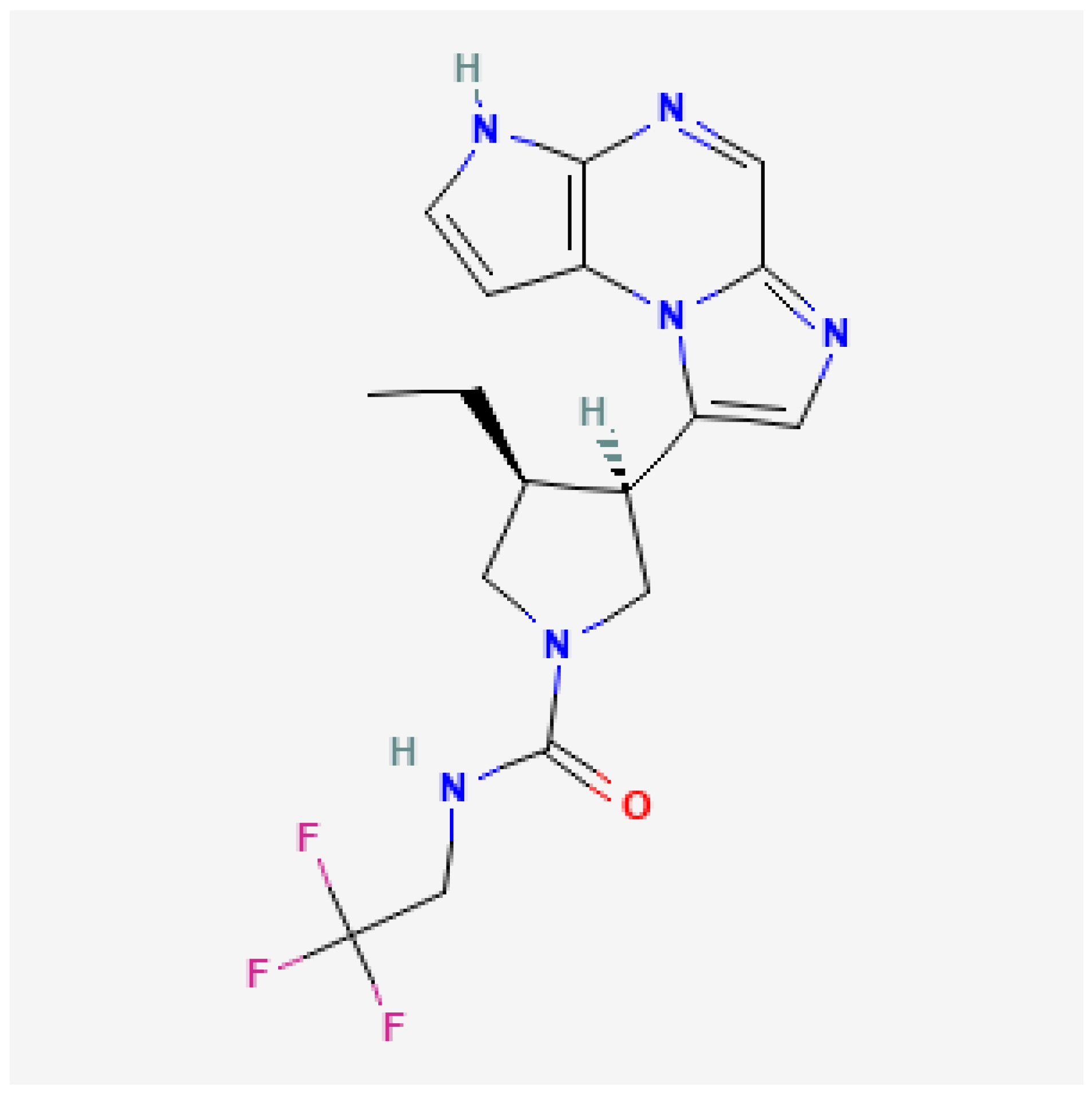
3. Chemical Structure and Pharmacokinetics
4. Efficacy
4.1. Phase 2 Studies with Upadacitinib and Dose Selection for the Phase 3 Program
4.2. The SELECT Phase 3 Trial Program with Upadacitinib in Patients with RA
4.2.1. Primary Efficacy Results
Upadacitinib in Patients with an Inadequate Response to Methotrexate or Other DMARDs
- Placebo-Controlled Trials
- Active-Controlled Trials
- Upadacitinib as Monotherapy in Patients with Inadequate Response to csDMARDs
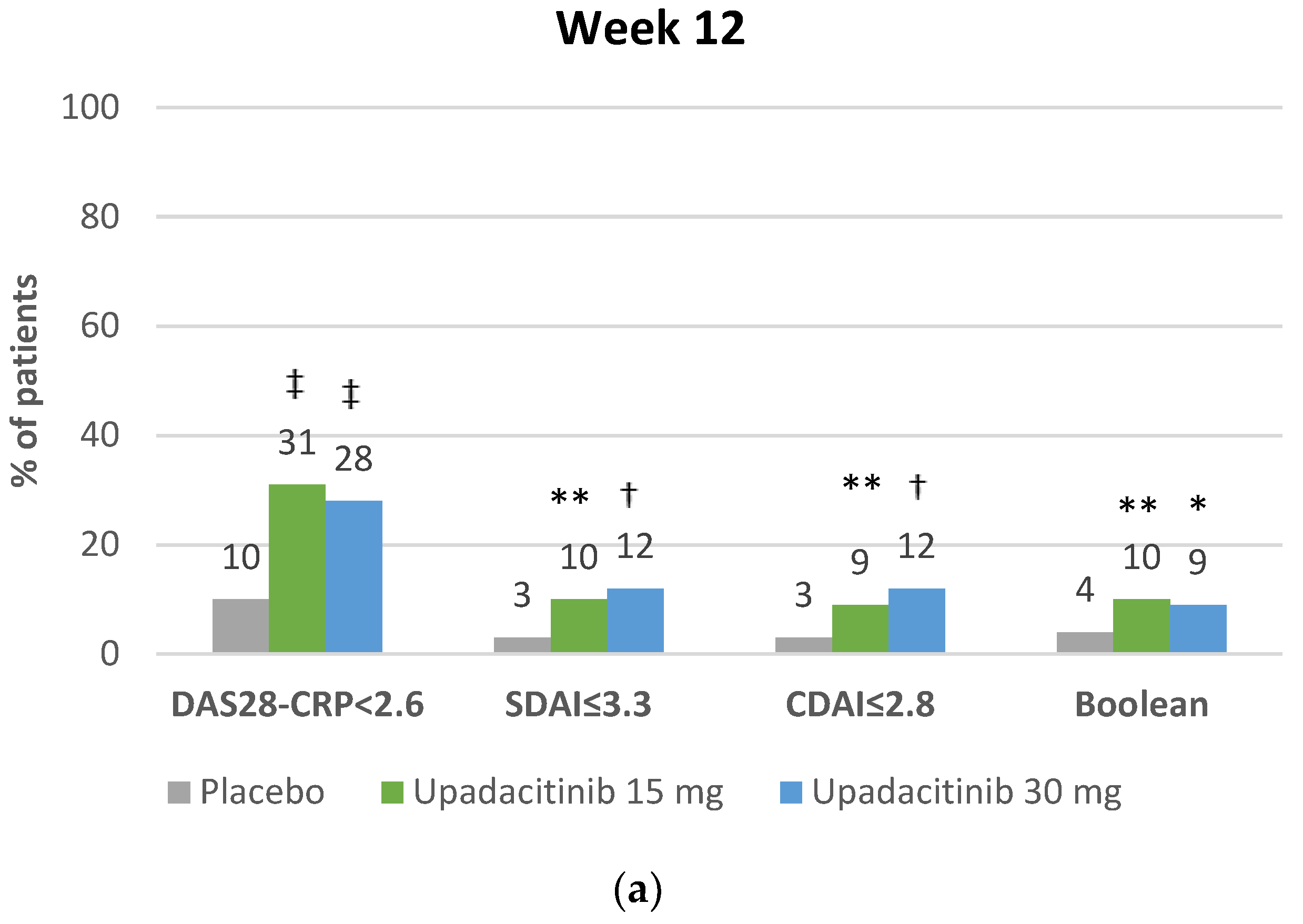
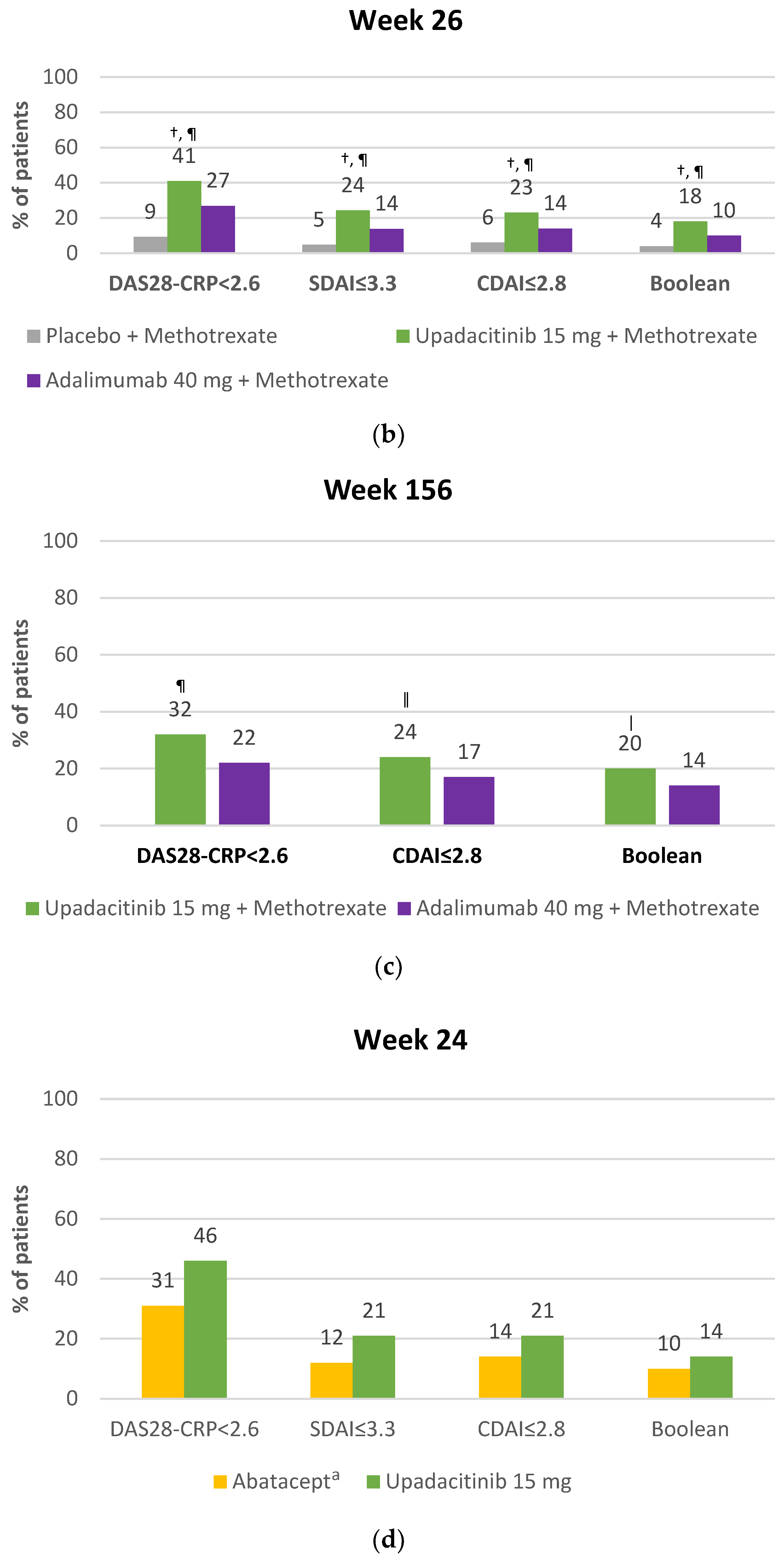
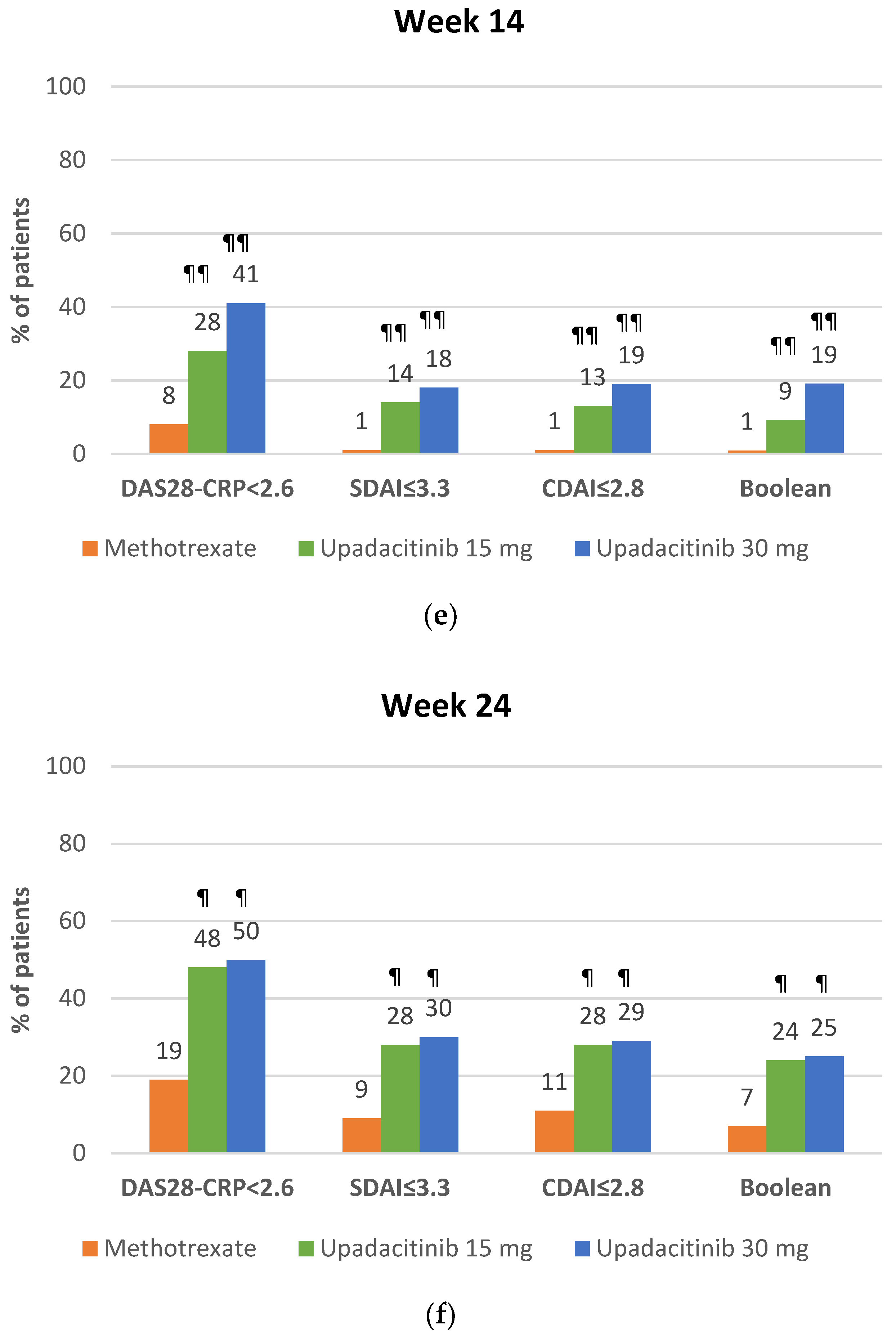
4.2.2. Clinical Remission with Upadacitinib
4.2.3. Impact of Upadacitinib on Patient-Reported Outcomes
4.2.4. Radiographic Outcomes with Upadacitinib
4.3. Comparative Efficacy of Janus Kinase Inhibitors
4.4. Real-World Data with Upadacitinib
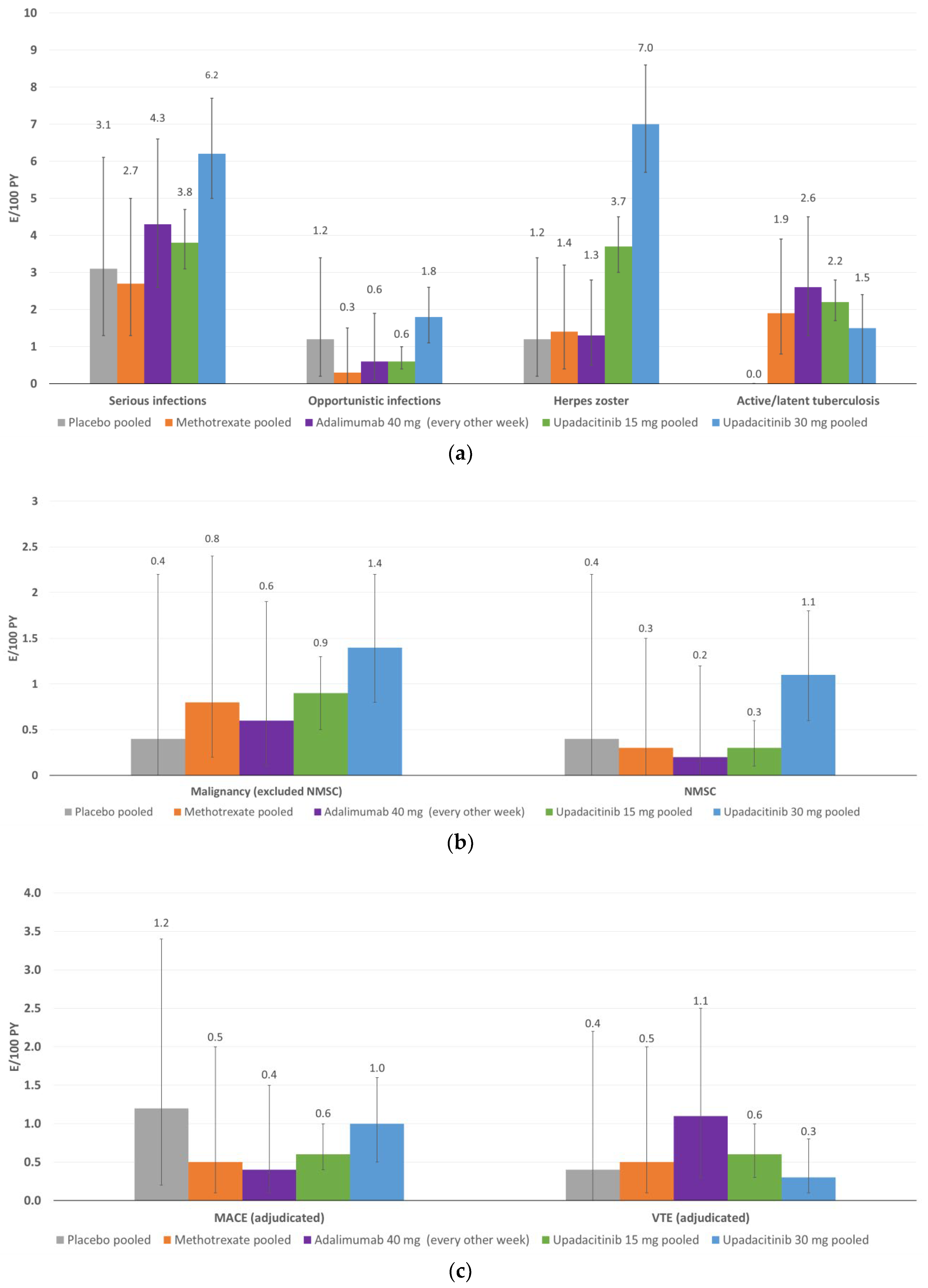
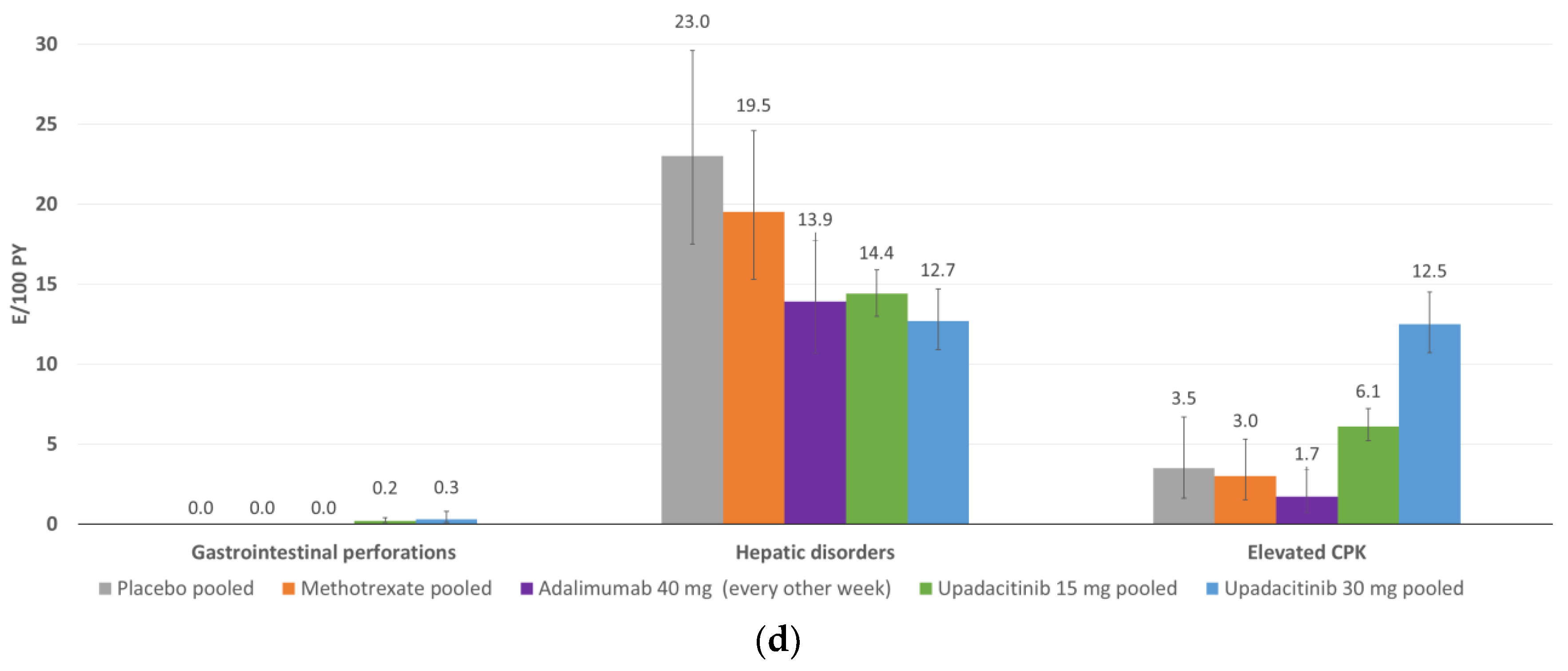
5. Safety
5.1. Overall Exposure and Tolerability/Safety Data
5.2. Infection
5.3. Malignancies
5.4. Cardiovascular Events
5.5. Other Events of Special Interest
5.6. To Date, Integrated Safety Analysis of Upadacitinib
6. A Clinician’s Perspective on Upadacitinib
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aletaha, D.; Smolen, J.S. Diagnosis and management of rheumatoid arthritis: A review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef]
- Baig, S.; DiRenzo, D.D. Complementary and Alternative Medicine Use in Rheumatoid Arthritis. Curr. Rheumatol. Rep. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Fragoulis, G.E.; Brock, J.; Basu, N.; McInnes, I.B.; Siebert, S. The role for JAK inhibitors in the treatment of immune-mediated rheumatic and related conditions. J. Allergy Clin. Immunol. 2021, 148, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, F.R.; Meylan, F.; O’Shea, J.J.; Gadina, M. JAK inhibitors: Ten years after. Eur. J. Immunol. 2021, 51, 1615–1627. [Google Scholar] [CrossRef]
- European Medicines Agency. RINVOQ. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/rinvoq-epar-product-information_en.pdf (accessed on 22 November 2021).
- European Medicines Agency. JYSELECA. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/jyseleca-epar-product-information_en.pdf (accessed on 22 November 2021).
- Nash, P. Clinical use of JAK 1 inhibitors for rheumatoid arthritis. Rheumatology 2021, 60, ii31–ii38. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef]
- Rocha, C.M.; Alves, A.M.; Bettanin, B.F.; Majolo, F.; Gehringer, M.; Laufer, S.; Goettert, M.I. Current jakinibs for the treatment of rheumatoid arthritis: A systematic review. Inflammopharmacology 2021, 29, 595–615. [Google Scholar] [CrossRef]
- Fleischmann, R. Recent issues in JAK inhibitor safety: Perspective for the clinician. Expert. Rev. Clin. Immunol. 2022, 18, 295–307. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef]
- Morinobu, A. JAK inhibitors for the treatment of rheumatoid arthritis. Immunol. Med. 2020, 43, 148–155. [Google Scholar] [CrossRef]
- Parmentier, J.M.; Voss, J.; Graff, C.; Schwartz, A.; Argiriadi, M.; Friedman, M.; Camp, H.S.; Padley, R.J.; George, J.S.; Hyland, D.; et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494). BMC Rheumatol. 2018, 2, 23. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 58557659, Upadacitinib. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Upadacitinib (accessed on 18 November 2021).
- Mohamed, M.F.; Klünder, B.; Othman, A.A. Clinical pharmacokinetics of upadacitinib: Review of data relevant to the rheumatoid arthritis indication. Clin. Pharmacokinet. 2020, 59, 531–544. [Google Scholar] [CrossRef]
- Kremer, J.M.; Emery, P.; Camp, H.S.; Friedman, A.; Wang, L.; Othman, A.A.; Khan, N.; Pangan, A.L.; Jungerwirth, S.; Keystone, E.C. A phase IIb study of ABT-494, a selective JAK-1 inhibitor, in patients with rheumatoid arthritis and an inadequate response to anti-tumor necrosis factor therapy. Arthritis Rheumatol. 2016, 68, 2867–2877. [Google Scholar] [CrossRef]
- Genovese, M.C.; Smolen, J.S.; Weinblatt, M.E.; Burmester, G.R.; Meerwein, S.; Camp, H.S.; Wang, L.; Othman, A.A.; Khan, N.; Pangan, A.L.; et al. Efficacy and safety of ABT-494, a selective JAK-1 inhibitor, in a phase IIb study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheumatol. 2016, 68, 2857–2866. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Klünder, B.; Camp, H.S.; Othman, A.A. Exposure-response analyses of upadacitinib efficacy in phase II trials in rheumatoid arthritis and basis for phase III dose selection. Clin. Pharmacol. Ther. 2019, 106, 1319–1327. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research. Guidance for Industry Rheumatoid Arthritis: Developing Drug Products for Treatment. Draft Guidance; U.S. Department of Health and Human Services: Washington, DC, USA, 2013.
- European Medicines Agency. Guideline on Clinical Investigation of Medicinal Products for the Treatment of Rheumatoid Arthritis; European Medicines Agency: Amsterdam, The Netherlands, 2017.
- Burmester, G.R.; Kremer, J.M.; Van den Bosch, F.; Kivitz, A.; Bessette, L.; Li, Y.; Zhou, Y.; Othman, A.A.; Pangan, A.L.; Camp, H.S. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018, 391, 2503–2512. [Google Scholar] [CrossRef] [PubMed]
- Genovese, M.C.; Fleischmann, R.; Combe, B.; Hall, S.; Rubbert-Roth, A.; Zhang, Y.; Zhou, Y.; Mohamed, M.F.; Meerwein, S.; Pangan, A.L. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): A double-blind, randomised controlled phase 3 trial. Lancet 2018, 391, 2513–2524. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, R.; Pangan, A.L.; Song, I.H.; Mysler, E.; Bessette, L.; Peterfy, C.; Durez, P.; Ostor, A.J.; Li, Y.; Zhou, Y.; et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: Results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol. 2019, 71, 1788–1800. [Google Scholar] [CrossRef] [PubMed]
- Rubbert-Roth, A.; Enejosa, J.; Pangan, A.L.; Haraoui, B.; Rischmueller, M.; Khan, N.; Zhang, Y.; Martin, N.; Xavier, R.M. Trial of upadacitinib or abatacept in rheumatoid arthritis. N. Engl. J. Med. 2020, 383, 1511–1521. [Google Scholar] [CrossRef]
- Smolen, J.S.; Pangan, A.L.; Emery, P.; Rigby, W.; Tanaka, Y.; Vargas, J.I.; Zhang, Y.; Damjanov, N.; Friedman, A.; Othman, A.A.; et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): A randomised, placebo-controlled, double-blind phase 3 study. Lancet 2019, 393, 2303–2311. [Google Scholar] [CrossRef]
- van Vollenhoven, R.; Takeuchi, T.; Pangan, A.L.; Friedman, A.; Mohamed, M.F.; Chen, S.; Rischmueller, M.; Blanco, R.; Xavier, R.M.; Strand, V. Efficacy and safety of upadacitinib monotherapy in methotrexate-naive patients with moderately-to-severely active rheumatoid arthritis (SELECT-EARLY): A multicenter, multi-country, randomized, double-blind, active comparator-controlled trial. Arthritis Rheumatol. 2020, 72, 1607–1620. [Google Scholar] [CrossRef] [PubMed]
- Citrome, L. Quantifying clinical relevance. Innov. Clin. Neurosci. 2014, 11, 26–30. [Google Scholar] [PubMed]
- Rendas-Baum, R.; Wallenstein, G.V.; Koncz, T.; Kosinski, M.; Yang, M.; Bradley, J.; Zwillich, S.H. Evaluating the efficacy of sequential biologic therapies for rheumatoid arthritis patients with an inadequate response to tumor necrosis factor-α inhibitors. Arthritis Res. Ther. 2011, 13, R25. [Google Scholar] [CrossRef] [PubMed]
- Schmalzing, M.; Behrens, F.; Schwaneck, E.C.; Koehm, M.; Greger, G.; Gnann, H.; Burkhardt, H.; Tony, H.P. Does concomitant methotrexate confer clinical benefits in patients treated with prior biologic therapy? Analysis of data from a noninterventional study of rheumatoid arthritis patients initiating treatment with adalimumab. Medicine 2020, 99, e20201. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.C.; Keystone, E.C.; van der Heijde, D.; Weinblatt, M.E.; Del Carmen Morales, L.; Reyes Gonzaga, J.; Yakushin, S.; Ishii, T.; Emoto, K.; Beattie, S.; et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N. Engl. J. Med. 2017, 376, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Keystone, E.C.; Kavanaugh, A.F.; Sharp, J.T.; Tannenbaum, H.; Hua, Y.; Teoh, L.S.; Fischkoff, S.A.; Chartash, E.K. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: A randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004, 50, 1400–1411. [Google Scholar] [CrossRef] [PubMed]
- Emery, P.; Sebba, A.; Huizinga, T.W. Biologic and oral disease-modifying antirheumatic drug monotherapy in rheumatoid arthritis. Ann. Rheum. Dis. 2013, 72, 1897–1904. [Google Scholar] [CrossRef]
- Thomas, K.; Kaltsonoudis, E.; Drosos, A.; Papalopoulos, I.; Sidiropoulos, P.; Katsimbri, P.; Boumpas, D.; Tsatsani, P.; Gazi, S.; Grika, E.; et al. SAT0176 patterns of biologic dmard monotherapy in a large nationwide rheumatoid arthritis cohort: Data from 1036 patients. Ann. Rheum. Dis. 2017, 76, 836–837. [Google Scholar] [CrossRef]
- Choy, E.; Aletaha, D.; Behrens, F.; Finckh, A.; Gomez-Reino, J.; Gottenberg, J.-E.; Schuch, F.; Rubbert-Roth, A. Monotherapy with biologic disease-modifying anti-rheumatic drugs in rheumatoid arthritis. Rheumatology 2016, 56, 689–697. [Google Scholar] [CrossRef]
- Fraenkel, L.; Bathon, J.M.; England, B.R.; St Clair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2021, 73, 1108–1123. [Google Scholar] [CrossRef]
- Fleischmann, R.; Mysler, E.; Bessette, L.; Peterfy, C.G.; Durez, P.; Tanaka, Y.; Swierkot, J.; Khan, N.; Bu, X.; Li, Y.; et al. Long-term safety and efficacy of upadacitinib or adalimumab in patients with rheumatoid arthritis: Results through 3 years from the SELECT-COMPARE study. RMD Open 2022, 8, e002012. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.M.; Kanno, Y.; Villarino, A.; Ward, M.; Gadina, M.; O’Shea, J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017, 16, 843–862. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T.; Smolen, J.S.; Wells, G.; Zhang, B.; van Tuyl, L.H.; Funovits, J.; Aletaha, D.; Allaart, C.F.; Bathon, J.; Bombardieri, S.; et al. American college of rheumatology/European league against rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum. 2011, 63, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Britsemmer, K.; van Schaardenburg, D.; Boers, M.; De Cock, D.; Verschueren, P.; Radner, H.; Smolen, J.S.; van Tuyl, L.H.D. Prevalence and validity of ACR/EULAR remission in four European early rheumatoid arthritis cohorts. Clin. Exp. Rheumatol. 2018, 36, 362–370. [Google Scholar]
- Shahouri, S.H.; Michaud, K.; Mikuls, T.R.; Caplan, L.; Shaver, T.S.; Anderson, J.D.; Weidensaul, D.N.; Busch, R.E.; Wang, S.; Wolfe, F. Remission of rheumatoid arthritis in clinical practice: Application of the American college of rheumatology/European league against rheumatism 2011 remission criteria. Arthritis Rheum. 2011, 63, 3204–3215. [Google Scholar] [CrossRef]
- Thiele, K.; Huscher, D.; Bischoff, S.; Späthling-Mestekemper, S.; Backhaus, M.; Aringer, M.; Kohlmann, T.; Zink, A. Performance of the 2011 ACR/EULAR preliminary remission criteria compared with DAS28 remission in unselected patients with rheumatoid arthritis. Ann. Rheum. Dis. 2013, 72, 1194–1199. [Google Scholar] [CrossRef]
- Fleischmann, R.M.; Genovese, M.C.; Enejosa, J.V.; Mysler, E.; Bessette, L.; Peterfy, C.; Durez, P.; Ostor, A.; Li, Y.; Song, I.H. Safety and effectiveness of upadacitinib or adalimumab plus methotrexate in patients with rheumatoid arthritis over 48 weeks with switch to alternate therapy in patients with insufficient response. Ann. Rheum. Dis. 2019, 78, 1454–1462. [Google Scholar] [CrossRef]
- Heiberg, T.; Kvien, T.K. Preferences for improved health examined in 1,024 patients with rheumatoid arthritis: Pain has highest priority. Arthritis Rheum. 2002, 47, 391–397. [Google Scholar] [CrossRef]
- Heiberg, T.; Finset, A.; Uhlig, T.; Kvien, T.K. Seven year changes in health status and priorities for improvement of health in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2005, 64, 191–195. [Google Scholar] [CrossRef]
- Hazes, J.M.; Taylor, P.; Strand, V.; Purcaru, O.; Coteur, G.; Mease, P. Physical function improvements and relief from fatigue and pain are associated with increased productivity at work and at home in rheumatoid arthritis patients treated with certolizumab pegol. Rheumatology 2010, 49, 1900–1910. [Google Scholar] [CrossRef]
- van Tuyl, L.H.; Sadlonova, M.; Hewlett, S.; Davis, B.; Flurey, C.; Goel, N.; Gossec, L.; Heegaard Brahe, C.; Hill, C.L.; Hoogland, W.; et al. The patient perspective on absence of disease activity in rheumatoid arthritis: A survey to identify key domains of patient-perceived remission. Ann. Rheum. Dis. 2017, 76, 855–861. [Google Scholar] [CrossRef] [PubMed]
- McGlothlin, A.E.; Lewis, R.J. Minimal clinically important difference: Defining what really matters to patients. JAMA 2014, 312, 1342–1343. [Google Scholar] [CrossRef] [PubMed]
- Strand, V.; Tundia, N.; Bergman, M.; Ostor, A.; Durez, P.; Song, I.H.; Enejosa, J.; Schlacher, C.; Song, Y.; Fleischmann, R. Upadacitinib improves patient-reported outcomes vs placebo or adalimumab in patients with rheumatoid arthritis: Results from SELECT-COMPARE. Rheumatology 2021, 60, 5583–5594. [Google Scholar] [CrossRef] [PubMed]
- van der Heijde, D.; Landewé, R. Should radiographic progression still be used as outcome in RA? Clin. Immunol. 2018, 186, 79–81. [Google Scholar] [CrossRef]
- Song, G.G.; Choi, S.J.; Lee, Y.H. Comparison of the efficacy and safety of tofacitinib and upadacitinib in patients with active rheumatoid arthritis: A bayesian network meta-analysis of randomized controlled trials. Int. J. Rheum. Dis. 2019, 22, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Ho Lee, Y.; Gyu Song, G. Comparative efficacy and safety of tofacitinib, baricitinib, upadacitinib, filgotinib and peficitinib as monotherapy for active rheumatoid arthritis. J. Clin. Pharm. Ther. 2020, 45, 674–681. [Google Scholar] [CrossRef]
- Pope, J.; Sawant, R.; Tundia, N.; Du, E.X.; Qi, C.Z.; Song, Y.; Tang, P.; Betts, K.A. Comparative efficacy of JAK inhibitors for moderate-to-severe rheumatoid arthritis: A network meta-analysis. Adv. Ther. 2020, 37, 2356–2372. [Google Scholar] [CrossRef]
- Lee, Y.H.; Song, G.G. Comparative efficacy and safety of tofacitinib, baricitinib, upadacitinib, and filgotinib in active rheumatoid arthritis refractory to biologic disease-modifying antirheumatic drugs. Z. Rheumatol. 2021, 80, 379–392. [Google Scholar] [CrossRef]
- Sung, Y.K.; Lee, Y.H. Comparative study of the efficacy and safety of tofacitinib, baricitinib, upadacitinib, and filgotinib versus methotrexate for disease-modifying antirheumatic drug-naïve patients with rheumatoid arthritis. Z. Rheumatol. 2021, 80, 889–898. [Google Scholar] [CrossRef]
- Weng, C.; Xue, L.; Wang, Q.; Lu, W.; Xu, J.; Liu, Z. Comparative efficacy and safety of Janus kinase inhibitors and biological disease-modifying antirheumatic drugs in rheumatoid arthritis: A systematic review and network meta-analysis. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720x21999564. [Google Scholar] [CrossRef]
- Best, J.H.; Kuang, Y.; Jiang, Y.; Singh, R.; Karabis, A.; Uyei, J.; Dang, J.; Reiss, W.G. Comparative efficacy (DAS28 remission) of targeted immune modulators for rheumatoid arthritis: A network meta-analysis. Rheumatol. Ther. 2021, 8, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Kremer, J.M.; Gibofsky, A.; Greenberg, J.D. The role of drug and disease registries in rheumatic disease epidemiology. Curr. Opin. Rheumatol. 2008, 20, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Pombo-Suarez, M.; Gomez-Reino, J. The role of registries in the treatment of rheumatoid arthritis with biologic disease-modifying anti-rheumatic drugs. Pharmacol. Res. 2019, 148, 104410. [Google Scholar] [CrossRef] [PubMed]
- Kremer, J.M.; Tundia, N.; McLean, R.; Blachley, T.; Maniccia, A.; Pappas, D.A. POS0435 characteristics and 6-month outcomes among real-world patients with rheumatoid arthritis initiating upadacitinib: Analysis from the corrona registry. Ann. Rheum. Dis. 2021, 80, 446. [Google Scholar] [CrossRef]
- Bergman, M.; Tundia, N.; Bryant, A.; Topuria, I.; Brecht, T.; Dunlap, K.; Gibofsky, A. POS0436 patient characteristics and outcomes in patients with rheumatoid arthritis treated with upadacitinib: The OM1 RA registry. Ann. Rheum. Dis. 2021, 80, 446–447. [Google Scholar] [CrossRef]
- Gibofsky, A.; Dhillon, B.; Pearson, M.E.; Tundia, N.; Song, Y.; Dunlap, K.; Wright, G. POS0666 treatment effectiveness of upadacitinib at 3 months in US patients with rheumatoid arthritis from the united rheumatology normalized integrated community evidence (NICE[TM]) real-world data. Ann. Rheum. Dis. 2021, 80, 575–576. [Google Scholar] [CrossRef]
- Witte, T.; Kiltz, U.; Haas, F.; Riechers, E.; Prothmann, U.; Adolf, D.; Holland, C.; Hecht, R.; Rössler, A.; Famulla, K.; et al. Effectiveness of Upadacitinib in Patients with Rheumatoid Arthritis in German Real-World Practice: Interim Results from a Post-Marketing Observational Study [abstract]. Arthritis Rheumatol. 2021, 73 (Suppl. S10), 578–581. Available online: https://acrabstracts.org/abstract/effectiveness-of-upadacitinib-in-patients-with-rheumatoid-arthritis-in-german-real-world-practice-interim-results-from-a-post-marketing-observational-study/ (accessed on 26 August 2022).
- Cohen, S.B.; van Vollenhoven, R.F.; Winthrop, K.L.; Zerbini, C.A.F.; Tanaka, Y.; Bessette, L.; Zhang, Y.; Khan, N.; Hendrickson, B.; Enejosa, J.V.; et al. Safety profile of upadacitinib in rheumatoid arthritis: Integrated analysis from the SELECT phase III clinical programme. Ann. Rheum. Dis. 2020, 80, 304–311. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2. 2021. Available online: https://training.cochrane.org/handbook/current (accessed on 31 May 2022).
- Winthrop, K.L.; Nash, P.; Yamaoka, K.; Mysler, E.; Khan, N.; Camp, H.S.; Song, Y.; Suboticki, J.L.; Curtis, J.R. Incidence and risk factors for herpes zoster in patients with rheumatoid arthritis receiving upadacitinib: A pooled analysis of six phase III clinical trials. Ann. Rheum. Dis. 2021, 81, 206–213. [Google Scholar] [CrossRef]
- Bechman, K.; Subesinghe, S.; Norton, S.; Atzeni, F.; Galli, M.; Cope, A.P.; Winthrop, K.L.; Galloway, J.B. A systematic review and meta-analysis of infection risk with small molecule JAK inhibitors in rheumatoid arthritis. Rheumatology 2019, 58, 1755–1766. [Google Scholar] [CrossRef]
- De Cock, D.; Hyrich, K. Malignancy and rheumatoid arthritis: Epidemiology, risk factors and management. Best Pract. Res. Clin. Rheumatol. 2018, 32, 869–886. [Google Scholar] [CrossRef] [PubMed]
- Inose, R.; Hosomi, K.; Takahashi, K.; Yokoyama, S.; Takada, M. Risk of malignant lymphoma in patients with rheumatoid arthritis treated with biological disease-modifying antirheumatic drugs and methotrexate. Int. J. Clin. Pharmacol. Ther. 2019, 57, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Inose, R.; Hashimoto, N.; Hosomi, K.; Yokoyama, S.; Takada, M. Association between malignancy and methotrexate and biological disease-modifying antirheumatic drugs in patients with rheumatoid arthritis. Int. J. Clin. Pharmacol. Ther. 2020, 58, 131–138. [Google Scholar] [CrossRef]
- Solipuram, V.; Mohan, A.; Patel, R.; Ni, R. Effect of janus kinase inhibitors and methotrexate combination on malignancy in patients with rheumatoid arthritis: A systematic review and meta-analysis of randomized controlled trials. Autoimmun. Highlights 2021, 12, 8. [Google Scholar] [CrossRef]
- Baldini, C.; Moriconi, F.R.; Galimberti, S.; Libby, P.; De Caterina, R. The JAK-STAT pathway: An emerging target for cardiovascular disease in rheumatoid arthritis and myeloproliferative neoplasms. Eur. Heart J. 2021, 42, 4389–4400. [Google Scholar] [CrossRef]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef]
- Thanigaimani, S.; Phie, J.; Krishna, S.M.; Moxon, J.; Golledge, J. Effect of disease modifying anti-rheumatic drugs on major cardiovascular events: A meta-analysis of randomized controlled trials. Sci. Rep. 2021, 11, 6627. [Google Scholar] [CrossRef]
- Xie, W.; Huang, Y.; Xiao, S.; Sun, X.; Fan, Y.; Zhang, Z. Impact of Janus kinase inhibitors on risk of cardiovascular events in patients with rheumatoid arthritis: Systematic review and meta-analysis of randomised controlled trials. Ann. Rheum. Dis. 2019, 78, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Yates, M.; Mootoo, A.; Adas, M.; Bechman, K.; Rampes, S.; Patel, V.; Qureshi, S.; Cope, A.P.; Norton, S.; Galloway, J.B. Venous thromboembolism risk with JAK inhibitors: A meta-analysis. Arthritis Rheumatol. 2021, 73, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Bilal, J.; Riaz, I.B.; Naqvi, S.A.A.; Bhattacharjee, S.; Obert, M.R.; Sadiq, M.; Abd El Aziz, M.A.; Nooman, Y.; Prokop, L.J.; Ge, L.; et al. Janus kinase inhibitors and risk of venous thromboembolism: A systematic review and meta-analysis. Mayo Clin. Proc. 2021, 96, 1861–1873. [Google Scholar] [CrossRef]
- Nash, P.; Lim, I.; Marabani, M. A comparison of janus kinase inhibitor safety in rheumatoid arthritis. Int. J. Rheum. Dis. 2021, 24, 3–14. [Google Scholar] [CrossRef]
- Cohen, S.B.; Van Vollenhoven, R.; Curtis, J.R.; Calabrese, L.; Zerbini, C.; Tanaka, Y.; Bessette, L.; Richez, C.; Lagunes-Galindo, I.; Liu, J.; et al. POS0220 integrated safety profile of upadacitinib with up to 4.5 years of exposure in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2021, 80, 328–329. [Google Scholar] [CrossRef]
- Fleischmann, R.; Mysler, E.; Bessette, L.; Peterfy, C.; Durez, P.; Tanaka, Y.; Swierkot, J.; Khan, N.; Bu, X.; LI, Y.; et al. POS0087 long-term safety and efficacy of upadacitinib or adalimumab in patients with rheumatoid arthritis: Results at 3 years from the select-compare study. Ann. Rheum. Dis. 2021, 80, 251–252. [Google Scholar] [CrossRef]
- Hall, S.; Takeuchi, T.; Thomson, G.; Emery, P.; Combe, B.; Everding, A.; Pavelka, K.; Song, Y.; Shaw, T.; Friedman, A.; et al. Characterization of Remission in Patients with Rheumatoid Arthritis Treated with Upadacitinib or Comparators [abstract]. Arthritis Rheumatol. 2019, 71 (Suppl. S10), 362. Available online: https://acrabstracts.org/abstract/characterization-of-remission-in-patients-with-rheumatoid-arthritis-treated-with-upadacitinib-or-comparators/ (accessed on 24 April 2022).
- Fleischmann, R.; Mysler, E.; Hall, S.; Kivitz, A.J.; Moots, R.J.; Luo, Z.; DeMasi, R.; Soma, K.; Zhang, R.; Takiya, L.; et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): A phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet 2017, 390, 457–468. [Google Scholar] [CrossRef]
- Combe, B.; Kivitz, A.; Tanaka, Y.; van der Heijde, D.; Simon, J.A.; Baraf, H.S.B.; Kumar, U.; Matzkies, F.; Bartok, B.; Ye, L.; et al. Filgotinib versus placebo or adalimumab in patients with rheumatoid arthritis and inadequate response to methotrexate: A phase III randomised clinical trial. Ann. Rheum. Dis. 2021, 80, 848–858. [Google Scholar] [CrossRef]
- van Vollenhoven, R.; Östör, A.; Mysler, E.; Damjanov, N.; Song, I.; Song, Y.; Suboticki, J.; Strand, V. The Impact of Upadacitinib versus Methotrexate or Adalimumab on Individual and Composite Disease Measures in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019, 71 (Suppl. S10), 651–652. Available online: https://acrabstracts.org/abstract/the-impact-of-upadacitinib-versus-methotrexate-or-adalimumab-on-individual-and-composite-disease-measures-in-patients-with-rheumatoid-arthritis/ (accessed on 24 April 2022).
- Taylor, P.C.; Lee, Y.C.; Fleischmann, R.; Takeuchi, T.; Perkins, E.L.; Fautrel, B.; Zhu, B.; Quebe, A.K.; Gaich, C.L.; Zhang, X.; et al. Achieving pain control in rheumatoid arthritis with baricitinib or adalimumab plus methotrexate: Results from the RA-BEAM trial. J. Clin. Med. 2019, 8, 831. [Google Scholar] [CrossRef]
- Rubbert-Roth, A.; Xavier, R.; Haraoui, B.; Baraf, H.S.B.; Rischmueller, M.; Martin, N.; Song, Y.; Suboticki, J.; Cush, J. POS0671 clinical responses to upadacitinib or abatacept in patients with rheumatoid arthritis by type of prior biologic disease-modifying antirheumatic drug: Data from the phase 3 select-choice study. Ann. Rheum. Dis. 2021, 80, 580. [Google Scholar] [CrossRef]
- Genovese, M.C.; Kremer, J.M.; Kartman, C.E.; Schlichting, D.E.; Xie, L.; Carmack, T.; Pantojas, C.; Sanchez Burson, J.; Tony, H.P.; Macias, W.L.; et al. Response to baricitinib based on prior biologic use in patients with refractory rheumatoid arthritis. Rheumatology 2018, 57, 900–908. [Google Scholar] [CrossRef]

| Characteristic * | ||
| Absorption | tmax, median: 2–4 h | |
| Distribution | Protein binding 52% | |
| Metabolism | Mainly CYP3A4 and minor contribution of CYP2D6 | |
| Elimination | Predominantly as the unchanged parent substance in urine (24%) and feces (38%). | |
| Terminal elimination half-life; mean: 9–14 h | ||
| Intrinsic factor | Effect | Recommendation |
| Age, sex, body weight, race and ethnicity | No clinically meaningful effect on upadacitinib exposure | No dose adjustment of upadacitinib is warranted based on these characteristics |
| Renal impairment | Upadacitinib AUC was 18%, 33% and 44% higher in subjects with mild, moderate and severe renal impairment, respectively, compared with subjects with normal renal function. Upadacitinib Cmax was similar in subjects with normal and impaired renal function. | No dose adjustment is required in patients with mild or moderate renal impairment. The recommended dose is 15 mg once daily for patients with severe renal impairment. |
| Hepatic impairment | Upadacitinib AUC was 28% and 24% higher in subjects with mild and moderate hepatic impairment, respectively, compared with subjects with normal liver function. Upadacitinib Cmax was unchanged in subjects with mild hepatic impairment and 43% higher in subjects with moderate hepatic impairment compared with subjects with normal liver function. | No dose adjustment is required in patients with mild (Child–Pugh A) or moderate (Child–Pugh B) hepatic impairment. |
| Upadacitinib was not studied in patients with severe (Child–Pugh C) hepatic impairment | Upadacitinib should not be used in patients with severe (Child–Pugh C) hepatic impairment. | |
| Inadequate Response to DMARDs | Naïve Patients | |||||
|---|---|---|---|---|---|---|
| NEXT | BEYOND | COMPARE § | CHOICE | MONOTHERAPY | EARLY | |
| Design | 12-wk, M, R, DB | 12-wk, M, R, DB | 26-wk, M, R, DB | 24-wk, M, R, DB | 14-wk, M, R, DB | 48-wk, M, R, DB |
| Population | csDMARD-IR | bDMARD-IR | Methotrexate -IR | bDMARD-IR | Methotrexate -IR | Naïve or limited exposure to methotrexate |
| Background therapy | csDMARD | csDMARD | Methotrexate | csDMARD | Not applicable | Not applicable |
| Upadacitinib arms | 15 mg QD 30 mg QD | 15 mg QD 30 mg QD | 15 mg QD | 15 mg QD | 15 mg QD 30 mg QD | 15 mg QD 30 mg QD |
| Comparator | Placebo | Placebo | Placebo Adalimumab 40 mg/2 wk | Abatacept | Methotrexate | Methotrexate |
| Type of treatment | Combination | Combination | Combination | Combination | Monotherapy | Monotherapy |
| Sample size | 661 | 499 | 1629 | 612 | 648 | 947 |
| Primary endpoint | ACR20 at wk 12 DAS28-CRP ≤ 3.2 at wk 12 | ACR20 at wk 12 DAS28-CRP ≤ 3.2 at wk 12 | ACR20 at wk 12 DAS28-CRP < 2.6 at wk 12 | ∆DAS28-CRP at week 12 (noninferiority) | ACR20 at wk 14 DAS28-CRP ≤ 3.2 at wk 14 | ACR50 at wk 12 DAS28-CRP < 2.6 at wk 24 |
| Confirmatory endpoints (FDA) | ∆DAS28-CRP ∆HAQ-DI ∆SF-36 PCS DAS28-CRP ≤ 3.2 DAS28-CRP < 2.6 CDAI ≤ 10 ∆Morning stiffness duration ∆FACIT-F | ∆DAS28-CRP ∆HAQ-DI DAS28-CRP ≤ 3.2 ∆SF-36 PCS | ∆DAS28-CRP ∆mTSS ∆HAQ-DI ACR50 | ∆SF-36 PCS DAS28-CRP ≤ 3.2 DAS28-CRP < 2.6 CDAI ≤ 10 ∆FACIT-F ∆Morning stiffness duration ACR50 ¶ ∆HAQ-DI ¶ ∆ Pain ¶ | ∆DAS28-CRP at week 12 (superiority) DAS28-CRP < 2.6 at week 12 (superiority) | ∆DAS28-CRP ∆HAQ-DI ∆SF-36 PCS DAS28-CRP ≤ 3.2 DAS28-CRP < 2.6 ∆Morning stiffness duration | ∆HAQ-DI ∆mTSS † DAS28-CRP ≤ 3.2 DAS28-CRP < 2.6 † ∆SF-36 PCS |
| Confirmatory endpoints (EMA) | ∆DAS28-CRP ∆HAQ-DI ACR20 ∆SF-36 PCS DAS28-CRP < 2.6 CDAI ≤ 10 ∆Morning stiffness duration ∆FACIT | ∆DAS28-CRP ACR20 ∆HAQ-DI ∆SF-36 PCS | ∆mTSS DAS28-CRP ≤ 3.2 ∆DAS28-CRP ∆HAQ-DI ACR20 DAS28-CRP ≤ 3.2 | ∆SF-36 PCS CDAI ≤ 10 ∆Morning stiffness duration ∆FACIT mTSS ≤ 0 ‡ | ∆DAS28-CRP ∆HAQ-DI ∆SF-36 PCS DAS28-CRP ≤ 3.2 DAS28-CRP < 2.6 ∆Morning stiffness duration | ∆DAS28-CRP † ∆HAQ-DI † ACR50 † ∆mTSS † DAS28-CRP ≤ 3.2 † ∆SF-36 PCS † % no radiographic progression: mTSS ≤ 0 † | |
| Study Name | Population | Primary Endpoints | UPA 15 mg | UPA 30 mg | PBO | MTX | ADA | ABA | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| NEXT [21] | csDMARD-IR | ACR20 at wk 12 | 64% ‡ | 66% ‡ | 36% | - | - | Both doses of UPA QD were superior to placebo. | |
| DAS28-CRP ≤ 3.2 at wk 12 | 48% ‡ | 48% ‡ | 17% | - | - | ||||
| BEYOND [22] | bDMARD-IR | ACR20 at wk 12 | 65% ‡ | 56% ‡ | 28% | - | - | Both doses of UPA QD were superior to placebo. | |
| DAS28-CRP ≤ 3.2 at wk 12 | 43% ‡ | 42% ‡ | 14% | - | - | ||||
| COMPARE [23] | Methotrexate -IR | ACR20 at wk 12 | 71% †,ǀ | - | 36% | - | 63% | UPA 15 mg QD was superior to adalimumab | |
| DAS28-CRP < 2.6 at wk 12 | 29% †,¶ | - | 6% | - | 18% | ||||
| CHOICE [24] | bDMARD-IR | Change from baseline in the DAS28-CRP (non-inf.) | −2.52 points ‖ | −2.00 points | UPA 15 mg QD was superior vs. abatacept | ||||
| MONOTHERAPY [25] | Methotrexate -IR | ACR20 at wk 14 | 68% ¶¶ | 71% ¶¶ | - | 41% | - | Both doses of UPA QD were superior to continuing MTX | |
| DAS28-CRP ≤ 3.2 at wk 14 | 45% ¶¶ | 53% ¶¶ | - | 19% | - | ||||
| EARLY [26] | Naïve or limited exposure to methotrexate | ACR50 at wk 12 | 52% ¶ | 56% ¶ | 28% | Both doses of UPA QD were superior to MTX | |||
| DAS28-CRP < 2.6 at wk 24 | 48% ¶ | 50% ¶ | 19% |
| Study | Outcome a | Week | Placebo | MTX | ABA | ADA | Upa 15 mg | Upa 30 mg |
|---|---|---|---|---|---|---|---|---|
| NEXT [21] | ΔVAS Pain | 12 | −10.3 | −29.9 ‡ | −31.7 ‡ | |||
| ΔFACIT-F | 12 | 3.0 | 7.9 ‡ | 7.7 ‡ | ||||
| ΔSF36-PCS | 12 | 3.0 | 7.6 ‡ | 8.0 ‡ | ||||
| ΔHAQ-DI | 12 | −0.26 | −0.61 ‡ | −0.55 ‡ | ||||
| BEYOND [22] | ΔSF36-PCS | 12 | 2.4 | 5.8 ‡ | 7.0 ‡ | |||
| 24 | NA | 7.2 | 8.0 | |||||
| ΔHAQ-DI | 12 | −0.16 | −0.41 ‡ | −0.44 ‡ | ||||
| COMPARE [23] | ΔVAS Pain | 12 | −15.7 | −25.6 | −32.1 †,¶ | |||
| ΔFACIT-F | 12 | 4.8 | 7.4 | 9.0 †,ǀ | ||||
| 26 | 5.5 | 8.2 | 9.7 †,ǀ | |||||
| ΔSF36-PCS | 12 | 3.6 | 6.3 | 7.9 †,ǁ | ||||
| 26 | 4.5 | 7.8 | 9.5 †,ǁ | |||||
| ΔHAQ-DI | 12 | −0.28 | −0.49 | −0.60 †,ǁ | ||||
| CHOICE [24] | ΔFACIT-F | 12 | 8.35 | 9.61 | ||||
| 24 | 10.32 | 10.73 | ||||||
| ΔSF36-PCS | 12 | 7.03 | 9.62 | |||||
| 24 | 9.40 | 10.97 | ||||||
| MONOTHERAPY [25] | ΔVAS Pain | 14 | −13.88 | −26.15 ¶¶ | −33.18 ¶¶ | |||
| ΔSF36-PCS | 14 | 4.3 | 8.3 ¶ | 10.2 ¶ | ||||
| ΔHAQ-DI | 14 | −0.32 | −0.65 ¶ | −0.73 ¶ | ||||
| EARLY [26] | ΔVAS Pain | 12 | −25.36 | −36.28 ¶ | −39.67 ¶ | |||
| 24 | −28.40 | −39.84 ¶ | −44.67 ¶ | |||||
| ΔFACIT-F | 12 | 6.80 | 10.01 ¶ | 9.57 ¶ | ||||
| 24 | 7.37 | 10.59 ¶ | 10.56 ¶ | |||||
| ΔSF36-PCS | 12 | 5.77 | 10.09 ¶ | 10.11 ¶ | ||||
| 24 | 6.99 | 10.97 ¶ | 11.69 ¶ | |||||
| ΔHAQ-DI | 12 | −0.49 | −0.83 ¶ | −0.86 ¶ | ||||
| 24 | −0.60 | −0.87 ¶ | −0.91 ¶ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanmartí, R.; Corominas, H. Upadacitinib for Patients with Rheumatoid Arthritis: A Comprehensive Review. J. Clin. Med. 2023, 12, 1734. https://doi.org/10.3390/jcm12051734
Sanmartí R, Corominas H. Upadacitinib for Patients with Rheumatoid Arthritis: A Comprehensive Review. Journal of Clinical Medicine. 2023; 12(5):1734. https://doi.org/10.3390/jcm12051734
Chicago/Turabian StyleSanmartí, Raimon, and Hèctor Corominas. 2023. "Upadacitinib for Patients with Rheumatoid Arthritis: A Comprehensive Review" Journal of Clinical Medicine 12, no. 5: 1734. https://doi.org/10.3390/jcm12051734
APA StyleSanmartí, R., & Corominas, H. (2023). Upadacitinib for Patients with Rheumatoid Arthritis: A Comprehensive Review. Journal of Clinical Medicine, 12(5), 1734. https://doi.org/10.3390/jcm12051734





