Abstract
Scoring systems for metabolic dysfunction-associated steatotic liver disease (MASLD) in individuals with prediabetes have not been extensively explored. This study aimed to investigate the prevalence of MASLD and to develop predictive tools for its detection in high cardiometabolic people with prediabetes. A cross-sectional study was conducted using baseline data from the prediabetes cohort. All participants underwent transient elastography to assess liver stiffness. MASLD was defined using a controlled attenuation parameter value > 275 dB/m and/or a liver stiffness measurement ≥ 7.0 kPa. Cases with secondary causes of hepatic steatosis were excluded. Out of 400 participants, 375 were included. The observed prevalence of MASLD in individuals with prediabetes was 35.7%. The most effective predictive model included FPG ≥ 110 mg/dL; HbA1c ≥ 6.0%; sex-specific cutoffs for HDL; ALT ≥ 30 IU/L; and BMI levels. This model demonstrated good predictive performance with an AUC of 0.80 (95% CI 0.73–0.86). At a cutoff value of 4.5, the sensitivity was 70.7%, the specificity was 72.3%, the PPV was 58.8%, and the NPV was 81.5%. Our predictive model is practical, easy to use, and relies on common parameters. The scoring system should aid clinicians in determining when further investigations of MASLD are warranted among individuals with prediabetes, especially in settings with limited resources.
1. Introduction
Prediabetes is a medical condition characterized by hyperglycemia levels above normal but below the threshold for diabetes. It poses a high risk for the development of diabetes, atherosclerotic cardiovascular disease, and associated metabolic complications [1]. Metabolic dysfunction-associated steatotic liver disease (MASLD) is a leading cause of chronic liver disease and is commonly associated with insulin resistance, metabolic syndrome, prediabetes, and type 2 diabetes [2]. Prediabetes and MASLD share similar risk factors, including being overweight, obese, or having central obesity. These factors contribute to systemic insulin resistance and increased levels of circulating free fatty acids, which are subsequently stored in the liver, resulting in MASLD. This hepatic fat accumulation promotes hepatic insulin resistance, activates inflammatory pathways, increases oxidative stress, and leads to hepatic fibrosis [3].
Previous studies have shown that individuals with both prediabetes and MASLD have a higher risk of developing type 2 diabetes than those with prediabetes alone [4]. Additionally, MASLD is associated with an increased risk of cirrhosis, cardiovascular disease, and cancer [5,6]. Typically, MASLD patients remain asymptomatic until severe liver disease manifests. Therefore, early detection of MASLD is crucial to prevent disease progression and associated complications. While liver biopsy is the gold standard for diagnosing metabolic dysfunction-associated steatohepatitis (MASH), its invasiveness and potential complications limit its widespread use [7]. Noninvasive approaches, such as transient elastography, have been developed and have shown good sensitivity in detecting MASLD and significant fibrosis [8,9,10,11]. However, the exact prevalence of MASLD is still ambiguous and varies depending on the screening methods used and the nature of the populations studied.
The prevalence of MASLD in individuals with prediabetes, particularly in the Asian population, has not been extensively studied. Therefore, this study sought to investigate the prevalence of MASLD, identify predictive risk factors, and develop a simple clinical predictive score for detecting MASLD in Thai individuals with prediabetes. Furthermore, we aimed to compare our predictive tool with previously published tools designed for predicting MASLD.
2. Materials and Methods
A cross-sectional analysis was conducted using the baseline data from a cohort of individuals with high cardiometabolic risk and prediabetes who attended the outpatient clinic of the Faculty of Medicine Siriraj Hospital between 2019 and 2022. Typically, attendees of this clinic are at high risk for diabetes or cardiovascular diseases, or they already have conditions such as hypertension, obesity, or multiple metabolic risk factors. The clinic provides education, prevention strategies, and appropriate medications such as blood pressure-lowering agents and cholesterol-lowering agents.
Participants included in the study were aged between 18 and 80 years and had experienced glucose levels within the prediabetes range on at least two occasions. Prediabetes was defined as a hemoglobin A1c (HbA1c) level of 39 to less than 48 mmol/mol (5.7% to less than 6.5%) and/or a fasting plasma glucose (FPG) level of 100 to less than 126 mg/dL [1]. All participants underwent transient elastography. Additionally, participants were required to have no previous diagnosis of diabetes, not be using hypoglycemic agents, and not be pregnant or breastfeeding.
Individuals with any secondary cause of hepatic steatosis were excluded, including those with a history of excessive alcohol intake (more than 30 g/day for men and 20 g/day for women), chronic viral hepatitis (hepatitis B or C), chronic liver disease, or drug-induced hepatitis. After applying these exclusion criteria, 375 participants were recruited for the present study.
The study protocol was approved by the Siriraj Institutional Review Board (certificate of approval [COA] number SI 495/2019 and COA number SI 447/2023).
2.1. Procedures and Measurements
All recruited individuals with prediabetes underwent various measurements and assessments. These included blood pressure and anthropometric measurements (such as height, weight, and waist circumference) which were conducted prior to performing the transient elastography. Transient elastography using the FibroScan Compact 530 (probe M+; Echosens, Paris, France) was also performed by one trained technician. Furthermore, the study obtained details of common predictors of MASLD through a literature review and assessed these parameters in the recruited individuals. All clinical and laboratory data were measured using standard techniques and recorded in the electronic database. Medical technicians who measured the laboratory data were blinded to the results of the transient elastography. Patient data were collected during recruitment through a case record form and a review of electronic medical records. In cases where data were missing, a multiple imputation method was used to increase the statistical power and allow for the development of a valid prediction model [12].
MASLD was characterized using either a controlled attenuation parameter (CAP) value > 275 dB/m and/or a liver stiffness measurement ≥ 7.0 kPa [11,13,14,15,16]. Advanced fibrosis and cirrhosis were defined as liver stiffness measurements ≥ 8 and ≥ 10.3 kPa, respectively. The selected CAP threshold of 275 dB/m was used in this analysis due to its high efficacy in detecting steatosis among high-risk individuals without secondary causes [11]. Atherosclerotic cardiovascular disease encompassed acute coronary syndrome [17], a history of myocardial infarction, stable or unstable angina, coronary or other arterial revascularization, stroke, transient ischemic attack, or peripheral artery disease of atherosclerotic origin. Metabolic syndrome was defined as the presence of prediabetes and at least two of the following: (1) a body mass index (BMI) ≥ 23 kg/m2, as per the Asian-specific BMI cutoff [18,19]; (2) documented hypertension; (3) a low, sex-specific, high-density lipoprotein cholesterol (HDL-c) level or documented dyslipidemia or statin use; and (4) hypertriglyceridemia (fasting triglycerides [TG] ≥ 150 mg/dL) or documented dyslipidemia or statin use [20,21].
2.2. Outcomes
The primary outcome was to ascertain the prevalence of MASLD detected through transient elastography and to develop a straightforward clinical predictive score for identifying MASLD in individuals with prediabetes at high cardiometabolic risk. Additionally, we compared the performance of our predictive score with previously published scores for predicting MASLD.
2.3. Statistical Analysis
Data Collection, Imputation, and Sample Size Calculations
The sample size was calculated based on the expected prevalence of MASLD in individuals with prediabetes, estimated at 59% [22]. To estimate the prevalence with 95% confidence, a minimum sample size of 372 participants was needed [23]. Regarding the sample size for developing the predictive score, ten outcome events per predictive variable were needed. The expected prevalence in our study was sufficient to develop the predictive model.
Clinical characteristics were compared between individuals with and without MASLD. Continuous variables with a normal distribution are presented as means ± standard deviations, while continuous variables with a skewed distribution are summarized as medians and interquartile ranges. Categorical variables are shown as numbers and percentages. Statistical significance between the MASLD and non-MASLD groups was assessed using unpaired t tests for normally distributed continuous variables, Mann–Whitney U tests for non-normally distributed variables, and chi-square tests for categorical variables.
2.4. Model Development
In the initial phase of model development, univariable statistical analysis was conducted to examine the association between the collected data and MASLD. Variables with a p value less than 0.1 were considered to have a significant difference. In cases where two or more similar parameters were found to be correlated, the one with the lowest p value was selected. To simplify the model, all continuous variables were categorized using clinically meaningful cutoff values commonly utilized in practice.
Forward and backward stepwise logistic regressions were then performed to select potential predictors for the prediction model. Predictors with p values less than 0.05 were incorporated into the model. Finally, using clinical judgment, five predictors were selected based on their practicality for use in daily practice. Weighted scores, derived from odds ratios from the logistic regression model, were assigned to all predictors.
2.5. Model Performance Assessment
The performance of the developed model was assessed in terms of discrimination using unpaired t tests. The model’s predictive accuracy was evaluated using the area under receiver operating characteristic (AuROC) curves, sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio. These measures were accompanied by 95% confidence intervals, calculated based on the appropriate cutoff value that aimed to achieve a balance between sensitivity and specificity. All data analyses were performed using PASW Statistics, version 18 (SPSS Inc., Chicago, IL, USA).
2.6. Comparison of Predictive Performance
To assess the accuracy of our scoring system in predicting the presence of MASLD in individuals with prediabetes, we compared it with five previously published noninvasive scoring systems used for MASLD detection. These were the fatty liver index (FLI) [24], the lipid accumulation product (LAP) index [25], the hepatic steatosis index (HSI) [26], the MASLD in metabolic syndrome patients score (NAFLD-MS) [27], and the NAFLD ridge score [28]. Supplementary Table S1 compares all scoring systems’ AuROCs, sensitivities, specificities, positive predictive values, negative predictive values, and likelihood ratios.
3. Results
3.1. Patient Characteristics
During the recruitment period, transient elastography was performed on 400 individuals with prediabetes. Of these, 25 were excluded from the analysis due to the presence of secondary causes of hepatic steatosis. Therefore, the present study analyzed data from 375 participants (Figure 1).
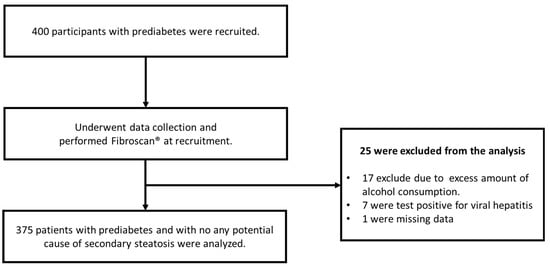
Figure 1.
Study flow chart. Data collection and transient elastography (FibroScan Compact 530) were performed on all 400 individuals during recruitment. Twenty-five patients were excluded due to potential secondary steatosis causes and incomplete medical records.
Of the 375 participants, 68% were female. The overall mean age was 62.1 ± 9.9 years, and the BMI was 26.3 ± 4.6 kg/m2. The proportion of participants with a BMI ≥ 25 kg/m2 was 56.0%, and 84.0% were diagnosed with metabolic syndrome. Additionally, dyslipidemia, hypertension, and atherosclerotic cardiovascular diseases were present in 80.0%, 69.9%, and 5.3% of the participants, respectively. Regarding clinically managed metabolic disturbances, 71.1% of the participants were on statin therapy, and 88.5% used antihypertensive medications. The overall prevalence of MASLD among individuals with prediabetes at a high risk of metabolic disturbance in our study was 35.7% (n = 134). Moreover, the prevalence of advanced fibrosis and cirrhosis in the entire cohort were 2.4% and 1.9%, respectively. There were substantial differences in most clinical characteristics between the MASLD and non-MASLD patients. In the MASLD group, compared to the non-MASLD group, adiposity parameters (BMI, body fat percentage, fat-free mass, waist circumference, and hip circumference), the proportion of metabolic syndrome, each metabolic syndrome parameter, glycemia (FPG and HbA1C), GGT, ALT, and uric acid levels were higher. Conversely, the MASLD group had a lower HDL-c level and mean age than the non-MASLD group. The participant characteristics are outlined in Table 1.

Table 1.
Clinical characteristics and evidence of differences (p values).
3.2. Model Development
Age, adiposity parameters, FPG, HbA1c, ALT, AST, SBP, DBP, TG, and HDL-c were identified as potential predictors through univariable statistical analysis. The cutoff values of continuous data, commonly used in clinical practice to indicate clinical significance, were applied (Supplementary Table S2). Five predictors were retained in the final model following multivariable logistic regression analysis and expert consultation. They were (1) BMI ≥ 23 kg/m2 and BMI ≥ 25 kg/m2; (2) FPG ≥ 110 mg/dL; (3) HbA1C ≥ 6.0%; (4) HDL-c < 40 mg/dL in males and HDL-c < 50 mg/dL in females; and (5) ALT ≥ 30 IU/L. The results of the multivariable logistic regression, including the regression coefficients, odds ratios, and assigned scores for the final model, are detailed in Table 2. The scoring system was named the “MASLD Pre-DM score”.

Table 2.
Final predictors from multivariable logistic regression, regression coefficients, odds ratios, and assigned scores.
3.3. Model Performance and Comparison of Predictive Performance of Scores in People with Prediabetes
The MASLD Pre-DM score demonstrated good discriminative power (p < 0.001). The parametric AuROC for the MASLD Pre-DM score was 0.80 (95% confidence interval 0.73–0.86). The sensitivity, specificity, likelihood ratio, and positive and negative predictive values at a cutoff of 4.5 are provided in Table 3.

Table 3.
Performance of noninvasive scoring systems in predicting MASLD among participants with prediabetes in our study.
Comparisons of the MASLD Pre-DM score with existing scores for predicting MASLD in prediabetic individuals yielded noteworthy insights. The AuROC values for the MASLD Pre-DM, FLI, LAP index, HSI, NAFLD-MS, and NAFLD ridge scores were recorded as 0.80, 0.84, 0.83, 0.68, 0.72, and 0.72, respectively. Specifically, within the prediabetic cohort, there were no statistically significant differences in the AuROC results between the MASLD Pre-DM score and the FLI (p = 0.3) or between the MASLD Pre-DM and LAP index (p = 0.6). However, the MASLD Pre-DM’s AuROC was notably superior to those of the HSI (p < 0.001), NAFLD-MS (p = 0.005), and the NAFLD ridge score (p < 0.001). The performance of each noninvasive scoring system in predicting MASLD among individuals with prediabetes in our study, including the AuROC, sensitivity, specificity, likelihood ratio, and positive and negative predictive values, is detailed in Table 3 and Figure 2.
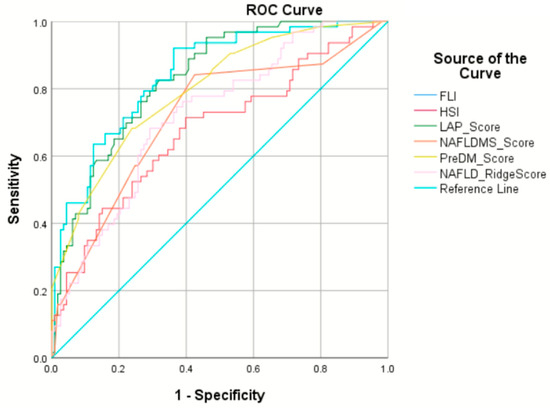
Figure 2.
Receiver operating characteristic curves of six noninvasive scoring systems. FLI—fatty liver index (light blue line); HSI—hepatic steatosis index (red line); LAP score—lipid accumulation product index (green line); NAFLD-MS score—NAFLD in metabolic syndrome patients score (orange line); PreDM score—MASLD Pre-DM score (yellow line); NAFLD ridge score (pink line); ROC—receiver operating characteristic curve.
4. Discussion
MASLD has become the leading cause of chronic liver disease and cardiovascular disease, affecting approximately one-quarter of the population [29]. In this cross-sectional study, we engaged 375 participants at risk for cardiometabolic diseases, specifically, those with impaired fasting glucose and/or elevated HbA1c levels indicating prediabetes. Our primary objective was to determine the prevalence of MASLD using transient elastography. Additionally, we aimed to develop and evaluate a clinical predictive score for detecting MASLD in individuals with prediabetes. The overall prevalence of MASLD in our study was 35.7%. We successfully developed a simple clinical risk scoring system called the “MASLD Pre-DM score”, which effectively distinguished the presence or absence of MASLD in individuals with prediabetes. The performance of the MASLD Pre-DM score was comparable to previous scoring systems used in the general population (FLI and LAP index) and superior to a previous predictive score used in Thai participants with metabolic syndrome (NAFLD-MS).
This study is the first to investigate the prevalence of MASLD detected using transient elastography in individuals with prediabetes who are at high cardiometabolic risk. In a previous study, the prevalence of MASLD detected using ultrasonography in Indian individuals with prediabetes of average risk was 59% [22]. Our study revealed a markedly lower prevalence of MASLD at 35.7%. In further parallel with our findings, a recent extensive systematic review and meta-analysis indicated a prevalence of 32.4% for MASLD in the general adult population. Notably, the large majority (86%) of the population studied in the meta-analysis was from Asia. However, the same review found a higher prevalence of 49.2% in individuals aged 50 years or older [30].
Several factors may account for the variations in prevalence compared to our study. For instance, our study employed transient elastography, whereas most other studies relied on ultrasonography, which is known to be highly operator dependent. Furthermore, differences in participant characteristics, such as adiposity, ethnicity, metabolic parameters, and liver enzyme levels, may contribute to the variations observed. Specifically, the levels of ALT and TG in individuals with prediabetes and MASLD reported in the Indian study were higher than the corresponding levels found in our study. Our investigation’s results align with previous research highlighting the significance of ALT and TG levels as indicators of MASLD [24,25,26,27]. Such observations suggest that factors beyond prediabetes, such as ALT and TG levels, may be as pivotal in predicting MASLD as prediabetes.
Only a limited number of studies have focused on developing a predictive tool for detecting MASLD in individuals at high risk for cardiometabolic conditions and with prediabetes [31]. Since MASLD is typically asymptomatic, early detection and effective management are essential to prevent disease progression and minimize liver and cardiovascular complications. To address this need, we developed a clinical scoring system to serve as a tool for detecting MASLD in this high-risk population. Compared to other scoring systems reported in the literature, our predictive scoring system demonstrated comparable performance to the FLI and LAP index and outperformed the HIS, NAFLD-MS, and NAFLD ridge scoring systems.
Although our MASLD Pre-DM score exhibits a lower sensitivity at lower cutoff values, it offers a higher specificity than the FLI and LAP index. Although the FLI and the NAFLD-MS may provide acceptable specificity at higher cutoff values (87.1% and 99.4%, respectively), their sensitivity is very poor (48.9% and 1.2%, respectively). In resource-limited settings, allocating resources wisely and minimizing the overuse of transient elastography is crucial. An ideal scoring system should strike a balance between sensitivity and specificity. Therefore, individuals with cumulative scores of less than 4.5 can be classified into a low-probability group, where transient elastography for MASLD diagnosis may not be necessary. For individuals in the high-probability group (MASLD Pre-DM scores ≥ 4.5), the score can aid clinicians in prioritizing further MASLD investigations or making informed decisions regarding MASLD management among individuals at high cardiometabolic risk with prediabetes.
Our analysis has strengths and limitations. To our knowledge, this is the first study to investigate the prevalence of MASLD using transient elastography and to develop a practical prediction model in high cardiometabolic risk individuals with prediabetes in the Thai population. However, a limitation of our study is that we did not perform a liver biopsy, which is considered the gold standard for diagnosing MASLD. Nevertheless, previous studies have shown good comparability between transient elastography and liver biopsy for MASLD diagnosis [32,33,34,35]. Another consideration is that our scoring system was developed using data from a specific subgroup of individuals with prediabetes and high cardiometabolic risk. External validation is necessary to confirm the performance of our scoring system in more extensive and diverse subgroups, such as lean individuals, those under the age of 50, and other ethnic groups. Additionally, the potential advantages of a sex-specific scoring system, incorporating sex-related variables, merit exploration. Our findings indicated no significant sex-based differences in MASLD prevalence or most characteristics, and we incorporated sex-specific cutoffs (Supplementary Table S3). Nevertheless, the limited sex representation in our sample size necessitates further investigation with larger, more diverse cohorts.
Additionally, our scoring system focuses on detecting the presence of MASLD rather than hepatic fibrosis, which may have more substantial clinical significance for prognosis in the future. However, hepatic fibrosis is rare in individuals with prediabetes. In our study, the prevalence of advanced fibrosis and cirrhosis was low (2.4% and 1.9%, respectively). Similar results were found in another study investigating the prevalence of hepatic fibrosis in individuals with prediabetes [36]. Therefore, developing a predictive score specifically for advanced hepatic fibrosis may not be feasible. Fortunately, early detection of MASLD can help minimize the progression to hepatic fibrosis [37,38].
5. Conclusions
Our practical scoring system, the MASLD Pre-DM score, demonstrated its capability to predict MASLD in high cardiometabolic risk individuals with prediabetes, particularly in resource-limited settings where noninvasive transient elastography may be limited. The MASLD Pre-DM score balances sensitivity and specificity and is a simple-to-use tool for preliminary screening. With appropriate cutoff values, the MASLD Pre-DM score can benefit clinicians in the early detection of patients at risk of MASLD and increase awareness among high cardiometabolic individuals with prediabetes, potentially leading to lifestyle modifications. However, further research is needed, including external validation of the scoring system, to confirm the benefits of implementing the MASLD Pre-DM score in routine MASLD screening.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12247617/s1, Table S1: Noninvasive scoring systems utilized for detecting MASLD. Table S2: Univariate and multivariable logistic regression analysis of risk factors associated with MASLD in individuals with prediabetes. Table S3: Clinical characteristics and evidence of differences by sex (p values).
Author Contributions
Conceptualization, C.W., C.K., R.T., P.C., P.P. and W.S.; methodology N.M., C.W., P.A., C.K., T.S., R.T., C.A., N.S., T.C., P.P., P.C. and W.S.; validation, N.M., C.W., C.K., T.S., R.T., T.C., P.P., P.C. and W.S.; formal analysis, C.K., C.W. and W.S.; investigation, N.M., C.W., C.K., T.S., R.T., T.C., P.P., P.C. and W.S.; data curation N.M., C.W., R.T., C.K. and W.S.; writing—original draft preparation, N.M., C.W., P.A., C.K., T.S., R.T., C.A., N.S., T.C., P.P., P.C. and W.S.; writing—review and editing, N.M., C.W., C.K., T.S., R.T., T.C., P.P., P.C. and W.S.; visualization, C.K., C.W., R.T. and W.S.; supervision, C.W., R.T., P.P., P.C. and W.S.; project administration, C.W. and W.S.; funding acquisition, C.W. and W.S. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by both the Faculty of Medicine Siriraj Hospital (R016233029) and Mahidol University (MU Strategic Research Fund): 2023 (R016620001).
Institutional Review Board Statement
The study protocol was authorized by the Siriraj Institutional Review Board (approval numbers SI 495/2019 and SI 447/2023).
Informed Consent Statement
Consent was obtained from all study participants.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical reasons.
Acknowledgments
We thank Euarat Mepramoon, Ambulatory Medicine Division, Department of Medicine, and the staff of the Division of Gastroenterology, Department of Medicine, Faculty of Medicine Siriraj Hospital, for their support with the transient elastography preparation and measurements. We are also indebted to David Park for the English-language editing of this paper.
Conflicts of Interest
All authors declare that there ia no competing interest that could be perceived as prejudicing the impartiality of the research reported.
References
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2013, 37, S81–S90. [Google Scholar] [CrossRef]
- Sepanlou, S.G.; Safiri, S.; Bisignano, C.; Ikuta, K.S.; Merat, S.; Saberifiroozi, M.; Poustchi, H.; Tsoi, D.; Colombara, D.V.; Abdoli, A.; et al. The Global, Regional, and National Burden of Cirrhosis by Cause in 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yu, R.; Xiong, Y.; Du, F.; Zhu, S. A Vicious Circle between Insulin Resistance and Inflammation in Nonalcoholic Fatty Liver Disease. Lipids Health Dis. 2017, 16, 203. [Google Scholar] [CrossRef] [PubMed]
- Nishi, T.; Babazono, A.; Maeda, T.; Imatoh, T.; Une, H. Evaluation of the Fatty Liver Index as a Predictor for the Development of Diabetes among Insurance Beneficiaries with Prediabetes. J. Diabetes Investig. 2015, 6, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global Burden of NAFLD and NASH: Trends, Predictions, Risk Factors and Prevention. Nat. Rev. Gastroenterol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease—Meta-analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Sumida, Y.; Nakajima, A.; Itoh, Y. Limitations of Liver Biopsy and Non-Invasive Diagnostic Tests for the Diagnosis of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. World J. Gastroenterol. 2014, 20, 475–485. [Google Scholar] [CrossRef]
- Geethakumari, P.; Kampa, P.; Parchuri, R.; Bhandari, R.; Alnasser, A.R.; Akram, A.; Kar, S.; Osman, F.; Mashat, G.D.; Tran, H.H.-V.; et al. Accuracy of Ultrasonography vs. Elastography in Patients with Non-Alcoholic Fatty Liver Disease: A Systematic Review. Cureus 2022, 14, e29967. [Google Scholar] [CrossRef]
- Sasso, M.; Miette, V.; Sandrin, L.; Beaugrand, M. The Controlled Attenuation Parameter (CAP): A Novel Tool for the Non-Invasive Evaluation of Steatosis Using Fibroscan®. Clin. Res. Hepatol. Gas. 2012, 36, 13–20. [Google Scholar] [CrossRef]
- Mikolasevic, I.; Orlic, L.; Franjic, N.; Hauser, G.; Stimac, D.; Milic, S. Transient Elastography (FibroScan®) with Controlled Attenuation Parameter in the Assessment of Liver Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease—Where Do We Stand? World J. Gastroenterol. 2016, 22, 7236–7251. [Google Scholar] [CrossRef]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef]
- Han, K.; Song, K.; Choi, B.W. How to Develop, Validate, and Compare Clinical Prediction Models Involving Radiological Parameters: Study Design and Statistical Methods. Korean J. Radiol. 2016, 17, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Liver, E.A. for the S. of the; Higado, A.L. para el E. del EASL-ALEH Clinical Practice Guidelines: Non-Invasive Tests for Evaluation of Liver Disease Severity and Prognosis. J. Hepatol. 2015, 63, 237–264. [Google Scholar] [CrossRef]
- Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M.; Petta, S.; Thiele, M. EASL Clinical Practice Guidelines on Non-Invasive Tests for Evaluation of Liver Disease Severity and Prognosis—2021 Update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Machado, M.V.; Cortez-Pinto, H. Non-Invasive Diagnosis of Non-Alcoholic Fatty Liver Disease. A Critical Appraisal. J. Hepatol. 2013, 58, 1007–1019. [Google Scholar] [CrossRef]
- Brunt, E.M.; Wong, V.W.-S.; Nobili, V.; Day, C.P.; Sookoian, S.; Maher, J.J.; Bugianesi, E.; Sirlin, C.B.; Neuschwander-Tetri, B.A.; Rinella, M.E. Nonalcoholic Fatty Liver Disease. Nat. Rev. Dis. Prim. 2015, 1, 15080. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Gaglia, J.L.; Hilliard, M.E.; Isaacs, D.; et al. 3. Prevention or Delay of Diabetes and Associated Comorbidities: Standards of Care in Diabetes—2023. Diabetes Care 2022, 46, S41–S48. [Google Scholar] [CrossRef]
- Snehalatha, C.; Viswanathan, V.; Ramachandran, A. Cutoff Values for Normal Anthropometric Variables in Asian Indian Adults. Diabetes Care 2003, 26, 1380–1384. [Google Scholar] [CrossRef] [PubMed]
- Consultation, W.E. Appropriate Body-Mass Index for Asian Populations and Its Implications for Policy and Intervention Strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C.; et al. Harmonizing the Metabolic Syndrome. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.; Zimmet, P.; Shaw, J. The Metabolic Syndrome—A New Worldwide Definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Rajput, R.; Ahlawat, P. Prevalence and Predictors of Non-Alcoholic Fatty Liver Disease in Prediabetes. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2957–2960. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mudgal, S.; Thakur, K.; Gaur, R. How to Calculate Sample Size for Observational and Experiential Nursing Research Studies? Natl. J. Physiol. Pharm. Pharmacol. 2019, 10, 1. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A Simple and Accurate Predictor of Hepatic Steatosis in the General Population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Kahn, H.S.; Bellentani, S.; Tiribelli, C. A Simple Index of Lipid Overaccumulation Is a Good Marker of Liver Steatosis. Bmc Gastroenterol. 2010, 10, 98. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, D.; Kim, H.J.; Lee, C.-H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.-H.; Cho, S.-H.; Sung, M.-W.; et al. Hepatic Steatosis Index: A Simple Screening Tool Reflecting Nonalcoholic Fatty Liver Disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Saokaew, S.; Kanchanasuwan, S.; Apisarnthanarak, P.; Charoensak, A.; Charatcharoenwitthaya, P.; Phisalprapa, P.; Chaiyakunapruk, N. Clinical Risk Scoring for Predicting Non-alcoholic Fatty Liver Disease in Metabolic Syndrome Patients (NAFLD-MS Score). Liver Int. 2017, 37, 1535–1543. [Google Scholar] [CrossRef]
- Yip, T.C.-F.; Ma, A.J.; Wong, V.W.-S.; Tse, Y.-K.; Chan, H.L.-Y.; Yuen, P.-C.; Wong, G.L.-H. Laboratory Parameter-based Machine Learning Model for Excluding Non-alcoholic Fatty Liver Disease (NAFLD) in the General Population. Aliment. Pharmacol. Ther. 2017, 46, 447–456. [Google Scholar] [CrossRef]
- Maurice, J.; Manousou, P. Non-Alcoholic Fatty Liver Disease. Clin. Med. 2018, 18, 245–250. [Google Scholar] [CrossRef]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.-A. The Prevalence and Incidence of NAFLD Worldwide: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef]
- Saokaew, S.; Kositamongkol, C.; Charatcharoenwitthaya, P.; Srivanichakorn, W.; Washirasaksiri, C.; Chaiyakunapruk, N.; Phisalprapa, P. Comparison of Noninvasive Scoring Systems for the Prediction of Nonalcoholic Fatty Liver Disease in Metabolic Syndrome Patients. Medicine 2020, 99, e23619. [Google Scholar] [CrossRef] [PubMed]
- Petroff, D.; Blank, V.; Newsome, P.N.; Shalimar; Voican, C.S.; Thiele, M.; de Lédinghen, V.; Baumeler, S.; Chan, W.K.; Perlemuter, G.; et al. Assessment of Hepatic Steatosis by Controlled Attenuation Parameter Using the M and XL Probes: An Individual Patient Data Meta-Analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 185–198. [Google Scholar] [CrossRef] [PubMed]
- de Lédinghen, V.; Wong, G.L.; Vergniol, J.; Chan, H.L.; Hiriart, J.; Chan, A.W.; Chermak, F.; Choi, P.C.; Foucher, J.; Chan, C.K.; et al. Controlled Attenuation Parameter for the Diagnosis of Steatosis in Non-alcoholic Fatty Liver Disease. J. Gastroenterol. Hepatol. 2016, 31, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Vuppalanchi, R.; Siddiqui, M.S.; Natta, M.L.V.; Hallinan, E.; Brandman, D.; Kowdley, K.; Neuschwander-Tetri, B.A.; Loomba, R.; Dasarathy, S.; Abdelmalek, M.; et al. Performance Characteristics of Vibration-controlled Transient Elastography for Evaluation of Nonalcoholic Fatty Liver Disease. Hepatology 2018, 67, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.S.; Vuppalanchi, R.; Natta, M.L.V.; Hallinan, E.; Kowdley, K.V.; Abdelmalek, M.; Neuschwander-Tetri, B.A.; Loomba, R.; Dasarathy, S.; Brandman, D.; et al. Vibration-Controlled Transient Elastography to Assess Fibrosis and Steatosis in Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2019, 17, 156–163.e2. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kwon, H.-J.; Sohn, W.; Cho, J.-Y.; Park, S.J.; Chang, Y.; Ryu, S.; Kim, B.I.; Cho, Y.K. Risk of Liver Fibrosis in Patients with Prediabetes and Diabetes Mellitus. PLoS ONE 2022, 17, e0269070. [Google Scholar] [CrossRef]
- Oliveira, C.P.; de Lima Sanches, P.; de Abreu-Silva, E.O.; Marcadenti, A. Nutrition and Physical Activity in Nonalcoholic Fatty Liver Disease. J. Diabetes Res. 2016, 2016, 4597246. [Google Scholar] [CrossRef]
- Tacke, F.; Weiskirchen, R. Non-Alcoholic Fatty Liver Disease (NAFLD)/Non-Alcoholic Steatohepatitis (NASH)-Related Liver Fibrosis: Mechanisms, Treatment and Prevention. Ann. Transl. Med. 2021, 9, 729. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).