Abstract
Sarcopenia and spinal spondylosis (SS) are important health challenges among older individuals; however, data regarding the effect of sarcopenia on SS are lacking. Hence, we aimed to organize the existing knowledge on the impact of sarcopenia on SS and explore potential issues in the available literature. We examined the trends and interventions regarding sarcopenia and SS, searching five databases (PubMed, Embase, CINHAL, Web of Science, and Cochrane Library) from inception to January 2023. Sarcopenia-related events were screened, selected, and reviewed, ultimately identifying 19 relevant studies. The identified reports were predominantly retrospective observational studies addressing lumbar degenerative spine disease (LDSD). Sarcopenia could negatively impact the quality of life and postoperative outcomes in several diseases, including cervical spondylotic myelopathy (CSM) and LDSD. However, there was no consensus among the studies regarding the relationship between sarcopenia and pain. These discrepancies were attributed to gaps in the assessment of sarcopenia, which the current study identifies as important challenges. This review identified several problems in the literature, including the limited number of studies examining CSM, adult spinal deformity (ASD) and scoliosis, and the retrospective study design of most reports. The further accumulation of quality research is needed to clarify the relationship between SS and sarcopenia.
1. Introduction
Sarcopenia is a progressive and generalized skeletal muscle disorder characterized by an accelerated loss of muscle mass and function. With respect to human health, sarcopenia increases the risk of falls and fractures; impairs the ability to perform activities of day-to-day living; and has been associated with cardiac disease, respiratory disease, and cognitive impairment. Sarcopenia leads to mobility disorders, resulting in a reduced quality of life (QOL), loss of independence or the need for long-term care placement, and death [1]. Therefore, sarcopenia is well-recognized as an important health challenge among the aging population. The prevalence of sarcopenia is higher among individuals with degenerative musculoskeletal diseases (e.g., osteoporosis, osteoarthritis (OA), and spinal spondylosis (SS)) than in the general older population. Sarcopenia is a risk factor for falls and fragility fractures [1], and its prevalence in patients with proximal femoral fractures and vertebral compression fractures is as high as 40%. Moreover, the presence of sarcopenia is a risk factor for postoperative complications and protracted pain after a fragility fracture, worsening the prognosis [2,3,4].
The prevalence of sarcopenia in patients with OA is approximately three times higher than that in the general older population [5]. In addition, the presence of sarcopenia in patients with OA is considered a risk factor for postoperative infections [6] and can hinder postoperative improvement in physical functions [7]. Similar to fragility fractures and OA, SS is also highly prevalent among older individuals, and patients with SS have an increased risk of developing sarcopenia, thereby necessitating appropriate countermeasures; however, reports assessing this issue remain scarce. To date, only lumbar degenerative spine disease (LDSD) has been examined in a meta-analysis, which found that LDSD was associated with a high prevalence of sarcopenia (approximately 25%) and could adversely impact QOL [8]. However, this meta-analysis had several issues, including a small number of included reports, predominantly cross-sectional and retrospective studies, with no high-quality prospective observational or interventional studies. Furthermore, the meta-analysis failed to address cervical spondylotic myelopathy (CSM) or adult spinal deformity (ASD). Accordingly, the association between sarcopenia and SS remains poorly explored. To discuss the need for sarcopenia countermeasures in SS, extensive and comprehensive data are critical to clarify the effects on prognosis and establish interventional strategies.
Therefore, we conducted the present literature review to organize the existing knowledge on the impact of sarcopenia on SS and explore the potential issues in the available literature.
2. Materials and Methods
2.1. Database Search
Data were collected in accordance with the procedures recommended in the extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews [9]. A comprehensive literature search was performed using five electronic databases, namely, PubMed, Embase, CINAHL, Web of Science, and Cochrane Reviews. For each database, the search range was set from the time of database inception to January 2023. Table 1 presents the keywords used in the search strategy. A manual search was performed using the citations listed in included articles, as needed.

Table 1.
Search strategy for study selection.
2.2. Study Eligibility
The clinical questions in this review were formulated as follows: patients (P): diagnosed with SS who had been evaluated for sarcopenia; exposure (E): sarcopenia defined according to the European Working Group on Sarcopenia in Older People, the Foundation for the National Institutes of Health, the Asian Working Group for Sarcopenia (AWGS), the Sarcopenia Definitions and Outcomes Consortium, and the International Working Group on Sarcopenia; comparison (C): non-sarcopenia; outcome (O): clinical outcomes (including QOL, physical function, and postoperative clinical outcomes; and study design (S): prospective and retrospective cohorts and interventional studies. To examine the relationship between SS and sarcopenia, only articles that met the above PECOS criteria were selected, and all others were excluded.
2.3. Data Collection
Relevant articles were not filtered out; two authors (Y.K. and T.W.) independently selected articles based on the eligibility criteria. Each author checked and screened the abstract and main text of the papers. In cases of disagreement, all the other authors evaluated the paper for eligibility. Two authors (Y.K. and T.W.) abstracted the final articles independently, and key elements were extracted using a predesigned template. The included articles were described with respect to the author information, study design, population (including nationality, disease, and sex), subject details, sarcopenia definition, and assessment methods. The primary endpoints of the studies were recorded as outcomes, and the actual data and results obtained were recorded as the key findings of the results.
3. Results
3.1. Search Results
After screening the titles and abstracts of articles identified in the database search, 59 articles fulfilled the eligibility criteria. Of these, 40 articles were excluded as patients lacked a sarcopenia evaluation (n = 18), the study design was not appropriate (n = 12), no comparison was performed with the sarcopenia cohort (n = 9), or the full text was unavailable (n = 1). Ultimately, 19 articles were included in the present study. Figure 1 presents the flowchart of the literature search.
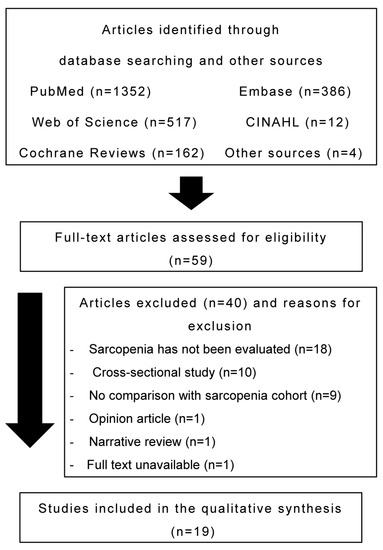
Figure 1.
Flow diagram of the search process.
3.2. Characteristics of Included Studies
3.2.1. Participant Characteristics
Most studies (n = 7 (36.8%) [10,11,12,13,14,15,16]) reported findings from Japan, while others reported findings from the United States (n = 5 [17,18,19,20,21]), Korea (n = 2 [22,23]), China (n = 2 [24,25]), Italy (n = 2 [26,27]), and Taiwan (n = 1 [8]). Table 2 summarizes the characteristics of the included articles. The target diseases were CSM (n = 2 [11,20]), LDSD (n = 9 [8,12,13,18,21,23,24,26,27]), lumbar spinal stenosis (LSS; n = 6 [10,14,15,16,22,25]), and ASD (n = 1 [19]). One study evaluated patients who underwent thoracolumbar surgery (n = 1 [17]).

Table 2.
Summary of extracted studies.
3.2.2. Study Characteristics
Regarding the publication language, excluding one report in Chinese [25], all the included articles were in English. Fifteen articles were retrospective observational studies [10,12,13,14,17,18,19,20,21,22,23,24,25,26,27], three were prospective observational studies [11,15,16], and one was a meta-analysis (LDSD) [8]. However, no intervention studies for SS and sarcopenia were available in any of the explored databases.
3.2.3. Sarcopenia Definition and Assessment Methods
All the identified studies assessed sarcopenia preoperatively. Regarding sarcopenia assessment in SS, five articles assessed muscle mass alone using dual-energy X-ray and bioelectrical impedance analysis [10,11,12,15,16]. The prevalence of sarcopenia, as defined by this assessment method, ranged between 20.3 and 47.4%.
Four articles evaluated the cross-sectional area of the psoas muscle [17,19,26,27]; the prevalence of sarcopenia ranged between 18.1 and 50.7%. Three articles evaluated sarcopenia according to the AWGS criteria [13,14,24], revealing that the prevalence of sarcopenia was 14.0, 20.0, and 23.1%, respectively. Two articles assessed sarcopenia based on the cross-sectional area of the erector spinae and psoas muscles, reporting a sarcopenia prevalence of 18.1 and 50.0%, respectively [18,25]. Another study defined sarcopenia by muscle quality, as assessed by magnetic resonance imaging (MRI), and reported a prevalence of 26.1% [20]. In addition, we included two articles that primarily examined physical function (grip strength) in patients with sarcopenia without assessing muscle mass, reporting prevalence rates of 38.2 and 50.0%, respectively [22,23]. One article examined sarcopenia using the International Statistical Classification of Diseases code and documented a sarcopenia prevalence of 4.5% [21].
3.2.4. Study Outcomes
Based on the studies included in the present review, the presence or absence of sarcopenia did not alter perioperative management. Five reports revealed that patients with SS and sarcopenia had decreased postoperative disease-specific QOL [10,12,20,22,24], whereas three reports found no significant postoperative differences between the sarcopenia and control groups [14,18,19]. A meta-analysis by Wu et al., which included several of the abovementioned studies, summarized the effects of sarcopenia on LDSD and found that the postoperative QOL was significantly lower in the sarcopenia group than that in the non-sarcopenia group [8].
Two articles reported a decrease in postoperative QOL in the sarcopenia group [11,22], whereas one detected no such difference [14]. One report documented poor improvement in postoperative pain changes in patients with LDSD in the sarcopenia group [25], and six found no differences in postoperative pain changes [10,12,13,14,18,24]. One report documented poor improvement in postoperative neck pain in patients with CSM in the sarcopenia group [20]. Additionally, two recorded a poor improvement in postoperative Japanese Orthopaedic Association (JOA) scores in the sarcopenia group [11,12], whereas one found no difference [13].
One article reported prolonged length of stay (LOS) after lumbar spine surgery in the sarcopenia group [23], and one found no such difference [19]. Two articles reported that the incidence of postoperative complications was significantly increased in the sarcopenia group after lumbar spine surgery [21,23], while three articles detected no difference [19,26,27]. Various other outcomes were evaluated after lumbar spine surgery, including postoperative costs [17], fall incidence [15], psychological factors [16], and motor function [22]; these outcomes were poorer in the sarcopenia group than in the non-sarcopenia group.
4. Discussion
Our literature review included 19 studies on sarcopenia and SS that examined postoperative outcomes as the main outcome measure. There were no articles on conservative management. Sarcopenia has been shown to negatively impact postoperative QOL in surgical patients. In addition, sarcopenia has a wide range of effects on surgical patients with SS, including increased risk of falls, deterioration of psychological factors, and impairment of postoperative physical function. Most studies were retrospective in design, and LDSD was the most frequently assessed disease. Imaging information derived from computed tomography (CT) and MRI is essential for the diagnosis and preoperative planning of LDSD; moreover, the associated muscle mass assessment is considered the gold standard [1]. LDSD was widely explored, and the number of publications on LDSD was the highest. This can be attributed to the ease of simultaneously performing disease-related and muscle mass assessments. Only two studies incorporated a sarcopenia cohort for CSM. One study included patients with ASD, while no study assessed patients with sarcopenia and scoliosis. At present, undertaking a systematic review of CSM, ASD, and scoliosis would be challenging owing to the limited number of reports. Additional studies on CSM, ASD, and scoliosis are needed to elucidate the relationship between SS and sarcopenia.
Among the articles included in the current review, several reported worse disease-specific QOL in patients with CSM and LDSD in the sarcopenia cohort [10,12,20,22,24], which is consistent with the results of the previous meta-analysis [8]. Several included articles reported similar adverse outcomes, considering the occurrence of postoperative complications and falls in the sarcopenia group [15,21,23]. Sarcopenia has been found to increase adverse outcomes and decrease QOL owing to several factors, including muscle weakness and decreased immunity [28]. This phenomenon may occur in SS, which largely affects older individuals, suggesting that sarcopenia may negatively affect SS outcomes.
However, the current literature review was complicated by several challenges that made it difficult to define the impact of sarcopenia on SS. First, sarcopenia assessment was performed using diverse methods, including those employing imaging information such as CT or MRI [17,18,19,20,25,26,27], those that follow the AWGS criteria [12,13,14,15,16,24], and those that rely solely on grip strength as an indicator [22,23]. A meta-analysis has reported that the prevalence of sarcopenia varied between 10.0 and 27.0% in the general older population, depending on the diagnostic method used [29]. Likewise, the prevalence of sarcopenia varied widely among the studies included in the current review, ranging between 4.5 and 50.0%. The use of distinct methods to assess sarcopenia may lead to an overestimation or underestimation of sarcopenia prevalence.
Furthermore, the differences in assessment methods may influence the prevalence of sarcopenia in SS and its associated outcomes. Although most studies detected no difference in postoperative pain, i.e., the main symptom, between patients with and without sarcopenia [10,12,13,14,18,24], some reported worse postoperative pain in the sarcopenia group [20,25]. These inconsistent findings may be attributed to the different approaches used to assess muscle mass, focusing either on limb muscles or assessing the spinal muscles and their quality. In particular, spinal muscles are essential for controlling spinal movements and may directly impact spine-related symptoms [30]. Accordingly, whether the sarcopenia assessment is based on the limb or spinal muscle mass could have a substantial impact in the context of SS. Thus, the different methods used to assess sarcopenia may have contributed to discrepancies in certain outcomes, including postoperative outcomes and complications.
Second, the available literature on the association between SS and sarcopenia is predominantly from Asian countries, which raises the issue of racial bias. The prevalence of sarcopenia is estimated to be the highest among Asian populations across all diagnostic criteria, considering the global definition of sarcopenia [29]. This high prevalence among Asian populations may be influenced by the substantial interest of researchers in this region and the availability of diagnostic criteria specifically tailored for these populations, e.g., the AWGS criteria. It is predicted that the availability and use of region-specific diagnostic criteria, such as the AWGS, may have influenced these findings.
Importantly, the standardization of diagnostic criteria for sarcopenia internationally could lead to a reduction in racial bias and ensure a more accurate representation of the prevalence and impact of sarcopenia in different populations.
Finally, challenges related to the study design need to be addressed. Most articles included in the current review were retrospective studies, resulting in limited evidence based on the impact of sarcopenia on SS at this stage. However, two prospective studies showed negative effects [11,16], suggesting that a higher quality review could be conducted by prospectively investigating the impact of sarcopenia on SS. There have been no interventional trials specifically targeting sarcopenia in SS. One randomized controlled trial [31] evaluated a potential interventional strategy, revealing that a nutritional intervention focused on protein intake could improve postoperative trunk muscle mass in patients undergoing lumbar fusion surgery. Another study reported improvements in physical function following strength training or surgical intervention for lumbar disorders [14,32]. These findings suggest potential strategies to address sarcopenia in SS. In the future, interventional studies aimed at improving sarcopenia in SS should be conducted to assess their impact on important outcomes such as pain and disease-specific QOL.
The follow-up periods after spinal surgery varied between studies. To systematically elucidate the association between SS and sarcopenia, a preplanned prospective observational study should be conducted. In addition, a follow-up period of 2 years or longer should be considered to examine long-term outcomes, as a 2-year follow-up is a known criterion for postoperative outcomes after SS surgery [33].
Regarding the current assessment methods for sarcopenia in SS, this review showed that the assessment of spinal muscle morphology through imaging, which reflects the main symptoms of pain and postoperative QOL, is clinically useful. Therefore, evaluation using imaging information from CT or MRI is considered useful for clinical purposes. However, regarding the evaluation method, it is crucial to assess the muscle mass of both limb skeletal muscles and spinal muscles, determine which strongly influences clinical outcomes, and establish a clinically valuable evaluation method.
This study had several limitations. No meta-analysis or other quantification was performed owing to the number of outcomes and diseases included in this review and the variability in sarcopenia assessment. Additionally, no bias assessment was performed; therefore, selection or publication bias may be present. Therefore, the objectivity of the results cannot be guaranteed. Finally, the literature search explored limited databases, and we excluded populations from Latin America, Oceania, and Africa, generating a potential language selection bias.
5. Conclusions
Our literature review focused on studies exploring sarcopenia and SS and found that sarcopenia can negatively impact the QOL and postoperative outcomes in several diseases, including CSM and LDSD. However, there was no consensus among studies regarding the relationship between sarcopenia and the main symptoms of SS, such as pain. These discrepancies were attributed to the gap in internationally standardized sarcopenia assessment methods, potential racial bias, and variations in research design, which were identified as important limitations of the current study. The further accumulation of quality research is needed to clarify the relationship between SS and sarcopenia.
Author Contributions
Conceptualization, Y.K., T.W. and H.H.; methodology, Y.K and T.W.; investigation, Y.K. and T.W; writing—original draft preparation, Y.K., T.W. and H.H.; writing—review and editing, Y.K., T.W., S.T., M.O., H.N. and H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. Public data sharing is not applicable to this article due to ethical considerations and privacy restrictions.
Acknowledgments
We are grateful to Ryoko Ikehara for providing secretarial assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Hida, T.; Shimokata, H.; Sakai, Y.; Ito, S.; Matsui, Y.; Takemura, M.; Kasai, T.; Ishiguro, N.; Harada, A. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur. Spine J. 2016, 25, 3424–3431. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.H.; Kuo, Y.J.; Chen, Y.P. The association between sarcopenia and postoperative outcomes among older adults with hip fracture: A systematic review. J. Appl. Gerontol. 2021, 40, 1903–1913. [Google Scholar] [CrossRef]
- Bo, J.; Zhao, X.; Hua, Z.; Li, J.; Qi, X.; Shen, Y. Impact of sarcopenia and sagittal parameters on the residual back pain after percutaneous vertebroplasty in patients with osteoporotic vertebral compression fracture. J. Orthop. Surg. Res. 2022, 17, 111. [Google Scholar] [CrossRef]
- Kemmler, W.; Teschler, M.; Goisser, S.; Bebenek, M.; von Stengel, S.; Bollheimer, L.C.; Sieber, C.C.; Freiberger, E. Prevalence of sarcopenia in Germany and the corresponding effect of osteoarthritis in females 70 years and older living in the community: Results of the FORMoSA study. Clin. Interv. Aging 2015, 10, 1565–1573. [Google Scholar] [CrossRef]
- Babu, J.M.; Kalagara, S.; Durand, W.; Antoci, V.; Deren, M.E.; Cohen, E. Sarcopenia as a risk factor for prosthetic infection after total hip or knee arthroplasty. J. Arthroplast. 2019, 34, 116–122. [Google Scholar] [CrossRef]
- Liao, C.D.; Chen, H.C.; Liou, T.H.; Lin, C.L.; Huang, S.W. Impact of sarcopenia and obesity on gait speed after total knee replacement. J. Am. Med. Dir. Assoc. 2022, 23, 631–637. [Google Scholar] [CrossRef]
- Wu, W.T.; Lee, T.M.; Han, D.S.; Chang, K.V. The prevalence of sarcopenia and its impact on clinical outcomes in lumbar degenerative spine disease—A systematic review and meta-analysis. J. Clin. Med. 2021, 10, 773. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Eguchi, Y.; Suzuki, M.; Yamanaka, H.; Tamai, H.; Kobayashi, T.; Orita, S.; Yamauchi, K.; Suzuki, M.; Inage, K.; Fujimoto, K.; et al. Influence of skeletal muscle mass and spinal alignment on surgical outcomes for lumbar spinal stenosis. Asian Spine J. 2018, 12, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Koshimizu, H.; Sakai, Y.; Harada, A.; Ito, S.; Ito, K.; Hida, T. The impact of sarcopenia on cervical spine sagittal alignment after cervical laminoplasty. Clin. Spine Surg. 2018, 31, E342–E346. [Google Scholar] [CrossRef] [PubMed]
- Inose, H.; Yamada, T.; Hirai, T.; Yoshii, T.; Abe, Y.; Okawa, A. The impact of sarcopenia on the results of lumbar spinal surgery. Osteoporos. Sarcopenia 2018, 4, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Hoshino, M.; Ohyama, S.; Terai, H.; Suzuki, A.; Yamada, K.; Takahashi, S.; Hayashi, K.; Tamai, K.; Hori, Y.; et al. Impact of sarcopenia on clinical outcomes of minimally invasive lumbar decompression surgery. Sci. Rep. 2019, 9, 16619. [Google Scholar] [CrossRef]
- Sakai, Y.; Wakao, N.; Matsui, H.; Tomita, K.; Watanabe, T.; Iida, H. Surgical results in older patients with lumbar spinal stenosis according to gait speed in relation to the diagnosis for sarcopenia. J. Orthop. Surg. 2020, 28, 2309499020918422. [Google Scholar] [CrossRef]
- Wada, T.; Tanishima, S.; Kitsuda, Y.; Osaki, M.; Nagashima, H.; Hagino, H. Preoperative low muscle mass is a predictor of falls within 12 months of surgery in patients with lumbar spinal stenosis. BMC Geriatr. 2020, 20, 516. [Google Scholar] [CrossRef]
- Wada, T.; Tanishima, S.; Kitsuda, Y.; Osaki, M.; Nagashima, H.; Hagino, H. Association between preoperative low muscle mass and psychological factors after surgery among patients with lumbar spinal stenosis: A longitudinal study. J. Clin. Neurosci. 2021, 89, 8–14. [Google Scholar] [CrossRef]
- Bokshan, S.L.; Han, A.; Depasse, J.M.; Marcaccio, S.E.; Eltorai, A.E.M.; Daniels, A.H. Inpatient costs and blood transfusion rates of sarcopenic patients following thoracolumbar spine surgery. J. Neurosurg. Spine 2017, 27, 676–680. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, J.C.; Wagner, S.C.; Sebastian, A.; Casper, D.S.; Mangan, J.; Stull, J.; Hilibrand, A.S.; Vaccaro, A.R.; Kepler, C. Sarcopenia does not affect clinical outcomes following lumbar fusion. J. Clin. Neurosci. 2019, 64, 150–154. [Google Scholar] [CrossRef]
- Akbik, O.S.; Al-Adli, N.; Pernik, M.N.; Hicks, W.H.; Hall, K.; Aoun, S.G.; Bagley, C.A. A comparative analysis of frailty, disability, and sarcopenia with patient characteristics and outcomes in adult spinal deformity surgery. Glob. Spine J. 2022, 21925682221082053. [Google Scholar] [CrossRef]
- Pinter, Z.W.; Salmons, H.I.t.; Townsley, S.; Omar, A.; Freedman, B.A.; Currier, B.L.; Elder, B.D.; Nassr, A.N.; Bydon, M.; Wagner, S.C.; et al. Multifidus sarcopenia is associated with worse patient-reported outcomes following posterior cervical decompression and fusion. Spine 2022, 47, 1426–1434. [Google Scholar] [CrossRef]
- Albright, J.A.; Chang, K.; Alsoof, D.; McDonald, C.L.; Diebo, B.G.; Daniels, A.H. Sarcopenia and postoperative complications, cost of care, and all-cause hospital readmission following lumbar spine arthrodesis: A propensity matched cohort study. World Neurosurg. 2023, 169, e131–e140. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.W.; Lee, B.H.; Lee, S.B.; Sung, S.; Lee, C.U.; Yang, J.H.; Park, M.S.; Byun, J.; Lee, H.M.; Moon, S.H. Hand grip strength can predict clinical outcomes and risk of falls after decompression and instrumented posterolateral fusion for lumbar spinal stenosis. Spine J. 2020, 20, 1960–1967. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Park, S.J.; Kim, N.Y.; Jeon, W.; Shin, D.A.; Kim, S.H. Influence of preoperative handgrip strength on length of stay after lumbar fusion surgery. J. Clin. Med. 2022, 11, 3928. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Ma, Y.; Li, F.; Xu, Z.; Chen, Q. The effect of sarcopenia in the clinical outcomes following stand-alone lateral lumbar interbody fusion. J. Back Musculoskelet. Rehabil. 2021, 34, 469–476. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, C.; Wang, H.; Yu, K.; Zhang, J.; Wang, Y. Impact of sarcopenia on effectiveness of lumbar decompression surgery in patients with lumbar spinal stenosis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2022, 36, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Ruffilli, A.; Manzetti, M.; Cerasoli, T.; Barile, F.; Viroli, G.; Traversari, M.; Salamanna, F.; Fini, M.; Faldini, C. Osteopenia and sarcopenia as potential risk factors for surgical site infection after posterior lumbar fusion: A retrospective study. Microorganisms 2022, 10, 1905. [Google Scholar] [CrossRef]
- Barile, F.; Ruffilli, A.; Fiore, M.; Manzetti, M.; Geraci, G.; Viroli, G.; Faldini, C. Is sarcopenia a risk factor for postoperative surgical site infection after posterior lumbar spinal fusion? Int. J. Spine Surg. 2022, 16, 735–739. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef]
- Jermy, J.E.; Copley, P.C.; Poon, M.T.C.; Demetriades, A.K. Does pre-operative multifidus morphology on MRI predict clinical outcomes in adults following surgical treatment for degenerative lumbar spine disease? A systematic review. Eur. Spine J. 2020, 29, 1318–1327. [Google Scholar] [CrossRef]
- Khalooeifard, R.; Shariatpanahi, Z.V.; Ahani, A.; Keykhaee, M.; Oraee-Yazdani, M.; Zali, A.; Oraee-Yazdani, S. Effect of protein supplement on paraspinal muscles in spine fusion surgery: A randomized, double-blind, placebo-controlled trial. Int. J. Spine Surg. 2021, 15, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Kernc, D.; Strojnik, V.; Vengust, R. Early initiation of a strength training based rehabilitation after lumbar spine fusion improves core muscle strength: A randomized controlled trial. J. Orthop. Surg. Res. 2018, 19, 151. [Google Scholar] [CrossRef] [PubMed]
- Zaina, F.; Tomkins-Lane, C.; Carragee, E.; Negrini, S. Surgical versus non-surgical treatment for lumbar spinal stenosis. Cochrane Database Syst. Rev. 2016, 2016, Cd010264. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).