Abstract
Chronic renal failure (CRF) is associated with the development of cardiovascular disease (CVD). Paraoxonase 1 (PON1), an antioxidant enzyme, shows cardioprotective properties and has been proposed as a therapeutic marker for CRF. A systematic analysis of the literature assessing the association between PON1 activity and renal replacement therapy (RRT) of CRF is currently lacking. Therefore, we set out to perform a meta-analysis of the available data on PON1 in RRT of CRF. We searched three electronic databases for studies on PON1 activity in CRF patients with RRT such as hemodialysis (HD), peritoneal dialysis (PD), or renal transplantation (RTx), published before June 2023. A random-effects and network meta-analysis were performed. A total of 53 studies were eligibly identified. Compared to CRF patients without RRT, RTx patients had higher paraoxonase activity (standard mean difference (SMD), 1.76, 95% confidence interval (CI), 0.76–2.75), followed by HD (SMD, 0.73; 95% CI, 0.02–1.45) and PD patients. Likewise, RTx patients had higher arylesterase activity (SMD, 1.84, 95% CI, 0.18–3.50), followed by HD and PD patients. Also, paraoxonase activity was increased after HD (SMD, 0.59, 95% CI, 0.16–1.03). In conclusion, the overall data demonstrated that PON1 activity is higher in CRF patients with RRT, particularly RTx, followed by that of HD and PD. Measuring PON1 activity can also be included to the paraclinical toolbox for the management of RRT, in addition to the understanding of CRF-related pathophysiology. Regarding the selection of RRT types and their potential to prevent CVD, more research is required.
1. Introduction
With a high incidence and being a leading cause of mortality, cardiovascular disease (CVD) is a global health concern; accordingly, numerous risk factors have been studied in an effort to lower the risk [1,2]. Disorders of low-density lipoprotein (LDL) and high-density lipoprotein (HDL) are some of the risk factors for the development of CVD, specifically their concentrations in blood and qualitative dysfunction [2].
Paraoxonase 1 (PON1) is an esterase and lactonase enzyme which participates in antioxidant defenses and that is synthesized primarily by the liver and released into the blood circulation linked to HDL [3,4]. PON1 has paraoxonase and arylesterase activity because it hydrolyzes aromatic carboxylic acid esters like phenylacetate and organophosphate compounds like paraoxon. Paraoxon for paraoxonase is a “discriminating” substrate (with a polymorphic distribution of activity), whereas phenylacetate for arylesterase is a “nondiscriminating” substrate; thus, PON1 activity is reported in the literature as paraoxonase or arylesterase [3,4], while PON1′s lactonizing/lactonase activity is presumed to be the important one physiologically. Along with its antioxidant capabilities, PON1 has antiatherogenic effects that prevent the development of macrophage foam cells, including decreased cholesterol and oxidized lipid input as well as facilitation of macrophage cholesterol efflux [3,4]. We can see evidence supporting the protection of PON1 activity on CVD [3,4].
Chronic kidney disease/chronic renal failure (CRF) is a common pathological condition of renal damage, and renal replacement therapy (RRT) (i.e., renal replacement therapy including hemodialysis [HD], peritoneal dialysis [PD], renal transplantation [RTx]) is applied to the end-stage kidney disease [5]. Cardiovascular disease is a frequent occurrence in CRF [5], and remarkably, PON1 activity is inversely related to CVD in CRF patients even when they are receiving RRT, suggesting the important role of antioxidant status in the pathophysiology of CVD in the management of CRF [2,4,6]. The impact of therapeutic interventions, such as RRT, on antioxidant defenses should be more clarified.
Our previous meta-analysis revealed that CRF patients without RRT had lower PON1 activity (arylesterase and paraoxonase) compared to healthy individuals, indicating PON1 may serve as a biomarker in CRF in relation to CVD [7]. However, an analysis of the current literature on the effects of RRT on PON1 activity in CRF patients remains needed. Hence, the aim of this study was to conduct a systematic meta-analysis of the available clinical studies on the effects of all RRT types on PON1 activity in such patients.
2. Materials and Methods
This study was performed following standard protocols according to the Preferred Reporting Items for Systematic Review and Meta-analysis 2020 [8]. The study protocol was registered in PROSPERO (CRD42023389430).
2.1. Eligibility Criteria
The studies included in the analysis met the following criteria: they were comparative studies by cohort, case-control, and cross-sectional designs, and reported the data of PON1 activity in healthy subjects aged 18 years and older, CRF patients without RRT, and CRF patients with HD, PD, or RTx. CRF without RRT was defined as the estimated glomerular filtration rate, eGFR < 30 mL/min/1.73 m2 (according to the international guidelines) without any corresponding RRT [9].
2.2. Information Source and Search
To identify studies investigating PON1 activity in CRF patients with RRT, a two-step strategy was employed. Firstly, comprehensive searches were conducted in databases of MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials. These were conducted until 1 June 2023 using the search engines of PubMed, Dialog, and Cochrane Library. The search terms included (“aryldialkylphosphatase” or “arylesterase” or “paraoxonase”) and (“renal insufficiency” or “chronic kidney failure” or “chronic renal insufficiency” or “kidney diseases” or “uremia” or “renal dialysis”) (Appendix A). There were no language restrictions applied during the search process.
2.3. Study Selection and Data Collection Process
In the screening process, independent reviewers (J.W. and K.K.) assessed the titles and abstracts of the identified studies. The eligibility assessment was then conducted based on the full texts of the selected studies, and the data were extracted. Any disagreements between the reviewers were resolved through discussions or by consulting an additional investigator (A.G.). The extracted data detailed the study design, study population, interventions, and outcomes. For the evaluation of risk of bias, independent reviewers (J.W. and K.K.) assessed the risk of bias using an 11-item checklist that was recommended by the Agency for Healthcare Research and Quality [10]. In case of any disagreements between the reviewers, they were discussed, and if necessary, a third reviewer (A.G.) acted as an arbiter.
2.4. Data Synthesis and Statistical Analysis
The network geometries are visually illustrated with circles representing the surgical procedure as nodes in the network, lines depicting direct comparisons using studies, and the width of the lines indicating the number of studies included in each comparison, which is also represented by corresponding numbers.
The standard mean differences (SMDs) and corresponding 95% confidence intervals (CIs) for paraoxonase and arylesterase activity, both continuous variables, were pooled in our analysis based on the Cochrane handbook [11]. Group-level data were utilized, and a random-effects Frequentist network meta-analysis model was employed to synthesize the effect sizes from the included studies. The normal likelihood was applied for continuous outcomes, and a normal prior was used. To account for correlations induced by multi-group studies, multivariable distributions were utilized. The variance in the random-effects distribution, referred to as the heterogeneity variance, was considered to capture the effects of both cross-study and within-comparison variability on treatment effects. The MetaInsight software version 4.2.0 was used for the analysis [12]. We also calculated the SMDs for paraoxonase activity before and after HD. The threshold of significance was p < 0.05.
3. Results
Figure 1 illustrates the process of study selection.
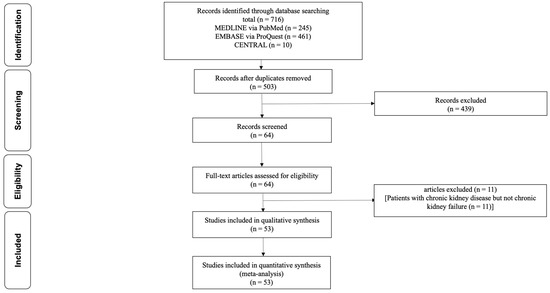
Figure 1.
Flow diagram of selection of articles that reported the PON1 in patients with chronic renal failure who were treated with hemodialysis, peritoneal dialysis, or renal transplantation. PON1, paraoxonase 1.
Following the removal of duplicate records, a total of 503 unique records were identified. After the initial screening process, 439 records were excluded based on predetermined criteria, resulting in 64 remaining records. During the secondary screening, 11 studies involving patients with CKD but not CRF (as eGFR ≥ 30 mL/min/1.73 m2) were excluded. Ultimately, a total of 53 studies were included in the analysis for further evaluation and synthesis [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65]. Of 53 studies, 47 studies reported PON1 activity in CRF patients with and without RRT. Two studies reported paraoxonase activity before and after HD, while four studies reported PON1 activity in CRF patients with and without RRT as well as paraoxonase activity before and after HD.
Table 1 shows a summary of the study characteristics in CRF patients with RRT. A total of 48 studies involving 7898 patients (53 years of mean age) reported paraoxonase activity, while 22 studies involving 3942 patients (49 years of mean age) reported arylesterase activity. The overall risk of bias was median 7, range 3–8, using an 11-item checklist by AHRQ in Appendix B.

Table 1.
Summary of the articles on PON1 activity included.
Figure 2 shows the forest plots for network meta-analysis and the network plot of all studies that measured paraoxonase activity. The network meta-analysis of paraoxonase activity was performed in 48 studies. Healthy controls had higher activity relative to CRF patients in accordance with the previous report [7]. Paraoxonase activity was significantly higher in CRF patients with RTx (SMD 1.76; 95% CI, 0.76–2.75) and HD (SMD 0.73; 95% CI, 0.02–1.45) compared to those without RRT. Paraoxonase activity tended to be high in CRF patients with PD compared to those without RRT (SMD 0.63; 95% CI, −0.36–1.62).
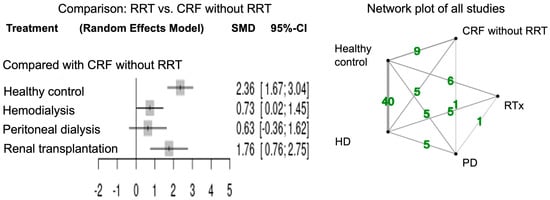
Figure 2.
Forest and network plots of paraoxonase activity in all studies compared with CRF without RRT. Green number indicates the study numbers. CRF, chronic renal failure; RRT, renal replacement therapy.
Figure 3 shows the forest plots for network meta-analysis and the network plot of all studies measuring arylesterase activity. The network meta-analysis of arylesterase activity was performed in 22 studies. Healthy controls had higher activity relative to CRF patients in agreement with the previous report [7]. Arylesterase activity was significantly higher in CRF patients with RTx (SMD 1.84; 95% CI, 0.18–3.50) compared to those without RRT. Arylesterase activity tended to be high in CRF patients with PD (SMD 1.17; 95% CI, −0.45–2.79) and HD (SMD 0.53; 95% CI, −0.60–1.67) compared to those without RRT.
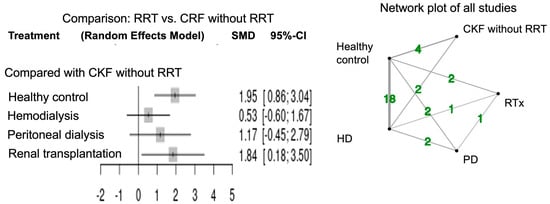
Figure 3.
Forest plot and network plots of arylesterase activity in all studies compared with CRF without RRT. Green number indicates the study numbers. CRF, chronic renal failure; RRT, renal replacement therapy.
Figure 4 shows the forest plots of paraoxonase activity in CRF patients before and after HD. In the meta-analysis, six studies reported paraoxonase activity in patients before and after HD. Paraoxonase activity was increased after HD (SMD, 0.59, 95% CI, 0.16–1.03) compared to that before HD.
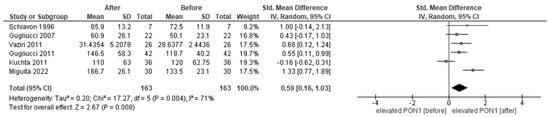
Figure 4.
Forest plot of paraoxonase activity before and after hemodialysis [29,33,34,51,65].
4. Discussion
Given the importance of PON1 for CVD in CRF patients with RRT, the present comprehensive meta-analysis of studies was carried out to assess the effect of various RRT types on PON1 activity [2,4,6]. The analysis corroborated the earlier finding that healthy controls have higher PON1 activities than CFK patients [7]. Importantly, we observed that paraoxonase and arylesterase activities were highest in CRF patients receiving RTx, followed by higher activity levels in patients under HD and PD as compared to those not receiving RRT. Additionally, paraoxonase activity was increased in CRF patients during HD when measured before and after this treatment modality. This might provide valuable insights into the use of PON1 activity in assessing the CRF-related pathophysiology as a surrogate marker of CVD and the potential guidance of therapeutic strategies for CRF patients.
As we summarize in Figure 5, “uremic” toxins, inflammatory cytokines, and hypertension with an active renin-angiotensin system cause excessive oxidative stress and decreased antioxidant defenses, which are generally recognized factors worsening CRF prognosis [66]. RRT can improve the situation by eliminating toxic substrates and alleviating a stressed-out system. The present meta-analysis results suggest that this may help to partially explain why PON1 activity was recovered. As seen in the present study, the degree of PON1 recovery differed depending on RRT types. Different mechanisms may be thus at work. For instance, the HD procedure by the dialysis machine [67] employs heparin to avoid clotting by contact with tubing and membranes. When heparin is employed, lipoprotein lipase is activated, which enhances very-low-density lipoprotein (VLDL) catabolism and an increase in HDL particles [68,69]. Along with the aggravating factors being removed, this may also contribute to an increase in PON1. On the other hand, the HD technique involves some aspects of the oxidative burden due to bioincompatible dialyzer, iron infusion, existence of anemia, and loss of antioxidants [70,71,72].
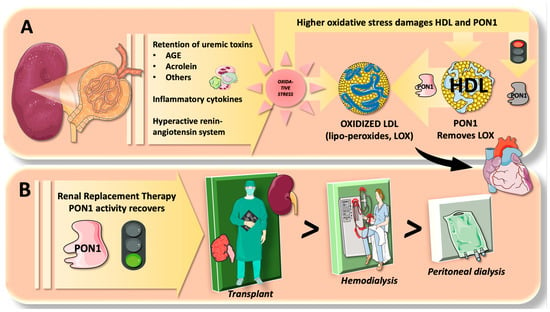
Figure 5.
Paraoxonase 1 dysfunction in chronic renal failure (CRF) and the effects of renal replacement therapy. (A) Deficient filtration in CRF leads to retention of uremic toxins such as advanced glycation endproducts (AGE), acrolein, and others, together with inflammatory cytokines which produce a burden of excessive oxidative stress. Lipoperoxides (LOX) are formed in low-density lipoprotein (LDL), which renders LDL more atherogenic. These are eliminated in part by paraoxonase 1 (PON1) in high-density lipoprotein (HDL). Excess oxidative stress, however, can impair PON1 activity; thus, PON1 activity is consistently lower in CRF. (B). The effect of renal replacement therapy (RRT) as shown in the present meta-analysis is positive to restore PON1 activity; namely, renal transplantation (RTx) exhibits the highest effect, followed by hemodialysis (HD) and peritoneal dialysis (PD). The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
Next, alternative to HD, PD utilizing the patient’s own peritoneum as a filter is a less invasive as a comparatively biocompatible type of RRT, which may show a reduction in oxidative stress mainly due to low pH, high lactate content, high osmolarity, high glucose concentration, and related degradation products [73]. However, because of the high glucose load and the consequent metabolic derangements associated with PD, there is still an elevated risk of CVD with the oxidative burden by this modality [74,75]. Being an HDL-associated molecule, PON1 can be affected by dysfunctional triglyceride-rich lipoprotein catabolism enhanced by the insulin resistance induced by the glucose load [3,4].
Finally, RTx shows the best results in PON1 activity recovery in the present meta-analysis. This appeared to be consistent with the lowest mortality rates with a high quality of life achieved by this modality compared to HD and PD [76]. Although there are not very many studies comparing the responses to oxidative stress and antioxidant defense across RRT types, one study comparing PD and HD found that PD provided better antioxidant protection than HD when measuring superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase, and glucose-6-phosphate dehydrogenase [77]. According to further research on RRT types, RTx removes oxidative stress-induced chemicals more effectively than HD and PD when measuring advanced glycation end products such as uremic toxins [78]. Despite a dearth of research evaluating PON1 activity across different RRT types, the results of our present meta-analysis, which found that CRF patients with RTx had the highest PON1 activity followed by those on HD and PD, are seemingly in agreement with some of those of earlier studies [77,78].
Some limitations apply to the present investigation. First, the methods of RRT (such as the devices and modes of HD) in the studies included on the meta-analysis might be heterogeneous since there were not unified study protocols. For instance, it remains to be a matter of consideration whether low- and high-flux membrane permeability of HD affects PON1 activity. Furthermore, the methods applicable to RRT are under continuous improvement. Second, the association of patient profiles related to RRT with PON1 activity was not fully described in the respective studies for the meta-analysis. For instance, time after the dialysis or transplantation can be informative as PON1 activity was reported to decrease with an increase in dialysis vintage (time on dialysis in years) [56,79]. Third, the association of lifestyle factors (e.g., diets) [80,81] and prescribed drugs [82] with PON1 activity was not explored in the included studies. In this case, therapies using antioxidants (e.g., vitamins B, C, D, and E, coenzyme Q10, L-carnitine, a-lipoic acid, curcumin, green tea, flavonoids, polyphenols, omega-3 polyunsaturated fatty acids, trace elements, N-acetylcysteine) might also be considered as the antioxidant supplementation is being studied to ameliorate oxidative stress in patients receiving HD and PD [83]. Forth, the effect of PON1 activity with RRT on cardiovascular events was not examined in the available studies. Finally, data on lactonase activity of PON1 was not included since the available data were so minimal. In future research, lactonase activity might be necessary to be included. Measurement assays of PON1 activity are not yet standardized (see units and measured values as shown in Table 1). Besides the need for their standardization, measuring PON1 activity in CRF patients with RRT may pave the way for more research along three axes: mechanistic studies, prospective cohort studies for outcomes, and intervention studies to boost PON1 function.
5. Conclusions
According to the overall findings, this meta-analysis provides robust information to attest that CRF patients with RRT, especially RTx, could have higher PON1 activity, followed by HD and PD. Measuring PON1 activity can be also included to the paraclinical toolbox for the management of RRT, in addition to the understanding of CRF-related pathophysiology. Further studies are thus warranted in relation to the choice of RRT types and the protective effect on CVD in this population.
Author Contributions
Conceptualization, J.W., K.K. and A.G.; Methodology, J.W. and K.K.; Software, J.W.; Validation, J.W. and K.K.; Formal Analysis, J.W.; Investigation, J.W. and K.K.; Data Curation, J.W. and K.K.; Writing—Original Draft Preparation, J.W.; Writing—Review and Editing, Y.I., K.K. and A.G.; Visualization, J.W.; Supervision, K.K. and A.G.; Project Administration, K.K.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
As this review did not involve animals, neither ethical review board approval nor patient consent was required.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data relevant to the study are included in the article.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
- MEDLINE via PubMed
- #1. “Aryldialkylphosphatase”[Mesh]
- #2. “aryldialkylphosphatase”[tiab]
- #3. “arylesterase”[tiab]
- #4. “paraoxonase”[tiab]
- #5. #1 OR #2 OR #3 OR #4
- #6. “Renal Insufficiency”[Mesh]
- #7. “Kidney Failure, Chronic”[Mesh]
- #8. “Renal Insufficiency, Chronic”[Mesh]
- #9. “Kidney Diseases”[Mesh]
- #10. “Uremia”[Mesh]
- #11. “end-stage renal”[tiab] OR “end-stage kidney”[tiab] OR “endstage renal”[tiab] OR “endstage kidney”[tiab]
- #12. “ESRF”[tiab] OR “ESKF”[tiab] OR “ESRD”[tiab] OR “ESKD”[tiab]
- #13. “chronic kidney”[tiab] OR “chronic renal”[tiab]
- #14. “CKF”[tiab] OR “CKD”[tiab] OR “CRF”[tiab] OR “CRD”[tiab]
- #15. “predialysis”[tiab] OR “predialysis”[tiab]
- #16. “Renal Dialysis”[Mesh]
- #17. ”hemodialysis”[tiab] OR “haemodialysis”[tiab]
- #18. ”hemofiltration”[tiab] OR “haemofiltration”[tiab]
- #19. ”hemodiafiltration”[tiab] OR “hemodiafiltration”[tiab]
- #20. ”dialysis”[tiab] OR “dialysis”[tiab]
- #21. “CAPD”[tiab] OR “CCPD”[tiab] OR “APD”[tiab]
- #22. #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21
- #17. #5 AND #22
- Embase via Proquest
- S1 EMB.EXACT.EXPLODE(“aryldialkylphosphatase”)
- S2 ab(aryldialkylphosphatase) OR ti(aryldialkylphosphatase)
- S3 ab(arylesterase) OR ti(arylesterase)
- S4 ab(paraoxonase) OR ti(paraoxonase)
- S5 S1 OR S2 OR S3 OR S4
- S6. EMB.EXACT.EXPLODE(“kidney failure”)
- S7. EMB.EXACT.EXPLODE(“chronic kidney failure”)
- S8. EMB.EXACT.EXPLODE(“chronic kidney failure”)
- S9. EMB.EXACT.EXPLODE(“kidney disease”)
- S10. EMB.EXACT.EXPLODE(“uremia”)
- S11. ab(end-stage renal) OR ti(end-stage renal) OR ab(end-stage kidney) OR ti(end-stage kidney) OR ab(endstage renal) OR ti(endstage renal) OR ab(endstage kidney) OR ti(endstage kidney)
- S12. ab(ESRF) OR ti(ESRF) OR ab(ESKF) OR ti(ESKF) OR ab(ESRD) OR ti(ESRD) OR ab(ESKD) OR ti(ESKD)
- S13. ab(chronic kidney) OR ti(chronic kidney) OR ab(chronic renal) OR ti(chronic renal)
- S14. ab(CKF) OR ti(CKF) OR ab(CKD) OR ti(CKD) OR ab(CRF) OR ti(CRF) OR ab(CRD) OR ti(CRD)
- S15. ab(predialysis) OR ti(predialysis) OR ab(predialysis) OR ti(predialysis)
- S16. EMB.EXACT.EXPLODE(“hemodialysis”)
- S17. ab(hemodialysis) OR ti(hemodialysis) OR ab(haemodialysis) OR ti(haemodialysis)
- S18. ab(hemofiltration) OR ti(hemofiltration) OR ab(haemofiltration) OR ti(haemofiltration)
- S19. ab(hemodiafiltration) OR ti(hemodiafiltration) OR ab(hemodiafiltration) OR ti(hemodiafiltration)
- S20. ab(dialysis) OR ti(dialysis) OR ab(dialysis) OR ti(dialysis)
- S21. ab(CAPD) OR ti(CAPD) OR ab(CCPD) OR ti(CCPD) OR ab(APD) OR ti(APD)
- S22. S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21
- S23. S5 AND S22
- CENTRAL via Cochrane Library
- #1. MeSH descriptor: [Aryldialkylphosphatase] explode all trees
- #2. aryldialkylphosphatase:ti,ab
- #3. arylesterase:ti,ab
- #4. paraoxonase:ti,ab
- #5. #1 OR #2 OR #3 OR #4
- #6. MeSH descriptor: [Renal Insufficiency] explode all trees
- #7. MeSH descriptor: [Kidney Failure, Chronic] explode all trees
- #8. MeSH descriptor: [Renal Insufficiency, Chronic] explode all trees
- #9. MeSH descriptor: [Kidney Diseases] explode all trees
- #10. MeSH descriptor: [Uremia] explode all trees
- #11. “end-stage renal”:ti,ab OR “end-stage kidney”:ti,ab OR “endstage renal”:ti,ab OR “endstage kidney”:ti,ab
- #12. ESRF:ti,ab OR ESKF:ti,ab OR ESRD:ti,ab OR ESKD:ti,ab
- #13. “chronic kidney”:ti,ab OR “chronic renal”:ti,ab
- #14. CKF:ti,ab OR CKD:ti,ab OR CRF:ti,ab OR CRD:ti,ab
- #15. predialysis:ti,ab OR predialysis:ti,ab
- #16. MeSH descriptor: [Renal Dialysis] explode all trees
- #17. hemodialysis:ti,ab OR haemodialysis:ti,ab
- #18. hemofiltration:ti,ab OR haemofiltration:ti,ab
- #19. hemodiafiltration:ti,ab OR hemodiafiltration:ti,ab
- #20. dialysis:ti,ab OR dialysis:ti,ab
- #21. “CAPD”[tiab] OR “CCPD”[tiab] OR “APD”[tiab]
- #22. #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21
- #17. #5 AND #22
Appendix B

Table A1.
Details of Risk of Bias Assessment.
Table A1.
Details of Risk of Bias Assessment.
| Authors [Reference No.] | Define the Source of Information | List Inclusion and Exclusion Criteria for Exposed and Unexposed Subjects | Indicate Time Period Used for Identifying Patients | Indicate Whether or Not Subjects Were Consecutive If Not Population-Based | Indicate if Evaluators of Subjective Components of Study Were Masked | Describe Any Assessments Undertaken for Quality Assurance Purposes | Explain Any Patient Exclusions from Analysis | Describe Any Assessments Undertaken for Quality Assurance Purpose | If Applicable, Explain How Missing Data Were Handled in the Analysis | Describe How Confounding Was Assessed and/or Controlled | Summarize Patient Response Rates and Completeness of Data Collection | Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schiavon [65] | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 5 |
| Hasselwander [64] | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 6 |
| Paragh [63] | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 5 |
| Itahara [62] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 8 |
| Juretić [61] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Ak [58] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Schiavon [59] | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 5 |
| Suehiro [60] | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 5 |
| Dirican [56] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Sutherland [57] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Kalogerakis [55] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Jurek [53] | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Krishnaswamy [54] | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 6 |
| Dirican [50] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Gugliucci [51] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Horoz [52] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Atamer [42] | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Dronca [43] | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | - | 1 | - | 5 |
| Ferretti [44] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Göçmen [45] | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Lahrach [46] | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 6 |
| Prakash [47] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Saeed [48] | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 6 |
| Senol [49] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Moradi [37] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Paragh [40] | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 6 |
| Varga [41] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Abdin [35] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Kannampuzha [36] | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 6 |
| Moradi [37] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Rajković [38] | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 6 |
| Emre [28] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Gugliucci [29] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Horoz [30] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 8 |
| Johnson-Davis [31] | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 6 |
| Kimak [32] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Kuchta [33] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Cacciagiú [20] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 8 |
| Gbandjaba [25] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Mahrooz [26] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Ribeiro [27] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Sztanek [15] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Kolarz [21] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Locsey [22] | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | - | 1 | - | 3 |
| Gugliucci [20] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Abdallah [19] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Coimbra [16] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
| Suematsu [18] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 8 |
| Sridevi [17] | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 6 |
| Jose [13] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 8 |
| Szentimrei [15] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | - | 1 | - | 7 |
References
- Campbell, N.R.C.; Ordunez, P.; Giraldo, G.; Rodriguez Morales, Y.A.; Lombardi, C.; Khan, T.; Padwal, R.; Tsuyuki, R.T.; Varghese, C. WHO HEARTS: A Global Program to Reduce Cardiovascular Disease Burden: Experience Implementing in the Americas and Opportunities in Canada. Can. J. Cardiol. 2021, 37, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Mineo, C.; Shaul, P.W. PON-Dering Differences in HDL Function in Coronary Artery Disease. J. Clin. Investig. 2011, 121, 2545–2548. [Google Scholar] [CrossRef] [PubMed]
- Mackness, M.I.; Arrol, S.; Durrington, P.N. Paraoxonase Prevents Accumulation of Lipoperoxides in Low-Density Lipoprotein. FEBS Lett. 1991, 286, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Vaya, J. Paraoxonase 1 Activities, Regulation, and Interactions with Atherosclerotic Lesion. Curr. Opin. Lipidol. 2013, 24, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Pavanello, C.; Ossoli, A. HDL and Chronic Kidney Disease. Atheroscler Plus 2023, 52, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Holzer, M.; Schilcher, G.; Curcic, S.; Trieb, M.; Ljubojevic, S.; Stojakovic, T.; Scharnagl, H.; Kopecky, C.M.; Rosenkranz, A.R.; Heinemann, A.; et al. Dialysis Modalities and HDL Composition and Function. J. Am. Soc. Nephrol. 2015, 26, 2267–2276. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Kotani, K.; Gugliucci, A. Paraoxonase 1 and Chronic Kidney Disease: A Meta-Analysis. J. Clin. Med. Res. 2023, 12, 1199. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Isakova, T.; Nickolas, T.L.; Denburg, M.; Yarlagadda, S.; Weiner, D.E.; Gutiérrez, O.M.; Bansal, V.; Rosas, S.E.; Nigwekar, S.; Yee, J.; et al. KDOQI US Commentary on the 2017 KDIGO Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Am. J. Kidney Dis. 2017, 70, 737–751. [Google Scholar] [CrossRef]
- Viswanathan, M.; Ansari, M.T.; Berkman, N.D.; Chang, S.; Hartling, L.; McPheeters, M.; Santaguida, P.L.; Shamliyan, T.; Singh, K.; Tsertsvadze, A. Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions. Available online: https://effectivehealthcare.ahrq.gov/ (accessed on 9 July 2023).
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3.2022. Available online: https://training.cochrane.org/handbook/current (accessed on 9 July 2023).
- Owen, R.K.; Bradbury, N.; Xin, Y.; Cooper, N.; Sutton, A. MetaInsight: An Interactive Web-Based Tool for Analyzing, Interrogating, and Visualizing Network Meta-Analyses Using R-Shiny and Netmeta. Res. Synth. Methods 2019, 10, 569–581. [Google Scholar] [CrossRef]
- Jose, S.; Kc, R.; Chandran, S. Oxidative Stress in Patients with Diabetic Nephropathy. Natl. J. Physiol. Pharm. Pharmacol. 2022, 12, 1. [Google Scholar] [CrossRef]
- Miguita, R., Jr.; Matsumoto, A.K.; Michelin, A.P.; Morelli, N.R.; Baltus, T.H.L.; Delfino, V.D.A.; Barbosa, D.S. Effect of Dialyzer Reuse on the Activity of Paraoxonase 1 in Patients on Hemodialysis. Indian J. Nephrol. 2022, 32, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Szentimrei, R.; Lőrincz, H.; Szentpéteri, A.; Varga, V.E.; Harangi, M.; Seres, I.; Szabó, R.P.; Nemes, B.; Paragh, G. Changes in Serum Pigment Epithelium-Derived Factor Levels after Kidney Transplantation in Patients with End-Stage Renal Disease. Ren. Fail. 2022, 44, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, S.; Reis, F.; Nunes, S.; Viana, S.; Valente, M.J.; Rocha, S.; Catarino, C.; Rocha-Pereira, P.; Bronze-da-Rocha, E.; Sameiro-Faria, M.; et al. The Protective Role of Adiponectin for Lipoproteins in End-Stage Renal Disease Patients: Relationship with Diabetes and Body Mass Index. Oxid. Med. Cell. Longev. 2019, 2019, 3021785. [Google Scholar] [CrossRef]
- Sridevi, C.; Sowjanya, U.; Kalai Selvi, V.S.; Rajakumari, D.M.M.; Babu, K. Serum Paraoxonase with HDL-C as a Predictor of Atherosclerosis in Patients of Chronic Kidney Disease. Biomedicine 2021, 40, 442–446. [Google Scholar] [CrossRef]
- Suematsu, Y.; Goto, M.; Park, C.; Nunes, A.C.F.; Jing, W.; Streja, E.; Rhee, C.M.; Cruz, S.; Kashyap, M.L.; Vaziri, N.D.; et al. Association of Serum Paraoxonase/Arylesterase Activity With All-Cause Mortality in Maintenance Hemodialysis Patients. J. Clin. Endocrinol. Metab. 2019, 104, 4848–4856. [Google Scholar] [CrossRef]
- Abdallah, E.; El-Shishtawy, S.; Sherif, N.; Ali, A.; El-Bendary, O. Assessment of the Relationship between Serum Paraoxonase Activity and Epicardial Adipose Tissue in Hemodialysis Patients. Int. Urol. Nephrol. 2017, 49, 329–335. [Google Scholar] [CrossRef]
- Gugliucci, A.; Kinugasa, E.; Ogata, H.; Caccavello, R.; Kimura, S. Activation of Paraoxonase 1 after Hemodialysis Is Associated with HDL Remodeling and Its Increase in the HDL2 Fraction and VLDL. Clin. Chim. Acta 2014, 430, 9–14. [Google Scholar] [CrossRef]
- Kolarz, M.; Małyszko, J.; Stompór, T.; Całka, A.; Undas, A.; Myśliwiec, M. Antibodies against Nε-Homocysteinylated Proteins in Patients on Different Methods of Renal Replacement Therapy. Clin. Chem. Lab. Med. 2013, 51, 1093–1099. [Google Scholar] [CrossRef]
- Locsey, L.; Seres, I.; Sztanek, F.; Harangi, M.; Padra, J.; Kovacs, D.; Fedor, R.; Asztalos, L.; Paragh, G. Relationship between Serum Paraoxonase and Homocysteine Thiolactonase Activity, Adipokines, and Asymmetric Dimethyl Arginine Concentrations in Renal Transplant Patients. Transplant. Proc. 2013, 45, 3685–3687. [Google Scholar] [CrossRef]
- Cacciagiú, L.D.; González, A.I.; Gomez Rosso, L.; Meroño, T.; De Marziani, G.; Elbert, A.; Berg, G.; Brites, F.; Schreier, L. HDL-Associated Enzymes and Proteins in Hemodialysis Patients. Clin. Biochem. 2012, 45, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Sztanek, F.; Seres, I.; Harangi, M.; Lőcsey, L.; Padra, J.; Paragh, G.J.R.; Asztalos, L.; Paragh, G. Decreased Paraoxonase 1 (PON1) Lactonase Activity in Hemodialyzed and Renal Transplanted Patients. A Novel Cardiovascular Biomarker in End-Stage Renal Disease. Nephrol. Dial. Transplant. 2012, 27, 2866–2872. [Google Scholar] [CrossRef] [PubMed]
- Gbandjaba, N.Y.; Ghalim, N.; Hassar, M.; Berrougui, H.; Labrazi, H.; Taki, H.; Saile, R.; Khalil, A. Paraoxonase Activity in Healthy, Diabetic, and Hemodialysis Patients. Clin. Biochem. 2012, 45, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Mahrooz, A.; Zargari, M.; Sedighi, O.; Shaygani, H.; Gohari, G. Increased Oxidized-LDL Levels and Arylesterase Activity/HDL Ratio in ESRD Patients Treated with Hemodialysis. Clin. Investig. Med. 2012, 35, E144–E151. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.; do Sameiro Faria, M.; Mascarenhas-Melo, F.; Freitas, I.; Mendonça, M.I.; Nascimento, H.; Rocha-Pereira, P.; Miranda, V.; Mendonça, D.; Quintanilha, A.; et al. Main Determinants of PON1 Activity in Hemodialysis Patients. Am. J. Nephrol. 2012, 36, 317–323. [Google Scholar] [CrossRef]
- Emre, H.; Keles, M.; Yildirim, S.; Uyanik, A.; Kara, F.; Tamer, F.; Aydinli, B. Comparison of the Oxidant-Antioxidant Parameters and Sialic Acid Levels in Renal Transplant Patients and Peritoneal Dialysis Patients. Transplant. Proc. 2011, 43, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Gugliucci, A.; Kinugasa, E.; Kotani, K.; Caccavello, R.; Kimura, S. Serum Paraoxonase 1 (PON1) Lactonase Activity Is Lower in End-Stage Renal Disease Patients than in Healthy Control Subjects and Increases after Hemodialysis. Clin. Chem. Lab. Med. 2011, 49, 61–67. [Google Scholar] [CrossRef]
- Horoz, M.; Kiykim, A.A.; Cimen, B.; Erdem, A. The Influence of Hemodialysis Membrane Permeability on Serum Paraoxonase-1 Activity and Oxidative Status Parameters. Artif. Organs 2011, 35, 923–929. [Google Scholar] [CrossRef]
- Johnson-Davis, K.L.; Fernelius, C.; Eliason, N.B.; Wilson, A.; Beddhu, S.; Roberts, W.L. Blood Enzymes and Oxidative Stress in Chronic Kidney Disease: A Cross Sectional Study. Ann. Clin. Lab. Sci. 2011, 41, 331–339. [Google Scholar]
- Kimak, E.; Hałabiś, M.; Baranowicz-Gąszczyk, I.; Solski, J.; Książek, A. Association between Moderately Oxidized Low-Density Lipoprotein and High-Density Lipoprotein Particle Subclass Distribution in Hemodialyzed and Post-Renal Transplant Patients. J. Zhejiang Univ. Sci. B 2011, 12, 365–371. [Google Scholar] [CrossRef]
- Kuchta, A.; Pacanis, A.; Kortas-Stempak, B.; Cwiklińska, A.; Ziętkiewicz, M.; Renke, M.; Rutkowski, B. Estimation of Oxidative Stress Markers in Chronic Kidney Disease. Kidney Blood Press. Res. 2011, 34, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Navab, K.; Gollapudi, P.; Moradi, H.; Pahl, M.V.; Barton, C.H.; Fogelman, A.M.; Navab, M. Salutary Effects of Hemodialysis on Low-Density Lipoprotein Proinflammatory and High-Density Lipoprotein Anti-Inflammatory Properties in Patient with End-Stage Renal Disease. J. Natl. Med. Assoc. 2011, 103, 524–533. [Google Scholar] [CrossRef]
- Abdin, A.A.; Hassanien, M.A.; Ibrahim, E.A.; El-Noeman, S.E.-D.A.A. Modulating Effect of Atorvastatin on Paraoxonase 1 Activity in Type 2 Diabetic Egyptian Patients with or without Nephropathy. J. Diabetes Complicat. 2010, 24, 325–333. [Google Scholar] [CrossRef]
- Kannampuzha, J.; Darling, P.B.; Maguire, G.F.; Donnelly, S.; McFarlane, P.; Chan, C.T.; Connelly, P.W. Paraoxonase 1 Arylesterase Activity and Mass Are Reduced and Inversely Related to C-Reactive Protein in Patients on Either Standard or Home Nocturnal Hemodialysis. Clin. Nephrol. 2010, 73, 131–138. [Google Scholar] [PubMed]
- Moradi, H.; Ganji, S.; Kamanna, V.; Pahl, M.V.; Vaziri, N.D. Increased Monocyte Adhesion-Promoting Capacity of Plasma in End-Stage Renal Disease–Response to Antioxidant Therapy. Clin. Nephrol. 2010, 74, 273–281. [Google Scholar] [CrossRef]
- Rajković, M.G.; Rumora, L.; Juretić, D.; Grubisić, T.Z.; Flegar-Mestrić, Z.; Vrkić, N.; Sinjeri, Z.; Barisić, K. Effect of Non-Genetic Factors on Paraoxonase 1 Activity in Patients Undergoing Hemodialysis. Clin. Biochem. 2010, 43, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Moradi, H.; Pahl, M.V.; Elahimehr, R.; Vaziri, N.D. Impaired Antioxidant Activity of High-Density Lipoprotein in Chronic Kidney Disease. Transl. Res. 2009, 153, 77–85. [Google Scholar] [CrossRef]
- Paragh, G.; Seres, I.; Harangi, M.; Pocsai, Z.; Asztalos, L.; Locsey, L.; Szeles, G.; Kardos, L.; Varga, E.; Karpati, I.; et al. Discordance in Human Paraoxonase-1 Gene between Phenotypes and Genotypes in Chronic Kidney Disease. Nephron Clin. Pract. 2009, 113, c46–c53. [Google Scholar] [CrossRef]
- Varga, E.; Seres, I.; Harangi, M.; Sztanek, F.; Asztalos, L.; Lõcsey, L.; Borbás, B.; Szegedi, J.; Kárpáti, I.; Paragh, G. Serum Cystatin C Is a Determinant of Paraoxonase Activity in Hemodialyzed and Renal Transplanted Patients. Dis. Markers 2009, 26, 141–148. [Google Scholar] [CrossRef]
- Atamer, A.; Kocyigit, Y.; Ecder, S.A.; Selek, S.; Ilhan, N.; Ecder, T.; Atamer, Y. Effect of Oxidative Stress on Antioxidant Enzyme Activities, Homocysteine and Lipoproteins in Chronic Kidney Disease. J. Nephrol. 2008, 21, 924–930. [Google Scholar]
- Dronca, M.; Paşca, S.P.; Nemeş, B.; Vlase, L.; Vladutiu, D. Serum Paraoxonase 1 Activities and Homocysteinemia in Hemodialysis Patients. Clin. Chem. Lab. Med. 2008, 46, 880–881. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Bacchetti, T.; Masciangelo, S.; Pallotta, G. Lipid Peroxidation in Hemodialysis Patients: Effect of Vitamin C Supplementation. Clin. Biochem. 2008, 41, 381–386. [Google Scholar] [CrossRef]
- Göçmen, A.Y.; Sahin, E.; Koçak, H.; Tuncer, M.; Gümüşlü, S. Levels of Asymmetric Dimethylarginine, Nitric Oxide and Lipid Peroxidation Markers in Patients with End-Stage Renal Disease Having Peritoneal Dialysis Treatment. Clin. Biochem. 2008, 41, 836–840. [Google Scholar] [PubMed]
- Lahrach, H.; Ghalim, N.; Taki, H.; Kettani, A.; Er-Rachdi, L.; Ramdani, B.; Saïle, R. Serum Paraoxonase Activity, High-Sensitivity C-Reactive Protein, and Lipoprotein Disturbances in End-Stage Renal Disease Patients on Long-Term Hemodialysis. J. Clin. Lipidol. 2008, 2, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Prakash, M.; Shetty, J.K.; Rao, L.; Sharma, S.; Rodrigues, A.; Prabhu, R. Serum Paraoxonase Activity and Protein Thiols in Chronic Renal Failure Patients. Indian J. Nephrol. 2008, 18, 13–16. [Google Scholar] [CrossRef]
- Saeed, S.A.; Elsharkawy, M.; Elsaeed, K.; Fooda, O. Paraoxonase-1 (PON1) Activity as a Risk Factor for Atherosclerosis in Chronic Renal Failure Patients. Hemodial. Int. 2008, 12, 471–479. [Google Scholar] [CrossRef]
- Senol, E.; Ersoy, A.; Erdinc, S.; Sarandol, E.; Yurtkuran, M. Oxidative Stress and Ferritin Levels in Haemodialysis Patients. Nephrol. Dial. Transplant. 2008, 23, 665–672. [Google Scholar] [CrossRef]
- Dirican, M.; Sarandol, E.; Serdar, Z.; Ocak, N.; Dilek, K. Oxidative Status and Prevalent Cardiovascular Disease in Patients with Chronic Renal Failure Treated by Hemodialysis. Clin. Nephrol. 2007, 68, 144–150. [Google Scholar] [CrossRef]
- Gugliucci, A.; Mehlhaff, K.; Kinugasa, E.; Ogata, H.; Hermo, R.; Schulze, J.; Kimura, S. Paraoxonase-1 Concentrations in End-Stage Renal Disease Patients Increase after Hemodialysis: Correlation with Low Molecular AGE Adduct Clearance. Clin. Chim. Acta 2007, 377, 213–220. [Google Scholar]
- Horoz, M.; Aslan, M.; Selek, S.; Koylu, A.O.; Bolukbas, C.; Bolukbas, F.F.; Celik, H.; Erel, O. PON1 Status in Haemodialysis Patients and the Impact of Hepatitis C Infection. Clin. Biochem. 2007, 40, 609–614. [Google Scholar] [CrossRef]
- Jurek, A.; Turyna, B.; Kubit, P.; Klein, A. LDL Susceptibility to Oxidation and HDL Antioxidant Capacity in Patients with Renal Failure. Clin. Biochem. 2006, 39, 19–27. [Google Scholar] [PubMed]
- Krishnaswamy, P.R.; Rao, A.; Murali, W.; Sudarshan Ballal, H. Paraoxonase Activity and Antibodies to Oxidized-LDL in Chronic Renal Failure Patients on Renal Replacement Therapy. Indian J. Clin. Biochem. 2006, 21, 173–176. [Google Scholar] [PubMed]
- Kalogerakis, G.; Baker, A.M.; Christov, S.; Rowley, K.G.; Dwyer, K.; Winterbourn, C.; Best, J.D.; Jenkins, A.J. Oxidative Stress and High-Density Lipoprotein Function in Type I Diabetes and End-Stage Renal Disease. Clin. Sci. 2005, 108, 497–506. [Google Scholar]
- Dirican, M.; Akca, R.; Sarandol, E.; Dilek, K. Serum Paraoxonase Activity in Uremic Predialysis and Hemodialysis Patients. J. Nephrol. 2004, 17, 813–818. [Google Scholar] [PubMed]
- Sutherland, W.H.F.; de Jong, S.A.; Walker, R.J. Hypochlorous Acid and Low Serum Paraoxonase Activity in Haemodialysis Patients: An in Vitro Study. Nephrol. Dial. Transplant. 2004, 19, 75–82. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ak, G.; Ozgönül, M.; Sözmen, E.Y.; Aslan, S.L.; Sözmen, B. Renal Cortical Thickness and PON1 Activity Both Decrease in Chronic Renal Failure. J. Nephrol. 2002, 15, 144–149. [Google Scholar]
- Schiavon, R.; Battaglia, P.; De Fanti, E.; Fasolin, A.; Biasioli, S.; Targa, L.; Guidi, G. HDL3-Related Decreased Serum Paraoxonase (PON) Activity in Uremic Patients: Comparison with the PON1 Allele Polymorphism. Clin. Chim. Acta 2002, 324, 39–44. [Google Scholar]
- Suehiro, T.; Ikeda, Y.; Shiinoki, T.; Inoue, M.; Kumon, Y.; Itahara, T.; Hashimoto, K. Serum Paraoxonase (PON1) Concentration in Patients Undergoing Hemodialysis. J. Atheroscler. Thromb. 2002, 9, 133–138. [Google Scholar]
- Juretić, D.; Tadijanović, M.; Rekić, B.; Simeon-Rudolf, V.; Reiner, E.; Baricić, M. Serum Paraoxonase Activities in Hemodialyzed Uremic Patients: Cohort Study. Croat. Med. J. 2001, 42, 146–150. [Google Scholar]
- Itahara, T.; Suehiro, T.; Ikeda, Y.; Inoue, M.; Nakamura, T.; Kumon, Y.; Kawada, M.; Hashimoto, K. Serum Paraoxonase and Arylesterase Activities in Hemodialysis Patients. J. Atheroscler. Thromb. 2000, 7, 152–158. [Google Scholar] [CrossRef][Green Version]
- Paragh, G.; Asztalos, L.; Seres, I.; Balogh, Z.; Löcsey, L.; Kárpáti, I.; Mátyus, J.; Katona, E.; Harangi, M.; Kakuk, G. Serum Paraoxonase Activity Changes in Uremic and Kidney-Transplanted Patients. Nephron 1999, 83, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Hasselwander, O.; McMaster, D.; Fogarty, D.G.; Maxwell, A.P.; Nicholls, D.P.; Young, I.S. Serum Paraoxonase and Platelet-Activating Factor Acetylhydrolase in Chronic Renal Failure. Clin. Chem. 1998, 44, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, R.; De Fanti, E.; Giavarina, D.; Biasioli, S.; Cavalcanti, G.; Guidi, G. Serum Paraoxonase Activity Is Decreased in Uremic Patients. Clin. Chim. Acta 1996, 247, 71–80. [Google Scholar] [PubMed]
- Ebert, T.; Neytchev, O.; Witasp, A.; Kublickiene, K.; Stenvinkel, P.; Shiels, P.G. Inflammation and Oxidative Stress in Chronic Kidney Disease and Dialysis Patients. Antioxid. Redox Signal. 2021, 35, 1426–1448. [Google Scholar]
- Colombo, G.; Reggiani, F.; Angelini, C.; Finazzi, S.; Astori, E.; Garavaglia, M.L.; Landoni, L.; Portinaro, N.M.; Giustarini, D.; Rossi, R.; et al. Plasma Protein Carbonyls as Biomarkers of Oxidative Stress in Chronic Kidney Disease, Dialysis, and Transplantation. Oxid. Med. Cell. Longev. 2020, 2020, 2975256. [Google Scholar]
- Gugliucci, A. Activation of Paraoxonase 1 Is Associated with HDL Remodeling Ex Vivo. Clin. Chim. Acta 2014, 429, 38–45. [Google Scholar]
- Umaerus, M.; Rosengren, B.; Fagerberg, B.; Hurt-Camejo, E.; Camejo, G. HDL2 Interferes with LDL Association with Arterial Proteoglycans: A Possible Athero-Protective Effect. Atherosclerosis 2012, 225, 115–120. [Google Scholar] [CrossRef]
- Moist, L.M.; Port, F.K.; Orzol, S.M.; Young, E.W.; Ostbye, T.; Wolfe, R.A.; Hulbert-Shearon, T.; Jones, C.A.; Bloembergen, W.E. Predictors of Loss of Residual Renal Function among New Dialysis Patients. J. Am. Soc. Nephrol. 2000, 11, 556–564. [Google Scholar] [CrossRef]
- Tetta, C.; Roy, T.; Gatti, E.; Cerutti, S. The Rise of Hemodialysis Machines: New Technologies in Minimizing Cardiovascular Complications. Expert Rev. Cardiovasc. Ther. 2011, 9, 155–164. [Google Scholar]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Dounousi, E.; Mertens, P.R. Oxidative Stress in Hemodialysis Patients: A Review of the Literature. Oxid. Med. Cell. Longev. 2017, 2017, 3081856. [Google Scholar]
- Krediet, R.T.; Zweers, M.M.; van Westrhenen, R.; Zegwaard, A.; Struijk, D.G. Effects of Reducing the Lactate and Glucose Content of PD Solutions on the Peritoneum. Is the Future GLAD? NDT Plus 2008, 1, iv56–iv62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kimak, E.; Solski, J.; Janicka, L.; Ksaziek, A.; Janicki, K. Concentration of Lp(a) and Other Apolipoproteins in Predialysis, Hemodialysis, Chronic Ambulatory Peritoneal Dialysis and Post-Transplant Patients. Clin. Chem. Lab. Med. 2000, 38, 421–425. [Google Scholar] [CrossRef]
- Johnson, D.W.; Armstrong, K.; Campbell, S.B.; Mudge, D.W.; Hawley, C.M.; Coombes, J.S.; Prins, J.B.; Isbel, N.M. Metabolic Syndrome in Severe Chronic Kidney Disease: Prevalence, Predictors, Prognostic Significance and Effects of Risk Factor Modification. Nephrology 2007, 12, 391–398. [Google Scholar] [PubMed]
- Voora, S.; Adey, D.B. Management of Kidney Transplant Recipients by General Nephrologists: Core Curriculum 2019. Am. J. Kidney Dis. 2019, 73, 866–879. [Google Scholar] [CrossRef] [PubMed]
- Stępniewska, J.; Dołęgowska, B.; Popińska, M.; Sałata, D.; Budkowska, M.; Gołembiewska, E.; Myślak, M.; Domański, M.; Marchelek-Myśliwiec, M.; Ciechanowski, K. Prooxidative-Antioxidative Balance of Cells in Different Types of Renal Replacement Therapy. Blood Purif. 2014, 37, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Dawnay, A.; Millar, D.J. The Pathogenesis and Consequences of AGE Formation in Uraemia and Its Treatment. Cell. Mol. Biol. 1998, 44, 1081–1094. [Google Scholar]
- Henning, B.F.; Holzhausen, H.; Tepel, M. Continuous Reduction of Plasma Paraoxonase Activity with Increasing Dialysis Vintage in Hemodialysis Patients. Ther. Apher. Dial. 2010, 14, 572–576. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Ikizler, T.A. Let Them Eat during Dialysis: An Overlooked Opportunity to Improve Outcomes in Maintenance Hemodialysis Patients. J. Ren. Nutr. 2013, 23, 157–163. [Google Scholar] [CrossRef]
- Kędzierska-Kapuza, K.; Szczuko, U.; Stolińska, H.; Bakaloudi, D.R.; Wierzba, W.; Szczuko, M. Demand for Water-Soluble Vitamins in a Group of Patients with CKD versus Interventions and Supplementation-A Systematic Review. Nutrients 2023, 15, 860. [Google Scholar] [CrossRef]
- Weir, M.R.; Fink, J.C. Safety of Medical Therapy in Patients with Chronic Kidney Disease and End-Stage Renal Disease. Curr. Opin. Nephrol. Hypertens. 2014, 23, 306–313. [Google Scholar] [CrossRef]
- Liakopoulos, V.; Roumeliotis, S.; Bozikas, A.; Eleftheriadis, T.; Dounousi, E. Antioxidant Supplementation in Renal Replacement Therapy Patients: Is There Evidence? Oxid. Med. Cell. Longev. 2019, 2019, 9109473. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).