Abstract
The aim of this analysis was to compare ventilation management and outcomes in invasively ventilated patients with acute hypoxemic respiratory failure due to coronavirus disease 2019 (COVID-19) between the first and second wave in the Netherlands. This is a post hoc analysis of two nationwide observational COVID-19 studies conducted in quick succession. The primary endpoint was ventilation management. Secondary endpoints were tracheostomy use, duration of ventilation, intensive care unit (ICU) and hospital length of stay (LOS), and mortality. We used propensity score matching to control for observed confounding factors. This analysis included 1122 patients from the first and 568 patients from the second wave. Patients in the second wave were sicker, had more comorbidities, and had worse oxygenation parameters. They were ventilated with lower positive end-expiratory pressure and higher fraction inspired oxygen, had a lower oxygen saturation, received neuromuscular blockade more often, and were less often tracheostomized. Duration of ventilation was shorter, but mortality rates were similar. After matching, the fraction of inspired oxygen was lower in the second wave. In patients with acute hypoxemic respiratory failure due to COVID-19, aspects of respiratory care and outcomes rapidly changed over the successive waves.
1. Introduction
The recurrent pandemic of coronavirus disease 2019 (COVID-19) has resulted in a tremendous health burden [1]. At the time of writing, more than 631 million confirmed COVID-19 cases and 6.6 million casualties have been reported worldwide [1]. Most, if not all, countries, including the Netherlands, have endured several waves of COVID-19 outbreaks, during which many patients with acute hypoxemic respiratory failure needed admission to an intensive care unit (ICU) for escalation of respiratory support, mostly invasive ventilation. The often hectic situation did not stop the ICU communities from providing excellent care with each wave, even though most healthcare workers struggled with many uncertainties, including how best to provide respiratory support in the early stages of the pandemic.
Due to changing hospital and ICU admission policies, modifications in national and international guidelines that involved the introduction or abundance of certain therapies, the care and outcomes of critically ill COVID-19 patients may have changed over successive outbreaks. Indeed, reports from countries worldwide suggest variances in care and trends towards a shorter length of hospital stay and even lower mortality rates in consecutive waves [2,3,4,5,6,7,8,9]. To date, few reports have described changes in care and outcomes in invasively ventilated COVID-19 patients within a single center, region, or country.
The aim of this analysis was to compare ventilation management and outcomes in critically ill invasively ventilated COVID-19 patients between the first and the second wave of the outbreak in the Netherlands. For this, we used the datasets of two nation-wide multicenter studies, known as the ‘PRactice of VENTilation in patients with COVID-19’ (PRoVENT–COVID) [10] and the ‘Practice of Adjunctive Treatments in intensive care unit patients with COVID-19’ (PRoAcT-COVID) [11]. While PRoVENT–COVID enrolled patients in the first wave of the national outbreak, PRoAcT-COVID enrolled patients in the second wave, which started within three months after the end of the first wave. We hypothesized that there would be important differences with regard to ventilation management between patients included in these two studies, conducted in quick successions. We used propensity score matching to control for observed confounding factors that could have affected these outcomes.
2. Materials and Methods
2.1. Design, Ethics and Patients
This is a post hoc analysis of PRoVENT–COVID and PRoAcT-COVID, two nationwide, multicenter, observational studies conducted in critically ill COVID-19 patients during the first and second wave of the national outbreak in the Netherlands [10,11]. The Institutional Review Board of the Amsterdam University Medical Centers, location AMC, Amsterdam, the Netherlands, approved the study protocols of PRoVENT–COVID (7 April 2020; W20_157 # 20,171) and PRoAcT-COVID (11 December 2020; W20_526 # 20,583). For both studies, the requirement of written informed consent was waived as the studies were purely observational and only captured data that were already collected as part of standard care. The studies were registered at clinicaltrials.gov (study identifiers NCT04346342 and NCT04719182). PRoVENT–COVID enrolled patients between 1 March and 1 June 2020 in 22 ICUs. PRoAcT-COVID enrolled patients between 1 September 2020 and 1 January 2021 in 16 ICUs. Fourteen centers participated in both studies.
Patients were eligible for participation in PRoVENT–COVID if: (1) aged 18 years or older; (2) admitted to one of the ICUs of a participating hospital; and (3) receiving invasive ventilation for COVID-19 that was confirmed by RT–PCR for SARS-CoV-2. The inclusion criteria in PRoAcT-COVID were similar, with the exception that this second study also enrolled patients under other forms of respiratory support, i.e., noninvasive ventilation and high-flow nasal oxygen therapy. For this current analysis, we excluded patients from PRoAcT-COVID that did not receive invasive ventilation.
2.2. Data Collected
Trained data collectors captured baseline characteristics, including age, sex, weight, height, comorbidities, home medication, kidney function, severity of acute respiratory distress syndrome (ARDS), and the Simplified Acute Physiology Score (SAPS) II. Ventilation mode, positive end-expiratory pressure (PEEP), fraction of inspired oxygen (FiO2), and oxygen saturation by pulse oximetry (SpO2) were collected at a fixed time in the morning until day 4. We also captured use of prone positioning, extracorporeal membrane oxygenation (ECMO), and neuromuscular blockade. In addition, we collected tracheostomy use till day 28, the last day of invasive ventilation, the last day of stay in the ICU and hospital, and life status at ICU and hospital discharge as well as at day 28 and 90.
2.3. Study Endpoints
The primary endpoint was ventilation management, consisting of PEEP, FiO2, SpO2, and the use of prone positioning, ECMO, or neuromuscular blockade. Secondary endpoints included use of tracheostomy, duration of invasive ventilation, ICU and hospital LOS, and ICU, hospital, and 28- and 90-day mortality.
2.4. Definitions
ARDS was defined according to the current definition [12]. Prone positioning was defined as turning a patient from supine to a (semi) prone position because of refractory hypoxemia. Neuromuscular blockade was defined as (incidental or continuous) use of a neuromuscular blocking agent (NMBA), wherein use to facilitate tracheal intubation was ignored. The number of days free from ventilation and alive was the number of days with unsupported breathing for at least 24 sequential hours up to day 28, wherein patients that died were counted as having zero days free from ventilation. For the purpose of this study, a patient was considered to have been weaned from invasive ventilation if invasive ventilation was not restarted within the timeframe of the study.
2.5. Power Calculation
We did not perform a power calculation. The summed number of patients present in the databases of PRoVENT–COVID and PRoAcT-COVID served as the sample size.
2.6. Statistical Analysis
Continuous variables are presented as medians (first quartile–third quartile) and categorical variables as numbers and percentages. Groups were compared using the Mann–Whitney U test for continuous variables and the Fisher exact or Chi-square tests for categorical variables. Distribution plots were constructed to visualize the distributions of PEEP and FiO2.
To test the impact of the first and second wave on ICU and hospital discharge, competing risk analysis was used, with mortality as competing risk factor.
Covariate propensity score balancing was used to match patients between the two waves [13]. If the number of missing values was <5%, multiple imputation by the chained equation method (MICE) was performed. If the number of missing values per variable crossed 5%, patients with missing data were excluded from this part of the analysis. Based on clinical relevance, the following baseline characteristics were added to the logistic regression model to calculate the propensity score: body mass index, age, severity of ARDS, comorbidities including hypertension, heart failure, diabetes, chronic kidney disease, liver cirrhosis, chronic obstructive pulmonary disease, active hematological neoplasia, active solid neoplasia, neuromuscular disease, immunosuppression, and home medication including systemic glucocorticosteroids, inhalation glucocorticosteroids, angiotensin II receptor blocker, beta-blockers, insulin, statins, and calcium channel blockers. The nearest neighbor matching without replacement strategy was used for 1:1 matching of patients from the first to the second COVID-19 wave. An initial caliper width of 0.1 standard deviation of the logit of the propensity score was used to match the patients. If the covariates remained imbalanced after matching, lower caliper widths (until 0.01) were tested until balance was achieved. Variables’ standardized mean differences were visualized in LOVE plots and used to assess matching performance. We aimed for standardized mean differences of ≤0.1 (10%) [14]. The propensity score and study sites were added to the logistic and linear regression models to compare length of stay in intensive care unit and hospital, and ICU, hospital, and 28- and 90-day mortality between patients in the two consecutive waves.
As a relatively high incidence of NMBA use was found, a post hoc analysis comparing mortality in patients with and without NMBA was performed.
R version 4.2.2 (R foundation for Statistical Computing, Vienna, Austria) was used for this analysis, and a p-value of <0.05 was considered statistically significant.
3. Results
3.1. Patients
The databases of PRoVENT–COVID and PRoAcT-COVID contain data of 1122 patients from 22 ICUs and 976 patients from 16 ICUs, respectively (Figure 1). For the current analysis, we used all patients in the database of PRoVENT–COVID, but only 568 patients from the the database of PRoAcT-COVID. The main reason for exclusion of patients in the second study was not having received invasive ventilation. Compared to patients in the first wave, patients in the second wave were more often obese, had more often comorbidities such as diabetes, chronic kidney disease, or neuromuscular disease, and were treated more frequently with systemic steroids, beta blocking agents, or insulin at home (Table 1). Patients in the second wave were also sicker according to the SAPS II score and had worse oxygenation parameters on ICU admission. In both waves, nearly all patients had ARDS, with a higher incidence of severe ARDS in the second wave.
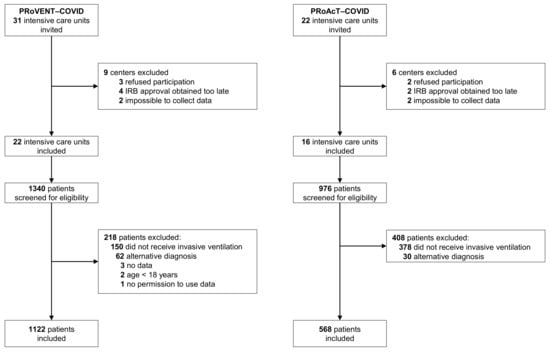
Figure 1.
CONSORTs of PRoVENT–COVID and PRoAcT-COVID.

Table 1.
Patient Characteristics and Medical History.
3.2. Unmatched Analysis
Compared to patients in the first wave, patients in the second wave received pressure control and pressure support more often (Table 2). Patients in the second wave were ventilated with a lower median PEEP and higher median FiO2, and median SpO2 was lower (Figure 2). Prone positioning and ECMO were equally used. Neuromuscular blockade was used more often in the second wave. Patients in the second wave received a tracheostomy less often (Table 3). Duration of ventilation as well as ICU and hospital length of stay in survivors was shorter in the second wave. Mortality rates were similar in the two waves (Table 3 and Figure 3).

Table 2.
Ventilation management and support treatments.
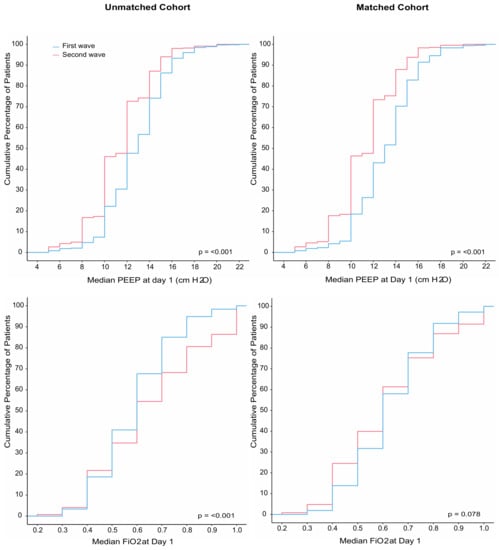
Figure 2.
(Left panel) Cumulative distribution of positive end-expiratory pressure (PEEP) and fraction inspired oxygen (FiO2) on the first day of ventilation in the unmatched analysis; (right panel) cumulative distribution of positive end-expiratory pressure (PEEP) and fraction inspired oxygen (FiO2) on the first day of ventilation in the matched analysis.

Table 3.
Clinical outcomes.
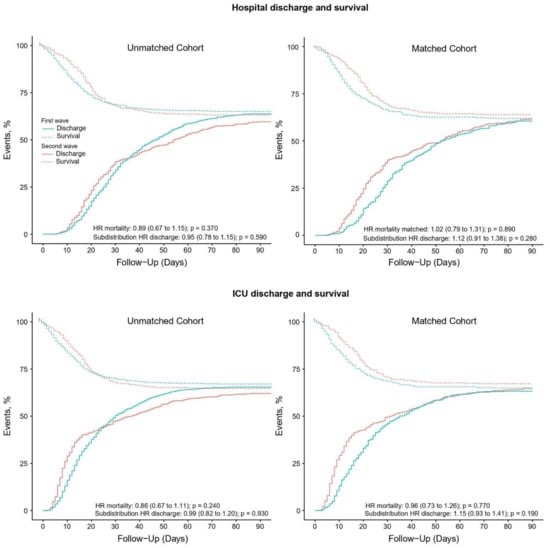
Figure 3.
(Left upper panel) Competing risk analysis of hospital survival and discharge in the unmatched analysis; (left lower panel) competing risk analysis of ICU survival and discharge in the unmatched analysis; (right upper panel) competing risk analysis of hospital survival and discharge in the matched analysis; (right lower panel) competing risk analysis of ICU survival and discharge in the matched analysis.
3.3. Matched Analysis
A total of 964 patients could be matched (Table S1, Figures S1–S5), with the baseline characteristics well balanced (Table 1). After propensity score matching, FiO2 was lower in the second wave (Table 2 and Figure 2). Differences in ICU and hospital LOS remained in the matched cohort but disappeared in multivariate models (Table 3 and Table S2). Mortality remained comparable between the two waves after matching (Table 3 and Table S3).
3.4. Post Hoc Analysis
In both the first wave and second wave, and in all patients combined, no differences in 90-day mortality were found between patients who received NMBA and those who did not (Figure 4).
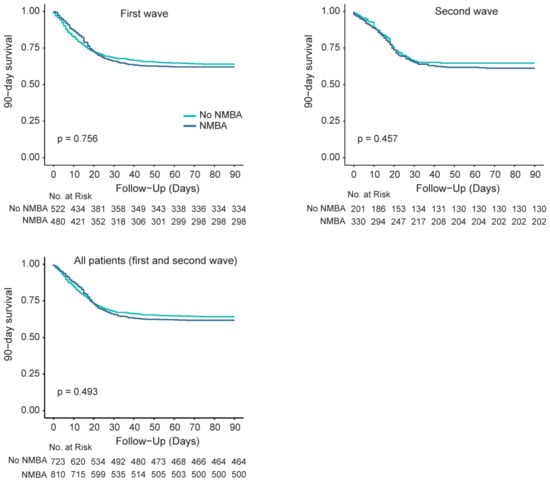
Figure 4.
(Left upper panel) 90-day mortality between patients with and without NMBA in the first wave; (right upper panel) 90-day mortality between patients with and without NMBA in the second wave; (left lower panel) 90-day mortality between patients with and without NMBA in all patients, both from the first and second wave.
4. Discussion
The main findings of this post hoc analysis using the individual patient data of two consecutive studies performed in quick succession in the first year of the COVID-19 outbreak in the Netherlands can be summarized as follows: (i) compared to patients in the first wave, patients in the second wave received invasive ventilation, with lower median PEEP and higher median FiO2, and (ii) received neuromuscular blockage more often. Patients in the second wave (iii) received tracheostomy less often, (iv) were weaned earlier from invasive ventilation, and (v) had shorter lengths of stay, but (vi) had a similar mortality rate as patients in the first wave.
This study has several strengths. First, both academic and non-academic and teaching and non-teaching hospitals participated in the two parent studies. This allows for a realistic national representation of respiratory care and outcomes during the two COVID-19 waves. In addition, most hospitals that participated in the first study also participated in the second study, minimizing the risk of finding differences related to variations in pre-existing practice between hospitals. To ensure the high quality of the data obtained, all data collectors were trained and provided with clear instructions before capturing the granular data. The parent studies had no exclusion criteria, and for this current analysis, we only excluded patients receiving noninvasive ventilatory support from the second study. Follow-up was near complete in both studies, and the analysis strictly followed a predefined analysis plan.
The most important difference regarding ventilation practice was the lower median PEEP in the second wave. The best PEEP level in patients with ARDS due to COVID-19 remains uncertain [15]. This is similar to patients with ARDS due to another cause [16]. During the two waves, a policy-driven shift in the study population that received invasive ventilation occurred. Early in the pandemic, there was a scarcity of noninvasive ventilation and HFNO as well as uncertainty regarding whether these strategies could increase the risk of infection of the healthcare workers. This led to a policy of early intubation, a strategy that was followed by most hospitals during the first wave. In the second wave, caregivers were more reluctant to intubate patients early. This probably resulted in a selection of sicker patients in the second wave—patients with a lower lung compliance. Consequently, the used level of PEEP was expected to be higher in the second wave. Our findings, however, show a lower median PEEP in the second wave. Thus, caregivers had developed a preference for lower PEEP.
We previously described an association between the use of higher PEEP and worse outcomes in COVID-19 patients [17]. In that study, we showed that while higher PEEP improves oxygenation, it has an association with a longer duration of ventilation [17]. While this study was published after the second wave, i.e., after the second study in which we used individual patient data, it must be noted that the findings were previously presented and thus known soon after the first wave in various meetings in the Netherlands; it could be that this caused a change with respect to PEEP. Of interest, in the second wave, with use of lower PEEP, we found a shorter duration of ventilation, in line with the study mentioned above [17].
Prone positioning appeared to be an effective measure to improve oxygenation in COVID-19 patients [18]. In our analysis, the reported incidence of prone positioning is high in both waves, which is in line with earlier research [19,20].
The overall reported use of NMBA of 47.6% in the first wave and 62.3% in the second wave in the unmatched cohort is considerably high compared to earlier non–COVID-19 ARDS studies. For example, in the LUNG SAFE study, a trial containing data of 2377 ARDS patients in 50 different countries, the overall incidence of NMBA use was only 21.7% [21]. In COVID-19 ARDS patients, the reported use of NMBA varies from 25% to 84% [19,22,23], and in some countries, there was even a temporary shortage of NMBA [24]. This broad variability in NMBA use might be explained by international variation in critical care and differences in study populations. Indeed, we only included patients receiving invasive ventilation, while other trials also included patients requiring non-invasive ventilation [21,22]. Consequently, the use of NMBA in those studies is expected to be lower. In addition, the definition for NMBA use varies by study. In this analysis, both incidental and continuous NMBA administration were defined as NMBA use. In other studies, only continuous NMBA administration for a certain period of time was considered as NMBA use [21]. Given the relatively high incidence of NMBA use, we did perform a post hoc analysis, in which we found no differences in 90-day mortality between patients who did and did not receive NMBA. Guidelines discourage long-term use of NMBA [25,26], but the exact role of NMBA in critically ill COVID-19 patients remains a matter of debate. Of note, despite the guidelines, we found that the use of NMBA actually increased from the first to the second wave. This might be caused by the sicker population of patients in the second wave. Those patients are prone to deep saturation dips when coughing or when patient–ventilator asynchronies occur. This could justify the higher use of NMBA observed in the second wave.
We also found a lower incidence of tracheostomy in the second wave. This is in line with earlier research showing that early tracheostomy does not improve patient outcome [27,28]. This analysis showed a shorter duration of ventilation and a shorter length of hospital and ICU stay in survivors in the second wave. As suggested above, the use of lower PEEP might have contributed to this finding. The introduction of new effective treatments such as HFNO, steroids, and anti-inflammatory strategies as well as the pursuit of a less positive fluid balance after the first wave is also expected to have had a positive effect on the clinical outcomes in patients from the second wave [29,30,31]. Indeed, earlier research showed that those new therapies resulted in a shorter duration of ventilation, hospital and ICU stay, and mortality.
We did not find improved survival in the second wave. This is not in line with earlier research [2,32]. The positive effects of new insight and treatments on mortality rates in COVID-19 patients during the second wave might have been masked by the earlier mentioned differences in disease severity in this analysis. We did, however, find a shorter duration of ventilation and length of hospital and ICU stay in the second wave. These are not only important patient-centered outcomes but also important economic and societal results. A shorter duration of ventilation and hospitalization saves expenses, but it also creates medical capacity for other patients requiring medical care.
This analysis implies that ICU care of invasively ventilated COVID-19 patients quickly changed from the first to the second wave. During the outbreak of the first COVID-19 pandemic, there were many uncertainties regarding the optimal treatment and mechanical ventilation strategy for these patients. This resulted in the initiation of a tremendous amount of research in a short period of time to improve the clinical care of patients [33]. This new knowledge provided great insight into the epidemiology and characteristics of the disease as well as the effective treatment strategies for COVID-19 patients. Effective treatments were rapidly implemented into standard care, which is confirmed by this analysis.
This analysis has several limitations. Unfortunately, data on ICU admission criteria and ‘Do Not Resuscitate’ (DNR) codes were not collected in the PRoVENT–COVID and PRoAcT-COVID study. This is a limitation, since DNR codes might have led to ‘door selection’ and thereby could have interfered with our study population and findings. Only critically ill COVID-19 patients receiving invasive ventilation were included in this analysis. Thus, our analysis only provides insights into this selection of critically ill COVID-19 patients, which is another limitation. The retrospective design of the two parent studies is also a limitation, since it only enables us to speak of associations and not causality between characteristics, treatment, and outcomes. As only Dutch ICUs participated in the PRoVENT-COVID and PRoAcT-COVID studies, the results of this analysis might only be extrapolated to hospitals with health care systems similar to the Netherlands. Another limitation is that we are restricted to the variables collected in the two studies. For example, tidal volume and peak pressure were not collected in the PRoAcT-COVID study. Next to those variables, modifications of SARS-CoV-2 as well as the introduction of other unrecorded treatments such as steroids and anti-inflammatory strategies may have influenced our findings. However, since these changes were not captured in the datasets, we could not quantify their effect on our findings and thus could not correct for them in our analysis. Of note, vaccination status is not relevant to this analysis, as the COVID-19 vaccine was not available in the Netherlands until early after the second COVID-19 wave—indeed, the inclusion of patients in PRoAcT-COVID stopped several weeks before the national vaccination campaign started.
5. Conclusions
This analysis shows that important aspects of invasive ventilation changed over time. It also shows that outcomes improved between the two waves, manifested by a shortened length of stay, but similar mortality rates. This emphasizes the importance of continuous re-evaluation of patient characteristics and treatment during pandemics. Therefore, in future COVID-19 waves, it is critical to remain vigilant regarding recognizing changes in patient and disease characteristics as well as aspects of care. To capture these changes, scientific research remains essential. Our findings could also imply that caution is warranted in the default use of high PEEP. However, future research considering potential confounding factors is needed to confirm these findings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12134507/s1, Figure S1: Distribution of missing data; Figure S2: Strip plot distribution imputed data (magenta) and observed data (blue); Figure S3: Histogram matched and unmatched cohort; Figure S4: Bal plot matched and unmatched cohort; Figure S5: Love plot matched and unmatched cohort; Table S1: Missing data for propensity score matching; Table S2: Multivariate model for length of hospital and ICU stay; Table S3: Multivariate model for mortality.
Author Contributions
Conceptualization, L.H., M.J.S., I.M.-L., D.M.P.v.M., A.S.N. and F.P.; methodology, L.H., M.J.S., I.M.-L., D.M.P.v.M., A.S.N. and F.P.; formal analysis, L.H., D.M.P.v.M. and A.S.N.; drafting the manuscript, L.H., M.J.S. and F.P. All authors have read and agreed to the published version of the manuscript.
Funding
PRoVENT–COVID and PRoAcT-COVID were funded by the Amsterdam UMC, location AMC, Amsterdam, the Netherlands, and by Zorgonderzoek Medische Wetenschappen (ZonMw), a collaboration between ‘Zorgonderzoek Nederland’ and ‘Medical Sciences of the Netherlands organization for Scientific Research’ (NWO-MW) (Number 10430102110008). The funder had no role in the design of the study, in the collection, analysis and interpretation of the data. The funder had no role in drafting the manuscript or in the decision to submit this manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki. The Institutional Review Board of the Amsterdam University Medical Centers, location AMC, Amsterdam, the Netherlands, approved the study protocols of PRoVENT–COVID (7 April 2020; W20_157 # 20,171) and PRoAcT-COVID (11 December 2020; W20_526 # 20,583).
Informed Consent Statement
Patient consent was waived, as the studies were purely observational and only captured data that were already collected as part of standard care.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the authors on reasonable request.
Acknowledgments
The authors would like to thank the PRoVENT–COVID and PRoAcT-COVID investigators (see Appendix A).
Conflicts of Interest
A.S.N. reports personal fees from Dräger, outside of the submitted work. M.S. reports personal fees from Hamilton Medical AG and Xenios/Novalung, outside of the submitted work; since January 2022 he has been employed by Hamilton Medical AG, Switzerland. The other authors declare no conflicts of interests.
Abbreviations
| ARDS | Acute Respiratory Distress Syndrome |
| BMI | Body Mass Index |
| COPD | Chronic Obstructive Pulmonary Disease |
| COVID-19 | Coronavirus disease 2019 |
| DNR | Do Not Resuscitate |
| ECMO | Extra Corporal Membrane Oxygenation |
| FiO2 | Fraction Inspired Oxygen |
| ICU | Intensive Care Unit |
| NMBA | Neuromuscular Blocking Agent |
| PEEP | Positive End-Expiratory Pressure |
| PRoVENT–COVID | PRactice of VENTilation in Patients with Novel Coronavirus disease |
| PRoAcT-COVID | Practice of adjunctive treatments in critically ill COVID-19 patients |
| SAPS | Simplified Acute Physiology Score |
| SPO2 | Oxygen Saturation |
Appendix A
PRoVENT–COVID investigators:
S. Ahuja; J.P. van Akkeren; A.G. Algera; C.K. Algoe; R.B. van Amstel; P. van de Berg; D.C. Bergmans; D.I. van den Bersselaar; F.A. Bertens; A.J. Bindels; J.S. Breel; C.L. Bruna; M.M. de Boer; S. den Boer; L.S. Boers; M. Bogerd; L.D. Bos; M. Botta; O.L. Baur; H. de Bruin; L.A. Buiteman-Kruizinga; O. Cremer; R.M. Determann; W. Dieperink; J. v. Dijk; D.A. Dongelmans; T.P. Dormans; M.J. de Graaff; M.S. Galekaldridge; L.A. Hagens; J.J. Haringman; S.T. van der Heide; P.L. van der Heiden; l.l. Hoeijmakers; L. Hol; M. W. Hollmann; J. Horn; R. van der Horst; E.L. Ie; D. Ivanov; N.P. Juffermans; E. Kho; E.S. de Klerk; A.W. Koopman; M. Koopmans; S. Kucukcelebi; M.A. Kuiper; D.W. de Lange; I. Martin–Loeches; G. Mazzinari; D.M van Meenen; N. van Mourik; S.G. Nijbroek; E.A. Oostdijk; F. Paulus; C.J. Pennartz; J. Pillay; I.M. Purmer; T.C. Rettig; O. Roca; J.P. Roozeman; M.J. Schultz; A. Serpa Neto; C. Sivakorn; G.S. Shrestha; M.E. Sleeswijk; P.E. Spronk; A.C. Strang; W. Stilma; P. Swart; A. Tri; A.M. Tsonas; C.M.A. Valk; A.P. Vlaar; L.I. Veldhuis; W.H. van der Ven; P. van Velzen; P. van Vliet; P. van der Voort; L. van Welie; B. van Wijk; T. Winters; W.Y. Wong; A.R. van Zanten.
PRoAcT-COVID investigators:
E. Aydeniz; P. van de Berg; D.C. Bergmans; M. Bevers; S. den Boer; L.S. Boers; L.D. Bos; M. Botta; L.A. Buiteman-Kruizinga; W. Coene; M. Delmte; Vincenzo Di Leo; D.A. Dongelmans; T.P. Dormans; L.M. Elting; A.A. Esmeijer; M. Gama de Abreu; A.R. Girbes; M.J. de Graaff; D.M. Go; R.L. Goossen; H.J. Hansen; J.J. Haringman; L. Hol; M.W. Hollmann; P.L. van der Heiden; J. Horn; L.E. van Ingen; N.P. Juffermans; M.A. Kuiper; L.J. Kuipers; E. Koornstra; A. Lokhorst; S.G. Nijbroek; I. Martin–Loeches; G. Mazzinari; S. Myatra; F. Paulus; M. Offermans; T. Pisters; A. Prins; P. van Oosten; J. Pillay; I.M. Purmer; A.S. Rezaee; T.C.D. Rettig; O. Roca; N.M. Rosenberg; N. Schavemaker; A.A. Sciascera, M.J. Schultz; A. Serpa Neto; M.E. Sleeswijk; G. Sherstha; W. Stilma; A.C. Strang; P.E. Spronk; P.R. Tuinman; A.M. Tsonas; C.M.A. Valk; M. Verboom; A.P. Vlaar; F.L. van der Ven; W. H. van der Ven; P. van Velzen; E.J. Verhoef; T.D. Vermeulen; P. van Vliet; J.J. J.S. Voorham; P.H. van der Voort; M. van der Woude; Weiner; N. Yaali; J.M. Zandvliet; A.R. van Zanten; T.Z. van Zijl; S.A. Zonneveld, I. Martin–Loeches (Dublin, Ireland).
References
- World Health Organization. Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 21 February 2023).
- Iftimie, S.; López-Azcona, A.F.; Vallverdú, I.; Hernández-Flix, S.; de Febrer, G.; Parra, S.; Hernández-Aguilera, A.; Riu, F.; Joven, J.; Andreychuk, N.; et al. First and second waves of coronavirus disease-19: A comparative study in hospitalized patients in Reus, Spain. PLoS ONE 2021, 16, e0248029. [Google Scholar] [CrossRef]
- Vahidy, F.S.; Drews, A.L.; Masud, F.N.; Schwartz, R.L.; Askary, B.B.; Boom, M.L.; Phillips, R.A. Characteristics and Outcomes of COVID-19 Patients During Initial Peak and Resurgence in the Houston Metropolitan Area. JAMA 2020, 324, 998–1000. [Google Scholar] [CrossRef]
- Saito, S.; Asai, Y.; Matsunaga, N.; Hayakawa, K.; Terada, M.; Ohtsu, H.; Tsuzuki, S.; Ohmagari, N. First and second COVID-19 waves in Japan: A comparison of disease severity and characteristics. J. Infect. 2021, 82, 84–123. [Google Scholar] [CrossRef]
- Borghesi, A.; Golemi, S.; Carapella, N.; Zigliani, A.; Farina, D.; Maroldi, R. Lombardy, Northern Italy: COVID-19 second wave less severe and deadly than the first? A preliminary investigation. Infect. Dis. 2021, 53, 370–375. [Google Scholar] [CrossRef]
- Portacci, A.; Carpagnano, G.E.; Tummolo, M.G.; Santomasi, C.; Palma, L.; Fasano, D.; Resta, E.; Lozupone, M.; Solfrizzi, V.; Panza, F.; et al. COVID-19 clinical phenotypes and short-term outcomes: Differences between the first and the second wave of pandemic in Italy. Expert Rev. Respir. Med. 2021, 15, 1619–1625. [Google Scholar] [CrossRef]
- Mughal, M.S.; Kaur, I.P.; Wang, C.; Alhashemi, R.; Buemio, A.; Patton, C.D.; Granet, K.M. Variation in clinical characteristics, outcomes, and mortality of hospitalized patients with COVID-19 during the second wave of the pandemic: A single-center experience. J. Investig. Med. 2021, 69, 1479–1482. [Google Scholar] [CrossRef] [PubMed]
- Atkin, C.; Kamwa, V.; Reddy-Kolanu, V.; Parekh, D.; Evison, F.; Nightingale, P.; Gallier, S.; Ball, S.; Sapey, E. The changing characteristics of COVID-19 presentations. A regional comparison of SARS-CoV-2 hospitalised patients during the first and second wave. Acute Med. 2021, 20, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Zuil, M.; Benítez, I.D.; Cabo-Gambín, R.; Manzano Senra, C.; Moncusí-Moix, A.; Gort-Paniello, C.; de Gonzalo-Calvo, D.; Molinero, M.; Vengoechea Aragoncillo, J.J.; Comella, T.; et al. Clinical management and outcome differences between first and second waves among COVID-19 hospitalized patients: A regional prospective observational cohort. PLoS ONE 2021, 16, e0258918. [Google Scholar] [CrossRef] [PubMed]
- Boers, N.S.; Botta, M.; Tsonas, A.M.; Algera, A.G.; Pillay, J.; Dongelmans, D.A.; Horn, J.; Vlaar, A.P.J.; Hollmann, M.W.; Bos, L.D.J.; et al. PRactice of VENTilation in Patients with Novel Coronavirus Disease (PRoVENT-COVID): Rationale and protocol for a national multicenter observational study in The Netherlands. Ann. Transl. Med. 2020, 8, 1251. [Google Scholar] [CrossRef]
- Valk, C.M.A.; Swart, P.; Boers, L.S.; Botta, M.; Bos, L.D.J.; de Abreu, M.G.; Hol, L.; Hollmann, M.W.; Horn, J.; Martin-Loeches, I.; et al. Practice of adjunctive treatments in critically ill COVID-19 patients-rational for the multicenter observational PRoAcT-COVID study in The Netherlands. Ann. Transl. Med. 2021, 9, 813. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Kosuke Imai, M.R. Covariate balancing propensity score. J. R. Stat. Soc. 2013, 76, 243–263. [Google Scholar] [CrossRef]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef] [PubMed]
- Alhazzani, W.; Møller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19). Crit. Care Med. 2020, 48, 854–887. [Google Scholar] [CrossRef] [PubMed]
- Santa Cruz, R.; Villarejo, F.; Irrazabal, C.; Ciapponi, A. High versus low positive end-expiratory pressure (PEEP) levels for mechanically ventilated adult patients with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst. Rev. 2021, 3, Cd009098. [Google Scholar] [CrossRef]
- Valk, C.M.A.; Tsonas, A.M.; Botta, M.; Bos, L.D.J.; Pillay, J.; Serpa Neto, A.; Schultz, M.J.; Paulus, F. Association of early positive end-expiratory pressure settings with ventilator-free days in patients with coronavirus disease 2019 acute respiratory distress syndrome: A secondary analysis of the Practice of VENTilation in COVID-19 study. Eur. J. Anaesthesiol. 2021, 38, 1274–1283. [Google Scholar] [CrossRef]
- Langer, T.; Brioni, M.; Guzzardella, A.; Carlesso, E.; Cabrini, L.; Castelli, G.; Dalla Corte, F.; De Robertis, E.; Favarato, M.; Forastieri, A.; et al. Prone position in intubated, mechanically ventilated patients with COVID-19: A multi-centric study of more than 1000 patients. Crit. Care 2021, 25, 128. [Google Scholar] [CrossRef]
- Ferrando, C.; Suarez-Sipmann, F.; Mellado-Artigas, R.; Hernández, M.; Gea, A.; Arruti, E.; Aldecoa, C.; Martínez-Pallí, G.; Martínez-González, M.A.; Slutsky, A.S.; et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. 2020, 46, 2200–2211. [Google Scholar] [CrossRef]
- Schmidt, M.; Hajage, D.; Demoule, A.; Pham, T.; Combes, A.; Dres, M.; Lebbah, S.; Kimmoun, A.; Mercat, A.; Beduneau, G.; et al. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensive Care Med. 2021, 47, 60–73. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef]
- Cummings, M.J.; Baldwin, M.R.; Abrams, D.; Jacobson, S.D.; Meyer, B.J.; Balough, E.M.; Aaron, J.G.; Claassen, J.; Rabbani, L.E.; Hastie, J.; et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 2020, 395, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Acosta, G.; Carrillo-Garcia, T.; Padrón-Espinosa, P.; Santana-Cabrera, L.; Blanco-López, J.J.; González-Martín, J.M.; Martín-Gonzalez, J.C. Differences between the first and the second wave of critically ill COVID-19 patients admitted to the intensive care units. Int. J. Crit. Illn. Inj. Sci. 2022, 12, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Ziatkowski-Michaud, J.; Mazard, T.; Delignette, M.-C.; Wallet, F.; Aubrun, F.; Dziadzko, M. Neuromuscular monitoring and neuromuscular blocking agent shortages when treating critically ill COVID-19 patients: A multicentre retrospective analysis. Br. J. Anaesth. 2021, 127, e73–e75. [Google Scholar] [CrossRef]
- Moss, M.; Huang, D.T.; Brower, R.G.; Ferguson, N.D.; Ginde, A.A.; Gong, M.N.; Grissom, C.K.; Gundel, S.; Hayden, D.; Hite, R.D.; et al. Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2019, 380, 1997–2008. [Google Scholar] [CrossRef]
- Nasa, P.; Azoulay, E.; Khanna, A.K.; Jain, R.; Gupta, S.; Javeri, Y.; Juneja, D.; Rangappa, P.; Sundararajan, K.; Alhazzani, W.; et al. Expert consensus statements for the management of COVID-19-related acute respiratory failure using a Delphi method. Crit. Care 2021, 25, 106. [Google Scholar] [CrossRef]
- Kwak, P.E.; Connors, J.R.; Benedict, P.A.; Timen, M.R.; Wang, B.; Zhang, Y.; Youlios, S.; Sureau, K.; Persky, M.J.; Rafeq, S.; et al. Early Outcomes from Early Tracheostomy for Patients With COVID-19. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 239–244. [Google Scholar] [CrossRef]
- Flinspach, A.N.; Booke, H.; Zacharowski, K.; Balaban, Ü.; Herrmann, E.; Adam, E.H. Association of mortality and early tracheostomy in patients with COVID-19: A retrospective analysis. Sci. Rep. 2022, 12, 15406. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Gordon, A.C.; Mouncey, P.R.; Al-Beidh, F.; Rowan, K.M.; Nichol, A.D.; Arabi, Y.M.; Annane, D.; Beane, A.; van Bentum-Puijk, W.; Berry, L.R.; et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 384, 1491–1502. [Google Scholar] [CrossRef]
- Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1637–1645. [CrossRef]
- Burrell, A.J.C.; Neto, A.S.; Broadley, T.; Trapani, T.; Begum, H.; Campbell, L.T.; Cheng, A.C.; Cheung, W.; Cooper, D.J.; Erickson, S.J.; et al. Comparison of baseline characteristics, treatment and clinical outcomes of critically ill COVID-19 patients admitted in the first and second waves in Australia. Crit. Care Resusc. 2021, 23, 308–319. [Google Scholar] [CrossRef]
- Riccaboni, M.; Verginer, L. The impact of the COVID-19 pandemic on scientific research in the life sciences. PLoS ONE 2022, 17, e0263001. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).