IL-6 Responsiveness of CD4+ and CD8+ T Cells after Allogeneic Stem Cell Transplantation Differs between Patients and Is Associated with Previous Acute Graft versus Host Disease and Pretransplant Antithymocyte Globulin Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Collection of Samples
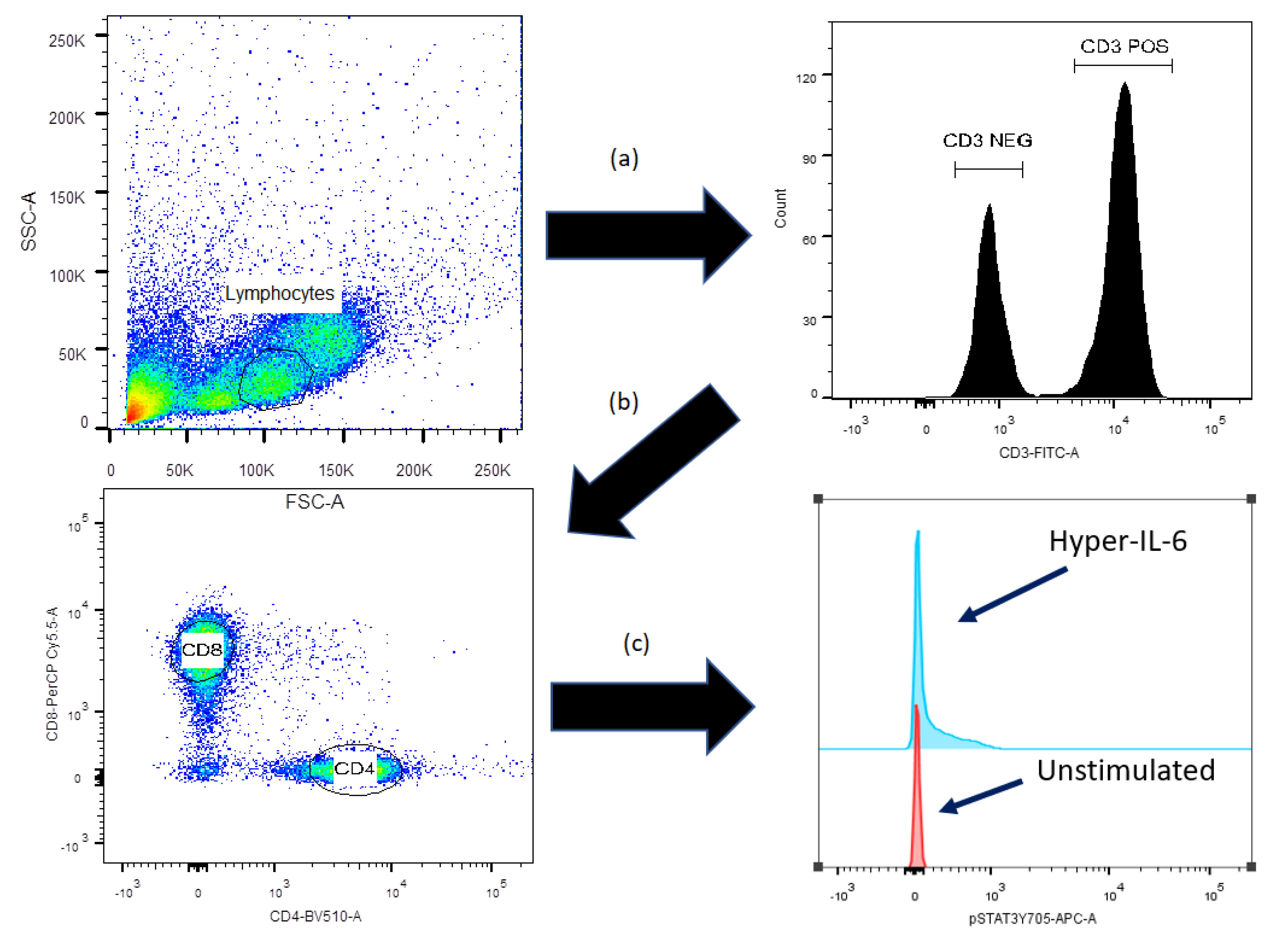
2.2. Flow-Cytometric Analysis of STAT3, AKT and mTOR Protein Phosphorylation
2.3. Flow-Cytometric Analysis of IL-6R Expression and Intracellular Cytokine Levels
2.4. Flow Cytometry and Statistical Analyses
3. Results
3.1. Patient and Donor Characteristics
3.2. Stimulation with Anti-CD3+Anti-CD28 Alone Increases AKT/mTOR Phosphorylation of Circulating CD3+CD4+ and CD3+CD8+ Post-transplant T Cells
3.3. PMA Increases Phosphorylation of STAT3 (Ser727), AKT (Thr308) and mTOR (Ser2448) for CD4+ and CD8+ T cells but Increases STAT3 (Tyr705) Phosphorylation Only for CD8+ T Cells
3.4. Strong T Cell AKT (Thr308), mTOR (Ser2448) and STAT3 (Ser727) Phosphoresponses to PMA Are Seen Especially for Allotransplant Recipients with Previous Acute GVHD
3.5. IL-6 Classical and Trans-Signaling Increase STAT (Tyr705) Phosphorylation of Resting CD3+CD4+ T Cells from Day +90 Post-Transplant
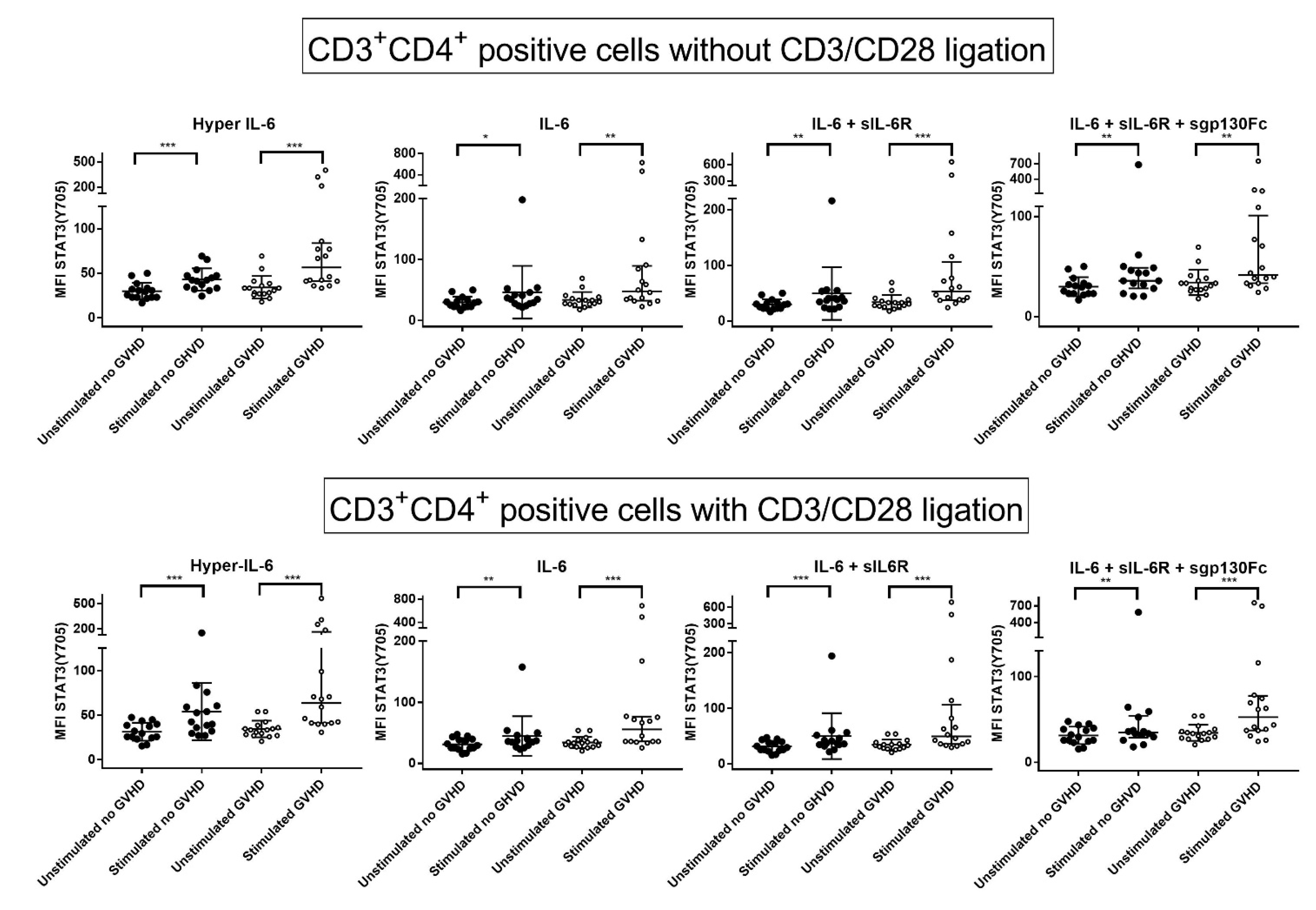
3.6. IL-6 Phosphoresponses of CD3+CD4+ T Cells Are Altered by TCR Activation: The STAT3 (Tyr705) Response Is Maintained While STAT (Ser727) and mTOR (Ser2448) Responses Increase
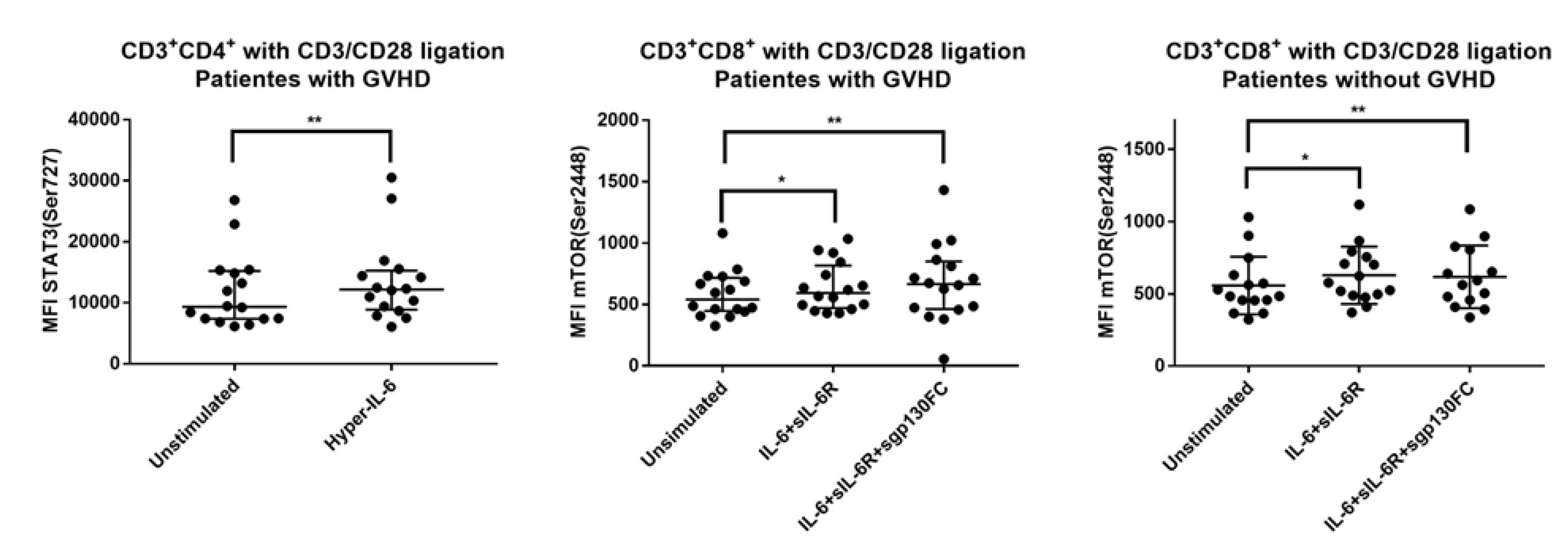
3.7. IL-6 Phosphoresponses of TCR Activated CD3+CD4+ Cells from Patients with and without Previous Acute GVHD; Similar STAT3 (Tyr705) but Different STAT3 (Ser727) and mTOR (Ser2448) Responses
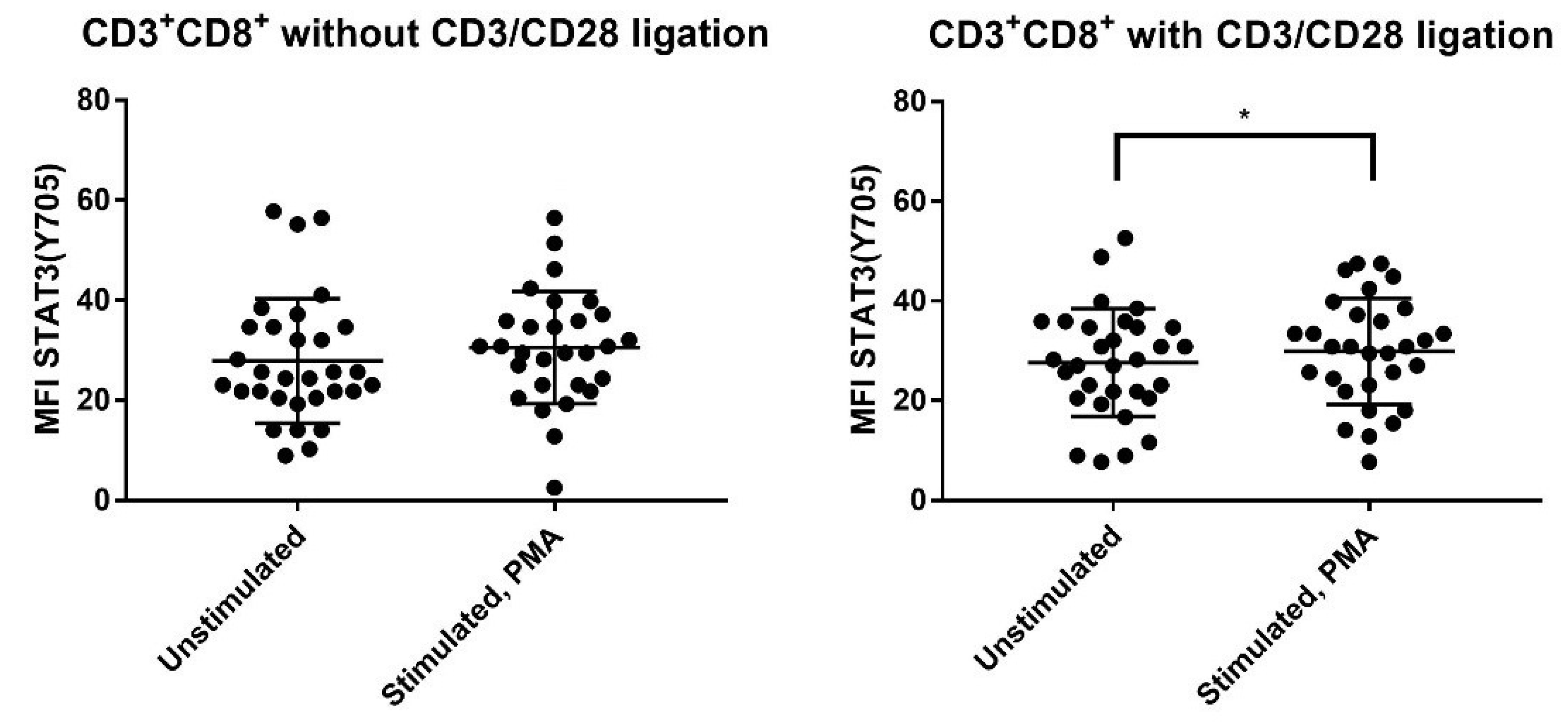
3.8. IL-6 Responsiveness of Post-Transplant CD3+CD8+ T Cells Increases during T Cell Activation
3.9. IL-6 Responsiveness of CD3+CD8+ T Cells: Only Resting but Not TCR Activated T Cells from Patients with and without Previous Acute GVHD Differ in Their IL-6 Responsiveness
3.10. The IL-6 Phosphoresponsiveness of Mononuclear CD3−-Cells
3.11. Effects of Prophylactic ATG on IL-6 Responsiveness of Circulating Post-Transplant T Cells
3.12. Circulating T Cells from Patients with and without Previous Acute GVHD Differ in the Levels of Circulating Th1 but Not in Th2 Levels, Th17 Levels and T Cell Expression of IL-6R
3.13. The IL-6 Responsiveness of day +90 Post-Transplant T Cells Does Not Predict the Probability to Later Wean off Systemic Immunosuppression
4. Discussion
- CD3+CD4+ T cells were generally more responsive to IL-6 than CD3+CD8+ T cells.
- IL-6-induced STAT3 phosphoresponses were most common, especially STAT3 (Tyr305) phosphorylation in CD3+CD4+ T cells, whereas mTOR (Ser2448) phosphoresponses differed between patients subsets (i.e., with/without acute GVHD or ATG).
- There was wide variation between patients with regard to pathway activation, and this variation was at least partly associated with/dependent on both previous ATG prophylaxis and previous acute GVHD.
- Patients with previous acute GVHD and/or without pretransplant ATG therapy differed from other patients especially with regard to IL-6-induced mTOR phosphoresponses, whereas the upstream AKT phosphoresponses did not differ.
- Differences between patients seem to involve both classical and IL-6 trans-signaling.
- It should be emphasized that an alternative experimental approach based on PMA-induced protein kinase C (PKC) activation confirmed that the downstream effects of T cell activation differed between patients with and without previous acute GVHD.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tvedt, T.H.A.; Ersvaer, E.; Tveita, A.A.; Bruserud, Ø. Interleukin-6 in Allogeneic Stem Cell Transplantation: Its Possible Importance for Immunoregulation and As a Therapeutic Target. Front. Immunol. 2017, 8, 667. [Google Scholar] [CrossRef]
- Drobyski, W.R.; Szabo, A.; Zhu, F.; Keever-Taylor, C.; Hebert, K.M.; Dunn, R.; Yim, S.; Johnson, B.; D’Souza, A.; Eapen, M.; et al. Tocilizumab, tacrolimus and methotrexate for the prevention of acute graft-versus-host disease: Low incidence of lower gastrointestinal tract disease. Haematologica 2018, 103, 717–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, G.A.; Varelias, A.; Vuckovic, S.; Le Texier, L.; Gartlan, K.H.; Zhang, P.; Thomas, G.; Anderson, L.; Boyle, G.; Cloonan, N.; et al. Addition of interleukin-6 inhibition with tocilizumab to standard graft-versus-host disease prophylaxis after allogeneic stem-cell transplantation: A phase 1/2 trial. Lancet Oncol. 2014, 15, 1451–1459. [Google Scholar] [CrossRef]
- von Bubnoff, N.; Ihorst, G.; Grishina, O.; Rothling, N.; Bertz, H.; Duyster, J.; Finke, J.; Zeiser, R. Ruxolitinib in GvHD (RIG) study: A multicenter, randomized phase 2 trial to determine the response rate of Ruxolitinib and best available treatment (BAT) versus BAT in steroid-refractory acute graft-versus-host disease (aGvHD) (NCT02396628). BMC Cancer 2018, 18, 1132. [Google Scholar] [CrossRef] [PubMed]
- Modemann, F.; Ayuk, F.; Wolschke, C.; Christopeit, M.; Janson, D.; von Pein, U.M.; Kröger, N. Ruxolitinib plus extracorporeal photopheresis (ECP) for steroid refractory acute graft-versus-host disease of lower GI-tract after allogeneic stem cell transplantation leads to increased regulatory T cell level. Bone Marrow Transplant. 2020, 55, 2286–2293. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, R.; von Bubnoff, N.; Butler, J.; Mohty, M.; Niederwieser, D.; Or, R.; Szer, J.; Wagner, E.M.; Zuckerman, T.; Mahuzier, B.; et al. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. N. Engl. J. Med. 2020, 382, 1800–1810. [Google Scholar] [CrossRef]
- Abedin, S.; McKenna, E.; Chhabra, S.; Pasquini, M.; Shah, N.N.; Jerkins, J.; Baim, A.; Runaas, L.; Longo, W.; Drobyski, W.; et al. Efficacy, Toxicity, and Infectious Complications in Ruxolitinib-Treated Patients with Corticosteroid-Refractory Graft-versus-Host Disease after Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 1689–1694. [Google Scholar] [CrossRef]
- Modi, B.; Hernandez-Henderson, M.; Yang, D.; Klein, J.; Dadwal, S.; Kopp, E.; Huelsman, K.; Mokhtari, S.; Ali, H.; Malki, M.M.A.; et al. Ruxolitinib as Salvage Therapy for Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2019, 25, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Kroger, N.; Shahnaz Syed Abd Kadir, S.; Zabelina, T.; Badbaran, A.; Christopeit, M.; Ayuk, F.; Wolschke, C. Peritransplantation Ruxolitinib Prevents Acute Graft-versus-Host Disease in Patients with Myelofibrosis Undergoing Allogenic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2018, 24, 2152–2156. [Google Scholar] [CrossRef] [Green Version]
- Zeiser, R.; Burchert, A.; Lengerke, C.; Verbeek, M.; Maas-Bauer, K.; Metzelder, S.K.; Spoerl, S.; Ditschkowski, M.; Ecsedi, M.; Sockel, K.; et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: A multicenter survey. Leukemia 2015, 29, 2062–2068. [Google Scholar] [CrossRef]
- Varelias, A.; Gartlan, K.H.; Kreijveld, E.; Olver, S.D.; Lor, M.; Kuns, R.D.; Lineburg, K.E.; Teal, B.E.; Raffelt, N.C.; Cheong, M.; et al. Lung parenchyma-derived IL-6 promotes IL-17A-dependent acute lung injury after allogeneic stem cell transplantation. Blood 2015, 125, 2435–2444. [Google Scholar] [CrossRef]
- Chen, X.; Das, R.; Komorowski, R.; Beres, A.; Hessner, M.J.; Mihara, M.; Drobyski, W.R. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood 2009, 114, 891–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noguchi, D.; Wakita, D.; Ohkuri, T.; Tajima, M.; Chamoto, K.; Kitamura, H.; Nishimura, T. Blockade of IL-6-signaling inhibits the pathogenesis of CD4+ T cell-mediated lethal graft-versus-host reaction against minor histocompatibility antigen. Immunol. Lett. 2011, 136, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Schaper, F.; Rose-John, S. Interleukin-6: Biology, signaling and strategies of blockade. Cytokine Growth Factor Rev. 2015, 26, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, A.; Rudra, D.; Treuting, P.; Samstein, R.M.; Liang, Y.; Kas, A.; Rudensky, A.Y. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 2009, 326, 986–991. [Google Scholar] [CrossRef] [Green Version]
- Fujino, M.; Li, X.K. Role of STAT3 in regulatory T lymphocyte plasticity during acute graft-vs.-host-disease. JAKSTAT 2013, 2, e24529. [Google Scholar] [CrossRef] [Green Version]
- Laurence, A.; Amarnath, S.; Mariotti, J.; Kim, Y.C.; Foley, J.; Eckhaus, M.; O’Shea, J.J.; Fowler, D.H. STAT3 transcription factor promotes instability of nTreg cells and limits generation of iTreg cells during acute murine graft-versus-host disease. Immunity 2012, 37, 209–222. [Google Scholar] [CrossRef] [Green Version]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef] [Green Version]
- Bruserud, Ø.; Nepstad, I.; Hauge, M.; Hatfield, K.J.; Reikvam, H. STAT3 as a possible therapeutic target in human malignancies: Lessons from acute myeloid leukemia. Expert Rev. Hematol. 2015, 8, 29–41. [Google Scholar] [CrossRef]
- Betts, B.C.; Sagatys, E.M.; Veerapathran, A.; Lloyd, M.C.; Beato, F.; Lawrence, H.R.; Yue, B.; Kim, J.; Sebti, S.M.; Anasetti, C.; et al. CD4+ T cell STAT3 phosphorylation precedes acute GVHD, and subsequent Th17 tissue invasion correlates with GVHD severity and therapeutic response. J. Leukoc. Biol. 2015, 97, 807–819. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Arras, D.; Rose-John, S. IL-6 pathway in the liver: From physiopathology to therapy. J. Hepatol. 2016, 64, 1403–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, K.A.; Manieri, N.A.; Liu, T.C.; Stappenbeck, T.S. IL-6 stimulates intestinal epithelial proliferation and repair after injury. PLoS ONE 2014, 9, e114195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, G.W.; McLoughlin, R.M.; Hammond, V.J.; Parker, C.R.; Williams, J.D.; Malhotra, R.; Scheller, J.; Williams, A.S.; Rose-John, S.; Topley, N.; et al. Loss of CD4+ T cell IL-6R expression during inflammation underlines a role for IL-6 trans signaling in the local maintenance of Th17 cells. J. Immunol. 2010, 184, 2130–2139. [Google Scholar] [CrossRef] [Green Version]
- Briso, E.M.; Dienz, O.; Rincon, M. Cutting edge: Soluble IL-6R is produced by IL-6R ectodomain shedding in activated CD4 T cells. J. Immunol. 2008, 180, 7102–7106. [Google Scholar] [CrossRef] [Green Version]
- Heink, S.; Yogev, N.; Garbers, C.; Herwerth, M.; Aly, L.; Gasperi, C.; Husterer, V.; Croxford, A.L.; Möller-Hackbarth, K.; Bartsch, H.S.; et al. Trans-presentation of IL-6 by dendritic cells is required for the priming of pathogenic TH17 cells. Nat. Immunol. 2017, 18, 74–85. [Google Scholar] [CrossRef] [Green Version]
- Gorentla, B.K.; Zhong, X.P. T cell Receptor Signal Transduction in T lymphocytes. J. Clin. Cell. Immunol. 2012, 2012 (Suppl. S12), 5. [Google Scholar]
- Filipovich, A.H.; Weisdorf, D.; Pavletic, S.; Socie, G.; Wingard, J.R.; Lee, S.J.; Martin, P.; Chien, J.; Przepiorka, D.; Couriel, D.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. Diagnosis and Staging Working Group Report. Biol. Blood Marrow Transplant. 2005, 11, 945–956. [Google Scholar] [CrossRef] [Green Version]
- Aigner, P.; Just, V.; Stoiber, D. STAT3 isoforms: Alternative fates in cancer? Cytokine 2019, 118, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Avalle, L.; Poli, V. Nucleus, Mitochondrion, or Reticulum? STAT3 a La Carte. Int. J. Mol. Sci. 2018, 19, 2820. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Morales, L.D.; Jang, I.S.; Cho, Y.Y.; Kim, D.J. Protein Tyrosine Phosphatases as Potential Regulators of STAT3 Signaling. Int. J. Mol. Sci. 2018, 19, 2708. [Google Scholar] [CrossRef] [Green Version]
- Eulenfeld, R.; Dittrich, A.; Khouri, C.; Muller, P.J.; Mutze, B.; Wolf, A.; Schaper, F. Interleukin-6 signalling: More than Jaks and STATs. Eur. J. Cell Biol. 2012, 91, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; August, A. The signaling symphony: T cell receptor tunes cytokine-mediated T cell differentiation. J. Leukoc. Biol. 2015, 97, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Sanchez, M.C.; Rodriguez-Serrano, C.; Almeida, J.; San Segundo, L.; Inoges, S.; Santos-Briz, A.; García-Briñón, J.; Corchete, L.A.; San Miguel, J.F.; Del Cañizo, C.; et al. Targeting of PI3K/AKT/mTOR pathway to inhibit T cell activation and prevent graft-versus-host disease development. J. Hematol. Oncol. 2016, 9, 113. [Google Scholar] [CrossRef] [Green Version]
- Zeiser, R.; Blazar, B.R. Acute Graft-versus-Host Disease-Biologic Process, Prevention, and Therapy. N. Engl. J. Med. 2017, 377, 2167–2179. [Google Scholar] [CrossRef]
- Ozawa, S.; Nakaseko, C.; Nishimura, M.; Maruta, A.; Cho, R.; Ohwada, C.; Sakamaki, H.; Sao, H.; Mori, S.; Okamoto, S.; et al. Chronic graft-versus-host disease after allogeneic bone marrow transplantation from an unrelated donor: Incidence, risk factors and association with relapse. A report from the Japan Marrow Donor Program. Br. J. Haematol. 2007, 137, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Storb, R.; Prentice, R.L.; Sullivan, K.M.; Shulman, H.M.; Deeg, H.J.; Doney, K.C.; Buckner, C.D.; Clift, R.A.; Witherspoon, R.P.; Appelbaum, F.A.; et al. Predictive factors in chronic graft-versus-host disease in patients with aplastic anemia treated by marrow transplantation from HLA-identical siblings. Ann. Intern. Med. 1983, 98, 461–466. [Google Scholar] [CrossRef]
- Betts, B.C.; Veerapathran, A.; Pidala, J.; Yu, X.Z.; Anasetti, C. STAT5 polarization promotes iTregs and suppresses human T-cell alloresponses while preserving CTL capacity. J. Leukoc. Biol. 2014, 95, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Fulton, L.M.; Carlson, M.J.; Coghill, J.M.; Ott, L.E.; West, M.L.; Panoskaltsis-Mortari, A.; Littman, D.R.; Blazar, B.R.; Serody, J.S. Attenuation of acute graft-versus-host disease in the absence of the transcription factor RORgammat. J. Immunol. 2012, 189, 1765–1772. [Google Scholar] [CrossRef]
- Kappel, L.W.; Goldberg, G.L.; King, C.G.; Suh, D.Y.; Smith, O.M.; Ligh, C.; Holland, A.M.; Grubin, J.; Mark, N.M.; Liu, C.; et al. IL-17 contributes to CD4-mediated graft-versus-host disease. Blood 2009, 113, 945–952. [Google Scholar] [CrossRef] [Green Version]
- Gagnon, J.; Ramanathan, S.; Leblanc, C.; Cloutier, A.; McDonald, P.P.; Ilangumaran, S. IL-6, in synergy with IL-7 or IL-15, stimulates TCR-independent proliferation and functional differentiation of CD8+ T lymphocytes. J. Immunol. 2008, 180, 7958–7968. [Google Scholar] [CrossRef] [Green Version]
- Ng, J.; Cantrell, D. STAT3 is a serine kinase target in T lymphocytes. Interleukin 2 and T cell antigen receptor signals converge upon serine 727. J. Biol. Chem. 1997, 272, 24542–24549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohtsuka, T.; Kaziro, Y.; Satoh, T. Analysis of the T-cell activation signaling pathway mediated by tyrosine kinases, protein kinase C, and Ras protein, which is modulated by intracellular cyclic AMP. Biochim. Biophys. Acta 1996, 1310, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Goel, G.; Makkar, H.P.; Francis, G.; Becker, K. Phorbol esters: Structure, biological activity, and toxicity in animals. Int. J. Toxicol. 2007, 26, 279–288. [Google Scholar] [CrossRef]
- Shin, S.Y.; Lee, D.H.; Lee, J.; Choi, C.; Kim, J.Y.; Nam, J.S.; Lim, Y.; Lee, Y.H. C-C motif chemokine receptor 1 (CCR1) is a target of the EGF-AKT-mTOR-STAT3 signaling axis in breast cancer cells. Oncotarget 2017, 8, 94591–94605. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Masters, A.R.; Fortner, K.A.; Champagne, D.P.; Yanguas-Casas, N.; Silberger, D.J.; Weaver, C.T.; Haynes, L.; Rincon, M. IL-6 promotes the differentiation of a subset of naive CD8+ T cells into IL-21-producing B helper CD8+ T cells. J. Exp. Med. 2016, 213, 2281–2291. [Google Scholar] [CrossRef] [PubMed]
- Dienz, O.; Rincon, M. The effects of IL-6 on CD4 T cell responses. Clin. Immunol. 2009, 130, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Diehl, S.; Rincon, M. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol. 2002, 39, 531–536. [Google Scholar] [CrossRef]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006, 126, 1121–1133. [Google Scholar] [CrossRef] [Green Version]
- Nurieva, R.I.; Chung, Y.; Hwang, D.; Yang, X.O.; Kang, H.S.; Ma, L.; Wang, Y.H.; Watowich, S.S.; Jetten, A.M.; Tian, Q.; et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 2008, 29, 138–149. [Google Scholar] [CrossRef] [Green Version]
- Diehl, S.A.; Schmidlin, H.; Nagasawa, M.; Blom, B.; Spits, H. IL-6 triggers IL-21 production by human CD4+ T cells to drive STAT3-dependent plasma cell differentiation in B cells. Immunol. Cell Biol. 2012, 90, 802–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottcher, J.P.; Schanz, O.; Garbers, C.; Zaremba, A.; Hegenbarth, S.; Kurts, C.; Beyer, M.; Schultze, J.L.; Kastenmüller, W.; Rose-John, S.; et al. IL-6 trans-signaling-dependent rapid development of cytotoxic CD8+ T cell function. Cell. Rep. 2014, 8, 1318–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hundhausen, C.; Roth, A.; Whalen, E.; Chen, J.; Schneider, A.; Long, S.A.; Wie, S.; Rawlings, R.; Kinsman, M.; Evanko, S.P.; et al. Enhanced T cell responses to IL-6 in type 1 diabetes are associated with early clinical disease and increased IL-6 receptor expression. Sci. Transl. Med. 2016, 8, 356ra119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron, F.; Mohty, M.; Blaise, D.; Socie, G.; Labopin, M.; Esteve, J.; Ciceri, F.; Giebel, S.; Gorin, N.C.; Savani, B.N.; et al. Anti-thymocyte globulin as graft-versus-host disease prevention in the setting of allogeneic peripheral blood stem cell transplantation: A review from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica 2017, 102, 224–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohty, M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia 2007, 21, 1387–1394. [Google Scholar] [CrossRef] [Green Version]
- Rahmat, L.T.; Logan, A.C. Ibrutinib for the treatment of patients with chronic graft-versus-host disease after failure of one or more lines of systemic therapy. Drugs Today 2018, 54, 305–313. [Google Scholar] [CrossRef]
- Miklos, D.; Cutler, C.S.; Arora, M.; Waller, E.K.; Jagasia, M.; Pusic, I.; Flowers, M.E.; Logan, A.C.; Nakamura, R.; Blazar, B.R.; et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood 2017, 130, 2243–2250. [Google Scholar] [CrossRef] [Green Version]
- Flynn, R.; Paz, K.; Du, J.; Reichenbach, D.K.; Taylor, P.A.; Panoskaltsis-Mortari, A.; Vulic, A.; Luznik, L.; MacDonald, K.K.; Hill, G.R.; et al. Targeted Rho-associated kinase 2 inhibition suppresses murine and human chronic GVHD through a Stat3-dependent mechanism. Blood 2016, 127, 2144–2154. [Google Scholar] [CrossRef] [Green Version]
- Schutt, S.D.; Fu, J.; Nguyen, H.; Bastian, D.; Heinrichs, J.; Wu, Y.; Liu, C.; McDonald, D.G.; Pidala, J.; Yu, X.Z. Inhibition of BTK and ITK with Ibrutinib Is Effective in the Prevention of Chronic Graft-versus-Host Disease in Mice. PLoS ONE 2015, 10, e0137641. [Google Scholar] [CrossRef] [Green Version]
- Flynn, R.; Allen, J.L.; Luznik, L.; MacDonald, K.P.; Paz, K.; Alexander, K.A.; Vulic, A.; Du, J.; Panoskaltsis-Mortari, A.; Taylor, P.A.; et al. Targeting Syk-activated B cells in murine and human chronic graft-versus-host disease. Blood 2015, 125, 4085–4094. [Google Scholar] [CrossRef] [Green Version]
- Iyengar, S.; Zhan, C.; Lu, J.; Korngold, R.; Schwartz, D.H. Treatment with a rho kinase inhibitor improves survival from graft-versus-host disease in mice after MHC-haploidentical hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2014, 20, 1104–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Recipient Characteristics (n = 31) | |
|---|---|
| Age, median and range (Years) | 59 (21–72) |
| Gender, Female/Male | 11/20 |
| Diagnosis (number) | |
| AML, de novo | 11 |
| Myelodysplastic syndrome, high-risk | 6 |
| Acute lymphoblastic leukemia | 3 |
| Chronic myeloid leukemia, myelofibrosis, chronic myelomonocytic leukemia, myeloproliferative neoplasia unspecified | 1 of each |
| Aplastic anemia | 2 |
| Conditioning regimes (number) | |
| Busulfan + cyclophosphamide (myeloablative condition) | 4 |
| Fludarabine + busulfan (reduced intensity conditioning) | 13 |
| Fludarabin + Treosulfan | 7 |
| Fludarabin + cyclophosphamide | 5 |
| Total body irradiation (TBI) + cyclophosphamide | 1 |
| Fludarabin + Thiotepa BU | 1 |
| GVHD prophylaxis (number) | |
| Cyclosporine A + methotrexate | 16 |
| Cyclosporine A + methotrexate + antithymocyte globulin | 10 |
| Cyclosporine A + sirolimus | 1 |
| Cyclosporine A + sirolimus + ATG | 3 |
| Post-transplant cyclophosphamide | 1 |
| Stem cell source (number) | |
| Peripheral blood mobilized stem cells | 23 |
| Bone marrow grafts | 8 |
| Ongoing immunosuppression on day +90 post-transplant (number) | |
| Cyclosporine | 31 |
| Corticosteroids | 5 |
| Others | 3 |
| Ongoing immunosuppression and alive on day +360 post-transplant (number) | |
| Cyclosporine | 6 |
| Corticosteroids | 4 |
| Others | 4 |
| Donor Characteristics | |
| Fully matched sibling/Matched unrelated donor/Haploidentical relative | 13/17/1 |
| CD3+CD4+ T Cells | CD3+CD8+ T Cells | CD3− | |||
|---|---|---|---|---|---|
| Phosphotarget | PMA Alone | PMA Anti-CD3 Anti-CD28 | PMA Alone | PMA Anti-CD3 Anti-CD28 | PMA Alone |
| All patients | |||||
| AKT (Thr308) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| mTOR (Ser2448) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| STAT3 (Ser727) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| STAT3 (Tyr705) | 0.03 | ||||
| Patients with previous acute GVHD (n = 16) | |||||
| AKT (Thr308) | <0.0001 | 0.0007 | 0.0009 | <0.0001 | 0.02 |
| mTOR (Ser2448) | 0.002 | 0.0003 | <0.0001 | 0.0006 | 0.001 |
| STAT3 (Ser727) | <0.0001 | 0.008 | 0.0006 | 0.04 | 0.0003 |
| STAT3 (Tyr705) | |||||
| Patients without previous acute GVHD (n = 15) | |||||
| AKT (Thr308) | 0.02 | .008 | 0.002 | ||
| mTOR (Ser2448) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| STAT3 (Ser727) | 0.02 | 0.03 | 0.004 | ||
| STAT3 (Tyr705) | |||||
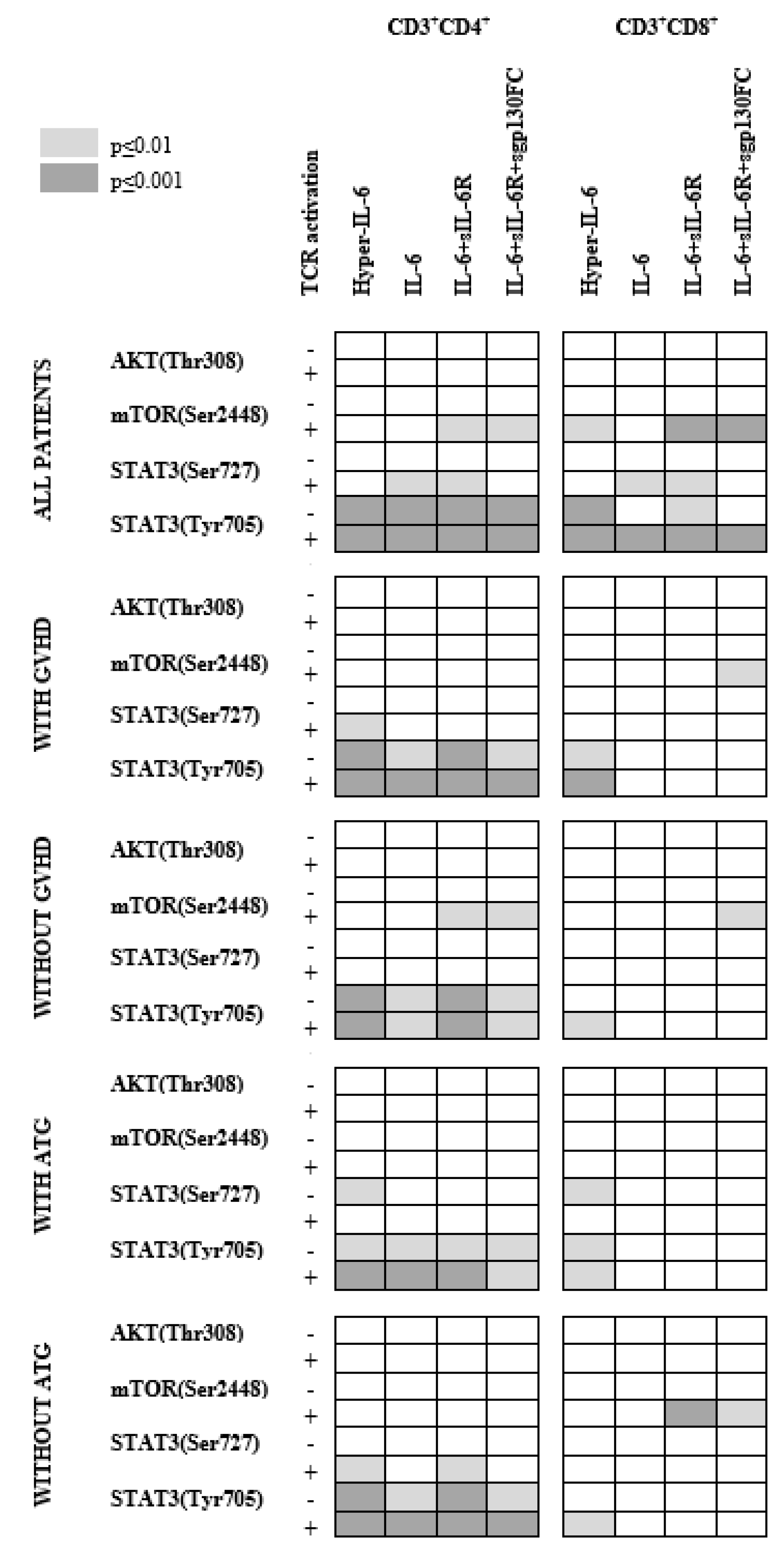
| Without TCR Ligation | With TCR Ligation | |||||||
|---|---|---|---|---|---|---|---|---|
| Phosphotarget | Hyper- IL-6 | IL-6 | IL-6 sIL-6R | IL-6 sIL-6R sgp130 | Hyper-IL-6 | IL-6 | IL6 sIL-6R | IL-6 sIL-6R sgp130 |
| All patients | ||||||||
| AKT (Thr308) | ||||||||
| mTOR (Ser2448) | 0.009 | 0.0056 | ||||||
| STAT3 (Ser727) | 0.03 | 0.02 | 0.007 | 0.007 | ||||
| STAT3 (Tyr705) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Patients with GVHD (n = 16) | ||||||||
| AKT (Thr308) | ||||||||
| mTOR (Ser2448) | 0.04 | |||||||
| STAT3 (Ser727) | 0.009 | |||||||
| STAT3 (Tyr705) | <0.0001 | 0.003 | 0.0008 | 0.004 | <0.0001 | 0.0002 | 0.0003 | 0.0008 |
| Patients without GVHD (n = 15) | ||||||||
| AKT (Thr308) | ||||||||
| mTOR (Ser2448) | 0.009 | 0.006 | ||||||
| STAT3 (Ser727) | ||||||||
| STAT3 (Tyr705) | <0.0001 | 0.002 | 0.001 | 0.004 | <0.0001 | 0.002 | 0.0005 | 0.006 |
| Without TCR Ligation | With TCR Ligation | |||||||
|---|---|---|---|---|---|---|---|---|
| Phosphotarget | Hyper- IL-6 | IL-6 | IL-6 sIL-6R | IL-6 sIL-6R sgp130 | Hyper- IL-6 | IL-6 | IL6 sIL-6R | IL-6 sIL-6R sgp130 |
| All patients | ||||||||
| AKT (Thr308) | 0.03 | |||||||
| mTOR (Ser2448) | 0.03 | 0.005 | 0.04 | <0.001 | <0.001 | |||
| STAT3 (Ser727) | 0.03 | 0.003 | 0.004 | |||||
| STAT3 (Tyr705) | <0.0001 | 0.03 | 0.004 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Patients with GVHD (n = 16) | ||||||||
| AKT (Thr308) | ||||||||
| mTOR (Ser2448) | 0.04 | 0.008 | ||||||
| STAT3 (Ser727) | 0.04 | |||||||
| STAT3 (Tyr705) | 0.003 | 0.0008 | ||||||
| Patients without GVHD (n = 15) | ||||||||
| AKT (Thr308) | ||||||||
| mTOR (Ser2448) | 0.03 | 0.005 | ||||||
| STAT3 (Ser727) | 0.02 | |||||||
| STAT3 (Tyr705) | 0.002 | |||||||
| All Patients Median (Range) | Patients with Previous Acute GVHD | Patients without Previous Acute GVHD | p-Value GVHD vs. No GVHD | |
|---|---|---|---|---|
| Circulating T cell subset | ||||
| CD3+CD4+(% of CD3+ cells) | 42 (10–73) | 50 (10–73) | 36 (18–54) | 0.06 |
| CD3+CD8+(% of CD3+ cells) | 48 (22–83) | 44 (22–83) | 51 (35–79) | 0.18 |
| Th1 (% of CD4+ T cells) | 11 (2–38) | 9 (5–19) | 15 (2–38) | 0.003 |
| Th2 (% of CD4+ T cells) | 5 (0.9–11) | 4.6 (1.5–9) | 4 (0.9–11) | 0.86 |
| Th17 (% of CD4+ T cells) | 0.9 (0.3–3.0) | 0.8 (0.3–3.0) | 1 (0.3–1.7) | 0.43 |
| IL-6R expression | ||||
| CD3+CD4+ | 241 (160–420) | 267 (160–420) | 223 (191–383) | 0.43 |
| CD3+CD8+ | 170 (134–261) | 179 (134–261) | 169 (142–226) | 0.37 |
| CD3− | 225 (194–366) | 227 (194–279) | 225 (204–336) | 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tvedt, T.H.A.; Rose-John, S.; Tsykunova, G.; Ahmed, A.B.; Gedde-Dahl, T.; Ersvær, E.; Bruserud, Ø. IL-6 Responsiveness of CD4+ and CD8+ T Cells after Allogeneic Stem Cell Transplantation Differs between Patients and Is Associated with Previous Acute Graft versus Host Disease and Pretransplant Antithymocyte Globulin Therapy. J. Clin. Med. 2022, 11, 2530. https://doi.org/10.3390/jcm11092530
Tvedt THA, Rose-John S, Tsykunova G, Ahmed AB, Gedde-Dahl T, Ersvær E, Bruserud Ø. IL-6 Responsiveness of CD4+ and CD8+ T Cells after Allogeneic Stem Cell Transplantation Differs between Patients and Is Associated with Previous Acute Graft versus Host Disease and Pretransplant Antithymocyte Globulin Therapy. Journal of Clinical Medicine. 2022; 11(9):2530. https://doi.org/10.3390/jcm11092530
Chicago/Turabian StyleTvedt, Tor Henrik Anderson, Stefan Rose-John, Galina Tsykunova, Aymen Bushra Ahmed, Tobias Gedde-Dahl, Elisabeth Ersvær, and Øystein Bruserud. 2022. "IL-6 Responsiveness of CD4+ and CD8+ T Cells after Allogeneic Stem Cell Transplantation Differs between Patients and Is Associated with Previous Acute Graft versus Host Disease and Pretransplant Antithymocyte Globulin Therapy" Journal of Clinical Medicine 11, no. 9: 2530. https://doi.org/10.3390/jcm11092530
APA StyleTvedt, T. H. A., Rose-John, S., Tsykunova, G., Ahmed, A. B., Gedde-Dahl, T., Ersvær, E., & Bruserud, Ø. (2022). IL-6 Responsiveness of CD4+ and CD8+ T Cells after Allogeneic Stem Cell Transplantation Differs between Patients and Is Associated with Previous Acute Graft versus Host Disease and Pretransplant Antithymocyte Globulin Therapy. Journal of Clinical Medicine, 11(9), 2530. https://doi.org/10.3390/jcm11092530







