Ultrasound-Guided Tru-Cut Biopsy in Gynecological and Non-Gynecological Pelvic Masses: A Single-Center Experience
Abstract
:1. Introduction
2. Material and Methods
- Adequacy was defined as the ability to obtain an amount of tissue able to determine the origin of the tumor and the ability to perform immunohistochemistry;
- Accuracy was classified as the concordance between the tru-cut needle ample and the postoperative histology;
- Safety was assessed based on complications rate.
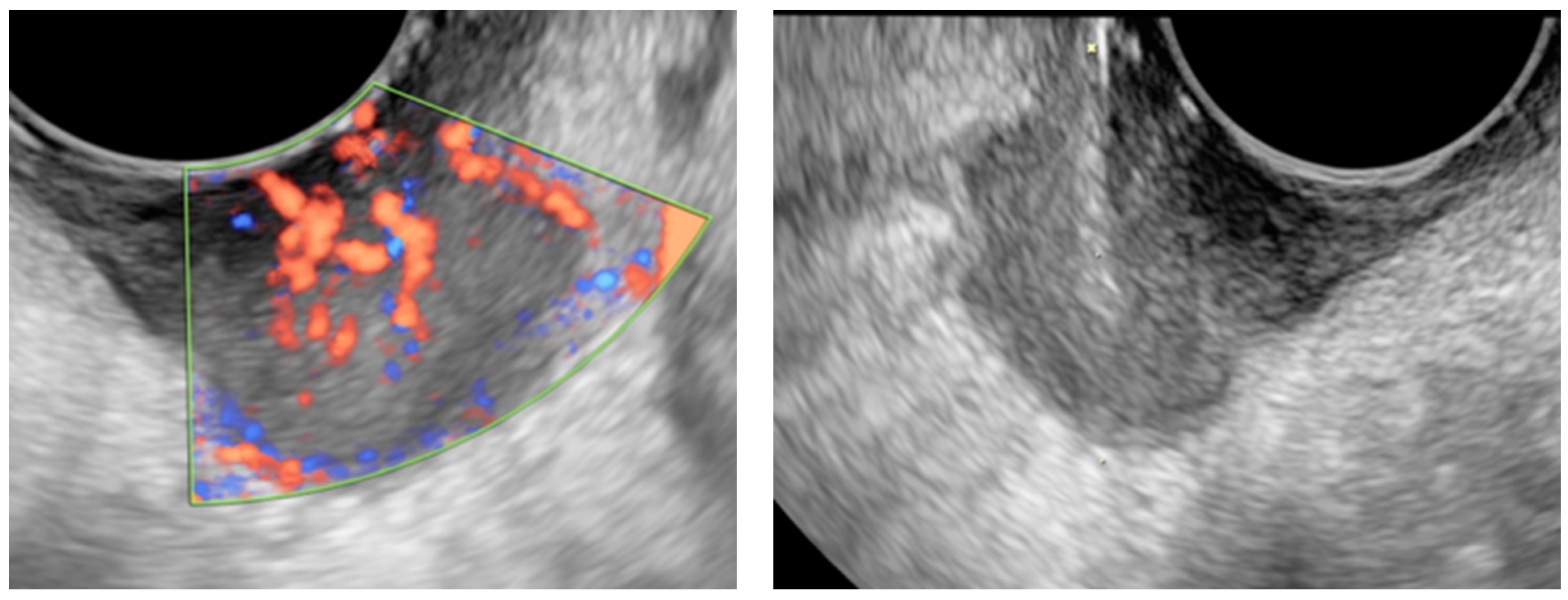
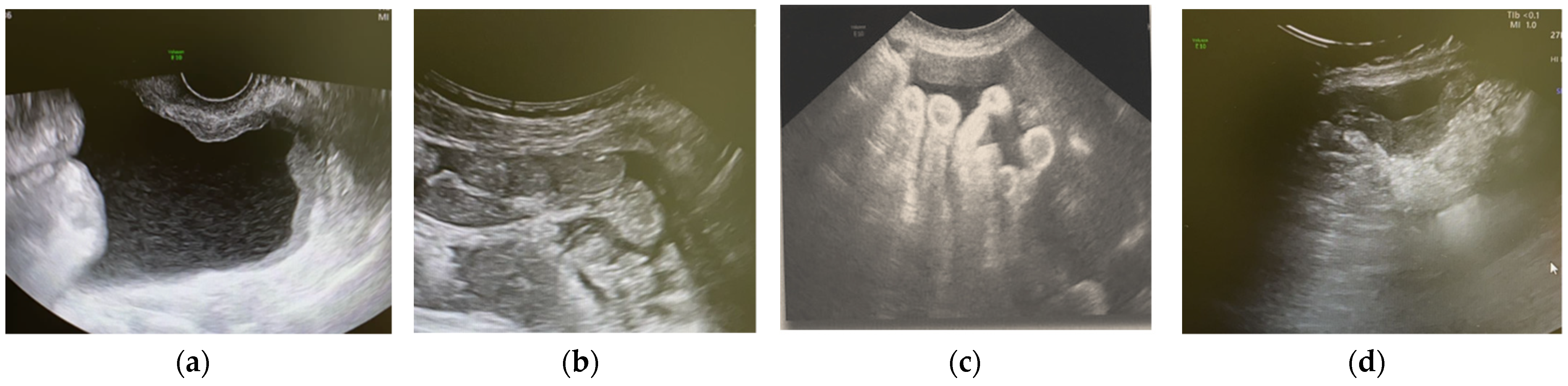
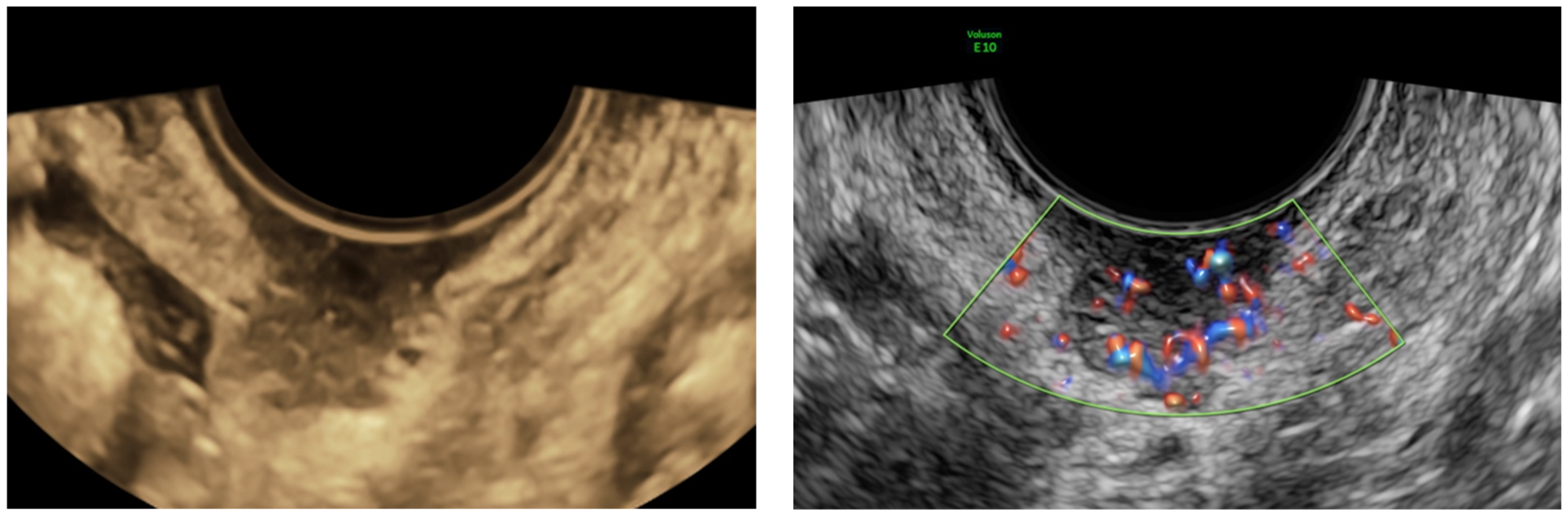
2.1. US-Guided Tru-Cut Biopsy
2.2. Histopathological Diagnosis
3. Statistical Analysis
4. Results
4.1. Ultrasound Examination and Ultrasound-Guided Tru-Cut Biopsies
4.2. Pathological Study
5. Cases of Primary Interest and Discussion
5.1. Non-Gynaecological Masses
5.2. TCBN in Recurrent Cancer
5.3. TBCN: Our False-Negative Results
5.4. TBCN in Advanced Ovarian Cancer
5.5. TCBN in Benign Masses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Fischerova, D.; Cibula, D. Ultrasound in gynecological cancer: Is it time for re-evaluation of its uses? Curr. Oncol. Rep. 2015, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J.R.; Coldewey, J.; Stewart, I.S. Comparison of fine needle aspiration cytology and needle core biopsy in the diagnosis of radiologically detected abdominal lesions. J. Clin. Pathol. 2002, 55, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Fischerova, D.; Cibula, D.; Dundr, P.; Zikan, M.; Calda, P.; Freitag, P.; Slama, J. Ultrasound-guided tru-cut biopsy in the management of advanced abdomino-pelvic tumors. Int. J. Gynecol. Cancer 2008, 18, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Oluwasola, A.G.; Adeoye, A.O.; Afolabi, A.O.; Adeniji-Sofoluwe, A.T.; Salami, A.; Ajani, M.A.; Ogundiran, T.O.; Obajimi, M.O. Diagnostic accuracy of tru-cut biopsy of breast lumps at University College Hospital, Ibadan. Afr. J. Med. Med. Sci. 2015, 44, 157–162. [Google Scholar]
- Omer, A.; Lamb, A.D. Optimizing prostate biopsy techniques. Curr. Opin. Urol. 2019, 29, 578–586. [Google Scholar] [CrossRef]
- Mascilini, F.; Quagliozzi, L.; Moro, F.; Moruzzi, M.C.; De Blasis, I.; Paris, V.; Scambia, G.; Fagotti, A.; Testa, A.C. Role of transvaginal ultrasound-guided biopsy in gynecology. Int. J. Gynecol. Cancer 2019, 30, 128–132. [Google Scholar] [CrossRef]
- Rikabi, A.; Hussain, S. Diagnostic Usefulness of Tru-Cut Biopsy in the Diagnosis of Breast Lesions. Oman Med. J. 2013, 28, 125–127. [Google Scholar] [CrossRef]
- Vlasak, P.; Bouda, J.; Kostun, J.; Berezovskiy, D.; Zikan, M.; Weinberger, V.; Ondic, O.; Rusavy, Z.; Kucera, R.; Topolcan, O.; et al. Diagnostic Reliability, Accuracy and Safety of Ultrasound-guided Biopsy and Ascites Puncture in Primarily Inoperable Ovarian Tumours. Anticancer Res. 2020, 40, 3527–3534. [Google Scholar] [CrossRef]
- Chojniak, R.; Isberner, R.K.; Viana, L.M.; Yu, L.S.; Aita, A.A.; Soares, F.A. Computed tomography guided needle biopsy: Experience from 1300 procedures. Sao Paulo Med. J. 2006, 124, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Zikan, M.; Fischerova, D.; Pinkavova, I.; Dundr, P.; Cibula, D. Ultrasound-guided tru-cut biopsy of abdominal and pelvic tumors in gynecology. Ultrasound Obstet. Gynecol. 2010, 36, 767–772. [Google Scholar] [CrossRef]
- Epstein, E.; Van Calster, B.; Timmerman, D.; Nikman, S. Subjective ultrasound assessment, the ADNEX model and ultrasound-guided tru-cut biopsy to differentiate disseminated primary ovarian cancer from metastatic non-ovarian cancer. Ultrasound Obstet. Gynecol. 2016, 47, 110–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verschuere, H.; Froyman, W.; Bosch, T.V.D.; Van Hoefs, M.; Kaijser, J.; Van Schoubroeck, D.; Van Rompuy, A.; Vergote, I.; Timmerman, D. Safety and efficiency of performing transvaginal ultrasound-guided tru-cut biopsy for pelvic masses. Gynecol. Oncol. 2021, 161, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.-H.; Lyu, G.-S. Abdominal Ultrasound-Guided Transvaginal Myometrial Core Needle Biopsy for the Definitive Diagnosis of Suspected Adenomyosis in 1032 Patients: A Retrospective Study. J. Minim. Invasive Gynecol. 2015, 22, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; Valentin, L.; Bourne, T.; Collins, W.P.; Verrelst, H.; Vergote, I. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: A consensus opinion from the International Ovarian Tumor Analysis (IOTA) group. Ultrasound Obstet. Gynecol. 2000, 16, 500–505. [Google Scholar] [CrossRef]
- Bosch, T.V.D.; Dueholm, M.; Leone, F.P.G.; Valentin, L.; Rasmussen, C.; Votino, A.; Van Schoubroeck, D.; Landolfo, C.; Installé, A.J.F.; Guerriero, S.; et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: A consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet. Gynecol. 2015, 46, 284–298. [Google Scholar] [CrossRef]
- Assem, H.; Rambau, P.F.; Lee, S.; Ogilvie, T.; Sienko, A.; Kelemen, L.E.; Köbel, M. High-grade Endometrioid Carcinoma of the Ovary. Am. J. Surg. Pathol. 2018, 42, 534–544. [Google Scholar] [CrossRef]
- Byrt, T.; Bishop, J.; Carlin, J.B. Bias, prevalence and kappa. J. Clin. Epidemiol. 1993, 46, 423–429. [Google Scholar] [CrossRef]
- Kho, R.M.; Abrao, M.S. Ovarian remnant syndrome. Curr. Opin. Obstet. Gynecol. 2012, 24, 210–214. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Guerriero, S.; Alcazar, J.L.; Pascual, M.; Ajossa, S.; Olartecoechea, B.; Hereter, L. Preoperative diagnosis of metastatic ovarian cancer is related to origin of primary tumor. Ultrasound Obstet. Gynecol. 2012, 39, 581–586. [Google Scholar] [CrossRef]
- Testa, A.C.; Ferrandina, G.; Timmerman, D.; Savelli, L.; Ludovisi, M.; Van Holsbeke, C.; Malaggese, M.; Scambia, G.; Valentin, L. Imaging in gynecological disease (1): Ultrasound features of metastases in the ovaries differ depending on the origin of the primary tumor. Ultrasound Obstet. Gynecol. 2007, 29, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Zikan, M.; Fischerova, D.; Pinkavova, I.; Dundr, P.; Cibula, D. Ultrasonographic appearance of metastatic non-gynecological pelvic tumors. Ultrasound Obstet. Gynecol. 2012, 39, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Xiong, Y.-H.; Yun, M.; Liu, L.-Z.; Zheng, W.; Lin, X.; Pei, X.-Q.; Li, A.-H. Transvaginal Ultrasound-Guided Core Needle Biopsy of Pelvic Masses. J. Ultrasound Med. 2017, 37, 453–461. [Google Scholar] [CrossRef] [Green Version]
- Meanwell, C.A.; Rolfe, E.B.; Blackledge, G.; Docker, M.F.; Lawton, F.G.; Mould, J.J. Recurrent female pelvic cancer: Assessment with transrectal ultrasonography. Radiology 1987, 162, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.Y.; Shen, A.J.; Yang, S.L.; Wu, M.F. Combination of Sonographic Morphology Score and Tumor Markers for Detecting Postoperative Recurrent Pelvic Ovarian Carcinoma. Ultrasound Q. 2019, 35, 45–53. [Google Scholar] [CrossRef]
- Sugiyama, T.; Nishida, T.; Komai, K.; Nishimura, H.; Yakushiji, M.; Nishimura, H. Comparison of CA 125 assays with abdominopelvic computed tomography and transvaginal ultrasound in monitoring of ovarian cancer. Int. J. Gynecol. Obstet. 1996, 54, 251–256. [Google Scholar] [CrossRef]
- Testa, A.; Fruscella, E.; Ludovisi, M.; De Vincenzo, R.; Malaggese, M.; Corrado, G.; Basso, D.; Scambia, G.; Ferrandina, G. The role of sonographic examination in the follow-up of gynecological neoplasms. Gynecol. Oncol. 2005, 99, 696–703. [Google Scholar] [CrossRef]
- Rosati, A.; Alletti, S.G.; Capozzi, V.A.; Mirandola, M.; Vargiu, V.; Fedele, C.; Uccella, S.; Vascone, C. Role of ultrasound in the detection of recurrent ovarian cancer: A review of the literature. Gland Surg. 2020, 9, 1092–1101. [Google Scholar] [CrossRef]
- Gao, C.; Wang, L.; Zhang, C.; Li, X. Transvaginal/transrectal ultrasound-guided aspiration biopsy for diagnosis of pelvic/pelvic floor tumors in females: A retrospective analysis. Exp. Ther. Med. 2019, 18, 352–357. [Google Scholar] [CrossRef] [Green Version]
- Van Calster, B.; Van Hoorde, K.; Froyman, W.; Kaijser, J.; Wynants, L.; Landolfo, C.; Anthoulakis, C.; Vergote, I.; Bourne, T.; Timmerman, D. Practical guidance for applying the ADNEX model from the IOTA group to discriminate between different subtypes of adnexal tumors. Facts Views Vis. ObGyn 2015, 7, 32–41. [Google Scholar]
- Heitz, F.; Ognjenovic, D.; Harter, P.; Kommoss, S.; Ewald-Riegler, N.; Haberstroh, M.; Gomez, R.; Barinoff, J.; Traut, A.; Du Bois, A. Abdominal Wall Metastases in Patients With Ovarian Cancer After Laparoscopic Surgery. Int. J. Gynecol. Cancer 2010, 20, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Querleu, D.; Leblanc, E.; Narducci, F.; Ferron, G. Low incidence of port-site metastases after laparoscopic staging of uterine cancer. Gynecol. Oncol. 2010, 118, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Marquette, S.; Amant, F.; Berteloot, P.; Neven, P. Port-site metastases after open laparoscopy: A study in 173 patients with advanced ovarian carcinoma. Int. J. Gynecol. Cancer 2005, 15, 776–779. [Google Scholar] [CrossRef] [PubMed]
- El Hachem, L.; Mathews, S.; Pereira, E.; Momeni, M.; Friedman, K.; Chuang, L.; Gretz, H. Tru-Cut Biopsy in Gynecologic Surgery. J. Minim. Invasive Gynecol. 2015, 22, S202. [Google Scholar] [CrossRef]
- Kawamura, N.; Ichimura, T.; Ito, F.; Shibata, S.; Takahashi, K.; Tsujimura, A.; Ishiko, O.; Haba, T.; Wakasa, K.; Ogita, S. Transcervical needle biopsy for the differential diagnosis between uterine sarcoma and leiomyoma. Cancer 2002, 94, 1713–1720. [Google Scholar] [CrossRef]
- Faulkner, R.L.; Mohiyiddeen, L.; McVey, R.; Kitchener, H.C. Transvaginal biopsy in the diagnosis of ovarian cancer. BJOG Int. J. Obstet. Gynaecol. 2005, 112, 991–993. [Google Scholar] [CrossRef]
- Lengyel, D.; Vereczkey, I.; Kőhalmy, K.; Bahrehmand, K.; Novák, Z. Transvaginal Ultrasound-Guided Core Biopsy—Experiences in a Comprehensive Cancer Centre. Cancers 2021, 13, 2590. [Google Scholar] [CrossRef]
- Fischerová, D.; Cibula, D. The role of ultrasound in primary workup of cervical cancer staging (ESGO, ESTRO, ESP cervical cancer guidelines). Ceska Gynekol. 2019, 84, 40–48. [Google Scholar]
- Timmerman, D.; Planchamp, F.; Bourne, T.; Landolfo, C.; du Bois, A.; Chiva, L.; Cibula, D.; Concin, N.; Fischerova, D.; Froyman, W.; et al. ESGO / ISUOG / IOTA / ESGE Consensus Statement on preoperative diagnosis of ovarian tumors. Ultrasound Obstet. Gynecol. 2021, 58, 148–168. [Google Scholar] [CrossRef]


| Variables | N = 42 Patients |
|---|---|
| Age (years): Median (min-max) | 72 (39–93) |
| Body Mass Index (Kg/m2): Median (min-max) | 24.9 (18.6–42.5) |
| CA-125 UI/L: Median (min-max) | 129 (3–2358.3) |
| Personal history of cancer (N, %) | 15 (35.7%) |
| Indications to the TCNB | |
| Inoperable advanced tumor (N, %) | 23 (54.8%) |
| Poor performance status (N, %) | 16 (38%) |
| Suspicion of recurrence (N, %) | 11 (26.1%) |
| Suspicion of metastases | 3 (7.1%) |
| Previously undefined malignancies (N, %) | 1 (2.3%) |
| Variables | N = 42 Patients |
|---|---|
| Largest diameter of the lesion (mm): median (min-max) | 51 (8–280) |
| Type of the tumor (N, %) | |
| Solid | 34 (81%) |
| Multilocular-solid | 8 (19 %) |
| Tumor margins (N, %) | |
| Irregular | 36 (85.7%) |
| Regular | 6 (14.3%) |
| Color score (N, %) | |
| 2 | 10 (23.8%) |
| 3 | 29 (69%) |
| 4 | 3 (7.1%) |
| Ascites (N, %) | 12 (28.6%) |
| Site of access (N, %) | |
| Transvaginal | 31 (73.8%) |
| Transabdominal | 11 (26.2%) |
| Site of biopsy (N, %) | |
| Lesion | 34 (81%) |
| Omental cake | 4 (10%) |
| Carcinosis | 4 (10%) |
| Histology (N, %) | |
| Benign | 2 (4.8%) |
| Malign | 40 (95.2%) |
| Primary advanced tumors | 27 (67.5%) |
| Advanced ovarian cancer | 19 (47.5%) |
| Advanced cervical cancer | 7 (17.5%) |
| Recurrent genital tumors | 10 (25%) |
| Recurrence of endometrial cancer | 4 (10%) |
| Recurrence of cervical cancer | 3 (7.5%) |
| Recurrence of ovarian cancer | 3 (7.5%) |
| Primary peritoneal cancer in an oncological patient (leiomyosarcoma) | 1 (2.5%) |
| Metastases or non-genital malignancy | 3 (7.5%) |
| Complications (mild) (N, %) | 1 (2.4%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buonomo, F.; Bussolaro, S.; de Almeida Fiorillo, C.; Oliveira de Souza, D.; Giudici, F.; Romano, F.; Romano, A.; Ricci, G. Ultrasound-Guided Tru-Cut Biopsy in Gynecological and Non-Gynecological Pelvic Masses: A Single-Center Experience. J. Clin. Med. 2022, 11, 2534. https://doi.org/10.3390/jcm11092534
Buonomo F, Bussolaro S, de Almeida Fiorillo C, Oliveira de Souza D, Giudici F, Romano F, Romano A, Ricci G. Ultrasound-Guided Tru-Cut Biopsy in Gynecological and Non-Gynecological Pelvic Masses: A Single-Center Experience. Journal of Clinical Medicine. 2022; 11(9):2534. https://doi.org/10.3390/jcm11092534
Chicago/Turabian StyleBuonomo, Francesca, Sofia Bussolaro, Clarice de Almeida Fiorillo, Danilo Oliveira de Souza, Fabiola Giudici, Federico Romano, Andrea Romano, and Giuseppe Ricci. 2022. "Ultrasound-Guided Tru-Cut Biopsy in Gynecological and Non-Gynecological Pelvic Masses: A Single-Center Experience" Journal of Clinical Medicine 11, no. 9: 2534. https://doi.org/10.3390/jcm11092534
APA StyleBuonomo, F., Bussolaro, S., de Almeida Fiorillo, C., Oliveira de Souza, D., Giudici, F., Romano, F., Romano, A., & Ricci, G. (2022). Ultrasound-Guided Tru-Cut Biopsy in Gynecological and Non-Gynecological Pelvic Masses: A Single-Center Experience. Journal of Clinical Medicine, 11(9), 2534. https://doi.org/10.3390/jcm11092534







