Abstract
We aimed to create percentile-based reference values of the umbilical cord blood insulin-like growth factor-1 (IGF-1) levels in Japanese newborns, as these values have not yet been established. A total of 259 newborns were classified into four gestational-age-at-birth (GA) groups: extremely preterm (<28 weeks); early preterm (28–33 weeks); late preterm (34–36 weeks); and term (≥37 weeks). They were further subclassified as small-for-gestational-age (SGA) or non-SGA. The 10th, 25th, 50th, 75th, and 90th percentiles of the umbilical cord blood IGF-1 levels were calculated and compared between the groups by using reference values of 9, 18, 33, 52, and 71 ng/mL, respectively. In the extremely preterm group, the IGF-1 levels were significantly lower than those in the early preterm, late preterm, and term groups (13.5, 24.0, 44.5, and 47.5 ng/mL, respectively; p < 0.001). The umbilical cord blood IGF-1 levels in the SGA newborns were significantly lower than those in the non-SGA newborns in all subgroups. In multivariate analyses, the GA and birth weight standard deviation scores were independent determinant factors for the umbilical cord blood IGF-1 levels. Thus, we established percentile-based reference values of umbilical cord blood IGF-1 in Japanese newborns; these reference values can be applied on the basis of the extent of prematurity and the SGA status.
1. Introduction
Insulin-like growth factor 1 (IGF-1) is a protein that is encoded by the IGF1 gene in humans [1,2]. IGF-1 is a single-stranded polypeptide that consists of 70 amino acids, with a molecular weight of 7649; it also has three disulfide bonds in its structure [3]. IGF-1 plays an important role in fetal and neonatal growth, as well as in cardiovascular functions, central nervous system functioning, and lung development [4,5,6]. It has been reported that long-term low serum IGF-1 levels from birth are associated with postnatal growth restriction, brain developmental disorders, retinopathy of prematurity (ROP), and chronic lung disease [7,8,9,10,11].
Previous studies have shown that the value of the IGF-1 at birth is correlated with the birth weight (BW) and the gestational age at birth (GA), and that the IGF-1 values in small-for-gestational-age (SGA) newborns are lower than those in non-SGA newborns [12,13]. The patients in previous reports with regard to the IGF-1 values were term or preterm newborns and term SGA newborns [14,15,16,17]. However, to date, no detailed studies have been conducted where extremely preterm-to-term newborns were included, especially when evaluating the IGF-1 reference values by using umbilical cord blood. In this study, we aimed to create percentile-based reference values for the umbilical cord blood IGF-1 levels in Japanese newborns on the basis of the sex, the GA, and the SGA or non-SGA status.
2. Materials and Methods
2.1. Study Design
A hospital-based retrospective cohort study was conducted at Nihon University Itabashi Hospital, Tokyo, Japan. This study was approved by the Ethics Committee of the Nihon University School of Medicine (approval no.: RK-190910-3; date: 20 November 2019). Written informed consent was obtained from the parents of all the enrolled newborns. This study was carried out in accordance with the relevant guidelines and regulations. Newborns that were born between 2019 and 2021 were enrolled and were then classified into one of the four GA groups: extremely preterm (<28 weeks); early preterm (28–33 weeks); late preterm (34–36 weeks); and term (≥37 weeks). Thereafter, the newborns were subclassified as either SGA or non-SGA. “SGA” and “non-SGA” were defined as the <10th percentile and the ≥10th percentile, respectively, for the BW, on the basis of Japanese neonatal anthropometric charts at birth for the GA, the sex, and the mother’s history of childbirth [18]. This study included not only singletons, but also twins and triplets. All newborns from whom the umbilical cord blood at birth was collected during the study period were included in this study.
2.2. Umbilical Cord Blood IGF-1 Levels
At birth, the umbilicus was double-clamped, and the cord blood was sampled from the umbilical vein. The median (50th percentile), 10th, 25th, 75th, and 90th percentiles of the umbilical cord blood IGF-1 levels were calculated in order to set up the reference values. The umbilical cord blood IGF-1 levels were measured by using the solid-phase radioimmunoassay (RIA) method, as described in a previous study [19,20]. The RIA is an immunological assay that was first developed as a method for measuring the amount of a hormone in the blood by using a radioisotope [21].
2.3. Study Methods
The umbilical cord blood IGF-1 levels were compared between the sexes and the four GA groups in all the SGA and non-SGA newborns. In each GA group, the umbilical cord blood IGF-1 levels were compared between the SGA and non-SGA newborns. To clarify the determinant clinical factors for the umbilical cord blood IGF-1 levels, the GA or anthropometric values at birth, such as the BW, the BW standard deviation score (SDS), the birth length (BL), the BL SDS, the birth head circumference, the birth head circumference SDS, and the birth chest circumference, were identified through regression and multivariate analyses.
2.4. Statistical Analyses
To compare the umbilical cord blood IGF-1 levels and other clinical factors at birth between the four GA groups, the Kruskal–Wallis test or a chi-squared test were used. The differences between the individual data sets were tested by the all-pairwise test by using the Steel–Dwass method. The correlation between the umbilical cord blood IGF-1 levels and each anthropometric value or GA was analyzed by a regression analysis (the correlation coefficients were calculated) and a multiple logistic regression analysis. Statistical analyses were performed using JMP, Version 14 (SAS Institute Inc., Tokyo, Japan). A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Umbilical Cord Blood IGF-1 Levels
A total of 259 newborns were enrolled, of which 36 were extremely preterm (SGA: 10; non-SGA: 26); 83 were early preterm (SGA: 28; non-SGA: 55); 90 were late preterm (SGA: 21; non-SGA: 69); and 50 were term (SGA: 19; non-SGA: 31). No statistical difference was observed in the umbilical cord blood IGF-1 levels between the sexes (male newborns: 35 [4–121]; female newborns: 32 [4–93]; p = 0.60; Figure 1).
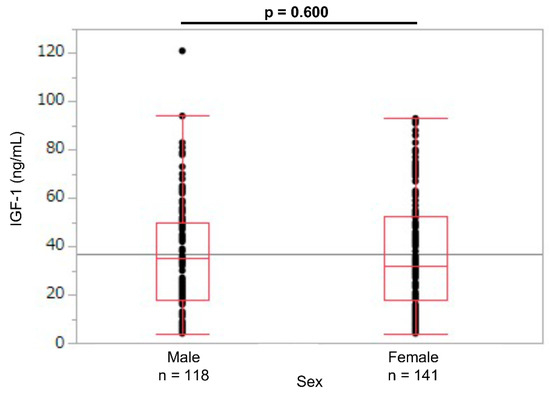
Figure 1.
Umbilical cord IGF-1 levels in male and female newborns. IGF-1: insulin-like growth factor 1.
The 10th, 25th, 50th, 75th, and 90th percentiles of the umbilical cord blood IGF-1 levels were calculated and compared between the groups by using reference values of 9, 18, 33, 52, and 71 ng/mL, respectively. The umbilical cord blood IGF-1 levels in the extremely preterm group were significantly lower than those of the early preterm, late preterm, and term groups (median values: 13.5, 24.0, 44.5, and 47.5 ng/mL, respectively; p < 0.01; Table 1; Figure 2). However, the IGF-1 levels were not significantly different between the late preterm and term groups (p = 0.722; Figure 2).

Table 1.
Umbilical cord blood IGF-1 levels classified by GA.
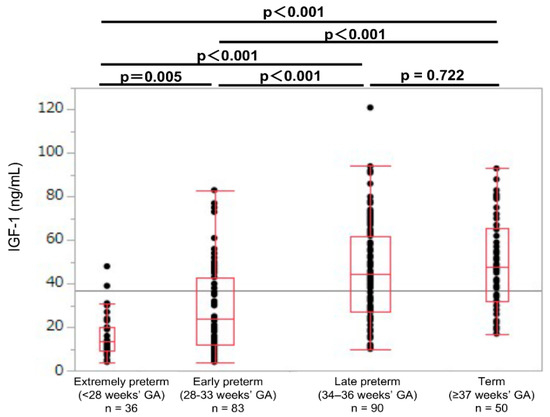
Figure 2.
Umbilical cord IGF-1 levels in extremely preterm, early preterm, late preterm, and term newborns. GA: gestational age at birth; IGF-1: insulin-like growth factor 1.
In each GA group, the umbilical cord blood IGF-1 levels were lower in the SGA newborns than in the non-SGA newborns (SGA: 9.5, 10.5, 25.0, and 38.0 ng/mL; non-SGA: 19.0, 38.0, 50.0, and 55.0 ng/mL, respectively; p < 0.01; Table 2). In the SGA newborns, the umbilical cord blood IGF-1 values showed no significant difference between the extremely preterm and early preterm newborns (median values: 9.5 and 10.5 ng/mL, respectively; p = 0.855; Figure 3). In the non-SGA newborns, the IGF-1 levels were significantly different between the extremely preterm and the early preterm newborns (median values: 19.0 and 38.0 ng/mL, respectively; p < 0.001; Figure 4).

Table 2.
Comparison of umbilical cord blood IGF-1 levels between SGA and non-SGA newborns.
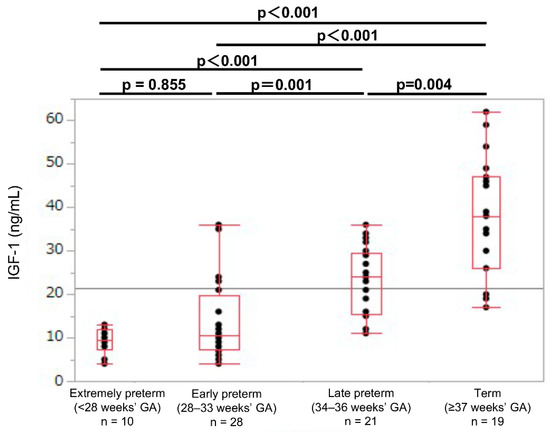
Figure 3.
Umbilical cord IGF-1 levels in extremely preterm, early preterm, late preterm, and term SGA newborns. GA: gestational age at birth; IGF-1: insulin-like growth factor 1; SGA: small-for-gestational-age.
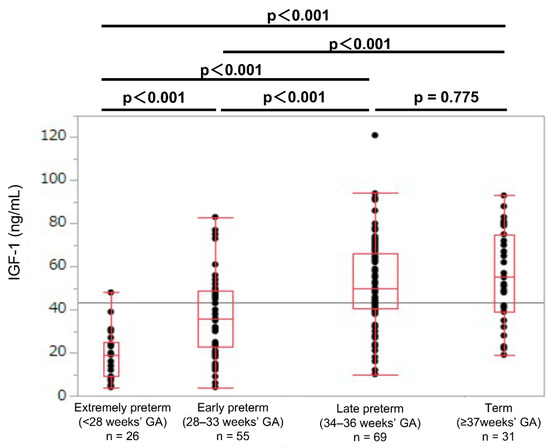
Figure 4.
Umbilical cord IGF-1 levels in extremely preterm, early preterm, late preterm, and term non-SGA newborns. GA: gestational age at birth; IGF-1: insulin-like growth factor 1; SGA: small-for-gestational-age.
3.2. Correlation between Umbilical Cord Blood IGF-1 Levels and Gestational Age or Anthropometric Measurements at Birth
The correlations between the umbilical cord blood IGF-1 levels and each anthropometric value or GA are shown in Table 3. All of the anthropometric values (GA, BW, BW SDS, BL, BL SDS, birth head circumference, birth head circumference SDS, birth chest circumference, and IGF-1 levels) showed significantly positive correlations (p <0.001; Table 3).

Table 3.
Association between insulin-like growth factor-1 levels and gestational age or anthropometric values at birth.
3.3. Multivariate Analyses
In the multivariate logistic regression analyses using the GA, BW SDS, BL SDS, and birth head circumference SDS, the GA and the BW SDS were the independent determinant factors for the umbilical cord blood IGF-1 levels (Table 4).

Table 4.
Multivariate analysis.
4. Discussion
In the present study, we established percentile-based reference values of umbilical cord blood IGF-1 in Japanese newborns. The median umbilical cord blood IGF-1 value was around 50 ng/mL at ≥34 weeks of GA, and it did not change between the late preterm and term newborns. In the non-SGA newborns, the umbilical cord blood IGF-1 levels showed a significant difference between the extremely preterm and early preterm newborns, but not in the SGA newborns. In the SGA newborns, the median umbilical cord blood IGF-1 values were extremely low, at approximately 10 ng/mL at <34 weeks of GA. It was rare for the SGA newborns to have higher IGF-1 levels than the non-SGA newborns. The GA and the BW SDS were the independent determinant factors for the umbilical cord blood IGF-1 levels.
4.1. IGF-1 Levels and Sex
In a report of the serum IGF-1 levels in preterm newborns at 33 weeks of corrected GA, the mean IGF-1 levels were 23.1 ng/mL (range: 15.44–39.75 ng/mL), with no difference between the male (23.1 ng/mL) and female infants (23.1 ng/mL) [15]. Consistent with these results, no umbilical cord blood IGF-1 level differences were observed according to sex in this study (Figure 1). Therefore, we created reference values for the umbilical cord blood IGF-1 for the male and female newborns combined.
4.2. IGF-1 in the Placenta
IGF-1, IGF-2, and their binding protein (IGFBP) are expressed in the placenta and regulate fetal growth by DNA methylation [22]. DNA methylation is an epigenetic mechanism that forms methylated cytosine-phosphate-guanine (CpG) dinucleotides that are known to suppress gene expression [23]. A study report analyzed the placenta of SGA newborns and found the mRNA and protein levels of IGF-1 to be lower than that of appropriate-for-gestational-age (AGA) newborns; however, no difference was observed between the AGA and the large-for-gestational-age newborns [24]. The CpG methylation of the promoter region of the IGF-1 gene was hypermethylated in the SGA newborns when compared with that of the AGA newborns [24]. In our present study, the SGA newborns had lower umbilical cord blood IGF-1 levels than the non-SGA newborns, and, therefore, the DNA methylation of the IGF-1 gene may be involved.
4.3. IGF-1 Levels in SGA Newborns
The production of IGF-1 during the fetal period is regulated by nutritional substances that are supplied by the mother, such as amino acids and glucose [25,26]. Previous reports of cord blood IGF-1 levels are strongly associated with the birth weight, the leptin level, the fat mass, and the body fat percentage, which indicate that IGF-1 is an important factor in the fetal fat accumulation in the uterus [27]. Another previous study reports that newborns who are born to mothers with pregnancy hypertension had lower cord blood IGF-1 levels compared with those born to mothers without pregnancy hypertension, and the SGA newborns showed low cord blood IGF-1 levels [28]. Consistent with the results, in our present study, the cord blood IGF-1 levels were lower in the SGA newborns compared to those of the non-SGA newborns in all four GA groups. The umbilical cord blood IGF-1 levels in the preterm SGA newborns who were born at 28–33 weeks of GA were as low as the extremely preterm newborns who were born at <28 weeks of GA. SGA was also an important determinant factor for the cord blood IGF-1 levels. In the SGA newborns, the umbilical cord blood IGF-1 levels were remarkably low in extremely preterm and early preterm newborns who were born at <34 weeks of GA, when compared with those of late preterm newborns who were born at 34–36 weeks of GA, and term newborns who were born at ≥37 weeks of GA. We previously reported that preterm SGA newborns had higher rates of short stature at three years of life than late preterm or term SGA newborns [29]. A reason for this difference in the incidence of short stature in early childhood may be related to the blood IGF-1 levels at birth.
4.4. IGF-1 Levels in Extremely Preterm Newborns
In our present study, in the extremely preterm newborns, the median IGF-1 values were as low as 13.5 ng/mL, which may indicate a low nutrition status. A clinical study in 87 newborns born between 24 and 32 weeks of GA evaluated the relationship between the serum IGF-1 levels and the nutrition up to 36 weeks of corrected GA, and it reveals that the parenteral nutrition and the IGF-1 levels at 2 weeks of age were inversely proportional [14]. The total energy intake was positively associated with the serum IGF-1 levels, especially at 30–33 weeks of corrected GA [14]. After birth, extremely preterm newborns lose nutrition from their mother and become dependent on parenteral nutrition for several weeks, which may result in low levels of IGF-1 for a prolonged period.
4.5. Association between IGF-1 Levels and Neonatal Diseases
In a study of preterm newborns that weighed under 1251 g in the United States, the mean IGF-1 value at 28–33 weeks of corrected GA without ROP was 20.0 ng/mL, and, at Stage 1 or 2, the ROP was 18.0 ng/mL, and, at Stage 3, the ROP was 17.0 ng/mL, which suggests that the IGF-1 values were associated with the ROP development and severity [30]. Therefore, newborns with low serum IGF-1 levels after birth may be predisposed to ROP and may require treatment. It has also been reported that low serum IGF-1 levels were strongly associated with various neonatal complications in animal models (periventricular leukomalacia model rat; cerebral hypoxic ischemia model rat; primary IGF-1-deficient mice; and a necrotizing enterocolitis model) [31,32,33,34] and humans (poor weight gain and brain weight, and the development of ROP and bronchopulmonary dysplasia) [7,9,10,11]. Early IGF-1 supplementation, such as the intravenous supplementation of human recombinant IGF-1 to attain normal serum levels, may reduce these complications and promote growth and development in extremely preterm newborns with/without SGA. Clinical trials are currently underway to see if early IGF-1 supplementation can improve and reduce preterm newborn morbidity [35,36]. A comparison of our cord blood IGF-1 reference values may be useful for the prediction of complications in extremely preterm newborns. The IGF-1 reference values change slightly with the various measurement methods that are available [19,20]. Therefore, it is important to carefully consider the measurement method when using the reference values. In the future, we expect to obtain the therapeutic effect of IGF-1 supplementation in extremely preterm newborns with low cord blood IGF-1 levels.
4.6. Limitations
This study has some limitations. First, this study was conducted with a small cohort in a single Japanese center. Second, this study included only Japanese newborns. It will be necessary to clarify the difference in the IGF-1 values between the different races. Third, we did not investigate the clinical information, such as neonatal diseases, the nutritional status, the insulin levels, the body weight gain, the smoking of the mother, and gestational diabetes mellitus. Finally, the postnatal serum IGF-1 levels were not measured. However, our results supply evidence for the newborn’s umbilical cord IGF-1 reference values, not only in all newborns, but also in newborns, classified by sex, GA, and SGA or non-SGA status.
5. Conclusions
We established percentile-based reference values of umbilical cord blood IGF-1 levels in Japanese newborns. These reference values can be applied on the basis of the extent of the gestational age or the SGA status. A comparison of our cord blood IGF-1 reference values may be useful for predicting neonatal diseases in preterm newborns.
Author Contributions
N.N. and I.M. conceived and designed the study; acquired, analyzed, and interpreted the data; and drafted the manuscript. D.K., K.H., Y.S., S.T., M.A. and R.A. were involved in the implementation of the research and the data collection. All authors critically revised the manuscript and gave final approval on the version to be submitted. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Nihon University School of Medicine Alumni Association’s 60th anniversary fund research grant (20N601), and Grants-in-Aid for Scientific Research (C) (21K11582) of JSPS KAKENHI and The Japanese Society for Pediatric Endocrinology Future Development Grant, supported by Novo Nordisk Pharma Ltd. (N.N.).
Institutional Review Board Statement
This study was approved by the Ethics Committee of the Nihon University School of Medicine (approval no.: RK-190910-3; date 20 November 2019). This study was carried out in accordance with the relevant guidelines and regulations.
Informed Consent Statement
Written informed consent was obtained from the parents of all enrolled newborns for publication.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that there are no competing interests in this study.
References
- Höppener, J.W.; de Pagter-Holthuizen, P.; Geurts van Kessel, A.H.; Jansen, M.; Kittur, S.D.; Antonarakis, S.E.; Lips, C.J.; Sussenbach, J.S. The Human Gene Encoding Insulin-Like Growth Factor I Is Located on Chromosome 12. Hum. Genet. 1985, 69, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.; van Schaik, F.M.; Ricker, A.T.; Bullock, B.; Woods, D.E.; Gabbay, K.H.; Nussbaum, A.L.; Sussenbach, J.S.; Van den Brande, J.L. Sequence of cDNA Encoding Human Insulin-Like Growth Factor I Precursor. Nature 1983, 306, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Rinderknecht, E.; Humbel, R.E. The Amino Acid Sequence of Human Insulin-Like Growth Factor I and Its Structural Homology with Proinsulin. J. Biol. Chem. 1978, 253, 2769–2776. [Google Scholar] [CrossRef]
- Murray, P.G.; Clayton, P.E. Endocrine Control of Growth. Am. J. Med. Genet. C Semin. Med. Genet. 2013, 163C, 76–85. [Google Scholar] [CrossRef]
- Ng, P.C.; Lam, C.W.; Lee, C.H.; Wong, G.W.; Fok, T.F.; Chan, I.H.; Ma, K.C.; Wong, E. Leptin and Metabolic Hormones in Preterm Newborns. Arch. Dis. Child. Fetal Neonatal Ed. 2000, 83, F198–F202. [Google Scholar] [CrossRef] [Green Version]
- Hellström, A.; Ley, D.; Hansen-Pupp, I.; Hallberg, B.; Löfqvist, C.; van Marter, L.; van Weissenbruch, M.; Ramenghi, L.A.; Beardsall, K.; Dunger, D.; et al. Insulin-Like Growth Factor 1 Has Multisystem Effects on Foetal and Preterm Infant Development. Acta Paediatr. 2016, 105, 576–586. [Google Scholar] [CrossRef] [Green Version]
- Kajantie, E.; Dunkel, L.; Rutanen, E.M.; Seppälä, M.; Koistinen, R.; Sarnesto, A.; Andersson, S. IGF-I, IGF Binding Protein (IGFBP)-3, Phosphoisoforms of IGFBP-1, and Postnatal Growth in Very Low Birth Weight Infants. J. Clin. Endocrinol. Metab. 2002, 87, 2171–2179. [Google Scholar] [CrossRef]
- Löfqvist, C.; Engström, E.; Sigurdsson, J.; Hård, A.L.; Niklasson, A.; Ewald, U.; Holmström, G.; Smith, L.E.; Hellström, A. Postnatal Head Growth Deficit Among Premature Infants Parallels Retinopathy of Prematurity and Insulin-Like Growth Factor-1 Deficit. Pediatrics 2006, 117, 1930–1938. [Google Scholar] [CrossRef] [Green Version]
- Hansen-Pupp, I.; Hövel, H.; Hellström, A.; Hellström-Westas, L.; Löfqvist, C.; Larsson, E.M.; Lazeyras, F.; Fellman, V.; Hüppi, P.S.; Ley, D. Postnatal Decrease in Circulating Insulin-Like Growth Factor-I and Low Brain Volumes in Very Preterm Infants. J. Clin. Endocrinol. Metab. 2011, 96, 1129–1135. [Google Scholar] [CrossRef]
- Pérez-Muñuzuri, A.; Fernández-Lorenzo, J.R.; Couce-Pico, M.L.; Blanco-Teijeiro, M.J.; Fraga-Bermúdez, J.M. Serum Levels of IGFl Are a Useful Predictor of Retinopathy of Prematurity. Acta Paediatr. 2010, 99, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Löfqvist, C.; Hellgren, G.; Niklasson, A.; Engström, E.; Ley, D.; Hansen-Pupp, I. WINROP Consortium Low Postnatal Serum IGF-I Levels Are Associated with Bronchopulmonary Dysplasia (BPD). Acta Paediatr. 2012, 101, 1211–1216. [Google Scholar] [CrossRef] [Green Version]
- Verhaeghe, J.; Van Herck, E.; Billen, J.; Moerman, P.; Van Assche, F.A.; Giudice, L.C. Regulation of Insulin-Like Growth Factor-I and Insulin-Like Growth Factor Binding Protein-1 Concentrations in Preterm Fetuses. Am. J. Obstet. Gynecol. 2003, 188, 485–491. [Google Scholar] [CrossRef]
- Yang, S.W.; Yu, J.S. Relationship of Insulin-Like Growth Factor-Ⅰ, Insulin-Like Growth Factor Binding protein-3, Insulin, Growth Hormone in Cord Blood and Maternal Factors with Birth Height and Birthweight. Pediatr. Int. 2000, 42, 31–36. [Google Scholar] [CrossRef]
- Yumani, D.F.J.; Calor, A.K.; van Weissenbruch, M.M. The Course of IGF-1 Levels and Nutrient Intake in Extremely and Very Preterm Infants During Hospitalisation. Nutrients 2020, 12, 675. [Google Scholar] [CrossRef] [Green Version]
- Banjac, L.; Kotur-Stevuljević, J.; Gojković, T.; Bokan-Mirković, V.; Banjac, G.; Banjac, G. Relationship Between Insulin-Like Growth Factor Type 1 and Intrauterine Growth. Acta Clin. Croat. 2020, 59, 91–96. [Google Scholar] [CrossRef]
- Rustogi, D.; Yadav, S.; Ramji, S.; Mishra, T.K. Growth Patterns in Small for Gestational Age Babies and Correlation with Insulin-Like Growth Factor-1 Levels. Indian Pediatr. 2018, 55, 975–978. [Google Scholar] [CrossRef]
- Dizdarer, C.; Korkmaz, H.A.; Büyükocak, Ö.M.; Tarancı, S.M.; Coban, A. Impact of Insulin Resistance on Insulin-Like Growth Factor-1/Insulin Like Growth Factor-Binding Protein-3 Axis and on Early Weight Gain in Small for Gestational Age Infants. J. Clin. Res. Pediatr. Endocrinol. 2013, 5, 104–109. [Google Scholar] [CrossRef]
- Itabashi, K.; Miura, F.; Uehara, R.; Nakamura, Y. New Japanese Neonatal Anthropometric Charts for Gestational Age at Birth. Pediatr. Int. 2014, 56, 702–708. [Google Scholar] [CrossRef]
- Chanson, P.; Arnoux, A.; Mavromati, M.; Brailly-Tabard, S.; Massart, C.; Young, J.; Piketty, M.L.; Souberbielle, J.C. VARIETE Investigators Reference Values for IGF-I Serum Concentrations: Comparison of Six Immunoassays. J. Clin. Endocrinol. Metab. 2016, 101, 3450–3458. [Google Scholar] [CrossRef] [Green Version]
- Frystyk, J.; Freda, P.; Clemmons, D.R. The Current Status of IGF-I Assays—A 2009 Update. Growth Horm. IGF Res. 2010, 20, 8–18. [Google Scholar] [CrossRef]
- Berson, S.A.; Yalow, R.S. Radioimmunoassays of Peptide Hormones in Plasma. N. Engl. J. Med. 1967, 20, 640–647. [Google Scholar] [CrossRef]
- Han, V.K.; Bassett, N.; Walton, J.; Challis, J.R. The Expression of Insulin-Like Growth Factor (IGF) and IGF-Binding Protein (IGFBP) Genes in the Human Placenta and Membranes: Evidence for IGF-IGFBP Interactions at the Feto-Maternal Interface. J. Clin. Endocrinol. Metab. 1996, 81, 2680–2693. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [Green Version]
- Le Stunff, C.; Castell, A.L.; Todd, N.; Mille, C.; Belot, M.P.; Frament, N.; Brailly-Tabard, S.; Benachi, A.; Fradin, D.; Bougnères, P. Fetal Growth Is Associated with CpG Methylation in the P2 Promoter of the IGF1 Gene. Clin. Epigenetics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Battaglia, F.C.; Meschia, G. Principal Substrates of Fetal Metabolism. Physiol. Rev. 1978, 58, 499–527. [Google Scholar] [CrossRef]
- Rao, P.N.S.; Shashidhar, A.; Ashok, C. In Utero Fuel Homeostasis: Lessons for a Clinician. Indian J. Endocrinol. Metab. 2013, 17, 60–68. [Google Scholar] [CrossRef]
- Kadakia, R.; Ma, M.; Josefson, J.L. Neonatal Adiposity Increases with Rising Cord Blood IGF-1 Levels. Clin. Endocrinol. 2016, 85, 70–75. [Google Scholar] [CrossRef]
- Gazy, P.K.; Marciniak, S.; Slawska, H.; Olejek, A.; Mazur, B. The Concentration of Insulin-Like Growth Factor-1 in Pregnancies Complicated by Pregnancy-Induced Hypertension and/or Intrauterine Hypotrophy. Ginekol. Pol. 2020, 91, 544–548. [Google Scholar] [CrossRef]
- Matsumoto, M.; Nagano, N.; Awano, H.; Ohyama, S.; Fujioka, K.; Iwatani, S.; Urakami, T.; Iijima, K.; Morioka, I. Incidence and Neonatal Risk Factors of Short Stature and Growth Hormone Treatment in Japanese Preterm Infants Born Small for Gestational Age. Sci. Rep. 2019, 9, 12238. [Google Scholar] [CrossRef]
- Jensen, A.K.; Ying, G.S.; Huang, J.; Quinn, G.E.; Binenbaum, G. Postnatal Serum Insulin-Like Growth Factor I and Retinopathy of Prematurity. Retina 2017, 37, 867–872. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.J.; Cho, S.Y.; Kim, S.U.; Jo, D.W.; Hwang, H.I.; Shin, H.K.; Jun, H.Y. IGF-1 Protects Neurons in the Cortex and Subventricular Zone in a Periventricular Leucomalacia Model. In Vivo 2021, 35, 307–312. [Google Scholar] [CrossRef]
- Lin, S.; Fan, L.W.; Rhodes, P.G.; Cai, Z. Intranasal Administration of IGF-1 Attenuates Hypoxic-Ischemic Brain Injury in Neonatal Rats. Exp. Neurol. 2009, 217, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Elis, S.; Courtland, H.W.; Wu, Y.; Rosen, C.J.; Sun, H.; Jepsen, K.J.; Majeska, R.J.; Yakar, S. Elevated Serum Levels of IGF-1 Are Sufficient to Establish Normal Body Size and Skeletal Properties Even in the Absence of Tissue IGF-1. J. Bone Miner. Res. 2010, 25, 1257–1266. [Google Scholar] [CrossRef] [Green Version]
- Holgersen, K.; Gao, X.; Narayanan, R.; Gaur, T.; Carey, G.; Barton, N.; Pan, X.; Muk, T.; Thymann, T.; Sangild, P.T. Supplemental Insulin-Like Growth Factor-1 and Necrotizing Enterocolitis in Preterm Pigs. Front. Pediatr. 2021, 8, 602047. [Google Scholar] [CrossRef]
- Hellström, A.; Ley, D.; Hansen-Pupp, I.; Hallberg, B.; Ramenghi, L.A.; Löfqvist, C.; Smith, L.E.; Hård, A.L. Role of Insulinlike Growth Factor 1 in Fetal Development and in the Early Postnatal Life of Premature Infants. Am. J. Perinatol. 2016, 33, 1067–1071. [Google Scholar] [CrossRef] [Green Version]
- Hellström, A.; Ley, D.; Hallberg, B.; Lofqvist, C.; Hansen-Pupp, I.; Ramenghi, L.A.; Borg, J.; Smith, L.E.H.; Hard, A.L. IGF-1 as a Drug for Preterm Infants: A Step-Wise Clinical Development. Curr. Pharm. Des. 2017, 23, 5964–5970. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).