Symptomatic Young Adults with ST-Segment Elevation—Acute Coronary Syndrome or Myocarditis: The Three-Factor Diagnostic Model
Abstract
:1. Introduction
2. Materials and Methods
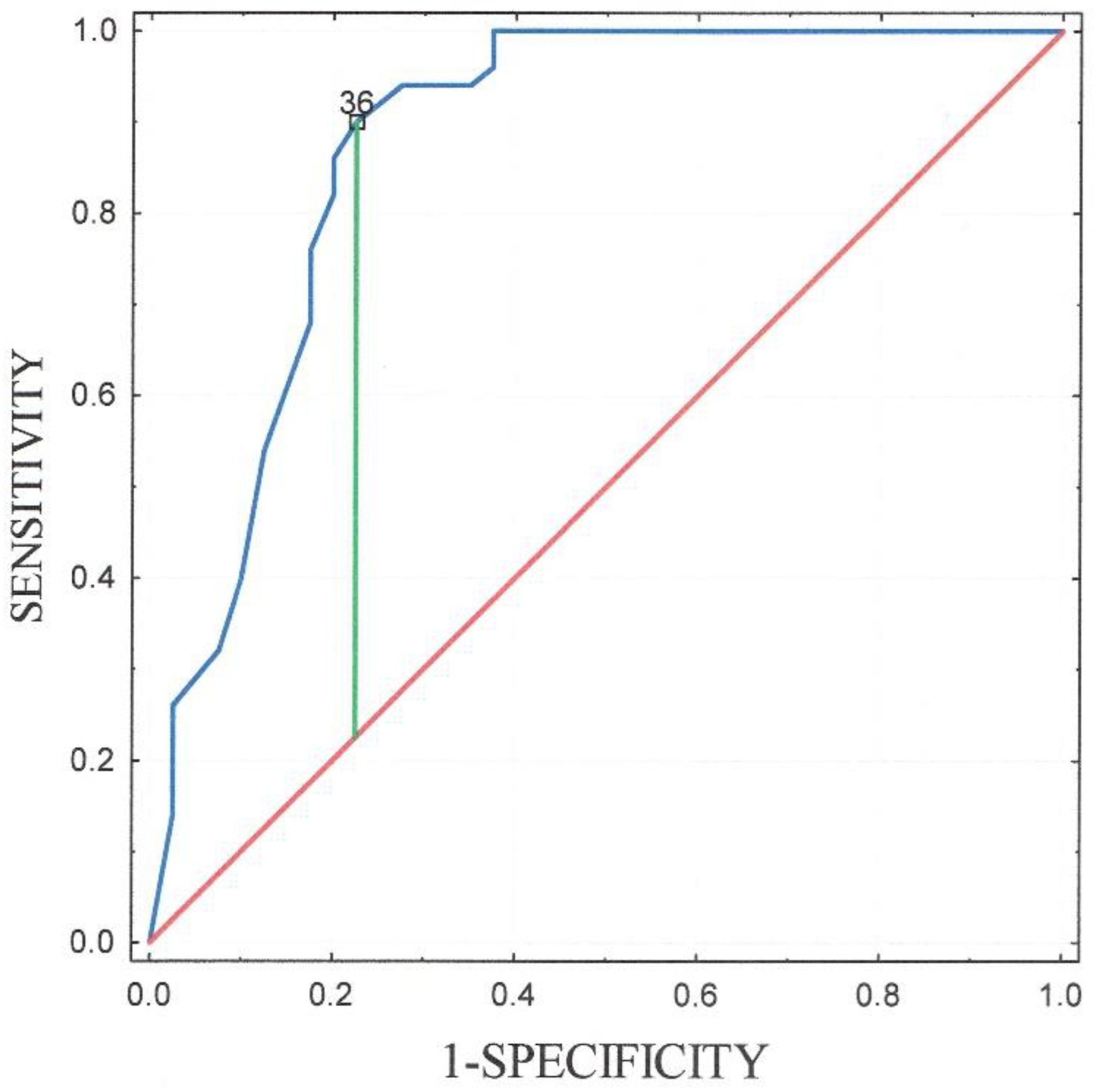
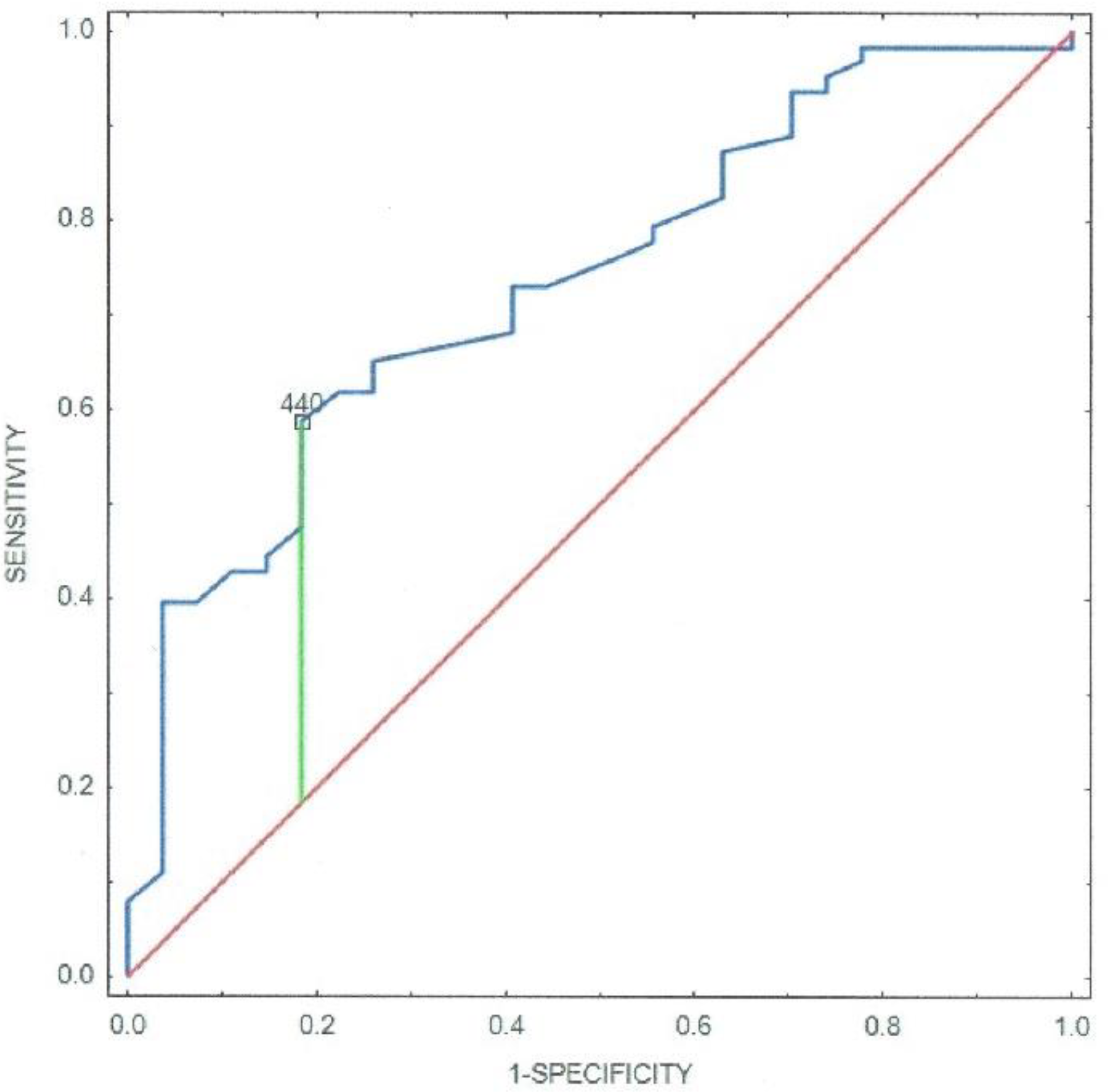
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Miranda, D.F.; Lobo, A.S.; Walsh, B.; Sandoval, Y.; Smith, S.W. New insight into the use of the 12-lead electrocardiogram for diagnosis acute myocardial infarction in the emergency department. Can. J. Cardiol. 2018, 34, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bliek, E.C. ST elevation: Differential diagnosis and caveats. A comprehensive review to help distinguish ST-elevation myocardial infarction from nonischemic etiologies of ST elevation. Turk. J. Emerg. Med. 2018, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stensaeth, K.H.; Fossum, E.; Hoffmann, P.; Mangschau, A.; Klow, N.E. Clinical characteristics and role of early cardiac magnetic resonance imaging in patients with suspected ST-elevation myocardial infarction and normal coronary arteries. Int. J. Cardiovasc. Imaging 2011, 27, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Hausvater, A.; Pasupathy, S.; Tornvall, P.; Gandhi, H.; Tavella, R.; Beltrame, J.; Agewall, S.; Ekenbäck, C.; Brolin, E.B.; Hochman, J.S.; et al. ST-segment elevation and cardiac magnetic resonance imaging findings in myocardial infarction with non-obstructive coronary arteries. Int. J. Cardiol. 2019, 287, 128–131. [Google Scholar] [CrossRef]
- Wu, C.-H.; Liu, P.-Y.; Tsai, T.-N.; Lin, C.-S.; Lin, W.-Y.; Cheng, C.-C.; Lin, W.-S.; Hsu, C.-H.; Liou, J.-T.; Cheng, S.-M.; et al. Clinical and prognostic correlates of ST-elevation myocardial infarction patients with normal coronary angiography. J. Med. Sci. 2015, 35, 135–140. [Google Scholar] [CrossRef]
- Widimsky, P.; Stellova, B.; Groch, L.; Aschermann, M.; Branny, M.; Zelizko, M.; Stasek, J.; Formanek, P. Prevalence of normal coronary angiography in the acute phase of suspected ST-elevation myocardial infarction: Experience from the PRAGUE studies. Can. J. Cardiol. 2006, 22, 1147–1152. [Google Scholar] [CrossRef] [Green Version]
- Pasupathy, S.; Tavella, R.; McRae, S.; Beltrame, J.F.; University of Adelaide; Central Adelaide Local Health Network; Pathology, A.S. Myocardial Infarction with Non-obstructive Coronary Arteries—Diagnosis and Management. Eur. Cardiol. Rev. 2015, 10, 79–82. [Google Scholar] [CrossRef]
- Saricam, E.; Saglam, Y.; Hazirolan, T. Clinical evaluation of myocardial involvement in acute myopericarditis in young adults. BMC Cardiovasc. Disord. 2017, 17, 129. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, Y.; Lin, H.; Wang, S.; Cai, X.; Gao, D. Early characteristics of fulminant myocarditis vs non-fulminant myocarditis. Medicine 2019, 98, e14697. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: Position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Fung, G.; Luo, H.; Qiu, Y.; Yang, D.; McManus, B. Myocarditis. Circ. Res. 2016, 118, 496–514. [Google Scholar] [CrossRef] [PubMed]
- Mosebach, C.M.; Tandon, V.; Kumar, M. Acute Myocarditis Presenting as Acute Coronary Syndrome. Cureus 2019, 11, e5212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costantini, M.; Tritto, C.; Licci, E.; Sticchi, G.; Capone, S.; Montinaro, A.; Bruno, A.; Nuzzaci, G.; Picano, E. Myocarditis with ST-elevation Myocardial Infarction presentation in young man. A case series of 11 patients. Int. J. Cardiol. 2005, 101, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Freixa, X.; Sionis, A.; Castel, A.; Guasch, E.; Loma-Osorio, P.; Arzamendi, D.; Roig, E.; Perez-Villa, F. Low Troponin-I Levels on Admission Are Associated with Worse Prognosis in Patients With Fulminant Myocarditis. Transplant. Proc. 2009, 41, 2234–2236. [Google Scholar] [CrossRef]
- Leitman, M.; Vered, Z.; Tyomkin, V.; Macogon, B.; Moravsky, G.; Peleg, E.; Copel, L. Speckle tracking imaging in inflammatory heart diseases. Int. J. Cardiovasc. Imaging 2018, 34, 787–792. [Google Scholar] [CrossRef]
- Pellaton, C.; Monney, P.; Ludman, A.J.; Schwitter, J.; Eeckhout, E.; Hugli, O.; Muller, O. Clinical features of myocardial infarction and myocarditis in young adults: A retrospective study. BMJ Open 2012, 2, e001571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heidecker, B.; Ruedi, G.; Baltensperger, N.; Gresser, E.; Kottwitz, J.; Berg, J.; Manka, R.; Landmesser, U.; Lüscher, T.F.; Patriki, D.; et al. Systematic use of cardiac magnetic resonance imaging in MINOCA led to a five-fold increase in the detection rate of myocarditis: A retrospective study. Swiss Med. Wkly. 2019, 149, w20098. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Meindl, C.; Paulus, M.; Poschenrieder, F.; Zeman, F.; Maier, L.S.; Debl, K. Patients with acute myocarditis and preserved systolic left ventricular function: Comparison of global and regional longitudinal strain imaging by echocardiography with quantification of late gadolinium enhancement by CMR. Clin. Res. Cardiol. 2021, 110, 1792–1800. [Google Scholar] [CrossRef]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H. European Guidelines for Obesity Management in Adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef]
- Rosselló, X.; Wiegerinck, R.F.; Alguersuari, J.; Bardají, A.; Worner, F.; Sutil, M.; Ferrero, A.; Cinca, J.; Sutil-Vega, M. New Electrocardiographic Criteria to Differentiate Acute Pericarditis and Myocardial Infarction. Am. J. Med. 2014, 127, 233–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meijboom, W.B.; van Mieghem, C.A.G.; Mollet, N.R.; Pugliese, F.; Weustink, A.C.; van Pelt, N.; Cademartiri, F.; Nieman, K.; Boersma, E.; de Jaegere, P.; et al. 64-slice computed tomography coronary angiography in patients with high, intermediate, or low pretest probability of significant coronary artery disease. J. Am. Coll. Cardiol. 2007, 50, 1469–1475. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, U.; Bamberg, F.; Chae, C.U.; Nichols, J.H.; Rogers, I.S.; Seneviratne, S.K.; Truong, Q.A.; Cury, R.C.; Abbara, S.; Shapiro, M.D.; et al. Coronary Computed Tomography Angiography for Early Triage of Patients With Acute Chest Pain: The ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) Trial. J. Am. Coll. Cardiol. 2009, 53, 1642–1650. [Google Scholar] [CrossRef] [Green Version]
- Scully, P.R.; Bastarrika, G.; Moon, J.C.; Treibel, T. Myocardial Extracellular Volume Quantification by Cardiovascular Magnetic Resonance and Computed Tomography. Curr. Cardiol. Rep. 2018, 20, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmisano, A.; Vignale, D.; Tadic, M.; Moroni, F.; De Stefano, D.; Gatti, M.; Boccia, E.; Faletti, R.; Oppizzi, M.; Peretto, G.; et al. Myocardial Late Contrast Enhancement CT in Troponin-Positive Acute Chest Pain Syndrome. Radiology 2021, 7, 211288. [Google Scholar] [CrossRef]
- Hahn, L.; Kligerman, S. Cardiac MRI Evaluation of myocarditis. Curr. Treat. Options Cardiovasc. Med. 2019, 21, 69. [Google Scholar] [CrossRef]
- Ferreira, V.; Piechnik, S.K.; Dall’Armellina, E.; Karamitsos, T.; Francis, J.M.; Ntusi, N.; Holloway, C.J.; Choudhury, R.P.; Kardos, A.; Robson, M.D.; et al. T1 Mapping for the Diagnosis of Acute Myocarditis Using CMR. JACC Cardiovasc. Imaging 2013, 6, 1048–1058. [Google Scholar] [CrossRef] [Green Version]
- Leiner, T.; Bogaert, J.; Friedrich, M.G.; Mohiaddin, R.; Muthurangu, V.; Myerson, S.; Powell, A.J.; Raman, S.V.; Pennell, D.J. SCMR Position Paper (2020) on clinical indications for cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2020, 22, 76. [Google Scholar] [CrossRef]
- Ammirati, E.; Cipriani, M.; Moro, C.; Raineri, C.; Pini, D.; Sormani, P.; Mantovani, R.; Varrenti, M.; Pedrotti, P.; Conca, C.; et al. Clinical Presentation and Outcome in a Contemporary Cohort of Patients with Acute Myocarditis. Circulation 2018, 138, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Barfuss, S.B.; Butts, R.; Knecht, K.R.; Prada-Ruiz, A.; Lal, A.K. Outcomes of Myocarditis in Patients with Normal Left Ventricular Systolic Function on Admission. Pediatr. Cardiol. 2019, 40, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Kanda, T.; Hasegawa, A.; Suzuki, T.; Kobayashi, I.; Nagai, R. C-reactive protein as a prognostic marker in lympphicytic myocarditis. Jpn. Heart J. 2000, 41, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauer, B.; Niederau, C.; Kühl, U.; Schannwell, M.; Pauschinger, M.; Strauer, B.-E.; Schultheiss, H.-P. Cardiac Troponin T in Patients with Clinically Suspected Myocarditis. J. Am. Coll. Cardiol. 1997, 30, 1354–1359. [Google Scholar] [CrossRef] [Green Version]
- Lauer, B.; Niederau, C.; Kühl, U.; Schannwell, M.; Pauschinger, M.; Strauer, B.E.; Schultheiss, H.P. Cardiac troponin T in the diagnosis and follow up of suspected myocarditis. Deutsche Medizinische Wochenschrift 1998, 123, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhao, R.C.; Gao, W.H.; Ciu, H.B. A risk predictor model for in-hospital mortality in patients with suspected myocarditis. Chin. Med. J. 2017, 130, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hoss, S.; Zeniou, V.; Shauer, A.; Admon, D.; Zwas, D.R.; Lotan, C.; Keren, A.; Gotsman, I. Electrocardiographic Predictors of Morbidity and Mortality in Patients with Acute Myocarditis: The Importance of QRS-T Angle. J. Card. Fail. 2018, 24, 3–8. [Google Scholar] [CrossRef]
- Fischer, K.; Marggraf, M.; Stark, A.W.; Kaneko, K.; Aghayev, A.; Guensch, D.P.; Huber, A.T.; Steigner, M.; Blankstein, R.; Reichlin, T.; et al. Association of ECG parameters with late gadolinium enhancement and outcome in patients with clinical suspicion of acute or subacute myocarditis referred for CMR imaging. PLoS ONE 2020, 15, e0227134. [Google Scholar] [CrossRef]
- Zlotoff, D.A.; Hassan, M.Z.O.; Zafar, A.; Alvi, R.M.; Awadalla, M.; Mahmood, S.S.; Zhang, L.; Chen, C.L.; Ederhy, S.; Barac, A.; et al. Electrocardiographic features of immune checkpoint inhibitor associated myocarditis. J. Immunother. Cancer 2021, 9, e002007. [Google Scholar] [CrossRef]

| Myocarditis N = 40 | |
|---|---|
| Ejection fraction (%) | 57 (52–59) |
| LV segments with LGE | 4 (4–6) |
| LV segments with oedema | 2 (1–2) |
| Regional increase of T2 signal intensity * | 40 (100%) |
| Global T2 signal intensity ratio ≥ 2 * | 0 |
| LGE non-ischeamic pattern * | 40 (100%) |
| Pericardial effusion/pericardial abnormalities | 0 |
| Myocarditis N = 40 | Myocardial Infarction N = 50 | p Value | |
|---|---|---|---|
| Age | 26 (21.5–34.5) | 41 (39–44) | <0.001 |
| Sex (male) | 37 (92.5%) | 39 (78%) | 0.593 |
| Smoking | 16 (40%) | 37 (74%) | <0.001 |
| Hypertension | 4 (10%) | 22 (44%) | <0.001 |
| Diabetes | 2 (5%) | 4 (8%) | 0.592 |
| Obesity BMI > 30 (kg/m2) | 5 (12.5%) | 18 (36%) | 0.011 |
| Family history of CAD | 32 (80%) | 36 (72%) | 0.331 |
| Symptoms | |||
| Chest pain | 38 (95%) | 47 (94%) | 0.837 |
| Dyspnea | 3 (7.5%) | 39 (78%) | 0.059 |
| Fever * | 14 (35%) | 1 (2%) | <0.001 |
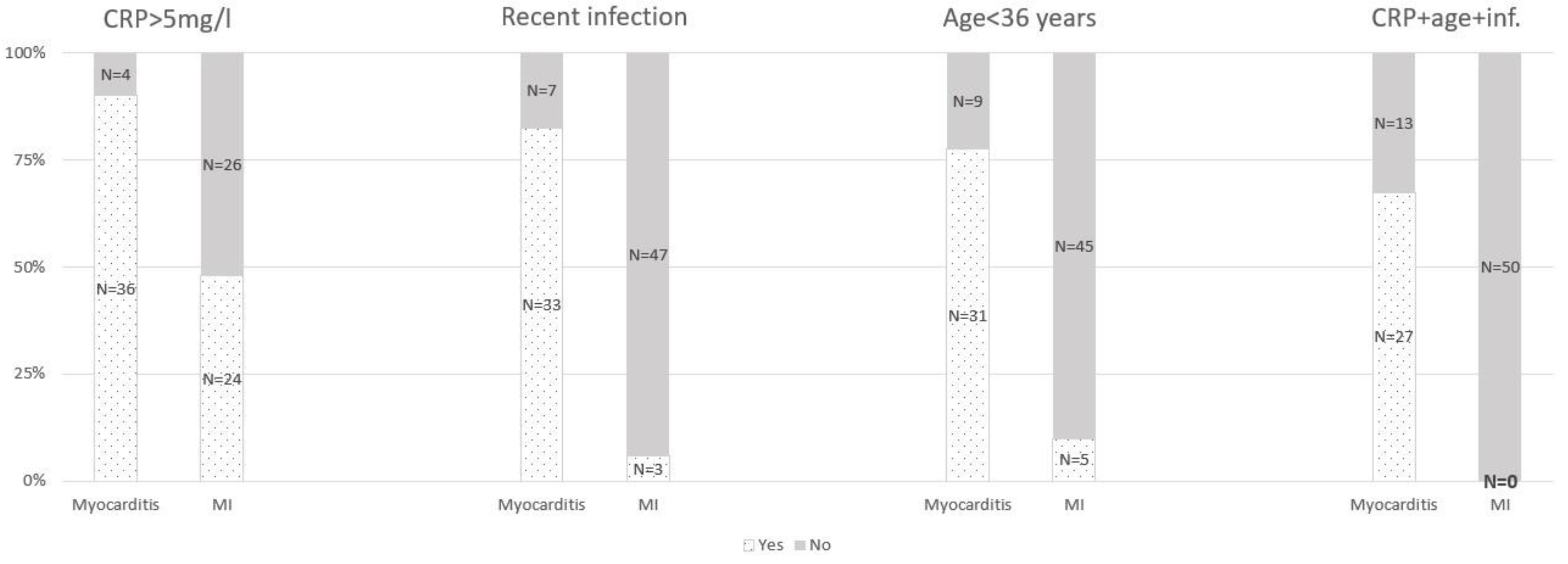
| Recent infection * | 33 (82.5%) | 3 (6%) | <0.001 |
| Antibiotic therapy * | 9 (22.5%) | 1 (2%) | 0.002 |
| Left ventricular ejection fraction (%) | 58 (53–60) | 50 (45–55) | <0.001 |
| Regional wall motion abnormality | 19 (47.5%) | 44 (88%) | <0.001 |
| Hospital mortality | 0 | 3 (6%) | 0.115 |
| Laboratory findings | |||
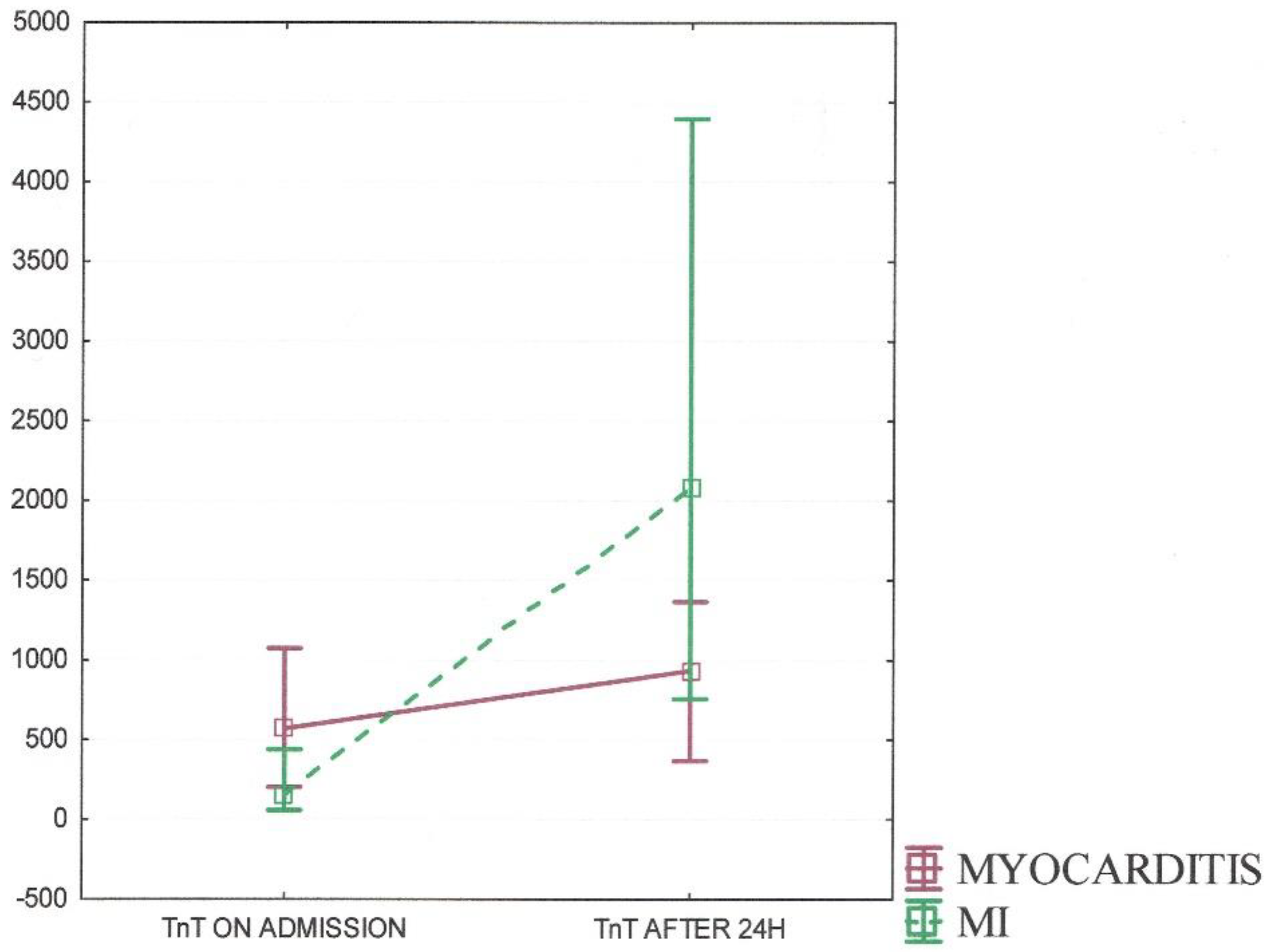
| TnT (on admission) (ng/L) | 569.5 (200–1074) | 150 (55–437) | 0.025 |
| TnT (after 24 h) (ng/L) | 936 (367–1364) | 2088.5 (757–4394) | <0.001 |
| CRP (on admission) (mg/L) | 45.9 (17.2–121) | 3.4 (1.8–9.8) | <0.001 |
| CRP (max.) (mg/L) | 47.9 (17.8–122.3) | 15.9 (2.2–89.7) | 0.014 |
| Total cholesterol (TC) (mmol/L) | 3.89 (3.6–4.66) | 5.95 (4.84–6.79) | <0.001 |
| LDL | 2.4 (1.83–2.86) | 3.44 (2.69–4.55) | <0.001 |
| HDL | 1.1 (0.87–1.29) | 1.1 (0.96–1.39) | 0.310 |
| Triglycerides (TG) | 1.19 (0.73–1.6) | 1.815 (1.34–2.59) | <0.001 |
| ECG parameters | |||
| QTc (ms) | 413 (319–561) | 452 (366–621) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieczorkiewicz, P.; Przybylak, K.; Supel, K.; Kidawa, M.; Zielinska, M. Symptomatic Young Adults with ST-Segment Elevation—Acute Coronary Syndrome or Myocarditis: The Three-Factor Diagnostic Model. J. Clin. Med. 2022, 11, 916. https://doi.org/10.3390/jcm11040916
Wieczorkiewicz P, Przybylak K, Supel K, Kidawa M, Zielinska M. Symptomatic Young Adults with ST-Segment Elevation—Acute Coronary Syndrome or Myocarditis: The Three-Factor Diagnostic Model. Journal of Clinical Medicine. 2022; 11(4):916. https://doi.org/10.3390/jcm11040916
Chicago/Turabian StyleWieczorkiewicz, Paulina, Katarzyna Przybylak, Karolina Supel, Michal Kidawa, and Marzenna Zielinska. 2022. "Symptomatic Young Adults with ST-Segment Elevation—Acute Coronary Syndrome or Myocarditis: The Three-Factor Diagnostic Model" Journal of Clinical Medicine 11, no. 4: 916. https://doi.org/10.3390/jcm11040916
APA StyleWieczorkiewicz, P., Przybylak, K., Supel, K., Kidawa, M., & Zielinska, M. (2022). Symptomatic Young Adults with ST-Segment Elevation—Acute Coronary Syndrome or Myocarditis: The Three-Factor Diagnostic Model. Journal of Clinical Medicine, 11(4), 916. https://doi.org/10.3390/jcm11040916






