Development of a Cryopreservation Technique for Xenogeneic Kidney Grafts: Evaluation Using a Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Transplanted Cells
2.3. Cryopreservation
2.3.1. Renal Progenitor Cell Cryopreservation
2.3.2. Fetal Kidney Tissue Cryopreservation
2.4. Cell Injection
2.5. Organ Culture
2.6. Transplantation of Metanephros
2.7. Whole-Mount Immunostaining
2.8. Hematoxylin-and-Eosin Staining and Immunostaining of Frozen Sections
2.9. Cap Mesenchyme Counting
3. Results
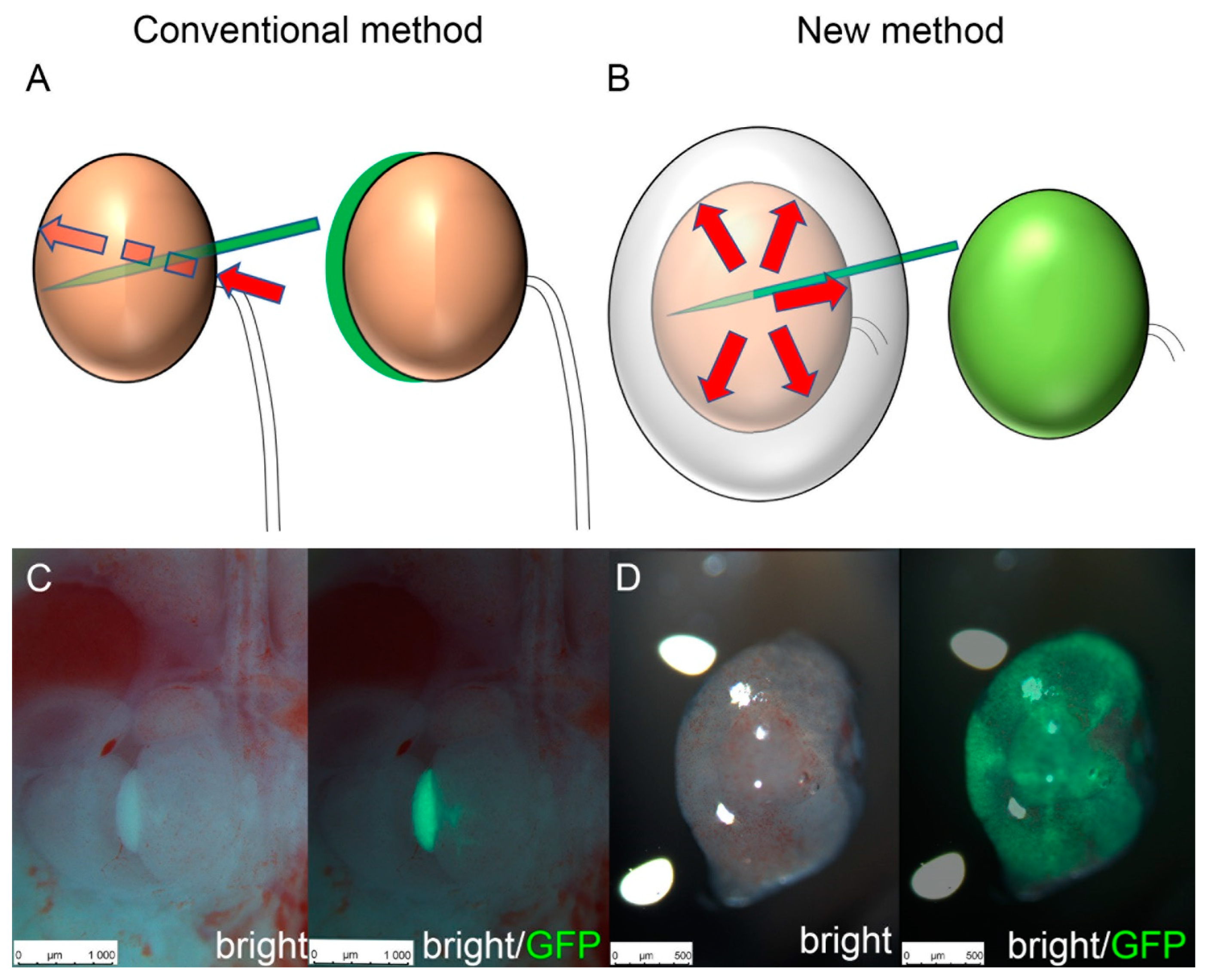
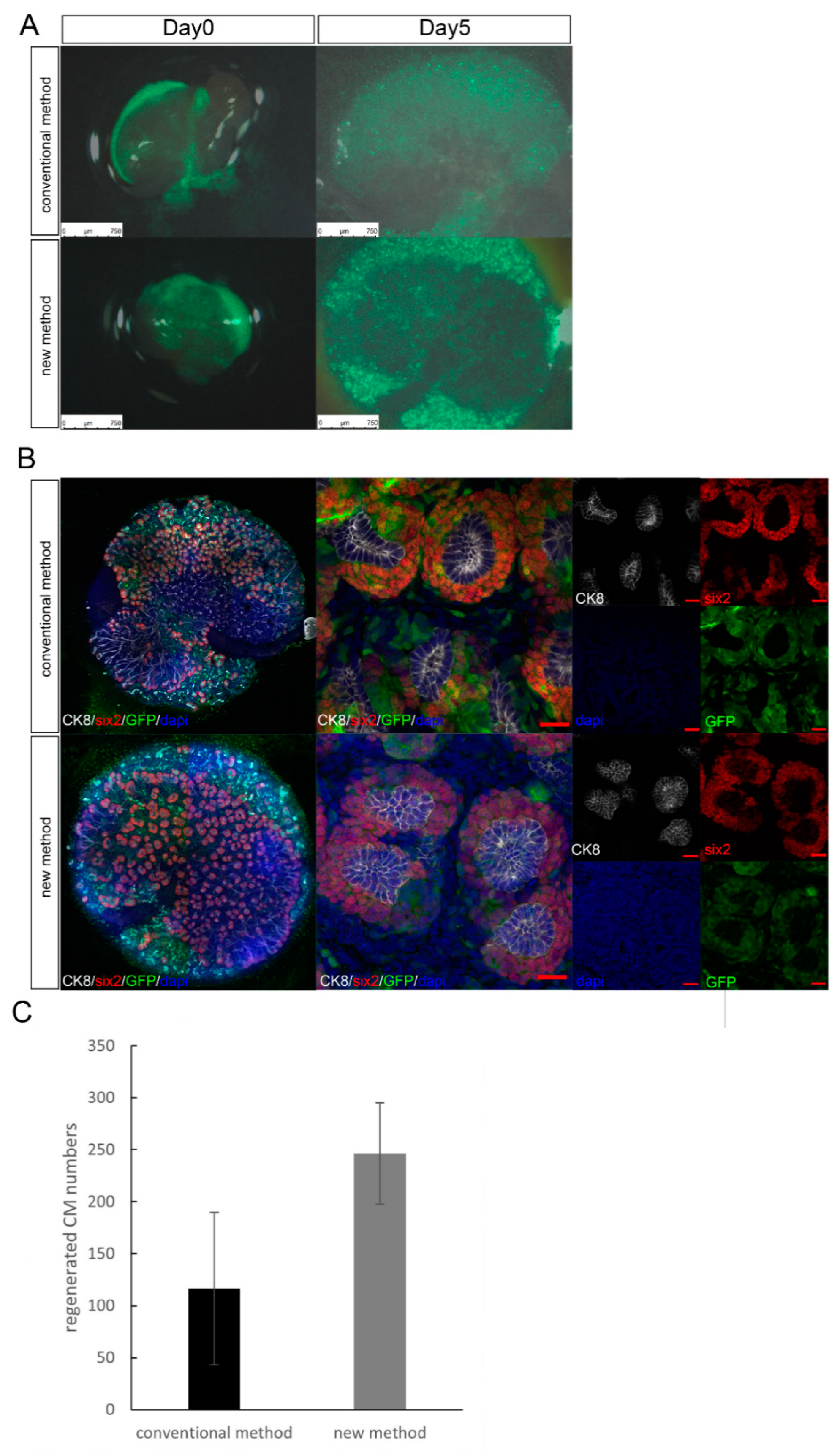
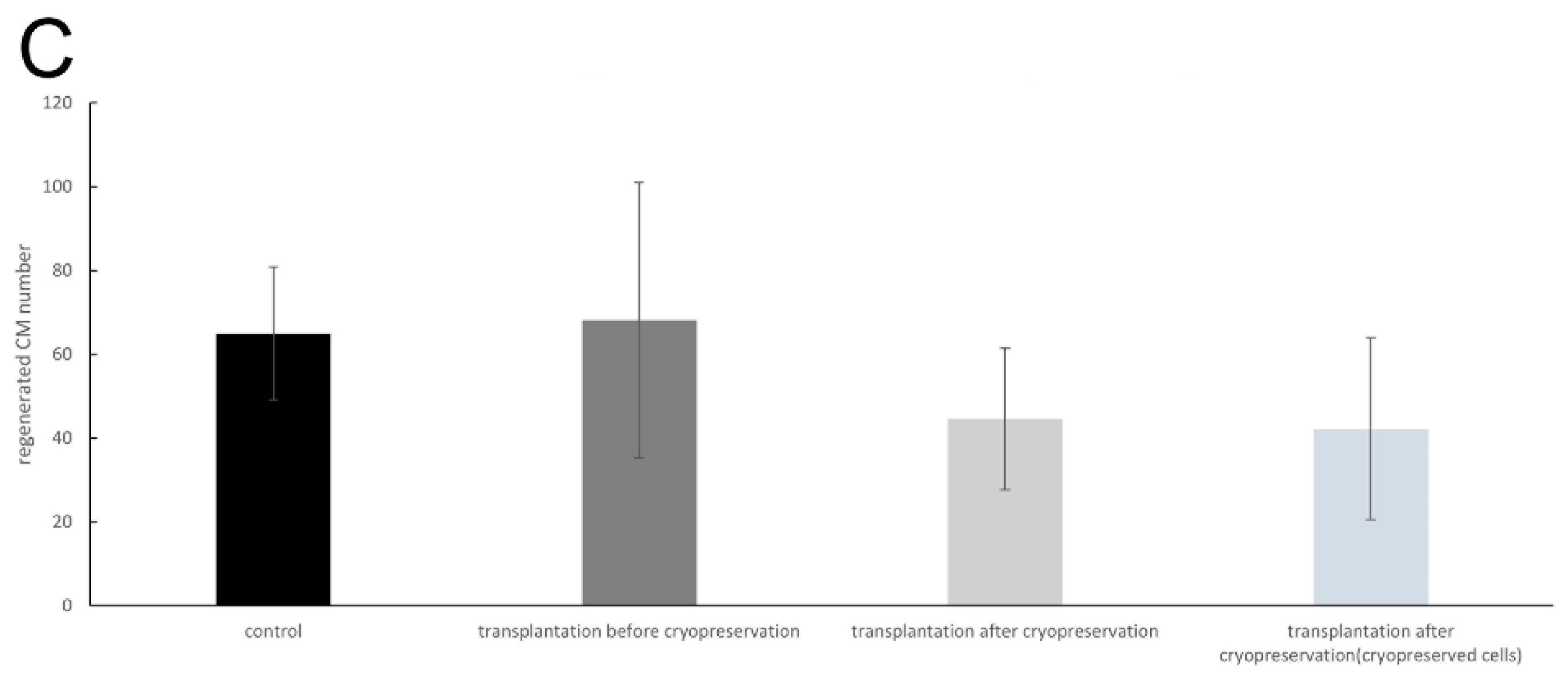
3.1. Experiment 1: Comparing the Transplantation Efficiency Using Different Cell Transplantation Methods
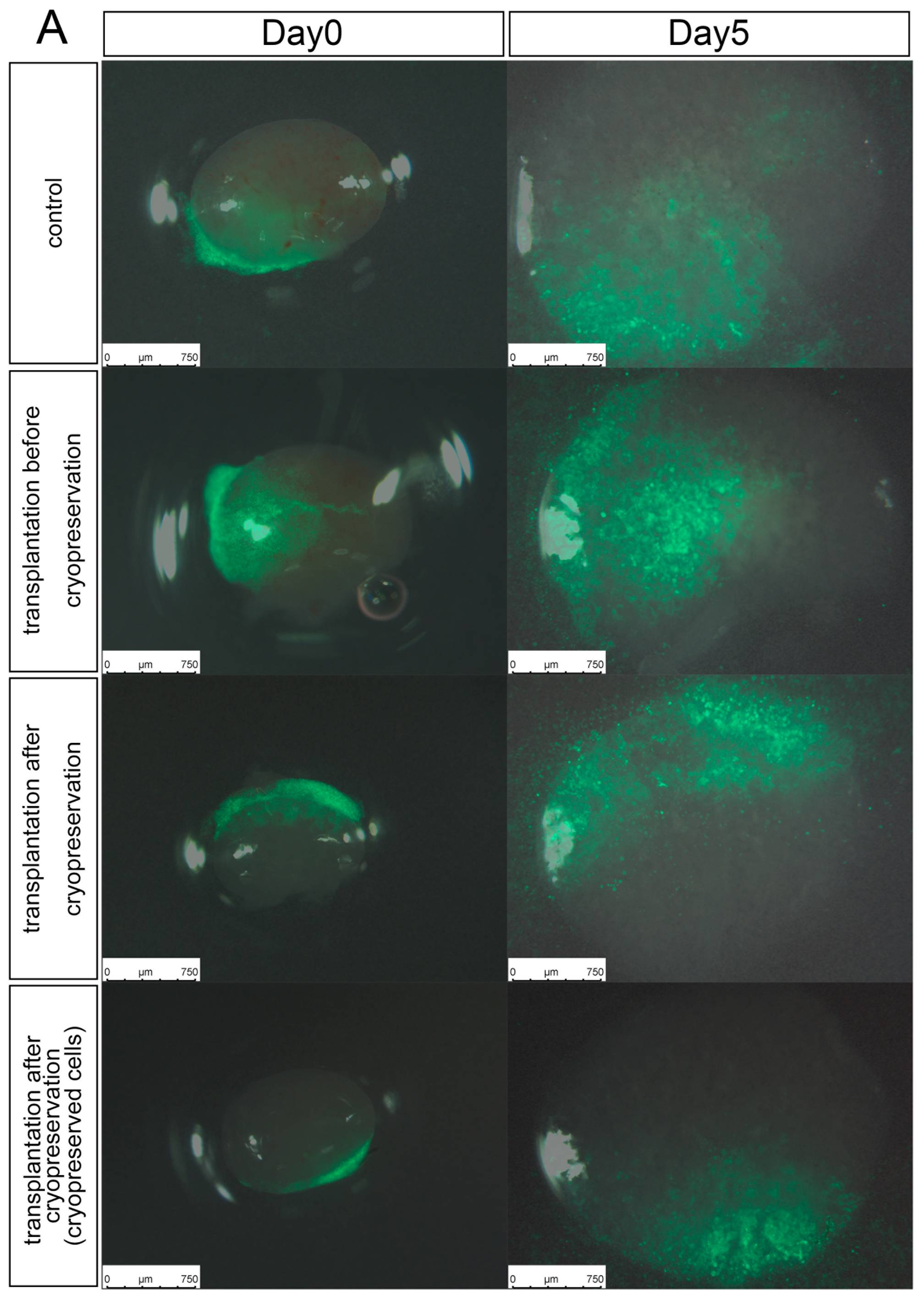
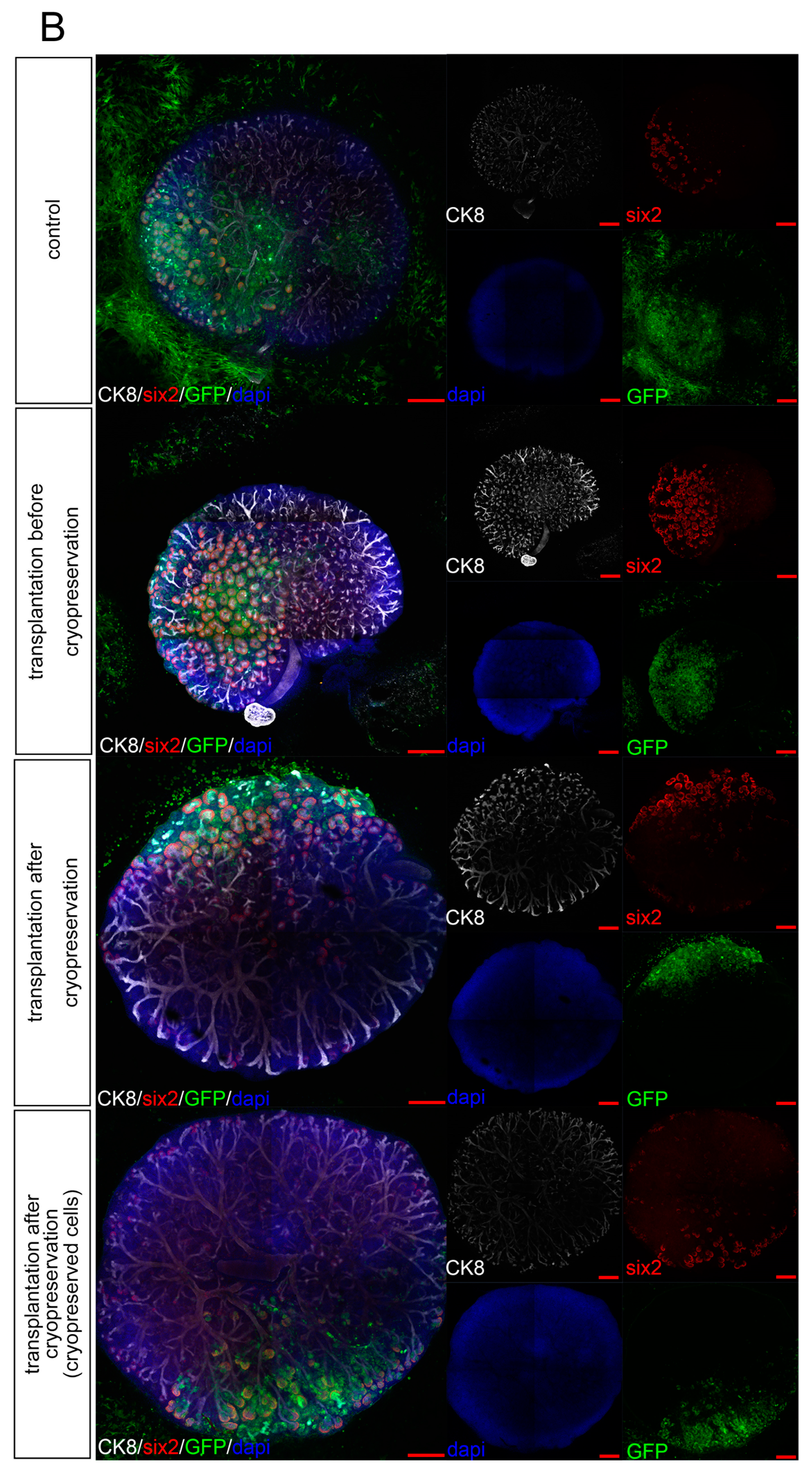
3.2. Experiment 2: Comparing the Transplantation Efficiency Using Different Cryopreserving Timings
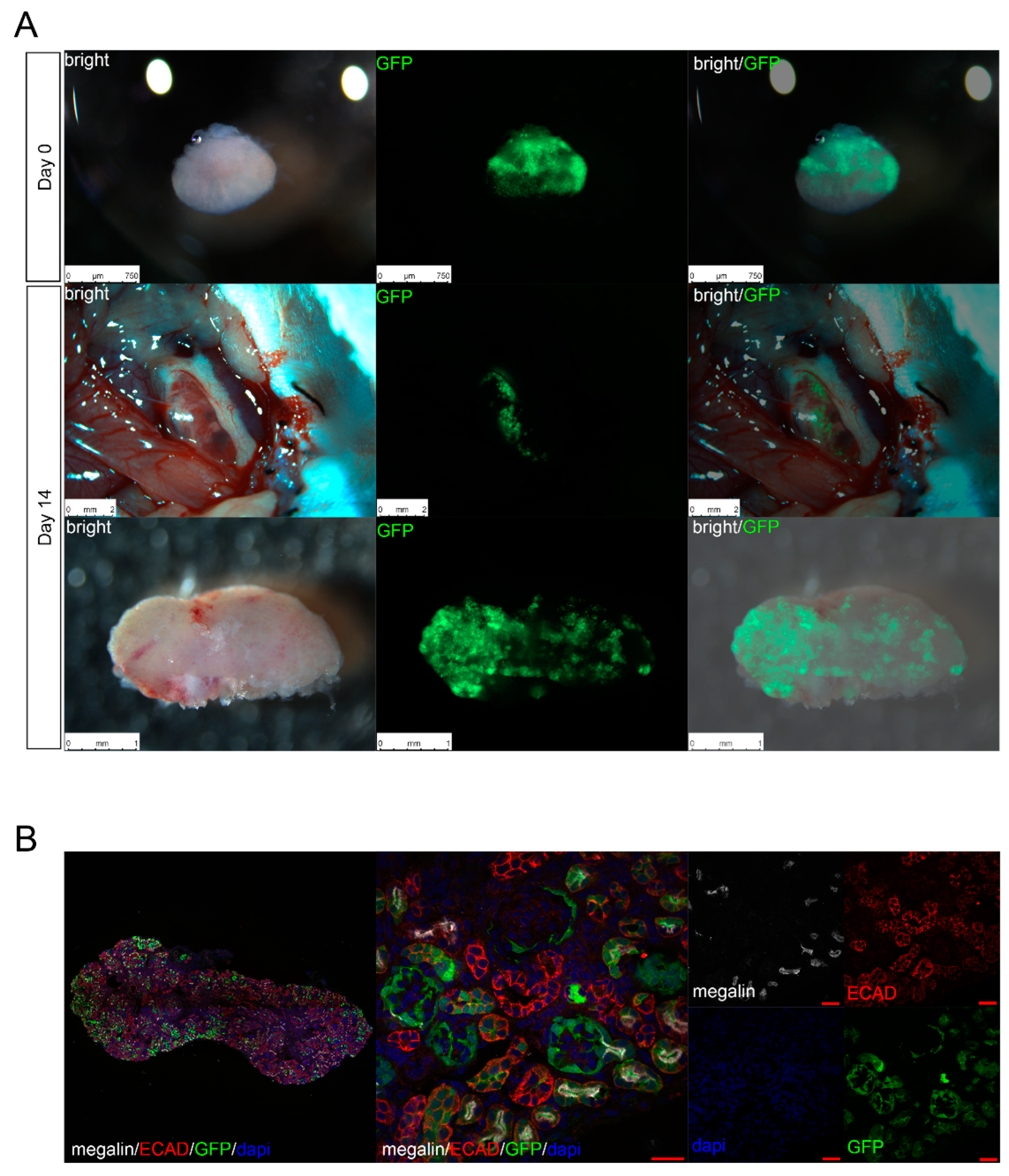
3.3. Experiment 3: In Vivo Function of Cryopreserved Cell-Implanted Metanephroi
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; van der Velde, M.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Chronic Kidney Disease Prognosis Consortium. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011, 79, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Nichols, G.A.; Ustyugova, A.; Déruaz-Luyet, A.; O’Keeffe-Rosetti, M.; Brodovicz, K.G. Health care costs by type of expenditure across eGFR stages among patients with and without diabetes, cardiovascular disease, and heart failure. J. Am. Soc. Nephrol. 2020, 31, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- de Vries, E.F.; Los, J.; de Wit, G.A.; Hakkaart-van Roijen, L. Patient, family and productivity costs of end-stage renal disease in the Netherlands; exposing non-healthcare related costs. BMC Nephrol. 2021, 22, 341. [Google Scholar] [CrossRef] [PubMed]
- Porrett, P.M.; Orandi, B.J.; Kumar, V.; Houp, J.; Anderson, D.; Cozette Killian, A.; Hauptfeld-Dolejsek, V.; Martin, D.E.; Macedon, S.; Budd, N.; et al. First clinical-grade porcine kidney xenotransplant using a human decedent model. Am. J. Transplant. 2022, 22, 1037–1053. [Google Scholar] [CrossRef]
- Montgomery, R.A.; Stern, J.M.; Lonze, B.E.; Tatapudi, V.S.; Mangiola, M.; Wu, M.; Weldon, E.; Lawson, N.; Deterville, C.; Dieter, R.A.; et al. Results of two cases of pig-to-human kidney xenotransplantation. N. Engl. J. Med. 2022, 386, 1889–1898. [Google Scholar] [CrossRef]
- Yokoo, T.; Yamanaka, S.; Kobayashi, E. Xeno-regenerative medicine: A novel concept for donor kidney fabrication. Xenotransplantation 2020, 27, e12622. [Google Scholar] [CrossRef] [PubMed]
- Takamura, T.; Matsui, K.; Matsumoto, N.; Saito, Y.; Fujimoto, T.; Tajiri, S.; Yamanaka, S.; Matsumoto, K.; Kobayashi, A.; Yamamoto, I.; et al. In vivo development of fetal pig kidneys in mature monkeys under clinically approved immunosuppressant drugs. Engineering 2022, 10, 65–73. [Google Scholar] [CrossRef]
- Saito, Y.; Matsumoto, N.; Yamanaka, S.; Yokoo, T.; Kobayashi, E. Beneficial impact of interspecies chimeric renal organoids against a xenogeneic immune response. Front. Immunol. 2022, 13, 848433. [Google Scholar] [CrossRef] [PubMed]
- Yokoo, T.; Ohashi, T.; Shen, J.S.; Sakurai, K.; Miyazaki, Y.; Utsunomiya, Y.; Takahashi, M.; Terada, Y.; Eto, Y.; Kawamura, T.; et al. Human mesenchymal stem cells in rodent whole-embryo culture are reprogrammed to contribute to kidney tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 3296–3300. [Google Scholar] [CrossRef]
- Yokoo, T.; Fukui, A.; Matsumoto, K.; Ohashi, T.; Sado, Y.; Suzuki, H.; Kawamura, T.; Okabe, M.; Hosoya, T.; Kobayashi, E. Generation of a transplantable erythropoietin-producer derived from human mesenchymal stem cells. Transplantation 2008, 85, 1654–1658. [Google Scholar] [CrossRef]
- Yamanaka, S.; Tajiri, S.; Fujimoto, T.; Matsumoto, K.; Fukunaga, S.; Kim, B.S.; Okano, H.J.; Yokoo, T. Generation of interspecies limited chimeric nephrons using a conditional nephron progenitor cell replacement system. Nat. Commun. 2017, 8, 1719. [Google Scholar] [CrossRef]
- Fujimoto, T.; Yamanaka, S.; Tajiri, S.; Takamura, T.; Saito, Y.; Matsumoto, N.; Matsumoto, K.; Tachibana, T.; Okano, H.J.; Yokoo, T. Generation of human renal vesicles in mouse organ niche using nephron progenitor cell replacement system. Cell Rep. 2020, 32, 108130. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Yamanaka, S.; Matsumoto, N.; Takamura, T.; Fujimoto, T.; Matsui, K.; Tajiri, S.; Matsumoto, K.; Kobayashi, E.; Yokoo, T. Generation of functional chimeric kidney containing exogenous progenitor-derived stroma and nephron via a conditional empty niche. Cell Rep. 2022, 39, 110933. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, A.; Kaku, Y.; Ohmori, T.; Sharmin, S.; Ogawa, M.; Sasaki, H.; Nishinakamura, R. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 2014, 14, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Morizane, R.; Lam, A.Q.; Freedman, B.S.; Kishi, S.; Valerius, M.T.; Bonventre, J.V. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol. 2015, 33, 1193–1200. [Google Scholar] [CrossRef]
- Takasato, M.; Er, P.X.; Chiu, H.S.; Maier, B.; Baillie, G.J.; Ferguson, C.; Parton, R.G.; Wolvetang, E.J.; Roost, M.S.; Chuva de Sousa Lopes, S.M.; et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 2015, 526, 564–568. [Google Scholar] [CrossRef]
- Matsumoto, K.; Yokoo, T.; Matsunari, H.; Iwai, S.; Yokote, S.; Teratani, T.; Gheisari, Y.; Tsuji, O.; Okano, H.; Utsunomiya, Y.; et al. Xenotransplanted embryonic kidney provides a niche for endogenous mesenchymal stem cell differentiation into erythropoietin-producing tissue. Stem Cells 2012, 30, 1228–1235. [Google Scholar] [CrossRef]
- Yamanaka, S.; Matsui, K.; Fujimoto, T.; Takamura, T.; Saito, Y.; Matsumoto, N.; Tajiri, S.; Matsumoto, K.; Yokoo, T. In vivo regeneration of neo-nephrons in rodents by renal progenitor cell transplantation. Star Protoc. 2021, 2, 100314. [Google Scholar] [CrossRef]
- Matsumoto, N.; Matsui, K.; Saitou, Y.; Takamura, T.; Yamanaka, S.; Yokoo, T.; Kobayashi, E. Techniques of fragile renal organoids transplantation in mice. Acta Cirúrgica Bras. 2021, 36, e361102. [Google Scholar] [CrossRef]
- Davies, J.A.; Unbekandt, M.; Ineson, J.; Lusis, M.; Little, M.H. Dissociation of embryonic kidney followed by re-aggregation as a method for chimeric analysis. Methods Mol. Biol. 2012, 886, 135–146. [Google Scholar] [CrossRef]
- Nakagawa, M.; Taniguchi, Y.; Senda, S.; Takizawa, N.; Ichisaka, T.; Asano, K.; Morizane, A.; Doi, D.; Takahashi, J.; Nishizawa, M.; et al. A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci. Rep. 2014, 4, 3594. [Google Scholar] [CrossRef]
- Kagawa, N.; Silber, S.; Kuwayama, M. Successful vitrification of bovine and human ovarian tissue. Reprod. Biomed. Online 2009, 18, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.H.; Park, S.C.; Yang, J.H.; Kim, J.Y.; Seok, J.H.; Park, U.S.; Choi, C.W.; Lee, S.R.; Han, J. Cryopreservation and its clinical applications. Integr. Med. Res. 2017, 6, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Yavin, S.; Arav, A. Measurement of essential physical properties of vitrification solutions. Theriogenology 2007, 67, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S.; Saito, Y.; Fujimoto, T.; Takamura, T.; Tajiri, S.; Matsumoto, K.; Yokoo, T. Kidney regeneration in later-stage mouse embryos via transplanted renal progenitor cells. J. Am. Soc. Nephrol. 2019, 30, 2293–2305. [Google Scholar] [CrossRef]
- Yokote, S.; Matsunari, H.; Iwai, S.; Yamanaka, S.; Uchikura, A.; Fujimoto, E.; Matsumoto, K.; Nagashima, H.; Kobayashi, E.; Yokoo, T. Urine excretion strategy for stem cell-generated embryonic kidneys. Proc. Natl. Acad. Sci. USA 2015, 112, 12980–12985. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takamura, T.; Nagashima, H.; Matsunari, H.; Yamanaka, S.; Saito, Y.; Kinoshita, Y.; Fujimoto, T.; Matsumoto, K.; Nakano, K.; Okano, H.J.; et al. Development of a Cryopreservation Technique for Xenogeneic Kidney Grafts: Evaluation Using a Mouse Model. J. Clin. Med. 2022, 11, 7237. https://doi.org/10.3390/jcm11237237
Takamura T, Nagashima H, Matsunari H, Yamanaka S, Saito Y, Kinoshita Y, Fujimoto T, Matsumoto K, Nakano K, Okano HJ, et al. Development of a Cryopreservation Technique for Xenogeneic Kidney Grafts: Evaluation Using a Mouse Model. Journal of Clinical Medicine. 2022; 11(23):7237. https://doi.org/10.3390/jcm11237237
Chicago/Turabian StyleTakamura, Tsuyoshi, Hiroshi Nagashima, Hitomi Matsunari, Shuichiro Yamanaka, Yatsumu Saito, Yoshitaka Kinoshita, Toshinari Fujimoto, Kei Matsumoto, Kazuaki Nakano, Hirotaka James Okano, and et al. 2022. "Development of a Cryopreservation Technique for Xenogeneic Kidney Grafts: Evaluation Using a Mouse Model" Journal of Clinical Medicine 11, no. 23: 7237. https://doi.org/10.3390/jcm11237237
APA StyleTakamura, T., Nagashima, H., Matsunari, H., Yamanaka, S., Saito, Y., Kinoshita, Y., Fujimoto, T., Matsumoto, K., Nakano, K., Okano, H. J., Kobayashi, E., & Yokoo, T. (2022). Development of a Cryopreservation Technique for Xenogeneic Kidney Grafts: Evaluation Using a Mouse Model. Journal of Clinical Medicine, 11(23), 7237. https://doi.org/10.3390/jcm11237237




