SARS-CoV-2 Infection and Outcomes in Children with Inflammatory Bowel Diseases: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy—Databases
2.2. Selection Criteria
2.3. Study Selection and Data Extraction
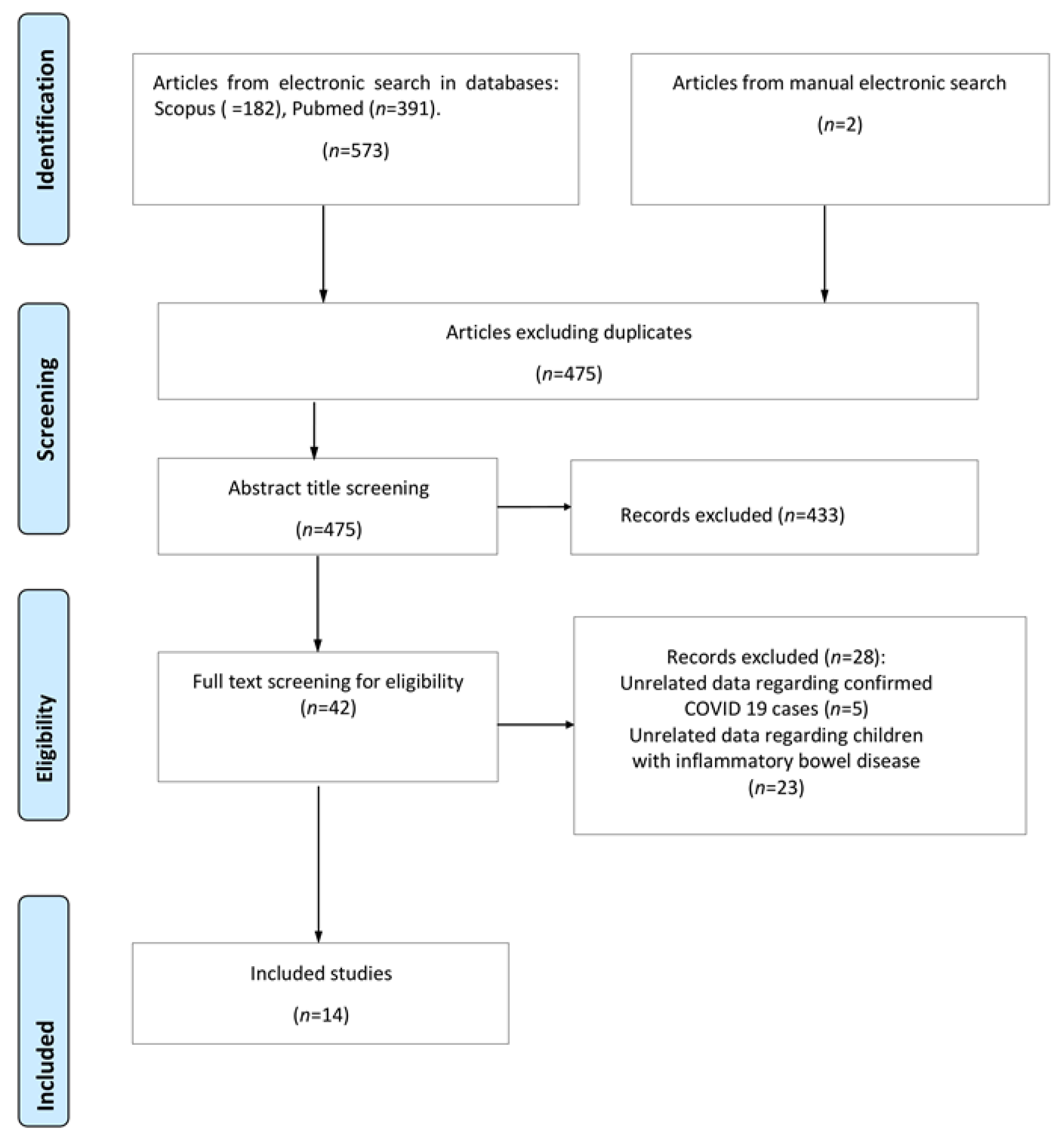
3. Results
3.1. Study Characteristics
3.2. IBD Disease and COVID-19
3.3. IBD Disease Activity and COVID-19
3.4. IBD Medication and COVID-19
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 27 October 2022).
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Rinaldi, E.; Zusi, C.; Beatrice, G.; Saccomani, M.D.; Dalbeni, A. Coronavirus disease 2019 (COVID-19) in children and/or adolescents: A meta-analysis. Pediatr. Res. 2021, 89, 733–737. [Google Scholar] [CrossRef]
- RCPCH. Guidance: Paediatric Multisystem Inflammatory Syndrome Temporally Associated with COVID-19. Available online: https://www.rcpch.ac.uk/resources/paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims-guidance (accessed on 13 October 2022).
- Conrad, M.A.; Rosh, J.R. Pediatric Inflammatory Bowel Disease. Pediatr. Clin. N. Am. 2017, 64, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.J.; Dhawan, A.; Saeed, S.A. Inflammatory Bowel Disease in Children and Adolescents. JAMA Pediatr. 2015, 169, 1053–1060. [Google Scholar] [CrossRef]
- Ashton, J.J.; Barakat, F.M.; Barnes, C.; Coelho, T.A.F.; Batra, A.; Afzal, N.A.; Beattie, R.M. Incidence and Prevalence of Paediatric Inflammatory Bowel Disease Continues to Increase in the South of England. J. Pediatr. Gastroenterol. Nutr. 2022, 75, e20–e24. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Nguyen, H.; Wyatt, A.; Ihekweazu, F.; Vartabedian, B.S.; Karam, L.; Walsh, S.; Kellermayer, R. High Seroconversion Rate Against Severe Acute Respiratory Syndrome Coronavirus 2 in Symptomatic Pediatric Inflammatory Bowel Disease Patients. J. Pediatr. Gastroenterol. Nutr. 2021, 73, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Hisamatsu, T. Management of inflammatory bowel disease during the COVID-19 pandemic. Immunol. Med. 2022, 45, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.A.; Clarke, M.; Rovers, M.; Riley, R.D.; Simmonds, M.; Stewart, G.; Tierney, J.F. Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: The PRISMA-IPD Statement. Jama 2015, 313, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, S.; Alvisi, P.; Banzato, C.; Bramuzzo, M.; Celano, R.; Civitelli, F.; D′Arcangelo, G.; Dilillo, A.; Dipasquale, V.; Felici, E.; et al. Impact of COVID-19 pandemic on the management of paediatric inflammatory bowel disease: An Italian multicentre study on behalf of the SIGENP IBD Group. Dig. Liver Dis. 2021, 53, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Brenner, E.J.; Pigneur, B.; Focht, G.; Zhang, X.; Ungaro, R.C.; Colombel, J.F.; Turner, D.; Kappelman, M.D.; Ruemmele, F.M. Benign Evolution of SARS-Cov2 Infections in Children With Inflammatory Bowel Disease: Results From Two International Databases. Clin. Gastroenterol. Hepatol. 2021, 19, 394–396.e395. [Google Scholar] [CrossRef] [PubMed]
- Brenner, E.J.; Ungaro, R.C.; Gearry, R.B.; Kaplan, G.G.; Kissous-Hunt, M.; Lewis, J.D.; Ng, S.C.; Rahier, J.F.; Reinisch, W.; Ruemmele, F.M.; et al. Corticosteroids, But Not TNF Antagonists, Are Associated With Adverse COVID-19 Outcomes in Patients With Inflammatory Bowel Diseases: Results From an International Registry. Gastroenterology 2020, 159, 481–491.e483. [Google Scholar] [CrossRef] [PubMed]
- Bosa, L.; Di Chiara, C.; Gaio, P.; Cosma, C.; Padoan, A.; Cozzani, S.; Perilongo, G.; Plebani, M.; Giaquinto, C.; Donà, D.; et al. Protective SARS-CoV-2 Antibody Response in Children With Inflammatory Bowel Disease. Front. Pediatr. 2022, 10, 815857. [Google Scholar] [CrossRef] [PubMed]
- D′Arcangelo, G.; Distante, M.; Raso, T.; Rossetti, D.; Catassi, G.; Aloi, M. Safety of Biological Therapy in Children With Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 736–741. [Google Scholar] [CrossRef]
- Koletzko, L.; Klucker, E.; Le Thi, T.G.; Breiteneicher, S.; Rubio-Acero, R.; Neuhaus, L.; Stark, R.G.; Standl, M.; Wieser, A.; Török, H.; et al. Following Pediatric and Adult IBD Patients through the COVID-19 Pandemic: Changes in Psychosocial Burden and Perception of Infection Risk and Harm over Time. J. Clin. Med. 2021, 10, 4124. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, N.S.F.; Martins, C.D.A.; Quaresma, A.B.; Hino, A.A.F.; Steinwurz, F.; Ungaro, R.C.; Kotze, P.G. COVID-19 outcomes in patients with inflammatory bowel diseases in Latin America: Results from SECURE-IBD registry. J. Gastroenterol. Hepatol. 2021, 36, 3033–3040. [Google Scholar] [CrossRef] [PubMed]
- Spencer, E.A.; Klang, E.; Dolinger, M.; Pittman, N.; Dubinsky, M.C. Seroconversion Following SARS-CoV-2 Infection or Vaccination in Pediatric IBD Patients. Inflamm. Bowel Dis. 2021, 27, 1862–1864. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, T.; Granado, M.C.; Manuel, A.R.; Espinheira, M.D.C.; Trindade, E. Impact of COVID-19 in Pediatric Patients and Young Adults with Inflammatory Bowel Disease. GE Port. J. Gastroenterol. 2022, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, M.; Strisciuglio, C.; Fedele, F.; Miele, E.; Staiano, A. Clinical and Psychological Issues in Children with Inflammatory Bowel Disease During COVID-19 Pandemic. Inflamm. Bowel Dis. 2020, 26, e95–e96. [Google Scholar] [CrossRef] [PubMed]
- Sansotta, N.; Norsa, L.; Zuin, G.; Panceri, R.; Dilillo, D.; Pozzi, E.; Giacomo, C.; Moretti, C.; Celano, R.; Nuti, F.; et al. Children With Inflammatory Bowel Disease in the COVID-19 Main Endemic Focus: The Lombardy Experience. Front. Pediatr. 2021, 9, 607285. [Google Scholar] [CrossRef]
- Dolinger, M.T.; Person, H.; Smith, R.; Jarchin, L.; Pittman, N.; Dubinsky, M.C.; Lai, J. Pediatric Crohn Disease and Multisystem Inflammatory Syndrome in Children (MIS-C) and COVID-19 Treated With Infliximab. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Huang, Y.; Martín-de-Carpi, J.; Aloi, M.; Focht, G.; Kang, B.; Zhou, Y.; Sanchez, C.; Kappelman, M.D.; Uhlig, H.H.; et al. Corona Virus Disease 2019 and Paediatric Inflammatory Bowel Diseases: Global Experience and Provisional Guidance (March 2020) from the Paediatric IBD Porto Group of European Society of Paediatric Gastroenterology, Hepatology, and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, K.; Godoy Brewer, G.; Thu Nguyen, M.; Singh, Y.; Saleh Ismail, M.; Sauk, J.S.; Parian, A.M.; Limketkai, B.N. COVID-19 and Outcomes in Patients With Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2022, 28, 1265–1279. [Google Scholar] [CrossRef]
- Gilissen, L.P.L.; Heinen, S.G.H.; Rijpma-Jacobs, L.; Schoon, E.; Schreuder, R.M.; Wensing, A.M.; van der Ende-van Loon, M.C.M.; Bloemen, J.G.; Stapelbroek, J.M.; Stronkhorst, A. Neither inflammatory bowel disease nor immunosuppressants are associated with an increased risk of severe COVID-19: An observational Dutch cohort study. Clin. Exp. Med. 2022, 22, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Body-Malapel, M.; Djouina, M.; Waxin, C.; Langlois, A.; Gower-Rousseau, C.; Zerbib, P.; Schmidt, A.M.; Desreumaux, P.; Boulanger, E.; Vignal, C. The RAGE signaling pathway is involved in intestinal inflammation and represents a promising therapeutic target for Inflammatory Bowel Diseases. Mucosal Immunol. 2019, 12, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Schneider, I.; Lindner, C.; Gonzalez, I.; Morales, M. Receptor for advanced glycation end-products axis and coronavirus disease 2019 in inflammatory bowel diseases: A dangerous liaison? World J. Gastroenterol. 2021, 27, 2270–2280. [Google Scholar] [CrossRef]
- Alrashed, F.; Alasfour, H.; Shehab, M. Impact of biologics and small molecules for inflammatory bowel disease on COVID-19-related hospitalization and mortality: A systematic review and meta-analysis. JGH Open 2022, 6, 241–250. [Google Scholar] [CrossRef]
- Coldewey, S.M.; Neu, C.; Bloos, F.; Baumbach, P.; Schumacher, U.; Bauer, M.; Reuken, P.; Stallmach, A. Infliximab in the treatment of patients with severe COVID-19 (INFLIXCOVID): Protocol for a randomised, controlled, multicentre, open-label phase II clinical study. Trials 2022, 23, 737. [Google Scholar] [CrossRef]
- CDC. Multisystem Inflammatory Syndrome (MIS). Available online: https://www.cdc.gov/mis/index.html (accessed on 9 October 2022).
- Hoste, L.; Van Paemel, R.; Haerynck, F. Multisystem inflammatory syndrome in children related to COVID-19: A systematic review. Eur. J. Pediatr. 2021, 180, 2019–2034. [Google Scholar] [CrossRef]
- Penner, J.; Abdel-Mannan, O.; Grant, K.; Maillard, S.; Kucera, F.; Hassell, J.; Eyre, M.; Berger, Z.; Hacohen, Y.; Moshal, K. 6-month multidisciplinary follow-up and outcomes of patients with paediatric inflammatory multisystem syndrome (PIMS-TS) at a UK tertiary paediatric hospital: A retrospective cohort study. Lancet. Child Adolesc. Health 2021, 5, 473–482. [Google Scholar] [CrossRef]
- Krawiec, P.; Opoka-Winiarska, V.; Pac-Kożuchowska, E. Is It Inflammatory Bowel Disease Flare or Pediatric Inflammatory Multisystem Syndrome Temporally Associated with COVID-19? J. Clin. Med. 2022, 11, 2765. [Google Scholar] [CrossRef] [PubMed]
- Sweeny, K.F.; Zhang, Y.J.; Crume, B.; Martz, C.A.; Blessing, M.M.; Kahn, S.A. Inflammatory Bowel Disease Presenting With Concurrent COVID-19 Multisystem Inflammatory Syndrome. Pediatrics 2021, 147, e2020027763. [Google Scholar] [CrossRef]
- Gavrilescu, O.; Prelipcean, C.C.; Dranga, M.; Popa, I.V.; Mihai, C. Impact of COVID-19 Pandemic on the Quality of Life of IBD Patients. Medicina 2022, 58, 562. [Google Scholar] [CrossRef] [PubMed]
- Fedele, F.; Martinelli, M.; Strisciuglio, C.; Dolce, P.; Giugliano, F.P.; Scarpato, E.; Staiano, A.; Miele, E. Health related quality of life in pediatric Inflammatory bowel disease during COVID-19 pandemic: A prospective study. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, L.; Nassar, R.; Binjamin Ohana, D.; Oseran, I.; Matar, M.; Shamir, R.; Assa, A. Pediatric inflammatory bowel disease and the effect of COVID-19 pandemic on treatment adherence and patients’ behavior. Pediatr. Res. 2021, 90, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Trindade, I.A.; Ferreira, N.B. COVID-19 Pandemic′s Effects on Disease and Psychological Outcomes of People With Inflammatory Bowel Disease in Portugal: A Preliminary Research. Inflamm. Bowel Dis. 2021, 27, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Beg, S.; Jain, A.; Bhatnagar, S. Paediatric COVID-19 and the GUT. Indian J. Med. Microbiol. 2020, 38, 261–264. [Google Scholar] [CrossRef]
- Lev-Tzion, R.; Focht, G.; Lujan, R.; Mendelovici, A.; Friss, C.; Greenfeld, S.; Kariv, R.; Ben-Tov, A.; Matz, E.; Nevo, D.; et al. COVID-19 Vaccine Is Effective in Inflammatory Bowel Disease Patients and Is Not Associated With Disease Exacerbation. Clin. Gastroenterol. Hepatol. 2022, 20, e1263–e1282. [Google Scholar] [CrossRef] [PubMed]
- Weaver, K.N.; Zhang, X.; Dai, X.; Watkins, R.; Adler, J.; Dubinsky, M.C.; Kastl, A.; Bousvaros, A.; Strople, J.A.; Cross, R.K.; et al. Impact of SARS-CoV-2 Vaccination on Inflammatory Bowel Disease Activity and Development of Vaccine-Related Adverse Events: Results From PREVENT-COVID. Inflamm. Bowel Dis. 2022, 28, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Weaver, K.N.; Kappelman, M.D.; Long, M.D. COVID-19 Vaccination Among Individuals With Inflammatory Bowel Disease: Perception, Efficacy, and Safety Gastroenterol. Hepatol. 2022, 18, 388. [Google Scholar]
- Cotugno, N.; Franzese, E.; Angelino, G.; Amodio, D.; Romeo, E.F.; Rea, F.; Faraci, S.; Tambucci, R.; Profeti, E.; Manno, E.C.; et al. Evaluation of Safety and Immunogenicity of BNT162B2 mRNA COVID-19 Vaccine in IBD Pediatric Population with Distinct Immune Suppressive Regimens. Vaccines 2022, 10, 1109. [Google Scholar] [CrossRef]
- Lee, K.J.; Choi, S.Y.; Lee, Y.M.; Kim, H.W. Neutralizing Antibody Response, Safety, and Efficacy of mRNA COVID-19 Vaccines in Pediatric Patients with Inflammatory Bowel Disease: A Prospective Multicenter Case-Control Study. Vaccines 2022, 10, 1265. [Google Scholar] [CrossRef] [PubMed]
- Kastl, A.J.; Weaver, K.N.; Zhang, X.; Strople, J.A.; Adler, J.; Dubinsky, M.C.; Bousvaros, A.; Watkins, R.; Dai, X.; Chen, W.; et al. Humoral Immune Response and Safety of SARS-CoV-2 Vaccination in Pediatric Inflammatory Bowel Disease. Am. J. Gastroenterol. 2022. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, S.E.; Park, Y.E.; Chang, J.Y.; Song, H.J.; Kim, D.H.; Yang, Y.J.; Kim, B.C.; Lee, J.G.; Yang, H.C.; et al. SARS-CoV-2 vaccination for adult patients with inflammatory bowel disease: Expert consensus statement by KASID. Intest. Res. 2022, 20, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Siegel, C.A.; Melmed, G.Y.; McGovern, D.P.; Rai, V.; Krammer, F.; Rubin, D.T.; Abreu, M.T.; Dubinsky, M.C. SARS-CoV-2 vaccination for patients with inflammatory bowel diseases: Recommendations from an international consensus meeting. Gut 2021, 70, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Cascini, F.; Pantovic, A.; Al-Ajlouni, Y.; Failla, G.; Ricciardi, W. Attitudes, acceptance and hesitancy among the general population worldwide to receive the COVID-19 vaccines and their contributing factors: A systematic review. EClinicalMedicine 2021, 40, 101113. [Google Scholar] [CrossRef] [PubMed]
- Crespo, R.F.; Shafat, M.; Melas-Kyriazi, N.; Gould, L.; Jones, S.; Neves, A.L.; Leis, M.S.; Maheswaran, H.; Darzi, A. International attitudes on COVID-19 vaccination: Repeat national cross-sectional surveys across 15 countries. medRxiv 2021. [Google Scholar] [CrossRef]
- Dalal, R.S.; McClure, E.; Marcus, J.; Winter, R.W.; Hamilton, M.J.; Allegretti, J.R. COVID-19 Vaccination Intent and Perceptions Among Patients With Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2021, 19, 1730–1732.e1732. [Google Scholar] [CrossRef]
- Costantino, A.; Noviello, D.; Conforti, F.S.; Aloi, M.; Armuzzi, A.; Bossa, F.; Ficari, F.; Leone, S.; Manguso, F.; Mocci, G.; et al. COVID-19 Vaccination Willingness and Hesitancy in Patients With Inflammatory Bowel Diseases: Analysis of Determinants in a National Survey of the Italian IBD Patients’ Association. Inflamm. Bowel Dis. 2022, 28, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lin, J.; Buch, H.; Ding, Q.; Zhang, F.; Cui, B.; Ji, G. The COVID-19 Vaccination Hesitancy Among the People With Inflammatory Bowel Disease in China: A Questionnaire Study. Front. Public Health 2021, 9, 731578. [Google Scholar] [CrossRef] [PubMed]
- Drouin, O.; Fontaine, P.; Arnaud, Y.; Montmarquette, C.; Prud’homme, A.; Da Silva, R.B. Parental decision and intent towards COVID-19 vaccination in children with asthma: An econometric analysis. BMC Public Health 2022, 22, 1547. [Google Scholar] [CrossRef] [PubMed]
- Temsah, M.H.; Alhuzaimi, A.N.; Aljamaan, F.; Bahkali, F.; Al-Eyadhy, A.; Alrabiaah, A.; Alhaboob, A.; Bashiri, F.A.; Alshaer, A.; Temsah, O.; et al. Parental Attitudes and Hesitancy About COVID-19 vs. Routine Childhood Vaccinations: A National Survey. Front. Public Health 2021, 9, 752323. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, M.; Sahin, M.K. Parents’ willingness and attitudes concerning the COVID-19 vaccine: A cross-sectional study. Int. J. Clin. Pract. 2021, 75, e14364. [Google Scholar] [CrossRef]
- Huang, Y.; Su, X.; Xiao, W.; Wang, H.; Si, M.; Wang, W.; Gu, X.; Ma, L.; Li, L.; Zhang, S.; et al. COVID-19 vaccine hesitancy among different population groups in China: A national multicenter online survey. BMC Infect. Dis. 2022, 22, 153. [Google Scholar] [CrossRef] [PubMed]
- Tangcharoensathien, V.; Calleja, N.; Nguyen, T.; Purnat, T.; D′Agostino, M.; Garcia-Saiso, S.; Landry, M.; Rashidian, A.; Hamilton, C.; AbdAllah, A.; et al. Framework for Managing the COVID-19 Infodemic: Methods and Results of an Online, Crowdsourced WHO Technical Consultation. J. Med. Internet Res. 2020, 22, e19659. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Cao, B.; Xiong, Y.; Tang, W. Digital Media’s Role in the COVID-19 Pandemic. JMIR mHealth uHealth 2020, 8, e20156. [Google Scholar] [CrossRef]
| Author | Country | Total IBD Patient (No) | Total IBD Patient with COVID-19 (No) | No Need for Hospitalization (No) | Hospitalization (No) | ICU Admission (No) | Deaths (No) | CD (No) | UC (No) | Mean/ Median, Age (yrs) | Mean Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arrigo et al., 2020, [12] | Italy | 2291 | 6 | 5 | 1 | 0 | 0 | 2 | 4 | 13.5 | Mild symptoms—1 severe infection |
| Brenner et al., 2021 [13] | 23 countries—USA, France, Italy, Argentina, UK, | 209 | 209 | 195 | 14 | 2 | 0 | 138 | 61 | 15/16 | Mild symptoms—14 hospitalized—2 PICU |
| Brenner et al., 2020 [14] | 33 countries | 29 | 29 | 26 | 3 | 0 | 0 | N/A | N/A | 0–19 | Mild symptoms—3 severe infection |
| D’Arcangelo et al., 2021 [16] | Italy | 185 | 4 | 4 | 0 | 0 | 0 | 2 | 2 | N/A | 2 asymptomatic—2 mild symptoms |
| Bosa et al., 2022 [15] | Italy | 84 | 12 | 12 | 0 | 0 | 0 | 9 | 1 | 14/1–18 | 4 asymptomatic—8 mild symptoms |
| Koletzko et al., 2021 [17] | Germany | 89 | 25 | 25 | 0 | 0 | 0 | 44 | 34 | 6–20 | Mild symptoms |
| Queiroz et al., 2021 [18] | Latin America (13 countries) | 18 | 18 | 17 | 1 | 0 | 0 | N/A | N/A | 0–18 | Mild symptoms |
| Dolinger et al., 2020 [23] | USA | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 14 | Severe symptoms |
| Spencer et al., 2021 [19] | USA | 340 | 51 | N/A | N/A | 0 | 0 | 39 | 9 | 0–21 | N/A |
| Ruan et al., 2021 [8] | USA | 14 | 14 | 14 | 0 | 0 | 0 | 10 | 4 | N/A | 7 transient symptoms—5 asymptomatic |
| Μagalhaes et al., 2021 [20] | Portugal | 268 | 11 | 11 | 0 | 0 | 0 | 7 | 4 | 15 (7–18) | 100% mild disease—no complication |
| Turner et al., 2020 [24] | Portugal-Global study | 8 | 8 | 8 | 0 | 0 | 5 | 2 | N/A | 100% mild disease—no complication | |
| Martinelli et al., 2020 [21] | Italy | 180 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | No COVID-19 cases | |
| Sansotta et al., 2021 [22] | Italy | 290 | 2 | 2 | 0 | 0 | 0 | 0 | 2 | 15.2 (2–18) | 100% mild disease—no complication |
| Arrigo et al., 2020, [12] | Brenner et al., 2020 [14] | Brenner et al., 2021 [13] | Dolinger et al., 2020 [23] | Queiroz et al., 2021 [18] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total IBD Patient (No) | 2291 | 29 | 209 | 1 | 18 | ||||||||
| Total IBD patient with COVID-19 (No) | 6 | 29 | 209 | 1 | 18 | ||||||||
| No need for hospitalization (No) | 5 | 26 | 195 | 0 | 17 | ||||||||
| Hospitalization (No) | 1 | 3 | 14 | 1 | 1 | ||||||||
| IBD activity | Treatment | IBD activity | Treatment | IBD activity | Treatment | IBD activity | Treatment | IBD activity | Treatment | ||||
| remission | azathioprine | N/A | N/A | mild | 3 | Sulfasalazine/mesalamine | 8 | severe | infliximab | N/A | N.A | ||
| moderate | 8 | Steroids | 4 | ||||||||||
| severe | 1 | 6MP/azathioprine monotherapyc | 2 | ||||||||||
| remission | 1 | Methotrexate monotherapyc | 0 | ||||||||||
| unknown | 1 | TNF antagonist | 1 | ||||||||||
| TNF antagonist/ 6MP/AZA/MTX | 2 | ||||||||||||
| Anti-integrin (vedolizumab) | 2 | ||||||||||||
| IL12/23 inhibitor | 2 | ||||||||||||
| Janus kinase inhibitor | 0 | ||||||||||||
| Other IBD medication(s) | 1 | ||||||||||||
| No IBD medication | 1 | ||||||||||||
| ICU admission (No) | 0 | 0 | 2 | 0 | 0 | ||||||||
| IBD activity | Treatment | IBD activity | Treatment | IBD activity | Treatment | IBD activity | Treatment | IBD activity | Treatment | ||||
| - | - | - | - | unknown | 1 | Sulfasalazine/Mesalamine | 2 | - | - | - | - | ||
| moderate | 1 | ||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batsiou, A.; Mantzios, P.; Piovani, D.; Tsantes, A.G.; Kopanou Taliaka, P.; Liakou, P.; Iacovidou, N.; Tsantes, A.E.; Bonovas, S.; Sokou, R. SARS-CoV-2 Infection and Outcomes in Children with Inflammatory Bowel Diseases: A Systematic Review. J. Clin. Med. 2022, 11, 7238. https://doi.org/10.3390/jcm11237238
Batsiou A, Mantzios P, Piovani D, Tsantes AG, Kopanou Taliaka P, Liakou P, Iacovidou N, Tsantes AE, Bonovas S, Sokou R. SARS-CoV-2 Infection and Outcomes in Children with Inflammatory Bowel Diseases: A Systematic Review. Journal of Clinical Medicine. 2022; 11(23):7238. https://doi.org/10.3390/jcm11237238
Chicago/Turabian StyleBatsiou, Anastasia, Petros Mantzios, Daniele Piovani, Andreas G. Tsantes, Paschalia Kopanou Taliaka, Paraskevi Liakou, Nicoletta Iacovidou, Argirios E. Tsantes, Stefanos Bonovas, and Rozeta Sokou. 2022. "SARS-CoV-2 Infection and Outcomes in Children with Inflammatory Bowel Diseases: A Systematic Review" Journal of Clinical Medicine 11, no. 23: 7238. https://doi.org/10.3390/jcm11237238
APA StyleBatsiou, A., Mantzios, P., Piovani, D., Tsantes, A. G., Kopanou Taliaka, P., Liakou, P., Iacovidou, N., Tsantes, A. E., Bonovas, S., & Sokou, R. (2022). SARS-CoV-2 Infection and Outcomes in Children with Inflammatory Bowel Diseases: A Systematic Review. Journal of Clinical Medicine, 11(23), 7238. https://doi.org/10.3390/jcm11237238











