Starting Home Telemonitoring and Oxygen Therapy Directly after Emergency Department Assessment Appears to Be Safe in COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
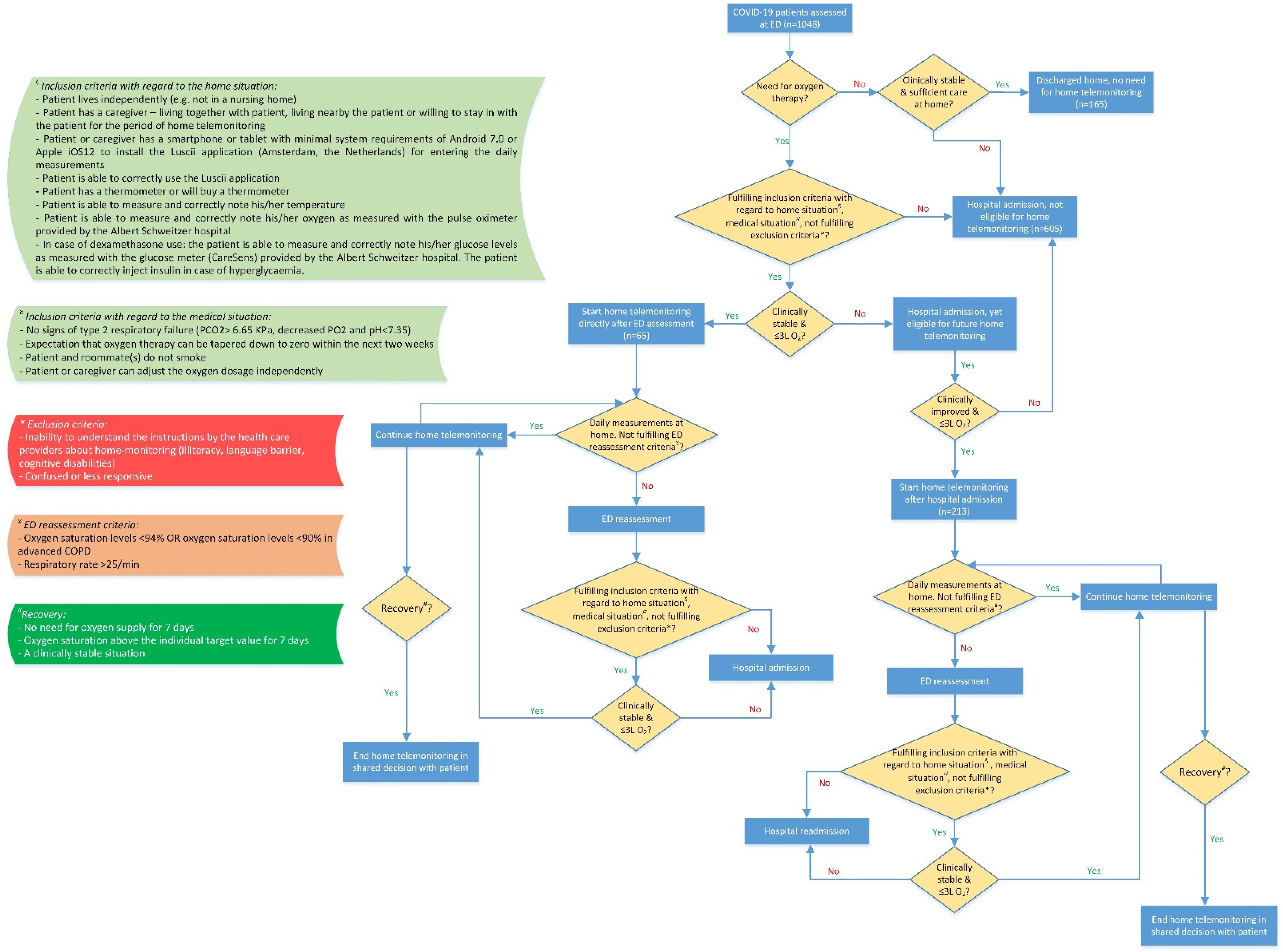
2.1. Patients
2.2. Home Telemonitoring
2.2.1. Continuation of Home Telemonitoring
2.2.2. Ending Home Telemonitoring
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Safety of Home Telemonitoring
3.2. Effectiveness of Home Telemonitoring
3.3. Experience and Satisfaction
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dirikgil, E.; Roos, R.; Groeneveld, G.H.; Heringhaus, C.; Silven, A.V.; Petrus, A.H.J.; Villalobos-Quesada, M.; Tsonaka, R.; van der Boog, P.J.M.; Rabelink, T.J.; et al. Home monitoring reduced short stay admissions in suspected COVID-19 patients: COVID-box project. Eur. Respir. J. 2021, 58, 2100636. [Google Scholar] [CrossRef] [PubMed]
- Silven, A.V.; Petrus, A.H.J.; Villalobos-Quesada, M.; Dirikgil, E.; Oerlemans, C.R.; Landstra, C.P.; Boosman, H.; van Os, H.J.A.; Blanker, M.H.; Treskes, R.W.; et al. Telemonitoring for Patients With COVID-19: Recommendations for Design and Implementation. J. Med. Internet Res. 2020, 22, e20953. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Annis, T.; Pleasants, S.; Hultman, G.; Lindemann, E.; Thompson, J.A.; Billecke, S.; Badlani, S.; Melton, G.B. Rapid implementation of a COVID-19 remote patient monitoring program. J. Am. Med. Inform. Assoc. 2020, 27, 1326–1330. [Google Scholar] [CrossRef]
- Casariego-Vales, E.; Blanco-Lopez, R.; Roson-Calvo, B.; Suarez-Gil, R.; Santos-Guerra, F.; Dobao-Feijoo, M.J.; Ares-Rico, R.; Bal-Alvaredo, M.; On Behalf of the Telea-Covid Lugo Comanagement Team. Efficacy of Telemedicine and Telemonitoring in At-Home Monitoring of Patients with COVID-19. J. Clin. Med. 2021, 10, 2893. [Google Scholar] [CrossRef]
- Hollander, J.E.; Carr, B.G. Virtually Perfect? Telemedicine for COVID-19. N. Engl. J. Med. 2020, 382, 1679–1681. [Google Scholar] [CrossRef]
- Grutters, L.A.; Majoor, K.I.; Mattern, E.S.K.; Hardeman, J.A.; van Swol, C.F.P.; Vorselaars, A.D.M. Home telemonitoring makes early hospital discharge of COVID-19 patients possible. J. Am. Med. Inform. Assoc. 2020, 27, 1825–1827. [Google Scholar] [CrossRef]
- Van Herwerden, M.C.; van Steenkiste, J.; El Moussaoui, R.; den Hollander, J.G.; Helfrich, G.; Verberk, J.A.M. Home telemonitoring and oxygen therapy in COVID-19 patients: Safety, patient satisfaction, and cost-effectiveness. Ned. Tijdschr. Geneeskd. 2021, 165, D5740. [Google Scholar]
- Martinez-Garcia, M.; Bal-Alvarado, M.; Santos Guerra, F.; Ares-Rico, R.; Suarez-Gil, R.; Rodriguez-Alvarez, A.; Perez-Lopez, A.; Casariego-Vales, E.; en nombre del Equipo de Seguimiento Compartido TELEA-COVID Lugo; Equipo TELEA COVID-19 (Lugo). Monitoring of COVID-19 patients by telemedicine with telemonitoring. Rev. Clin. Esp. (Barc) 2020, 220, 472–479. [Google Scholar] [CrossRef]
- Gruwez, H.; Bakelants, E.; Dreesen, P.; Broekmans, J.; Criel, M.; Thomeer, M.; Vandervoort, P.; Ruttens, D. Remote patient monitoring in COVID-19: A critical appraisal. Eur. Respir. J. 2022, 59, 2102697. [Google Scholar] [CrossRef]
- Khalid, I.; Imran, M.; Imran, M.; Khan, S.; Akhtar, M.A.; Amanullah, K.; Khalid, T.J. Telemedicine monitoring of high-risk coronavirus disease 2019 (COVID-19) patients by family medicine service after discharge from the emergency department. J. Fam. Community Med. 2021, 28, 210–216. [Google Scholar] [CrossRef]
- Banerjee, J.; Canamar, C.P.; Voyageur, C.; Tangpraphaphorn, S.; Lemus, A.; Coffey, C., Jr.; Wald-Dickler, N.; Holtom, P.; Shoenberger, J.; Bowdish, M.; et al. Mortality and Readmission Rates Among Patients With COVID-19 After Discharge From Acute Care Setting With Supplemental Oxygen. JAMA Netw. Open 2021, 4, e213990. [Google Scholar] [CrossRef] [PubMed]
- Kyriakides, J.; Khani, A.; Kelly, C.; Coleman, R. Analysis of an ambulatory care pathway for patients with COVID-19 utilising remote pulse oximetry. Clin. Med. 2021, 21, 48–49. [Google Scholar] [CrossRef] [PubMed]
- Pedretti, A.; Marquez Fosser, S.; Pasquinelli, R.; Vallone, M.; Plazzotta, F.; Luna, D.; Martinez, B.; Rodriguez, P.; Grande Ratti, M.F. Risk of readmission to the emergency department in mild COVID-19 outpatients with telehealth follow-up. Rev. Fac Cien Med. Univ. Nac. Cordoba 2021, 78, 249–256. [Google Scholar] [CrossRef]
- Dinh, A.; Mercier, J.C.; Jaulmes, L.; Artigou, J.Y.; Juilliere, Y.; Yordanov, Y.; Jourdain, P.; Collaboration, A.P.-H.U.I.C.-R. Safe Discharge Home With Telemedicine of Patients Requiring Nasal Oxygen Therapy After COVID-19. Front. Med. 2021, 8, 703017. [Google Scholar] [CrossRef]
- Van Goor, H.M.R.; Breteler, M.J.M.; van Loon, K.; de Hond, T.A.P.; Reitsma, J.B.; Zwart, D.L.M.; Kalkman, C.J.; Kaasjager, K.A.H. Remote Hospital Care for Recovering COVID-19 Patients Using Telemedicine: A Randomised Controlled Trial. J. Clin. Med. 2021, 10, 5940. [Google Scholar] [CrossRef]
- Viel, S.; Markowicz, S.; Ait-Medjber, L.; Ouissa, R.; Delta, D.; Portecop, P.; Foucan, T.; Roger, P.M. Dedicated team to ambulatory care for patients with COVID-19 requiring oxygen: Low rate of hospital readmission. Int. J. Infect. Dis. 2022, 123, 92–96. [Google Scholar] [CrossRef]
- Terp, S.; Reichert, Z.; Burner, E.; Randhawa, J.; Axeen, S.; Messina, M.; Dworkis, D.A.; Menchine, M.; Lam, C.N.; Banerjee, J.; et al. Characteristics and Outcomes of 360 Consecutive COVID-19 Patients Discharged from the Emergency Department with Supplemental Oxygen. Ann. Emerg. Med. 2022. [Google Scholar] [CrossRef]
- Kuo, S.; Aledia, A.; O’Connell, R.; Rudkin, S.; Dangodara, A.A.; Amin, A.N. Implementation and impact on length of stay of a post-discharge remote patient monitoring program for acutely hospitalized COVID-19 pneumonia patients. JAMIA Open 2022, 5, ooac060. [Google Scholar] [CrossRef]
- Steel, P.A.D.; Siegal, J.; Zhang, Y.; Cato, K.; Greenwald, P.; Melville, L.D.; Gogia, K.; Smith, Z.; Sharma, R.; Romney, M. Telehealth follow up in emergency department patients discharged with COVID-like illness and exertional hypoxia. Am. J. Emerg. Med. 2021, 49, 426–430. [Google Scholar] [CrossRef]
- National Institute for Public Health and the Environment. Available online: https://www.rivm.nl/en/coronavirus-COVID-19 (accessed on 7 April 2020).
- Van Klaveren, D.; Rekkas, A.; Alsma, J.; Verdonschot, R.; Koning, D.; Kamps, M.J.A.; Dormans, T.; Stassen, R.; Weijer, S.; Arnold, K.S.; et al. COVID outcome prediction in the emergency department (COPE): Using retrospective Dutch hospital data to develop simple and valid models for predicting mortality and need for intensive care unit admission in patients who present at the emergency department with suspected COVID-19. BMJ Open 2021, 11, e051468. [Google Scholar] [CrossRef] [PubMed]
| ED-Group (n = 65) | Admission-Group (n = 213) | |
|---|---|---|
| Age (years), mean (SD) | 57.1 (12.4) | 59.9 (11.4) |
| Female, n (%) | 28 (43.1) | 82 (38.5) |
| BMI (kg/m2), mean (SD) | 29.8 (5.8) | 30.7 (6.9) |
| Normal BMI (18.5–25), n (%) | 6 (23.1) | 11 (13.4) |
| Overweight (25–30), n (%) | 10 (38.5) | 36 (43.9) |
| Obesity (≥30), n (%) | 10 (38.5) | 35 (42.7) |
| Comorbidities, n (%) | ||
| Asthma | 4 (6.2) | 15 (7.0) |
| (Auto-)immune disorder | 0 (0.0) | 2 (0.9) |
| Chronic cardiovascular disease | 26 (40.0) | 69 (32.4) |
| Chronic kidney disease | 1 (1.5) | 7 (3.3) |
| Chronic liver disease | 1 (1.5) | 6 (2.8) |
| Chronic neurological disorder | 7 (10.8) | 19 (8.9) |
| Chronic pulmonary disease | 0 (0.0) | 12 (5.6) |
| Diabetes mellitus | 7 (10.8) | 18 (8.5) |
| Malignancy | 12 (18.5) | 32 (15.0) |
| COPE score death within 28 days (%) *, median (IQR) (min-max) | 5.4 (2.7–9.8) (0.8–21.4) (n = 61) | 5.3 (3.4–10.0) (0.9–24.5) (n = 87) |
| COPE score ICU admission within 28 days (%) *, median (IQR) (min-max) | 14.2 (9.8–19.6) (4.9–29.6) (n = 61) | 14.0 (11.0–19.8) (5.2–31.8) (n = 87) |
| Medical therapy (during admission/at discharge), n (%) | ||
| Antibiotics | 21 (32.3) | 168 (78.9) |
| Anticoagulants | 21 (32.3) | 198 (93.0) |
| Steroids | 24 (36.9) | 197 (92.5) |
| Immunosuppressive drugs | 1 (1.5) | 8 (3.8) |
| Tocilizumab | 3 (4.6) | 34 (16.0) |
| Regen-cov | - | 3 (1.4) |
| Remdesivir | - | 0 (0.0) |
| Length of stay in days, median (IQR) (min-max) | - | 4 (2–7) (0–27) ** |
| Admission to intensive care, n (%) | - | 12 (5.6) |
| Oxygen therapy during hospitalization, n (%) | ||
| Non-invasive mechanical ventilation of high nasal flow oxygen therapy | 0 (0.0) | 13 (6.1) |
| Intubation | 4 (6.2) | 15 (7.0) |
| ED-Group (n = 65) | Admission-Group (n = 213) | |
|---|---|---|
| Duration of home telemonitoring in days | 14 (9–18) (2–52) ¥ | 14 (10–20) (1–91) ¥ |
| Number of measurements | ||
| Respiratory rate | 24 (10–43) (1–131) | 23 (14–42) (1–207) |
| O2 saturation | 27 (14–45) (2–134) | 27 (16–45) (1–204) |
| Number of alerts | ||
| Respiratory rate | 2 (0–10) (0–28) | 1 (0–8) (0–95) |
| O2 saturation | 3 (1–6) (0–36) | 1 (0–4) (0–40) |
| Patients appreciating to have had an additional consultation with the physician (not urgent) | 4 (1–13) (1–63) | 11.5 (3–24) (1–139) |
| Number of telephone contacts health care provider–patient | 9 (7–12) (0–27) | 9 (7–12) (0–38) |
| Oxygen flow at start (L/min) | 2 (1–2) (1–4) | 2 (2–3) (1–5) |
| Duration of oxygen therapy in days | 9 (7–13) (2–52) | 10 (6–16) (1–91) |
| Reassessments at ED, n (%) | 15 (23.8) | 37 (15.8) |
| Hospital (re)admissions after reassessment, n (%) | 10 (15.9) | 14 (6.5) |
| ICU (re)admissions after reassessment, n (%) | 5 (7.7) | 5 (2.4) |
| Length of readmission in days | 6.5 (1–8) (1–27) | 5 (2–8) (0–81) |
| All-cause mortality, n (%) | 2 (3.1) | 8 (3.8) |
| n = 58 | ||
|---|---|---|
| I received sufficient information, so I knew what I could expect. | Did not agree Agreed a bit Partly agreed Largely agreed Agreed | 1.9 1.9 5.8 19.2 71.2 |
| I received clear instructions on how to use the pulse oximeter. | Did not agree Agreed a bit Partly agreed Largely agreed Agreed | 0.0 2.0 3.9 13.7 80.4 |
| I received clear instructions on how to use the glucose meter. | Did not agree Agreed a bit Partly agreed Largely agreed Agreed Not applicable | 19.1 0.0 0.0 9.5 61.9 9.5 |
| I received clear instructions on how to install the Luscii app on my mobile device. | Did not agree Agreed a bit Partly agreed Largely agreed Agreed | 3.9 0.0 2.0 9.8 84.3 |
| The Luscii app was easy to use. | Did not agree Agreed a bit Partly agreed Largely agreed Agreed | 2.0 2.0 0.0 9.8 86.3 |
| I received clear instructions on how to reach the healthcare providers. | Did not agree Agreed a bit Partly agreed Largely agreed Agreed | 2.0 0.0 5.9 9.8 82.4 |
| I could reach the healthcare providers when needed. | Did not agree Agreed a bit Partly agreed Largely agreed Agreed | 2.1 2.1 2.1 10.6 83.0 |
| Healthcare providers listened carefully to my concerns and physical complaints. | Did not agree Agreed a bit Partly agreed Largely agreed Agreed | 2.0 0.0 4.0 10.0 84.0 |
| Healthcare providers paid attention to my caregiver. | Did not agree Agreed a bit Partly agreed Largely agreed Agreed I do not know | 7.5 5.0 12.5 15.0 52.5 7.5 |
| Healthcare providers had sufficient time for me and my caregiver. | Did not agree Agreed a bit Partly agreed Largely agreed Agreed I do not know | 6.7 0.0 4.4 0.0 62.2 8.9 |
| The number of contacts with healthcare providers was sufficient. | Did not agree Agreed a bit Partly agreed Largely agreed Agreed | 2.0 0.0 5.9 13.7 78.4 |
| I have confidence in the expertise of healthcare providers. | Did not agree Agreed a bit Partly agreed Largely agreed Agreed | 2.0 2.0 2.0 9.8 84.3 |
| I felt safe recovering at home. | Did not agree Agreed a bit Partly agreed Largely agreed Agreed | 2.0 0.0 4.0 8.0 86.0 |
| Retrospectively, I am satisfied that I could recover at home. | Did not agree Agreed a bit Partly agreed Largely agreed Agreed | 0.0 2.0 0.0 4.0 94.0 |
| Score (0–10), median (IQR) | 9 (8–10) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van den Berg, R.; Meccanici, C.; de Graaf, N.; van Thiel, E.; Schol-Gelok, S. Starting Home Telemonitoring and Oxygen Therapy Directly after Emergency Department Assessment Appears to Be Safe in COVID-19 Patients. J. Clin. Med. 2022, 11, 7236. https://doi.org/10.3390/jcm11237236
van den Berg R, Meccanici C, de Graaf N, van Thiel E, Schol-Gelok S. Starting Home Telemonitoring and Oxygen Therapy Directly after Emergency Department Assessment Appears to Be Safe in COVID-19 Patients. Journal of Clinical Medicine. 2022; 11(23):7236. https://doi.org/10.3390/jcm11237236
Chicago/Turabian Stylevan den Berg, Rosaline, Celisa Meccanici, Netty de Graaf, Eric van Thiel, and Suzanne Schol-Gelok. 2022. "Starting Home Telemonitoring and Oxygen Therapy Directly after Emergency Department Assessment Appears to Be Safe in COVID-19 Patients" Journal of Clinical Medicine 11, no. 23: 7236. https://doi.org/10.3390/jcm11237236
APA Stylevan den Berg, R., Meccanici, C., de Graaf, N., van Thiel, E., & Schol-Gelok, S. (2022). Starting Home Telemonitoring and Oxygen Therapy Directly after Emergency Department Assessment Appears to Be Safe in COVID-19 Patients. Journal of Clinical Medicine, 11(23), 7236. https://doi.org/10.3390/jcm11237236








