Abstract
Contradicting evidence exists regarding the role of lipids in outcomes following intravenous (IV) thrombolysis with tissue plasminogen activator (tPA). Restricted cubic spline curves and adjusted logistic regression were used to evaluate associations of low-density lipoprotein cholesterol (LDL-C), non-high-density lipoprotein cholesterol (non-HDL-C), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and LDL-C/HDL-C ratio with poor functional outcome, symptomatic intracranial hemorrhage (SICH) and 90-day mortality, among 1004 acute ischemic stroke (AIS) patients who received IV tPA in a comprehensive stroke center. Quartile (Q) 1, Q2 and Q3 of HDL-C were associated with increased odds of poor functional outcome (adjusted odds ratio (adjOR) 1.66, 95% CI 1.06–2.60, p = 0.028, adjOR 1.63, 95% CI 1.05–2.53, p = 0.027, adjOR 1.56, 95% CI 1.01–2.44, p = 0.048) compared to Q4. Q2 and Q4 of non-HDL-C were associated with increased odds of SICH (adjOR 4.28, 95% CI 1.36–18.90, p = 0.025, adjOR 5.17, 95% CI 1.64–22.81, p = 0.011) compared to Q3. Q1 and Q2 of LDL-C was associated with increased odds of mortality (adjOR 2.57, 95% CI 1.27–5.57, p = 0.011 and adjOR 2.28, 95% CI 1.10–5.02, p = 0.032) compared to Q3. In AIS patients who received IV tPA, low LDL-C was associated with increased odds of mortality while HDL-C may be protective against poor functional outcome.
1. Introduction
Stroke incidence and stroke-related mortality increased substantially from 1990 to 2019, and the stroke burden will continue to increase globally, especially in underdeveloped countries [1]. In Singapore, a study reported an increased prevalence of stroke risk factors and the increased crude incidence rate of stroke among those younger than 65 [2]. There has been conflicting evidence on how lipid parameters affect post-thrombolysis outcomes [3,4], with non-traditional lipid parameters like non-HDL-C suggested to have similar functions as LDL-C in predicting hemorrhagic transformation (HT) [5]. Pre-stroke statin use was also shown to influence ischemic stroke outcomes [6].Hence, we aimed to explore the general associations of selected traditional and non-traditional lipid parameters with post-thrombolysis outcomes, to compare their clinical utility in prognostication. One significance of this study is to facilitate determination of cholesterol target levels in achieving optimal post-stroke recovery. Lastly, there are varied results on the relationship between lipid parameters and large artery atherosclerosis (LAA) stroke subtype. Bang et al. found that higher non-HDL-C and TG, but not LDL-C, was associated with LAA. Several studies have also shown that LDL-C may not best predict atherosclerotic vascular risk [7]. However, Hindy et al. suggested LDL-C lowering likely reduces LAA risk [8]. These should be further assessed to evaluate the utility of targeting lipid parameters for LAA stroke prevention.
2. Materials and Methods
2.1. Study Design
In this study, we included consecutive patients who received IV tPA from September 2006 to June 2018. All these patients had no contraindications to IV tPA use. The study obtained ethics approval from the National Healthcare Group-Domain Specific Review Board (NHG DSRB Ref: 2010/00509). Patients were assessed by a neurologist for eligibility to receive intravenous thrombolysis according to institutional protocol and American Heart Association/American Stroke Association guidelines at a standard dose of 0.9 mg/kg body weight [9]. All thrombolysed stroke patients underwent standard non-contrast head computed tomography and computed tomography brain and neck angiography. Patients who were deemed as unsuitable for IV-tPA, or who underwent endovascular thrombectomy were excluded from the study. Patients that were included had valid lipid parameters of TC, HDL-C and TG collected in fasting conditions, in mmol/litre, that were taken within 24 h of their AIS admission as per our institution protocol (Figure 1).
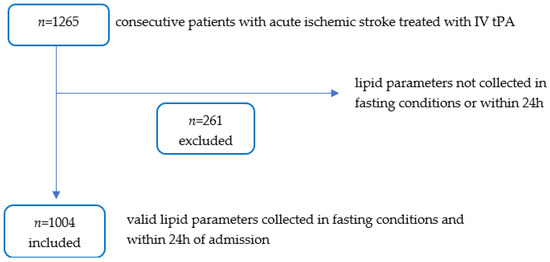
Figure 1.
Flow Diagram Illustrating Only Patients with Valid Lipid Parameters were Analysed.
LDL-C was calculated by the Friedewald equation (TC-HDL-C-TG/5) [10], while some had LDL-C collected directly. Non-HDL-C levels were calculated by subtracting HDL-C levels from TC. LDL-C/HDL-C ratio was calculated by dividing LDL-C by HDL-C. Other baseline demographics, clinical parameters and ischemic stroke characteristics were collected and tabulated within 24 h of AIS admission. Diabetes mellitus (DM) was defined as pre-existing diagnosis of diabetes mellitus or an admitting fasting blood glucose level greater than or equal to 7.0 mmol/L or an HbA1c greater than or equal to 6.5% [11]. The severity of stroke at presentation was assessed using the National Institute of Health Stroke Scale (NIHSS) [12]. This assessment was made by credentialed nurses as part of the acute stroke response team. The presence of Large Vessel Occlusion (LVO) was defined as occlusions of the first and second segment of the middle cerebral artery (MCA), M1 and M2, the Internal Carotid Artery (ICA) and as well as its terminus, tandem occlusions involving ICA-MCA, or occlusion of the basilar artery. The Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria was used by the treating stroke neurologist to classify stroke subtypes [13]. Investigations to evaluate the TOAST mechanism included: vessel imaging with either computed tomography angiography (CTA), magnetic resonance angiography (MRA), transcranial doppler (TCD), carotid duplex or a combination of these, as well as Holter monitoring and transthoracic echocardiography.
The primary outcome measured was poor functional outcome (90-day modified Rankin Scale (mRS) of 3–6). Secondary outcomes measured were symptomatic intracranial hemorrhage (SICH) and 90-day all-cause mortality. SICH was based on the European Cooperative Acute Stroke Study (ECASS) II definition [14]. The 90-day mRS was evaluated during follow-up visit to the stroke clinic. If not, mRS was evaluated via telephone call instead.
2.2. Data Analysis
Analyses were performed using SPSS for Windows version 27.0 (SPSS Inc, Chicago, IL, USA) and R version 4.0.5. Restricted cubic splines with 5 knots were plotted to visually assess the univariate associations between the lipid parameters and the three outcomes. These served qualitative and descriptive purposes only. Since non-linear (U-shaped/inverse U-shaped/J-shaped/reverse tick) associations were found, lipid parameters were divided into quartiles for analysis. Descriptive statistics for continuous variables were presented as mean (SD) when normality and homogeneity assumptions were satisfied, otherwise as median (interquartile range) (IQR), and n (%) for categorical variables. Differences in continuous variables were assessed using 2 sample t-test when normality and homogeneity assumptions were satisfied; otherwise, Mann-Whitney U test was used where data was not distributed normally. Chi-square or Fisher’s exact test was used for categorical variables. Covariates that were selected a priori for variable adjustment were gender, age, hypertension, atrial fibrillation, large vessel occlusion, diabetes mellitus, admitting NIHSS and admitting SBP. Logistic regression assessed the associations between lipid levels and outcomes, and with LAA. Results were presented as adjusted odds ratio (adjOR) with 95% confidence interval (CI). Statistical significance was set at two-sided p < 0.05.
3. Results
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
3.1. Baseline Characteristics and Associations of Lipid Parameters with LAA
1265 consecutive patients with ischemic stroke treated with IV tPA were analyzed and 1004 patients with valid lipid assessments were included in this study. Of 1004 patients, 589/986 (59.7%) were male, 586/883 (66.4%) were of Chinese ethnicity, 596/916 (65.1%) experienced a LVO and 190/1004 (18.9%) had AF. There were 657/1004 (65.4%) patients with history of with hypertension, 526/1004 (52.4%) with hyperlipidemia, and 306/1004 (30.5%) with diabetes mellitus. Median age was 66 (IQR 56–77) years while median admitting NIHSS was 15 (IQR 8–21). Median LDL-C, non-HDL-C, TC, HDL-C and LDL-C/HDL-C were 2.86 (IQR 2.18–3.50) mmol/L, 3.43 (IQR 2.70–4.19) mmol/L, 4.62 (IQR 3.86–5.36) mmol/L, 1.12 (IQR 0.95–1.32) mmol/L and 2.49 (IQR 1.84–3.30) respectively. In accordance with the TOAST classification, 322/975 (33.0%) had LAA stroke, 341/975 (35.0%) had cardioembolic (CE) stroke, 168/975 (17.2%) had small vessel occlusion (SVO), 10/975 (1.0%) had stroke of other determined etiology and 134/975 (13.7%) had cryptogenic stroke (Table 1).

Table 1.
Baseline Characteristics of Study Population.
Comparing LAA and non-LAA groups, median LDL-C, non-HDL-C, LDL-C/HDL-C ratio, as well as white blood cell (WBC) count, neutrophils, platelets, admitting NIHSS and presence of LVO were significantly higher in the LAA compared to non-LAA group (Table S1). Regarding lipid parameters and associations with LAA, after adjustment for gender, age, hypertension, atrial fibrillation, large vessel occlusion, diabetes mellitus, admitting NIHSS and admitting SBP, the following results were obtained. Q4 of LDL-C was associated with increased odds of LAA (adjusted odds ratio (adjOR) 1.69, 95% CI 1.07–2.69, p = 0.024) compared to Q1. Q2 and Q4 of non-HDL-C was significantly associated with increased odds of LAA (adjOR 1.64, 95% CI 1.04–2.60, p = 0.035 and adjOR 1.75, 95% CI 1.10–2.80, p = 0.018 respectively) compared to Q1. Q3 of HDL-C was associated with increased odds of LAA (adjOR 1.84, 95% CI 1.16–2.95, p = 0.010) compared to Q4. Lastly, Q3 and Q4 of LDL-C/HDL-C were associated with increased odds of LAA (adjOR 1.88, 95% CI 1.18–3.00, p = 0.008 and adjOR 1.71, 95% CI 1.07–2.77, p = 0.027 respectively) compared to Q1 (Table 2).

Table 2.
Lipid Parameters and Associations with LAA.
3.2. Stroke Outcomes
Of 1004 patients, 479/995 (48.1%) experienced poor functional outcomes (mRS 3–6), 48/1003 (4.8%) suffered SICH and 117/990 (11.8%) died. (Table 3) There was no statistically significant difference in prevalence of these three outcomes between the LAA and non-LAA group (Table S2).

Table 3.
Stroke Outcomes.
3.3. Associations of Lipid Parameters with Poor Functional Outcome, SICH and Mortality
When evaluating the following relationships between lipid parameters and outcomes measured, variables adjusted for in the multivariate model include gender, age, hypertension, atrial fibrillation, large vessel occlusion, diabetes mellitus, admitting NIHSS and admitting SBP.
3.3.1. Poor Functional Outcome
Restricted cubic spline curves showed a ‘reverse tick’ relationship between LDL-C, non-HDL-C and TC with poor functional outcome (Figure 2).
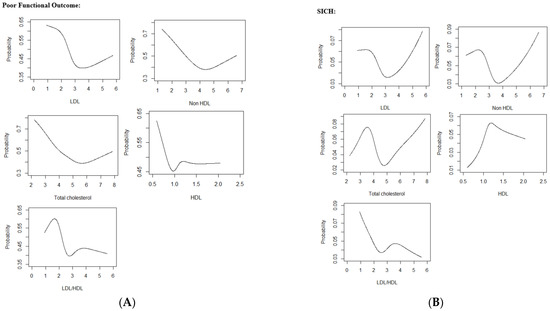
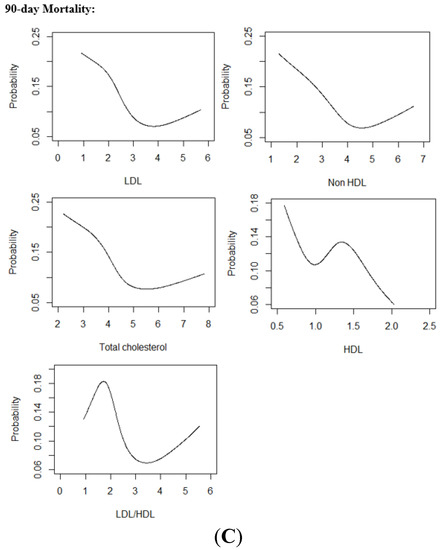
Figure 2.
(A) Restricted Cubic Spline Curves relating Lipid Parameters with Poor Functional Outcome (B) Restricted Cubic Spline Curves relating Lipid Parameters with SICH (C) Restricted Cubic Spline Curves relating Lipid Parameters with Mortality.Abbreviations: LDL low-density lipoprotein cholesterol, HDL high-density lipoprotein cholesterol, LDL/HDL low-density lipoprotein cholesterol/high-density lipoprotein cholesterol ratio, SICH symptomatic intracranial hemorrhage.
In multivariate analysis, Q1, Q2 and Q3 of HDL-C were significantly associated with increased odds of poor functional outcome (adjOR 1.66, 95% CI 1.06–2.60, p = 0.028, adjOR 1.63, 95% CI 1.05–2.53, p = 0.027 and OR 1.56, 95% CI 1.01–2.44, p = 0.048 respectively) when compared to Q4. Q2 and Q4 of LDL-C/HDL-C ratio were associated increased odds of poor functional outcome (adjOR 1.56, 95% CI 1.02–2.41, p = 0.043, OR: 1.78, 95% CI 1.16–2.76, p = 0.009 respectively) when compared to Q3 (Table 4).

Table 4.
Association of Lipid Parameters with Poor Functional Outcome, SICH and Mortality.
3.3.2. SICH
Restricted cubic spline curve showed a U-shaped association between non-HDL-C and LDL-C with SICH. (Figure 2) The lipid parameters that had significant non-linear relationships with SICH were non-HDL-C and TC. In multivariate analysis, Q2 and Q4 of non-HDL-C were significantly associated with SICH (adjOR 4.28, 95% CI 1.36–18.90, p = 0.025 and adjOR 5.17, 95% CI 1.64–22.81, p = 0.011 respectively) when compared to Q3. Similarly, Q2 and Q4 of TC were significantly associated with increased odds SICH (adjOR 4.46, 95% CI 1.43–19.59, p = 0.021 and adjOR 5.29, 95% CI 1.69–23.33, p = 0.010 respectively) when compared to Q3. Q2 of HDL-C remained significantly associated with increased odds of SICH (adjOR 3.07, 95% CI 1.23–8.75, p = 0.022) when compared to Q1 (Table 4).
3.3.3. 90-Day Mortality
Restricted cubic spline curves showed a ‘reverse tick’ relationship of LDL-C, non-HDL-C and TC with mortality. (Figure 2) In multivariate analysis, Q1 and Q2 of LDL-C were significantly associated with increased odds of mortality (adjOR 2.57, 95% CI 1.27–5.57, p = 0.011 and adjOR 2.28, 95% CI 1.10–5.02, p = 0.032) when compared to Q3. Q2 of LDL-C/HDL-C was associated with increased odds of mortality (adjOR 2.51, 95% CI 1.30–5.05, p = 0.008) when compared to Q3. No significant associations were found to relate non-HDL-C, TC and HDL-C with mortality on multivariate analysis (Table 4). Results of logistic regressions that adjusted for age and gender only, and age, gender, admitting NIHSS and LVO, are illustrated in the Supplementary Material (Tables S4 and S5).
4. Discussion
It was found that firstly, low HDL-C was associated with poor functional outcome, and secondly, a U-shaped relationship was found between non-HDL-C and SICH. Finally, low LDL-C was also found to be associated with increased mortality.
4.1. Lipid Parameters and Functional Neurological Outcome
We found that high HDL-C tended to be protective against poor functional outcome. This corroborates with a past Japanese study that found higher HDL-C and increased odds of favourable functional outcome after tPA thrombolysis [15]. This can be explained by how HDL reduces neuronal injury after ischemic stroke, possibly through anti-oxidative or anti-inflammatory pathways [16]. By suppressing inflammatory responses, HDL-C could promote neurological recovery because inflammatory cells and responses like neutrophils and neutrophil accumulation have been shown to cause poorer neurological outcome [17]. Next, our study found that the relationship between LDL-C/HDL-C and functional outcome may be non-linear, expanding on a previous Chinese study of 763 AIS patients treated with tPA, that demonstrated a LDL-C/HDL-C ratio cut-off of <2.71 was associated with higher risk of poor outcome [18]. Higher LDL-C/HDL-C was also found to be protective against mRS >2 at 3 months [19]. However, the relationship between LDL-C/HDL-C and functional outcome after ischemic stroke thrombolysis continues to be inconclusive.
4.2. Lipid Parameters and SICH
When investigating relationships with SICH, we found a U-shaped and non-linear relationship of non-HDL-C and TC with SICH respectively. Previously, a Chinese study found that lower non-HDL-C resulted in greater risk of hemorrhagic transformation [5], but other studies were unable to validate this association [3,20]. Conclusions on associations between TC and SICH were also varied [21,22]. Our finding of a U-shaped relationship between non-HDL-C and SICH where increased risk of SICH occurs at either moderately low or high non-HDL concentrations could explain the discrepancy in prior studies’ results. Postulated mechanisms include how low cholesterol reflects poor general health and undernourishment that predisposes individuals to hemorrhagic transformation unrelated to cholesterol pathways [23]. High non-HDL-C could increase risk of SICH through the development of arterial stiffness, which independently increases the risk of hemorrhagic transformation in thrombolysis-treated stroke patients [24]. Next, some studies have suggested a correlation between lower LDL-C and increased SICH risk [25,26]. In our study, non-HDL-C, but not LDL-C, was significantly associated with SICH. This is a surprising finding because LDL-C is a major component of non-HDL-C. However, other lipoproteins which contribute to non-HDL-C, such as very-low-density lipoproteins, intermediate-density lipoproteins and lipoprotein(a) could affect SICH occurrence. For instance, lipoprotein(a) was found to reduce bleeding risk in the brain due to its hemostatic properties [27]. Past studies have also demonstrated significant differences in the predictive ability of non-HDL-C and LDL-C on major adverse cardiac events (MACE) [28], reinstating the different roles of non-HDL-C and LDL-C in cardiovascular and cerebrovascular events.
4.3. LDL-C and Mortality
Our study found low LDL-C was significantly associated with increased odds of 90-day all-cause mortality after multivariate adjustment. This association has also been previously suggested outside of stroke—in a systematic review involving 68094 elderly patients, an inverse association between LDL-C and all-cause mortality was found, hypothesized by how low LDL-C increased vulnerability to fatal illnesses [29]. In our study, patients with low LDL-C had more comorbidities, (Table S3) which likely increased mortality predisposition. Hence, it was proposed that low LDL-C is an indirect indicator of severe illness rather than the cause of increased mortality [30]. Another explanation, although speculative, is the interaction between low LDL-C with dysbiosis and changes to bile acid metabolism that eventually leads to mortality [31]. The finding of low LDL-C increasing mortality may also be attributed to AIS patients without prior statin use, supported by Cheng et al. who found that low LDL-C was associated with higher mortality rates in statin-naïve acute ischemic stroke [32]. This finding is therefore generalized without comparison between statin, non-statin or proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor use.
4.4. Analysis of Lipid Parameters with LAA Subtype
This study also found that LDL-C, non-HDL-C, HDL-C and LDL-C/HDL-C were significantly associated with LAA after multivariate adjustment. Previous studies found elevated LDL-C a risk factor for atherothrombotic infarct, and LDL-C higher in LAA than other stroke subtypes [33,34]. Our finding of high LDL-C and increased odds of LAA can be explained by LDL-C’s association with intracranial and extracranial stenosis [35]. Next, our study found that higher HDL-C may be more desirable than moderate HDL-C levels in protection against LAA, reasoned by how HDL-C increases LDL-C reverse transport, delivers antioxidants to LDL-C and decreases susceptibility of LDL-C to oxidation in endothelium, slowing the process of atherosclerosis [36]. Lastly, our finding of high LDL-C/HDL-C ratio and association with increased odds of LAA can be explained by the atherogenicity of LDL-C and atheroprotection by HDL-C, supporting the previous finding of LDL-C/HDL-C ratio and its association with increased intima-media thickness, a measure of subclinical atherosclerosis [37]. Thus, high LDL-C/HDL-C ratio may be a useful indicator of atherosclerosis to help identify patients at higher risk of LAA stroke.
5. Strengths and Limitations
Our study is a comprehensive report detailing associations of selected lipid parameters with post-thrombolysis outcomes, representing a relatively large cohort size (1004 patients analysed) compared to previous thrombolysis studies. However, this study was a single institution retrospective cohort study that solely evaluated intravenous thrombolysis patients. This may limit the generalisability of results to other cohorts, warranting more prospective studies on lipid parameters and ischemic stroke outcomes. We would like to acknowledge the possibility of Type I error in the multivariate analyses in which significant associations may no longer hold true after Bonferroni correction. Excessive correction of statistical level of significance may also increase the likelihood of Type II error as a trade-off to reduce Type I error, which may increase false negatives. The multivariate relationship between HDL-C and poor functional outcome is to be interpreted with caution as no significant relationship was found on univariate analysis, in which the possible reasons can be explained statistically by Lo et al. and Wang et al. [38,39]. Hence further studies in this area are required to confirm this association. Our study did not distinguish statin and non-statin users or evaluated use of other lipid-lowering agents, nor compare antithrombotic drug use which could theoretically affect SICH risk. Comorbidities like chronic kidney disease could be a confounder that should be explored in future studies. We would suggest future studies compare ischemic stroke outcomes between intravenous thrombolysis, intra-arterial thrombolysis and mechanical thrombectomy cohorts, and evaluate the role of other non-traditional lipid measures like TG/HDL-C and TC/HDL-C ratio in these treatment options.
6. Conclusions
In AIS patients who received IV tPA, low LDL-C was associated with increased odds of mortality while HDL-C may be protective against poor functional outcome.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11237148/s1: Table S1: Baseline Characteristics of LAA vs. non-LAA Stroke Mechanisms; Table S2: Stroke Outcomes of LAA vs. non-LAA Mechanisms; Table S3: Baseline Characteristics of Study Population by LDL-C Quartiles; Table S4: Association of Lipid Parameters with Poor Functional Outcome, SICH and Mortality (Adjustment for Age and Gender only); Table S5: Association of Lipid Parameters with Poor Functional Outcome, SICH and Mortality (Adjustment for Age, Gender, Admitting NIHSS and Large Vessel Occlusion).
Author Contributions
Conceptualization, C.-H.S., A.F.W.H., A.Y.L.L. and B.Y.Q.T.; methodology, C.M., E.M.S.T. and Q.V.Y.; software, C.M., E.M.S.T., Q.V.Y. and Y.H.C.; formal analysis, C.M., E.M.S.T., Q.V.Y. and Y.H.C.; investigation, C.-H.S., F.A.N., A.Y.L.L. and B.Y.Q.T.; resources, B.Y.Q.T.; data curation, Q.V.Y., C.-H.S. and B.Y.Q.T.; writing—original draft preparation, C.M., E.M.S.T., Q.V.Y.; writing—review and editing, C.M., E.M.S.T., L.L.L.Y., C.-H.S., A.F.W.H., F.A.N., A.Y.L.L. and B.Y.Q.T.; visualization, L.L.L.Y., A.Y.L.L. and B.Y.Q.T.; supervision, L.L.L.Y., C.-H.S., A.F.W.H., Y.H.C., A.Y.L.L. and B.Y.Q.T.; project administration, B.Y.Q.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the study obtained ethics approval from the National Healthcare Group-Domain Specific Review Board (NHG DSRB Ref: 2010/00509).
Informed Consent Statement
Due to the retrospective conduct of this study, patient informed consent was waived, and this waiver was approved by NHG DSRB.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.Y.Q.; Tan, J.T.C.; Cheah, D.; Zheng, H.; Pek, P.P.; De Silva, D.A.; Ahmad, A.; Chan, B.P.L.; Chang, H.M.; Kong, K.H.; et al. Long-Term Trends in Ischemic Stroke Incidence and Risk Factors: Perspectives from an Asian Stroke Registry. J. Stroke 2020, 22, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Rocco, A.; Sykora, M.; Ringleb, P.; Diedler, J. Impact of statin use and lipid profile on symptomatic intracerebral haemorrhage, outcome and mortality after intravenous thrombolysis in acute stroke. Cerebrovasc. Dis. 2012, 33, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Nardi, K.; Engelter, S.; Strbian, D.; Sarikaya, H.; Arnold, M.; Casoni, F.; Ford, G.A.; Cordonnier, C.; Lyrer, P.; Bordet, R.; et al. Lipid Profile in Thrombolysis Study, G. Lipid profiles and outcome in patients treated by intravenous thrombolysis for cerebral ischemia. Neurology 2012, 79, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, Q.; Cheng, Y.; Wei, C.; Ye, C.; Liu, J.; Wu, B.; Liu, M. Association between non-high-density lipoprotein cholesterol and haemorrhagic transformation in patients with acute ischaemic stroke. BMC Neurol. 2020, 20, 47. [Google Scholar] [CrossRef]
- Hong, K.S.; Lee, J.S. Statins in Acute Ischemic Stroke: A Systematic Review. J. Stroke 2015, 17, 282–301. [Google Scholar] [CrossRef]
- Bang, O.Y.; Saver, J.L.; Liebeskind, D.S.; Pineda, S.; Ovbiagele, B. Association of serum lipid indices with large artery atherosclerotic stroke. Neurology 2008, 70, 841–847. [Google Scholar] [CrossRef]
- Hindy, G.; Engstrom, G.; Larsson, S.C.; Traylor, M.; Markus, H.S.; Melander, O.; Orho-Melander, M.; Stroke Genetics, N. Role of Blood Lipids in the Development of Ischemic Stroke and its Subtypes: A Mendelian Randomization Study. Stroke 2018, 49, 820–827. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- American Diabetes, A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44 (Suppl. 1), S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Lyden, P.; Brott, T.; Tilley, B.; Welch, K.M.; Mascha, E.J.; Levine, S.; Haley, E.C.; Grotta, J.; Marler, J. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke 1994, 25, 2220–2226. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Hacke, W.; Kaste, M.; Fieschi, C.; von Kummer, R.; Davalos, A.; Meier, D.; Larrue, V.; Bluhmki, E.; Davis, S.; Donnan, G.; et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 1998, 352, 1245–1251. [Google Scholar] [CrossRef]
- Makihara, N.; Okada, Y.; Koga, M.; Shiokawa, Y.; Nakagawara, J.; Furui, E.; Kimura, K.; Yamagami, H.; Hasegawa, Y.; Kario, K.; et al. Effect of serum lipid levels on stroke outcome after rt-PA therapy: SAMURAI rt-PA registry. Cerebrovasc. Dis. 2012, 33, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Paterno, R.; Ruocco, A.; Postiglione, A.; Hubsch, A.; Andresen, I.; Lang, M.G. Reconstituted high-density lipoprotein exhibits neuroprotection in two rat models of stroke. Cerebrovasc. Dis. 2004, 17, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Lambertsen, K.L.; Finsen, B.; Clausen, B.H. Post-stroke inflammation-target or tool for therapy? Acta Neuropathol. 2019, 137, 693–714. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, J.; Yan, X.L.; Jin, H.; Sun, X.; Guo, Z.N.; Yang, Y. Association of Non-Traditional Lipid Parameters with Hemorrhagic Transformation and Clinical Outcome After Thrombolysis in Ischemic Stroke Patients. Curr. Neurovasc. Res. 2020, 17, 736–744. [Google Scholar] [CrossRef]
- Liu, L.; Yin, P.; Lu, C.; Li, J.; Zang, Z.; Liu, Y.; Liu, S.; Wei, Y. Association of LDL-C/HDL-C Ratio with Stroke Outcomes Within 1 Year After Onset: A Hospital-Based Follow-Up Study. Front. Neurol. 2020, 11, 408. [Google Scholar] [CrossRef]
- Wang, G.; Jing, J.; Wang, A.; Zhang, X.; Zhao, X.; Li, Z.; Wang, C.; Li, H.; Liu, L.; Wang, Y.; et al. China National Stroke Registry, I.I.I. Non-High-Density Lipoprotein Cholesterol Predicts Adverse Outcomes in Acute Ischemic Stroke. Stroke 2021, 52, 2035–2042. [Google Scholar] [CrossRef]
- Lv, G.; Wang, G.Q.; Xia, Z.X.; Wang, H.X.; Liu, N.; Wei, W.; Huang, Y.H.; Zhang, W.W. Influences of blood lipids on the occurrence and prognosis of hemorrhagic transformation after acute cerebral infarction: A case-control study of 732 patients. Mil. Med. Res. 2019, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Messe, S.R.; Pervez, M.A.; Smith, E.E.; Siddique, K.A.; Hellkamp, A.S.; Saver, J.L.; Bhatt, D.L.; Fonarow, G.C.; Peterson, E.D.; Schwamm, L.H. Lipid profile, lipid-lowering medications, and intracerebral hemorrhage after tPA in get with the guidelines-stroke. Stroke 2013, 44, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Bang, O.Y.; Saver, J.L.; Liebeskind, D.S.; Starkman, S.; Villablanca, P.; Salamon, N.; Buck, B.; Ali, L.; Restrepo, L.; Vinuela, F.; et al. Cholesterol level and symptomatic hemorrhagic transformation after ischemic stroke thrombolysis. Neurology 2007, 68, 737–742. [Google Scholar] [CrossRef]
- Acampa, M.; Camarri, S.; Lazzerini, P.E.; Guideri, F.; Tassi, R.; Valenti, R.; Cartocci, A.; Martini, G. Increased arterial stiffness is an independent risk factor for hemorrhagic transformation in ischemic stroke undergoing thrombolysis. Int. J. Cardiol. 2017, 243, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.F.; Chao, A.C.; Hu, H.H.; Lin, R.T.; Chen, C.H.; Chan, L.; Lin, H.J.; Sun, Y.; Lin, Y.Y.; Chen, P.L.; et al. Taiwan Thrombolytic Therapy for Acute Ischemic Stroke Study, G. Low Cholesterol Levels Increase Symptomatic Intracranial Hemorrhage Rates After Intravenous Thrombolysis: A Multicenter Cohort Validation Study. J. Atheroscler. Thromb. 2019, 26, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Lin, M.; Wang, B.G.; Zeng, W.Y.; He, Y.F.; Peng, H.Y.; Zeng, J.; Wu, Z.Y.; Zhong, Y. Low level of low-density lipoprotein cholesterol is related with increased hemorrhagic transformation after acute ischemic cerebral infarction. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 673–678. [Google Scholar]
- Langsted, A.; Kamstrup, P.R.; Nordestgaard, B.G. High Lipoprotein(a) and Low Risk of Major Bleeding in Brain and Airways in the General Population: A Mendelian Randomization Study. Clin. Chem. 2017, 63, 1714–1723. [Google Scholar] [CrossRef]
- Wongcharoen, W.; Sutthiwutthichai, S.; Gunaparn, S.; Phrommintikul, A. Is non-HDL-cholesterol a better predictor of long-term outcome in patients after acute myocardial infarction compared to LDL-cholesterol?: A retrospective study. BMC Cardiovasc. Disord. 2017, 17, 10. [Google Scholar] [CrossRef]
- Ravnskov, U.; Diamond, D.M.; Hama, R.; Hamazaki, T.; Hammarskjold, B.; Hynes, N.; Kendrick, M.; Langsjoen, P.H.; Malhotra, A.; Mascitelli, L.; et al. Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: A systematic review. BMJ Open 2016, 6, e010401. [Google Scholar] [CrossRef]
- Johannesen, C.D.L.; Langsted, A.; Mortensen, M.B.; Nordestgaard, B.G. Association between low density lipoprotein and all cause and cause specific mortality in Denmark: Prospective cohort study. BMJ 2020, 371, m4266. [Google Scholar] [CrossRef]
- Sung, K.C.; Huh, J.H.; Ryu, S.; Lee, J.Y.; Scorletti, E.; Byrne, C.D.; Kim, J.Y.; Hyun, D.S.; Ko, S.B. Low Levels of Low-Density Lipoprotein Cholesterol and Mortality Outcomes in Non-Statin Users. J. Clin. Med. 2019, 8, 1571. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.H.; Lin, J.R.; Anderson, C.S.; Lai, W.T.; Lee, T.H.; Group, S. Lipid Paradox in Statin-Naive Acute Ischemic Stroke but Not Hemorrhagic Stroke. Front. Neurol. 2018, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Doi, Y.; Arima, H.; Yonemoto, K.; Hata, J.; Kubo, M.; Tanizaki, Y.; Ibayashi, S.; Iida, M.; Kiyohara, Y. LDL cholesterol and the development of stroke subtypes and coronary heart disease in a general Japanese population: The Hisayama study. Stroke 2009, 40, 382–388. [Google Scholar] [CrossRef]
- Lv, P.; Jin, H.; Liu, Y.; Cui, W.; Peng, Q.; Liu, R.; Sun, W.; Fan, C.; Teng, Y.; Sun, W.; et al. Comparison of Risk Factor between Lacunar Stroke and Large Artery Atherosclerosis Stroke: A Cross-Sectional Study in China. PLoS ONE 2016, 11, e0149605. [Google Scholar] [CrossRef]
- Lei, C.; Wu, B.; Liu, M.; Chen, Y. Risk factors and clinical outcomes associated with intracranial and extracranial atherosclerotic stenosis acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2014, 23, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Mackness, M.I.; Abbott, C.; Arrol, S.; Durrington, P.N. The role of high-density lipoprotein and lipid-soluble antioxidant vitamins in inhibiting low-density lipoprotein oxidation. Biochem. J. 1993, 294 Pt 3, 829–834. [Google Scholar] [CrossRef]
- Enomoto, M.; Adachi, H.; Hirai, Y.; Fukami, A.; Satoh, A.; Otsuka, M.; Kumagae, S.; Nanjo, Y.; Yoshikawa, K.; Esaki, E.; et al. LDL-C/HDL-C Ratio Predicts Carotid Intima-Media Thickness Progression Better Than HDL-C or LDL-C Alone. J. Lipids 2011, 2011, 549137. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.K.; Li, I.T.; Tsou, T.S.; See, L. [Non-significant in univariate but significant in multivariate analysis: A discussion with examples]. Changgeng Yi Xue Za Zhi 1995, 18, 95–101. [Google Scholar] [PubMed]
- Wang, H.; Peng, J.; Wang, B.; Lu, X.; Zheng, J.Z.; Wang, K.; Tu, X.M.; Feng, C. Inconsistency Between Univariate and Multiple Logistic Regressions. Shanghai Arch. Psychiatry 2017, 29, 124–128. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).