Preoperative Risk Stratification of Increased MIB-1 Labeling Index in Pituitary Adenoma: A Newly Proposed Prognostic Scoring System
Abstract
1. Introduction
2. Materials and Methods
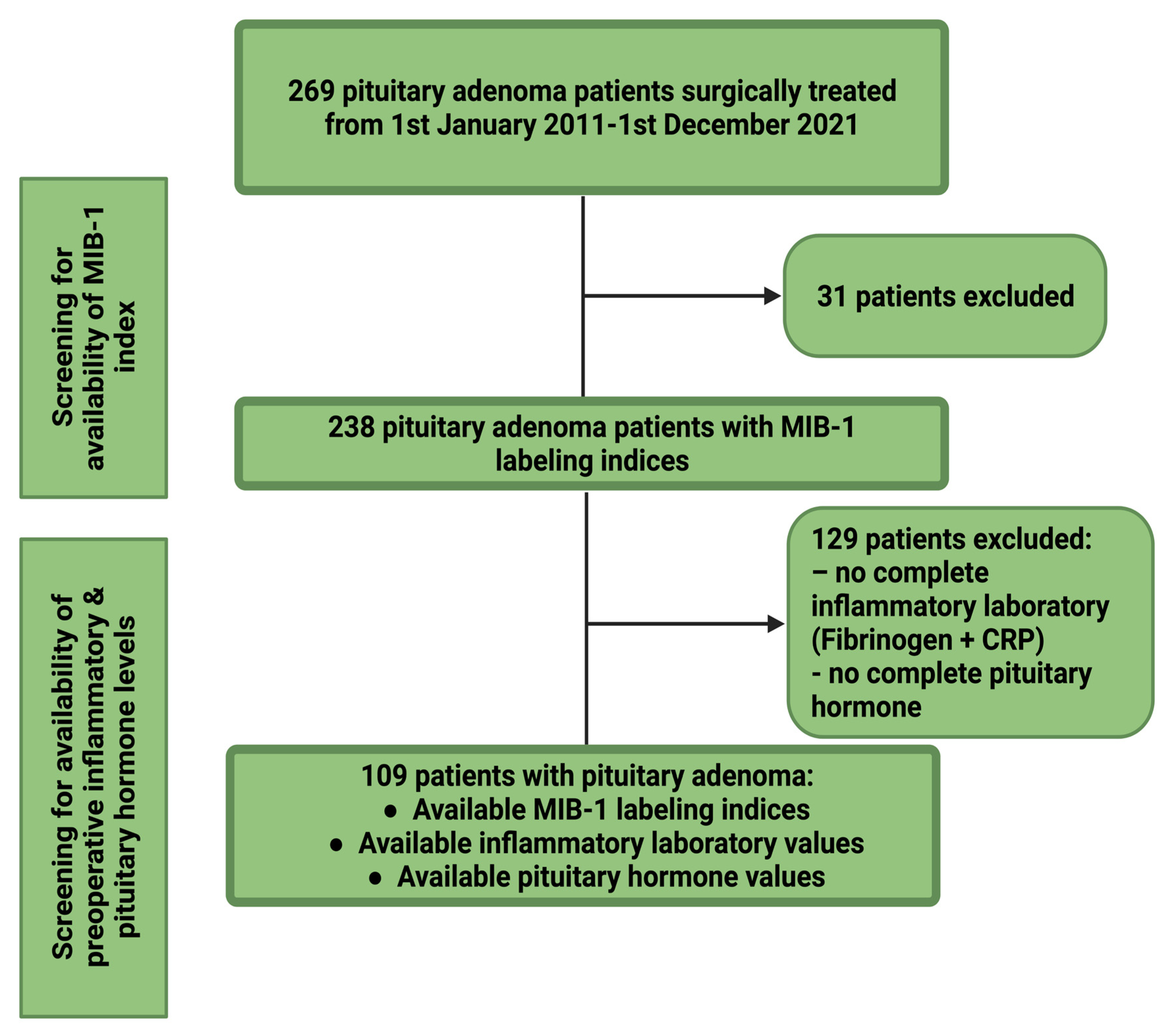
2.1. Study Design and Inclusion Criteria
2.2. Data Recording
2.3. Neuropathology
2.4. Follow-Up Scheduling
2.5. Statistical Analysis
3. Results
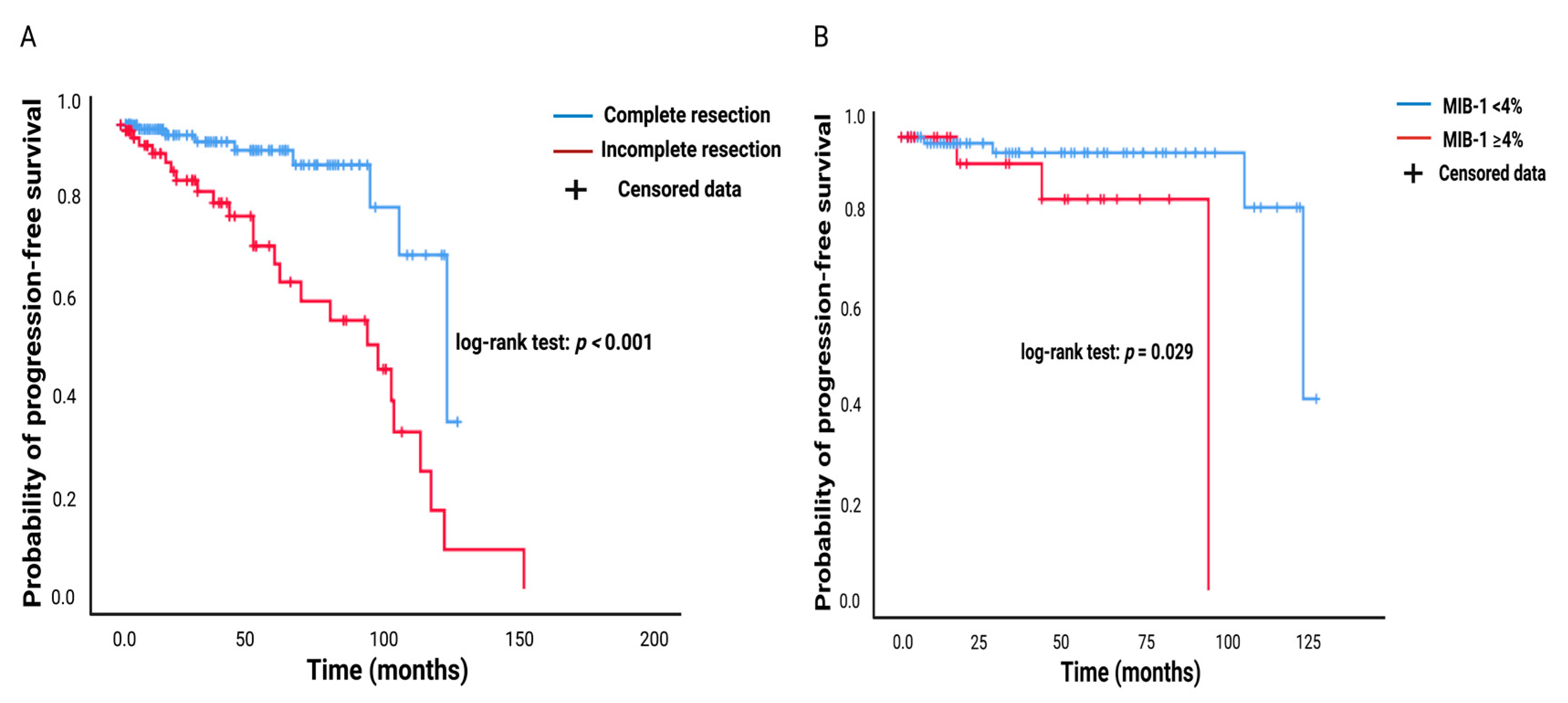
3.1. Probability of Progression-Free Survival in Pituitary Adenoma and Prognostic Value of MIB-1 Labeling Index in the Prediction of Recurrent Pituitary Adenoma
3.2. Patient Characteristics of the Screening group for Baseline Characteristics Being Associated with Elevated MIB-1 Labeling Index
3.3. Pituitary Adenoma Growth Pattern, Surgical Therapy, and Neuropathological Pituitary Adenoma Types
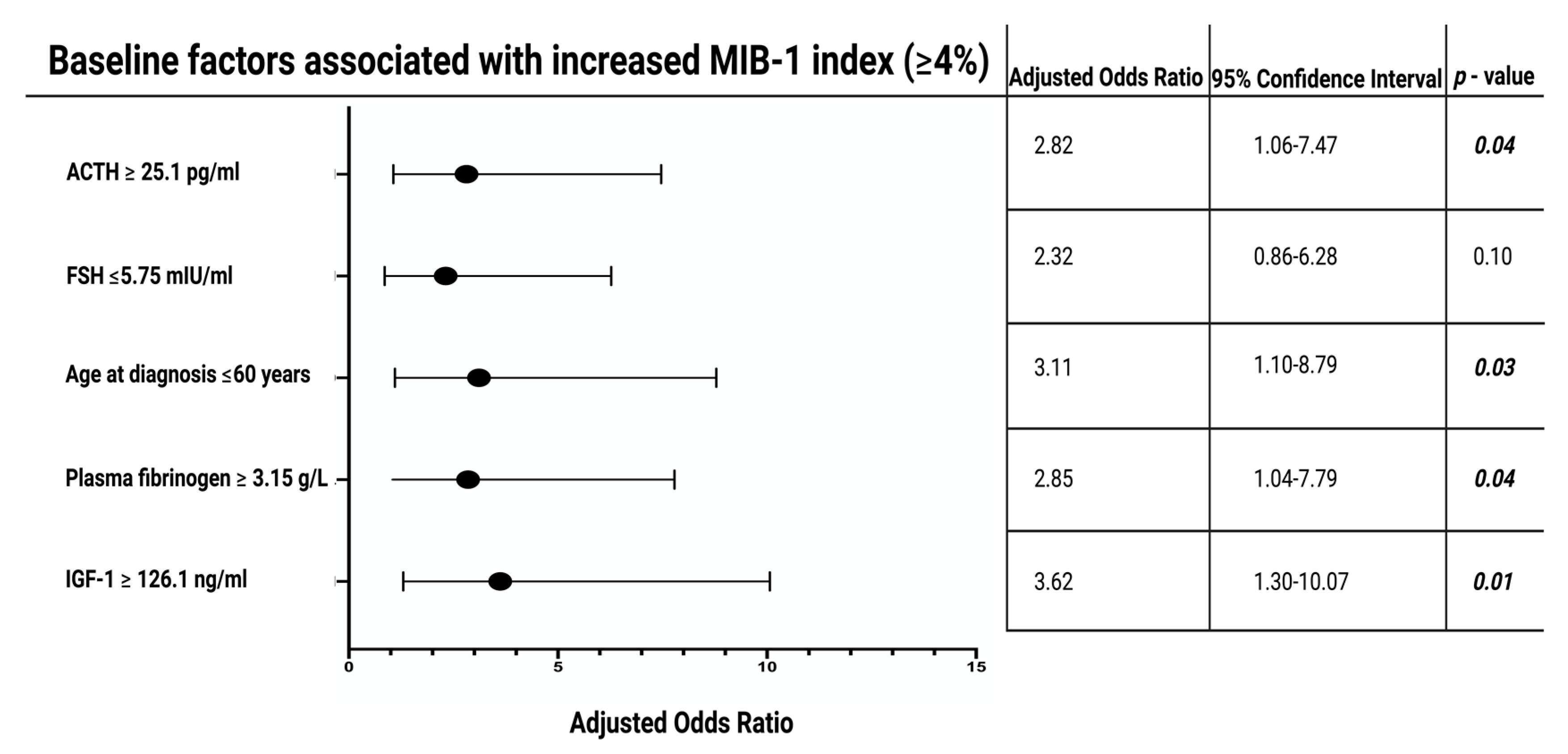
3.4. Screening for Associations between MIB-1 Labeling Index and Patient Characteristics
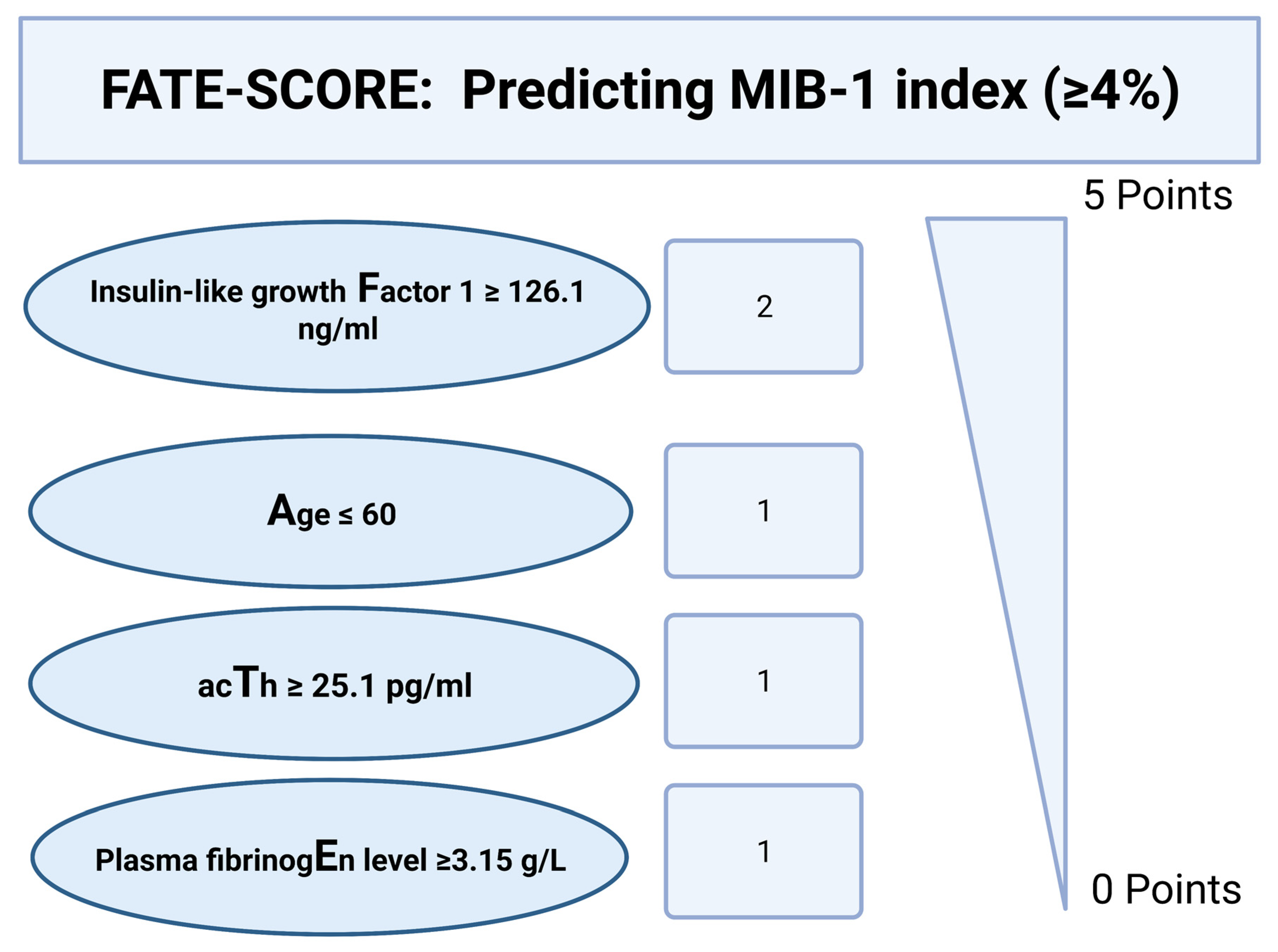
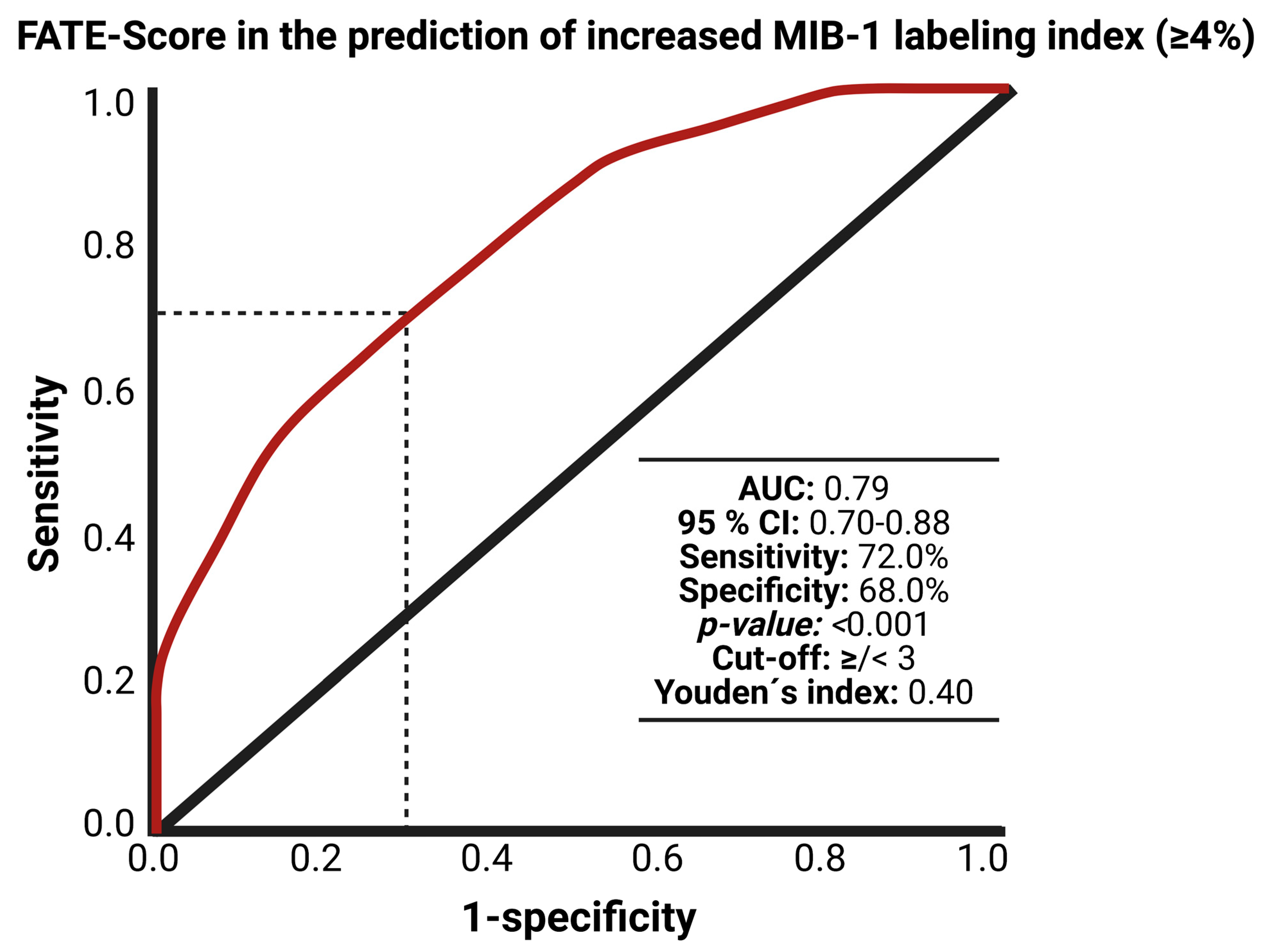
3.5. Scoring System
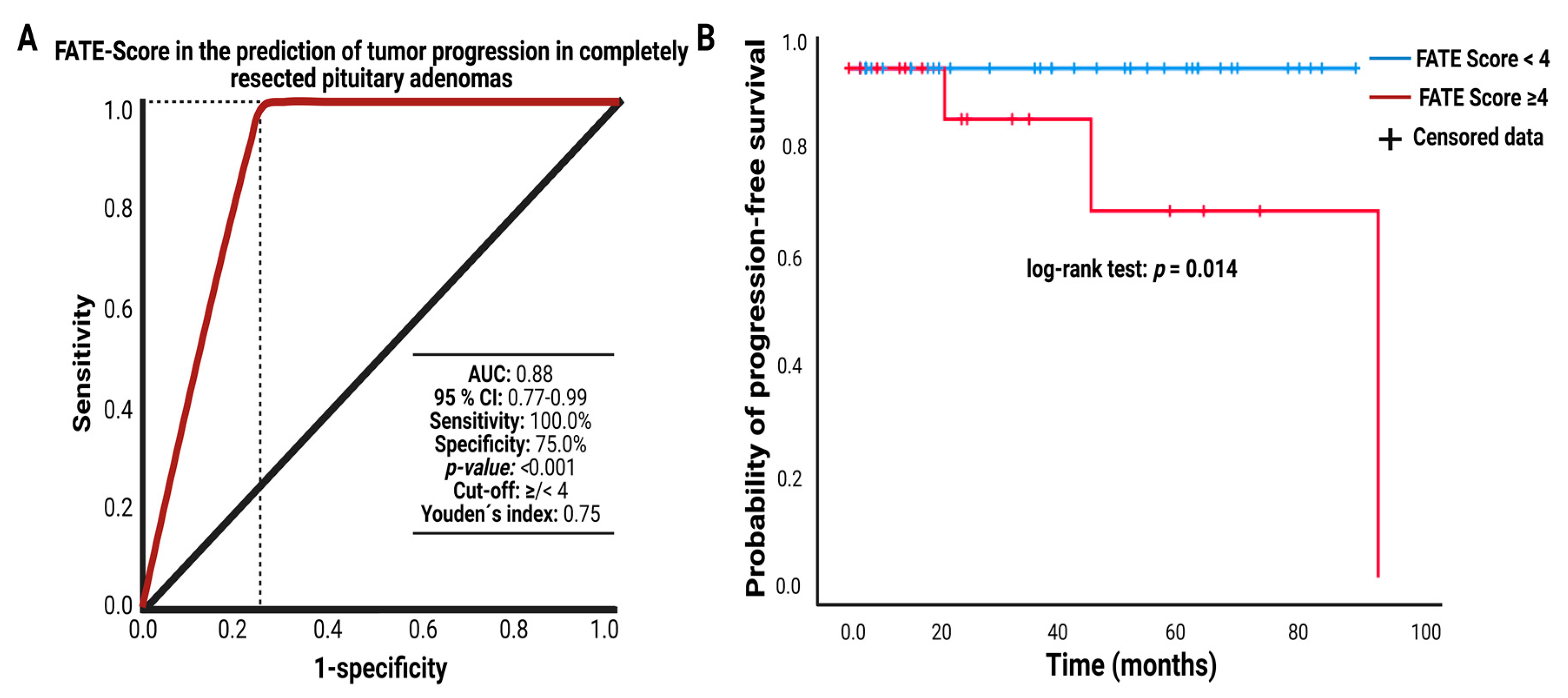
3.6. FATE Score and Progression-free Survival
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melmed, S. Pituitary-Tumor Endocrinopathies. N. Engl. J. Med. 2020, 382, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Dallapiazza, R.F.; Grober, Y.; Starke, R.M.; Laws, E.R., Jr.; Jane, J.A., Jr. Long-term results of endonasal endoscopic transsphenoidal resection of nonfunctioning pituitary macroadenomas. Neurosurgery 2015, 76, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Van Diest, P.J.; Brugal, G.; Baak, J.P. Proliferation markers in tumours: Interpretation and clinical value. J. Clin. Pathol. 1998, 51, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Wach, J.; Brandecker, S.; Güresir, A.; Schuss, P.; Vatter, H.; Güresir, E. The impact of the MIB-1 index on facial nerve outcomes in vestibular schwannoma surgery. Acta Neurochir. 2020, 162, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, J.; Schwab, U.; Lemke, H.; Stein, H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int. J. Cancer. 1983, 31, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Chiloiro, S.; Bianchi, A.; Doglietto, F.; de Waure, C.; Giampietro, A.; Fusco, A.; Iacovazzo, D.; Tartaglione, L.; Di Nardo, F.; Signorelli, F.; et al. Radically resected pituitary adenomas: Prognostic role of Ki 67 labeling index in a monocentric retrospective series and literature review. Pituitary 2014, 17, 267–276. [Google Scholar] [CrossRef]
- Almeida, J.P.; Tabasinejad, R.; Kalyvas, A.; Takami, H.; Mohan, N.; O’Halloran, P.J.; Sanchez, M.M.; Velasquez, C.; Zadeh, G.; Gentili, F. The Importance of Long Term Follow Up After Endoscopic Pituitary Surgery: Durability of Results and Tumor Recurrence. Neurol. India 2020, 68, S92–S100. [Google Scholar]
- Zheng, X.; Li, S.; Zhang, W.; Zang, Z.; Hu, J.; Yang, H. Current biomarkers of invasive sporadic pituitary adenomas. Ann. Endocrinol. 2016, 77, 658–667. [Google Scholar] [CrossRef]
- Ugga, L.; Cucolo, R.; Solari, D.; Guadagno, E.; D’Amico, A.; Somma, T.; Cappabianca, P.; Del Basso de Caro, M.L.; Cavallo, L.M.; Brunetti, A. Prediction of high proliferative index in pituitary macrodadenomas using MRI-based radiomics and machine learning. Neuroradiology 2019, 61, 1365–1373. [Google Scholar] [CrossRef]
- Cai, X.; Zhu, J.; Yang, J.; Tang, C.; Yuan, F.; Cong, Z.; Ma, C. A Nomogram for Preoperatively Predicting the Ki-67 index of a Pituitary Tumor: A Retrospective Cohort Study. Front. Oncol. 2021, 11, 687333. [Google Scholar] [CrossRef] [PubMed]
- Incandela, F.; Feraco, P.; Putorti, V.; Geraci, L.; Salvaggio, G.; Sarno, C.; La Tona, G.; Lasio, G.; Gagliardo, G. Malignancy course of pituitary adenoma in MEN1 syndrome: Clinical-Neuroradiological signs. Eur. J. Radiol. Open 2020, 7, 100242. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, J.; Labat-Moleur, F.; Sturm, N.; Kujas, M.; Heymann, M.F.; Figarella-Branger, D.; Patey, M.; Mazucca, M.; Decullier, E.; Verges, B.; et al. Pituitary tumors and hyperplasia in multiple endocrine neoplasia type 1 syndrome (MEN1): A case-control study in a series of 77 patients versus 2509 non-MEN1 patients. Am. J. Surg. Pathol. 2008, 32, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Henson, J.W.; Ulmer, S.; Harris, G.J. Brain tumor imaging in clinical trials. Am. J. Neuroradiol. 2008, 29, 419–424. [Google Scholar] [CrossRef]
- Wach, J.; Apallas, S.; Schneider, M.; Güresir, A.; Schuss, P.; Herrlinger, U.; Vatter, H.; Güresir, E. Baseline Serum C-Reactive Protein and Plasma Fibrinogen-Based Score in the Prediction of Survival in Glioblastoma. Front. Oncol. 2021, 11, 653614. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Majores, M.; Schick, V.; Engels, G.; Fassunke, J.; Elger, C.E.; Schramm, J.; Blumcke, I.; Becker, A.J. Mutational and immunohistochemical analysis of ezrin-, radixin-, moesin (ERM) molecules in epilepsy-associated glioneuronal lesions. Acta Neuropathol. 2005, 110, 537–546. [Google Scholar] [CrossRef]
- Majores, M.; von Lehe, M.; Fassunke, J.; Schramm, J.; Becker, A.J.; Simon, M. Tumor recurrence and malignant progression of gangliogliomas. Cancer 2008, 113, 3355–3363. [Google Scholar] [CrossRef]
- Gerges, M.M.; Rumalla, K.; Godil, S.S.; Younus, I.; Elshamy, W.; Dobri, G.A.; Kacker, A.; Tabee, A.; Anand, V.K.; Schwartz, T.H. Long-term outcomes after endoscopic endonasal surgery for nonfunctioning pituitary macroadenomas. J. Neurosurg. 2020, 31, 1–12. [Google Scholar] [CrossRef]
- Thapar, K.; Kovacs, K.; Scheithauer, B.W.; Stefaneau, L.; Horvath, E.; Pernicone, P.J.; Murray, D.; Laws, E.R., Jr. Proliferative activity and invasiveness among pituitary adenomas and carcinomas: An analysis using the MIB-1 antibody. Neurosurgery 1996, 38, 99–106, discussion 106–107. [Google Scholar] [CrossRef]
- Hlaváč, M.; Knoll, A.; Mayer, B.; Braun, M.; Karpel-Massler, G.; Etzrodt-Walter, G.; Coburger, J.; Wirtz, C.R.; Paľa, A. Ten years’ experience with intraoperative MRI-assisted transsphenoidal pituitary surgery. Neurosurg. Focus 2020, 48, E14. [Google Scholar] [CrossRef] [PubMed]
- Das, C.; Mondal, P.; Mukhopadhyay, M.; Mukhopadhyay, S.; Ghosh, I.; Handral, A. Evaluation of prognostic utility of Ki-67, P53, and O-6-methylguanine-DNA methyltransferase expression in pituitary tumors. J. Lab. Physicians 2019, 11, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Glebauskiene, B.; Liutkeviciene, R.; Vilkeviciute, A.; Gudinaviciene, I.; Rocyte, A.; Simonaviciute, D.; Mazetyte, R.; Kriauciuniene, L.; Zaliuniene, D. Association of Ki-67 Labelling Index and IL-17A with Pituitary Adenoma. BioMed Res. Int. 2018, 31, 7490585. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, J.; Jaffrain-Rea, M.L.; Vasiljevic, A.; Raverot, G.; Roncaroli, F.; Villa, C. How to Classify the Pituitary Neuroendocrine Tumors (PitNET)s in 2020. Cancers 2020, 12, 514. [Google Scholar] [CrossRef] [PubMed]
- Lugo, G.; Pena, L.; Cordido, F. Clinical manifestations and diagnosis of acromegaly. Int. J. Endocrinol. 2012, 2012, 540398. [Google Scholar] [CrossRef]
- Fusco, A.; Zatelli, M.C.; Bianchi, A.; Cimino, V.; Tilaro, L.; Veltri, F.; Angelini, F.; Lauriola, L.; Vellone, V.; Doglietto, F.; et al. Prognostic significance of the Ki-67 labeling index in growth hormone-secreting pituitary adenomas. J. Clin. Endocrinol. Metab. 2008, 93, 2746–2750. [Google Scholar] [CrossRef] [PubMed]
- Yakar, S.; Pennisi, P.; Zhao, H.; Zhang, Y.; LeLeroith, D. Circulating IGF-1 and Its Role in Cancer: Lessons from the IGF-1 Gene Deletion (LID) Mouse. Novartis Found. Symp. 2004, 262, 3–18. [Google Scholar] [PubMed]
- Yakar, S.; Leroith, D.; Brodt, P. The role of the growth hormone/insulin-like growth factor axis in tumor growth and progression: Lessons from animal models. Cytokine Growth Factor Rev. 2005, 16, 407–420. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, T.; Wu, J.; Huang, O.; Zhu, L.; He, J.; Li, Y.; Chen, W.; Chen, X.; Shen, K. Associations Between Circulating Insulin-Like Growth Factor 1 and Mortality in Women With Invasive Breast Cancer. Front. Oncol. 2020, 10, 1384. [Google Scholar] [CrossRef]
- Puche, J.E.; Castilla-Cortazar, I. Human conditions of insulin-like growth factor-I (IGF-I) deficiency. J. Transl. Med. 2012, 10, 224. [Google Scholar]
- Yeves, A.M.; Burgos, J.I.; Medina, A.J.; Villa-Abrille, M.C.; Ennis, I.L. Cardioprotective role of IGF-1 in the hypertrophied myocardium of the spontaneously hypertensive rats: A key effect on NHE-1 activity. Acta Physiol. 2018, 224, e13092. [Google Scholar] [CrossRef] [PubMed]
- Cittadini, A.; Ishiguro, Y.; Strömer, H.; Spindler, M.; Moses, A.C.; Clark, R.; Douglas, P.S.; Ingwall, J.S.; Morgan, J.P. Insulin-like growth factor-1 but not growth hormone augments mammalian myocardial contractility by sensitizing the myofilament to Ca2+ through a wortmannin-sensitive pathway. Circ. Res. 1998, 83, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, G.A.; Barrett-Connor, E.; Criqui, M.H.; Kritz-Silverstein, D. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: The Rancho Bernardo study. J. Clin. Endocrinol. Metab. 2004, 89, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Juul, A.; Scheike, T.; Davidsen, M.; Gyllenborg, J.; Jorgensen, T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease. Circulation 2002, 106, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Hongo, K.; Tada, T.; Sakai, K.; Kakizawa, Y.; Kobayashi, S. Growth pattern and rate in residual nonfunctioning pituitary adenomas: Correlations among tumor volume doubling time, patient age, and MIB-1 index. J. Neurosurg. 2003, 98, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, S.; Aboeerad, M.; Sharifi, F.; Tavangar, S.M.; Mohajeri-Tehrani, M. Associations of Ki-67 Labeling Index with Clinical and Paraclinical Features of Growth Hormone-Secreting Pituitary Adenomas: A Single Center Report from Iran. Int. J. Endocrinol. Metabol. 2019, 17, e81983. [Google Scholar] [CrossRef]
- Lyu, W.; Fei, X.; Chen, C.; Tang, Y. Normogram predictive model of post-operative recurrence in non-functioning pituitary adenoma. Gland Surg. 2021, 10, 807–815. [Google Scholar] [CrossRef]
- Pizzaro, C.B.; Oliveira, M.C.; Coutinho, L.B.; Ferreira, N.P. Measurement of Ki-67 antigen in 159 pituitary adenomas using the MIB-1 monoclonal antibody. Braz. J. Med. Biol. Res. 2004, 37, 235–243. [Google Scholar] [CrossRef]
- Mastronnardi, L.; Guiducci, A.; Spera, C.; Puzzilli, F.; Liberati, F.; Ruggeri, A.; Peciarolo, A. Adrenocorticotropic hormone secreting pituitary adenomas: Analysis of growth fraction using the MIB-1 antibody. Tumori 2000, 86, 229–232. [Google Scholar] [CrossRef]
- Kurotani, R.; Yasuda, M.; Oyama, K.; Egashira, N.; Sugaya, M.; Teramoto, A.; Osamura, R.Y. Expression of interleukin-6, interleukin-6 receptor (gp80), and the receptor’s signal-transducing subunit (gp130) in human normal pituitary glands and pituitary adenomas. Mod. Pathol. 2001, 14, 791–797. [Google Scholar] [CrossRef][Green Version]
- Marques, P.; Barry, S.; Carlsen, E.; Collier, D.; Ronaldson, A.; Awad, S.; Dorward, N.; Grieve, J.; Mendoza, N.; Muquit, S.; et al. Chemokins modulate the tumour microenvironment in pituitary neuroendocrine tumours. Acta Neuropathol. Commun. 2019, 7, 172. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, S.H.; Yang, M.; Chen, Z.H.; Li, S.T. The diagnostic value of preoperative inflammatory markers in craniopharyngioma: A multicenter cohort study. J. Neurooncol. 2018, 138, 113–122. [Google Scholar] [CrossRef]
- Ambrogio, A.G.; De Martin, M.; Ascoli, P.; Cavagnini, F.; Pecori Giraldi, F. Gender-dependent changes in haematological parameters in patients with Cushing´s disease before and after remission. Eur. J. Endocrinol. 2014, 170, 393–400. [Google Scholar] [CrossRef]
- Masri-Iraqi, H.; Robenshtok, E.; Tzvetov, G.; Manistersky, Y.; Shimon, I. Elevated white blood cell counts in Cushing´s disease: Association with hypercortisolism. Pituitary 2014, 17, 393–400. [Google Scholar] [CrossRef]
- Marques, P.; de Vries, F.; Dekkers, O.M.; van Furth, W.R.; Korbonits, M.; Biermasz, N.R.; Pereira, A.M. Pre-operative serum inflammation-based scores in patients with pituitary adenomas. Pituitary 2021, 24, 334–350. [Google Scholar] [CrossRef]
- Wei, L.; Yue, Z.; Wang, S. Immunopathological study of plurihormonal pituitary adenomas. Chin. J. Neurosurg. 2008, 13, 208. [Google Scholar]
- Ho, D.M.; Hsu, C.Y.; Ting, L.T.; Chiang, H. Plurihormonal pituitary adenomas: Immunostaining of all pituitary hormones is mandatory for correct classification. Histopathology 2001, 39, 310–319. [Google Scholar] [CrossRef]
- Shi, R.; Wan, X.; Yan, Z.; Tan, Z.; Liu, X.; Lei, T. Clinicopathological Characteristics of Plurihormonal Pituitary Adenoma. Front. Surg. 2022, 9, 826720. [Google Scholar] [CrossRef]
- Ozer, E.; Canda, M.S.; Ulukus, C.; Guray, M.; Erbayraktar, S. Expression of Bcl-2, Bax and p53 proteins in pituitary adenomas: An immunohistochemical study. Tumori 2003, 89, 54–59. [Google Scholar] [CrossRef]
- Zakir, J.C.; Casulari, L.A.; Rosa, J.W.; Rosa, J.W.; de Mello, P.A.; de Malhaes, A.V.; Naves, L.A. Prognostic Value of Invasion, Markers of Proliferation, and Classification of Giant Pituitary Tumors, in a Georeferred Cohort in Brazil of 50 Patients, with a Long-Term Postoperative Follow-Up. Int. J. Endocrinol. 2016, 2016, 7964523. [Google Scholar] [CrossRef]
- Jang, M.H.; Kim, H.J.; Chung, Y.R.; Lee, Y.; Park, S.Y. A comparison of Ki-67 counting methods in luminal Breast Cancer: The Average Method vs. the Hot Spot Method. PLoS ONE 2017, 12, e0172031. [Google Scholar] [CrossRef] [PubMed]
| Median Age (IQR) (In y) | 60 (47–70) |
|---|---|
| Sex Female Male | 49 (45.0%) 60 (55.0%) |
| Median preoperative KPS (IQR) | 90 (90–100) |
| Maximum diameter (mm) | 21 (14–27) |
| Cavernous sinus invasion | 43 (39.4%) |
| Pituitary apoplexy | 6 (5.5%) |
| Extent of resection Gross total resection Subtotal resection | 67 (61.5%) 42 (38.5%) |
| Pathological tumor types Gonadotroph adenoma Plurihormonal adenoma Null cell adenoma Corticotroph adenoma Somatotroph adenoma Prolactinoma Thyrotroph adenoma | 49 (45%) 35 (32.1%) 11 (10.1%) 8 (7.3%) 3 (2.8%) 2 (1.8%) 1 (0.9%) |
| Median MIB-1 labeling index (%), IQR | 3 (2–4) |
| p53 expression (available in 88 patients) | 44 (50.0%) |
| Variable | MIB-1 < 4% (n = 77) | MIB-1 ≥ 4% (n = 32) | p-Value |
|---|---|---|---|
| Sex Female Male | 38 (49.4%) 39 (50.6%) | 11 (34.3%) 21 (65.5%) | 0.21 |
| Age, mean ± SD | 61.4 ± 15.6 | 49.3 ± 15.9 | <0.001 |
| Preoperative KPS, mean ± SD | 90.9 ± 10.8 | 92.2 ± 8.7 | 0.55 |
| Body mass index, mean ± SD | 28.7 ± 5.8 | 30.8 ± 9.1 | 0.16 |
| Acromegaly Present Not present | 12 65 | 6 26 | 0.78 |
| Cushing´s disease Present Not present | 2 75 | 2 30 | 0.58 |
| Diabetes Present Not present | 8 (10.4%) 69 (89.6%) | 6 (18.8%) 26 (81.2%) | 0.35 |
| Acetylsalicylic acid intake Present Not present | 8 10.4%) 69 (89.6%) | 3 (18.8%) 29 (81.2%) | 0.99 |
| Pituitary apoplexy Present Not present | 4 (5.2%) 73 (94.8%) | 2 (6.3%) 30 (93.7%) | 0.99 |
| Maximum diameter (mm), mean ± SD | 21.8 ± 9.8 | 21.3 ± 9.0 | 0.81 |
| Microadenoma Macroadenoma | 13 (16.9%) 64 (83.1%) | 6 (18.7%) 26 (81.3%) | 0.79 |
| Plurihormonal (immunohistochemical) Present Not present | 24 (31.2%) 53 (61.8%) | 11 (34.4%) 21 (65.6%) | 0.82 |
| Cavernous sinus invasion Present Not present | 28 (36.4%) 49 (63.6%) | 15 (46.9%) 17 (53.1%) | 0.39 |
| Preoperative hormone replacement Hydrocortisone Levothyroxine Testosterone Levothyroxine and testosterone Hydrocortisone and levothyroxine Hydrocortisone and testosterone and levothyroxine None | 12 (15.6%) 11 (14.3%) 0 (0.0%) 1 (1.3%) 10 (13.0%) 1 (1.3%) 42 (54.5%) | 11 (34.4%) 5 (15.6%) 1 (3.1%) 0 (0.0%) 4 (12.5%) 0 (0.0%) 11 (34.4%) | 0.14 |
| p53 expression (available in 88 patients) present not present | 27 (46.55%) 31 (53.45%) | 17 (56.66%) 13 (43.33%) | 0.50 |
| Baseline Plasma fibrinogen (g/L), mean ± SD | 3.19 ± 0.89 | 3.55 ± 0.89 | 0.06 |
| Baseline Serum C-reactive protein (mg/I), mean ± SD | 4.76 ± 14.81 | 6.22 ± 11.55 | 0.62 |
| Baseline TSH (µU/mL), mean ± SD | 1.30 ± 0.83 | 1.44 ± 0.81 | 0.45 |
| Baseline IGF-1 (ng/mL), mean ± SD | 189.56 ± 238.51 | 293.46 ± 310.77 | 0.06 |
| Baseline ACTH (pg/mL), mean ± SD | 26.76 ± 20.34 | 39.54 ± 34.36 | 0.03 |
| Baseline LH (U/I), mean ± SD | 5.15 ± 6.40 | 3.56 ± 4.68 | 0.22 |
| Baseline FSH (mIU/mL), mean ± SD | 13.53 ± 18.47 | 8.07 ± 12.63 | 0.09 |
| Baseline prolactin (ng/mL), mean ± SD | 29.18 ± 33.00 | 28.48 ± 39.73 | 0.93 |
| Baseline estradiol (pg/mL), mean ± SD | 21.38 ± 25.77 | 47.84 ± 93.66 | 0.38 |
| Baseline testosterone (ng/mL), mean ± SD | 1.98 ± 1.85 | 1.53 ± 1.11 | 0.20 |
| Baseline growth hormone (ng/mL), mean ± SD | 5.15 ± 12.64 | 6.50 ± 12.24 | 0.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiseyeu, I.; Güresir, Á.; Vatter, H.; Herrlinger, U.; Becker, A.; Wach, J.; Güresir, E. Preoperative Risk Stratification of Increased MIB-1 Labeling Index in Pituitary Adenoma: A Newly Proposed Prognostic Scoring System. J. Clin. Med. 2022, 11, 7151. https://doi.org/10.3390/jcm11237151
Maiseyeu I, Güresir Á, Vatter H, Herrlinger U, Becker A, Wach J, Güresir E. Preoperative Risk Stratification of Increased MIB-1 Labeling Index in Pituitary Adenoma: A Newly Proposed Prognostic Scoring System. Journal of Clinical Medicine. 2022; 11(23):7151. https://doi.org/10.3390/jcm11237151
Chicago/Turabian StyleMaiseyeu, Ivan, Ági Güresir, Hartmut Vatter, Ulrich Herrlinger, Albert Becker, Johannes Wach, and Erdem Güresir. 2022. "Preoperative Risk Stratification of Increased MIB-1 Labeling Index in Pituitary Adenoma: A Newly Proposed Prognostic Scoring System" Journal of Clinical Medicine 11, no. 23: 7151. https://doi.org/10.3390/jcm11237151
APA StyleMaiseyeu, I., Güresir, Á., Vatter, H., Herrlinger, U., Becker, A., Wach, J., & Güresir, E. (2022). Preoperative Risk Stratification of Increased MIB-1 Labeling Index in Pituitary Adenoma: A Newly Proposed Prognostic Scoring System. Journal of Clinical Medicine, 11(23), 7151. https://doi.org/10.3390/jcm11237151








