Abstract
Background: As a preventive procedure, minimizing periprocedural risk is crucially important during left atrial appendage closure (LAAC). Methods: We included consecutive patients receiving LAAC at nine centres and assessed the relationship between baseline characteristics and the acute procedural outcome. Major procedural complications were defined as all complications requiring immediate invasive intervention or causing irreversible damage. Logistic regression was performed and included age and left-ventricular function. Furthermore, the association between acute complications and long-term outcomes was evaluated. Results: A total of 405 consecutive patients with a median age of 75 years (37% female) were included. 47% had a history of stroke. Median CHA2DS2-VASc score was 4 (interquartile range, 3–5) and the median HAS-BLED score was 3 (2–4). Major procedural complications occurred in 7% of cases. Low haemoglobin (OR 0.8, 95% CI 0.65–0.99 per g/dL, p = 0.040) and end-stage kidney disease (OR 13.0, CI 2.5–68.5, p = 0.002) remained significant in multivariate analysis. Anaemia (haemoglobin < 12 and < 13 g/dL in female and male patients) increased the risk of complications 2.2-fold. Conclusions: The major complication rate was low in this high-risk patient population undergoing LAAC. End-stage kidney disease and low baseline haemoglobin were independently associated with a higher major complication rate.
1. Introduction
Left atrial appendage closure (LAAC) is an interventional procedure for stroke prevention in patients with atrial fibrillation (AF). While oral anticoagulation (OAC) is considered standard treatment in most patients with an elevated stroke risk, LAAC may be performed as an alternative in patients with a high thromboembolic risk and are contraindicated to long-term OAC therapy (recommendation IIb by ACC and ESC guidelines) [1,2]. Another emerging indication is recurrent stroke despite adequate antithrombotic treatment [3]. Since the approval of LAAC by authorities, there has been some emerging experience with this technology in the western world [4,5].
While LAAC showed favourable results in patients who possess contraindications to OAC [6,7,8] and even compared to direct OAC (DOAC) therapy [9,10], it still represents a purely preventive procedure without proven acute benefit to the patient. Therefore, the avoidance of procedural complications is important. The identification of predictors for complications may improve the patient selection process and guide optimal procedural preparation.
The goal of this analysis was to identify predictors for acute procedural complications in consecutive patients undergoing LAAC and to evaluate the importance of centre experience on complications and long-term outcomes.
2. Materials and Methods
This is a subanalysis of the Austrian LAAC Registry (NCT03409159). The registry includes all patients, who underwent LAAC in Austria between 2010 and 2021. The study is approved by the institutional review board of the Medical University of Graz (29-355 ex 16/17).
2.1. Recruitment and Procedure
Details about the recruitment, indications, and outcomes in LAAC patients in Austria have been published elsewhere [11]. In short, patients with AF, a high thromboembolic risk, and either contraindications, intolerances, or ineffectiveness of OAC were evaluated at each of the referral centres in Austria. Both the decision to perform LAAC and the selection of device was left to the operators’ or institutes’ discretion, according to international guidelines [1] and the vendors’ standard operating protocol. Used devices were Watchman™, Watchman FLX™ (Boston Scientific, Marlborough, MA, USA), Amplatzer Cardiac Plug™, Amplatzer Amulet™ (Abbott Laboratories, North Chicago, IL, USA) or LAmbre™ (Lifetech Scientific, Shenzhen, China). Post-procedural management and antithrombotic treatment were left to the operators’ discretion, tailored according to the patients’ individual risk profiles. Follow up included transoesophageal echocardiography and regular ambulatory checks.
2.2. Data Collection
Clinical data of consecutive patients undergoing LAAC at participating centres were captured, considering recommendations from the European Heart Rhythm Association (EHRA) and the European Association of Percutaneous Cardiovascular Interventions (EAPCI) [5] by either a local representative or an external reviewer. Mortality data was included from the Austrian government’s population registry. Patient inclusion was complete in all centres until the end of 2019 and in 8/9 centres (88.9%) until the end of 2020.
2.3. Endpoints
Major procedural complications were defined as all complications requiring immediate invasive intervention. Cardiac tamponade was defined as pericardial effusion requiring pericardiocentesis or surgery. Access site complications were documented if they required invasive intervention. Ischaemic stroke was defined as clinically relevant ischaemic stroke according to current guidelines [12]. Other procedural complications were summarized as minor procedural complications. Bleeding requiring transfusion was explicitly defined as a minor procedural complication. Pre-procedural anaemia was defined as haemoglobin <12 mg/dL in female patients and <13 mg/dL in male patients [13]. For long-term outcome assessment, one-year survival was analysed.
2.4. Statistical Analysis
We expressed parameters as count (proportion), mean ± standard deviation, or median (interquartile range), as appropriate. Depending on the presence of a normal distribution, calculated by the Shapiro-Wilk test, either the student’s t-test or the Wilcoxon signed-rank test was used for univariable analysis. For outcome analyses, multivariable analysis was performed using logistics regression analysis, including all parameters with significant differences between patients with and without complications (p < 0.05), age, and left ventricular function. For the interdependent variables (e.g., haematocrit and haemoglobin), only one parameter with the highest significance was allowed into the multivariable analysis. Multiple imputation with five iterations was used for missing values. A two-sided p value < 0.05 was considered significant. For statistical analysis, R 4.2.0 (The R Project, Vienna, Austria) and RStudio 2022.02.1 Build 461 (RStudio PBC, Boston, MA, USA) were used.
3. Results
A total of 405 patients undergoing LAAC between November 2010 and December 2021 at 9 Austrian centres were included into this analysis.
3.1. Baseline Characteristics
The median age was 75 years and 37% were female (Table 1). The median (IQR) body mass index was 27 (24–30) kg/m2 and body surface area was 1.92 ± 0.21 m2. The median (IQR) CHA2DS2-VASc score was 4 (3–5) and HAS-BLED score was 3 (2–4). The most common comorbidities were arterial hypertension (88.4%), coronary artery disease (41%), and previous stroke (47%). Ischaemic stroke was present in 25%, haemorrhagic stroke in 26% and anaemia in 38%. Other comorbidities are summarized in Table 1.

Table 1.
Baseline characteristics.
The median haemoglobin was 12.5 g/dL and median creatinine was 1.1 mg/dL, leading to a median estimated glomerular filtration rate of 75 mL/min/1.73 m2.
3.2. Procedural Details and Outcome
The procedural details and outcomes until discharge was complete for 100% of patients. Patients received LAAC because of prior bleeding (66%), thromboembolism (10.0%) or other reasons (24%). An isolated LAAC procedure was planned in 90.0% of patients; combined procedures included closure of the patent foramen ovale or atrial septal defect (5%), cardiac ablation (3%), edge-to-edge mitral repair (2%), coronary angiography (1%), pacemaker implantation (0.2%), and transcutaneous aortic valve implantation (0.2%). The most common anticipated device was Amplatzer (56%), followed by Watchman (43%), and LAmbre (0.5%).
The median procedural duration was 70 (IQR 53–98) minutes with a median fluoroscopy time of 16 (11–22) minutes and a dose area product of 4433 µGym2 (Table 2). The device was successfully implanted in 97% of the cases.

Table 2.
Procedural outcome.
A complication occurred in 19% of patients and major procedural complications arose in 7%. Access site complications requiring intervention occurred in 3%, cardiac tamponade requiring intervention in 2%, and ischaemic stroke in 1% of patients (Table 2). Furthermore, the following complications occurred in the whole population: cardiopulmonary resuscitation (0.5%, n = 2), open heart surgery (surgical retrieval of an embolized and dislocated device and surgical treatment of cardiac perforation with tamponade; 0.5%, n = 2), and interventional retrieval of dislocated LAAC device (0.5%, n = 2; Table 2).
Periprocedural survival was 99.5%. Two deaths in association with the procedure occurred:
- One patient had dislocation of the LAAC device into the left atrium two days after the procedure, requiring cardiac surgery. The patient aspirated in the postoperative phase, developed a severe pneumonia, and died due to respiratory failure at day 27 after LAAC.
- One patient developed massive throat bleeding, probably caused by the insertion of the transoesophageal probe, leading to tracheal obstruction on the day of the LAAC. Despite an emergency tracheotomy and resuscitation, the patient deteriorated and passed away in the operating theatre.
Remaining patients were discharged after a median of 2 (IQR, 1–3) days.
Minor complications occurred in 16.8% of patients, including a transfer to the intensive care unit, catecholamine support (6% each), failure to implant the device (3%), bleeding requiring transfusion (3%), new pericardial effusion without intervention (5%), and prolonged hospital stay (4%).
3.3. Predictors for Complications
End-stage kidney injury requiring chronic dialysis was associated with major complications with a bivariate OR of 16 (95% CI 3–70, p = 0.001). The prevalence of end-stage kidney disease among patients with and without complications was 14% and 1%, respectively. Furthermore, low haemoglobin and low erythrocytes were associated with increased risk of complications (haemoglobin: 11.3 vs. 12.7 mg/dL in patients with vs. without complications, OR 0.8, 95% 0.6–0.9, p = 0.010; haematocrit: 35% vs. 38%, p = 0.016). Anaemic patients had a 2.2-fold increased risk of major complications (9.6% vs. 4.4%, p = 0.047).
There were no differences in major complications among patients receiving Amplatzer vs. Watchman devices (8% vs. 5%, n = 0.240). However, minor complications occurred significantly more often in the Amplatzer group (22% vs. 9%, p < 0.001), mostly due to a higher rate of shock (9% vs. 1%, p < 0.001). Other distinct complications were similar between both devices. Most notably, procedure duration (83 vs. 64 min, p = 0.001) and dose area product (6972 vs. 1750 µGym2, p < 0.001) were increased in patients receiving Amplatzer devices.
Neither minor complications, major complications, nor periprocedural death were correlated with low centre volume (p = 0.107 for major complications, Figure 1). The major complication rate was similar between low-volume centres (defined as total procedure count below the median, 3%) and high-volume centres (remaining centres, 8%, p = 0.268).
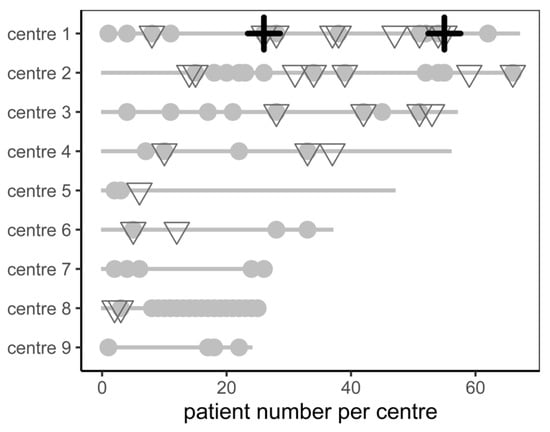
Figure 1.
Patient count, minor complications (grey dot), major complications (grey triangle), and periprocedural mortality (black cross) stratified by centre.
3.4. Multivariate Analysis
When adjusted for age and left ventricular function, both dialysis (OR 13, 95% confidence interval 2.5–68.5, p = 0.002) and haemoglobin (OR 0.80, 95% CI 0.65–0.99 per g/dL, p = 0.040) remained as independent predictors of major complications and death (Table 3).

Table 3.
Multivariate logistic regression analysis with the endpoint as major periprocedural complications.
3.5. Complications and Long-Term Outcomes
One-year follow up regarding mortality was complete in 78% of patients. Major complications were associated with reduced one-year survival (cumulative survival 85.0% vs. 94.2%, p = 0.040, Figure 2).
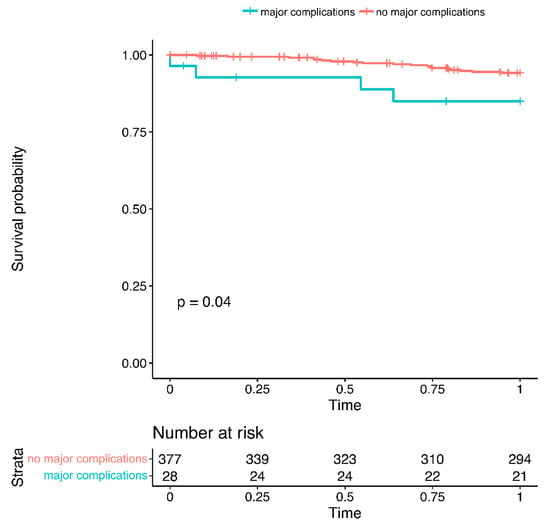
Figure 2.
One-year mortality in patients with vs. without major complications.
4. Discussion
This study shows that (1) major complications and procedural death occurred relatively rarely in a high-risk patient cohort receiving LAAC, (2) end-stage kidney failure and low haemoglobin were independently associated with a worse short-term outcome after LAAC, and (3) low centre experience was not associated with increased major complication rates, which themselves (4) were associated with higher long-term mortality.
Currently, patients undergoing LAAC in Austria represent a high-risk cohort with a mean CHA2DS2-VASc score of 4.4, a median HAS-BLED score of 3.1, and a history of stroke in almost half of patients (47%). Due to limitations in reimbursement, a dramatic ischaemic or haemorrhagic event usually precedes the decision to perform LAAC in most patients [11]. Baseline ischaemic and haemorrhagic risk is similar to the Left Atrial Appendage Closure Registry [4].
As LAAC is a preventive measure without any acute symptom improvement, any complication imposes a burden on a patient who otherwise would have been asymptomatic. Furthermore, the beneficial effect of LAAC cannot be predicted on an individual level. Therefore, the decision to perform LAAC must be evaluated thoroughly, and the complication rate must be kept at a low level. This is especially important as major complications were associated with long-term mortality in this cohort.
4.1. Types of Complications
The most common major complications are access site complications. The use of ultrasound-guided puncture and adequate post-procedural management must be emphasized, as well as vascular closure devices as appropriate [5].
Pericardial tamponade occurs in a substantial proportion of patients and requires quick action from the medical team. Fortunately, the prognosis after interventional pericardiocentesis is good. In this analysis, there was only one case requiring bailout heart surgery with a positive result. Emergency plans and standard operation procedures for handling acute cardiac tamponade are required to be in place, with fast transfer to a cardiac surgery department in the event of a failed interventional drainage.
Furthermore, the device dislocated in three cases, but interventional capture was possible in two of them. Unfortunately, ischaemic stroke also occurred in a proportion of patients during the procedure.
The reduction of periprocedural complications may be achieved through adequate patient selection, patient preparation, and optimized procedural management. The preprocedural workflow may be further supported by individual computational simulation technology [14].
Fortunately, “typical” complications of LAAC were treated very well without immediate mortality. Only one patient suffered from throat bleeding, a rare complication of LAAC, and died in the cath lab. Further deaths may have been prevented by adequate training of the responsible medical staff for common complications.
4.2. Predictors for Complications
Currently, there is no optimal treatment strategy for stroke prevention in patients on dialysis, as they have both a high ischaemic and bleeding risk, and the experience of DOACs in this patient population is limited [1]. On the one hand, these patients have a ten times elevated risk of stroke [15] and may therefore benefit most from LAAC. However, end-stage renal failure is also associated with competing complications that lead to morbidity and mortality, such as acute myocardial infarction, heart failure, and sudden cardiac death [16]. In this analysis, end-stage chronic kidney failure was independently associated with increased major complication rates. Therefore, the benefits and risks of LAAC have to be weighed carefully in this fragile patient population. These results are in conflict with Jamal et al. and Benini Tapias et al., who did not find a worse outcome in LAAC patients with chronic kidney disease [17,18]. In other cardiac interventions, such as percutaneous coronary intervention and transcatheter aortic valve implantation, end-stage renal disease was also associated with poorer acute outcomes [19,20].
A low haemoglobin level was independently associated with increased major complications in the multivariable analysis, although periprocedural bleeding requiring transfusion was not defined as a major complication. Of note, there was no association between the history of anaemia, previous bleeding, or previous blood transfusions and major complications. We hypothesise that suboptimal preparation before the procedure, for example, hypoferremia or uncorrected anaemia after a recent bleeding event, may be associated with increased complications. Patients with low haemoglobin levels undergoing LAAC may have fewer physiological resources to cope with acute stress on the cardiovascular system, leading to quicker deterioration and therefore a higher risk of complications. It can be speculated that appropriate preparation, such as control of the bleeding and iron supplementation, may have ameliorated short-term outcomes. While low haemoglobin levels were associated with poorer outcomes in elective non-cardiac surgery [21,22] and cardiac surgery [23], this is the first study to evaluate haemoglobin on acute outcome in LAAC. Despite low evidence from mainly retrospective analyses [24], there is general agreement that adequate preoperative preparation may be useful in patients with anaemia [22]. Laboratory parameters other than haemoglobin and haematocrit were not associated with the periprocedural complication rate.
Regarding procedural management, we did not find differences in major complications in patients receiving Amplatzer and Watchman devices. This is reassuring, as there was a significant difference described by Qiao et al. and Lakkireddy et al. [25,26]. However, the rate of minor complications was higher in Amplatzer devices. It is unclear to the authors if this difference is relevant as the administration of catecholamines in patients with low blood pressure and the threshold for transfer to the intensive care unit may vary between centres. Of note, minor complications were not spread equally across centres (Figure 1).
In our analysis, there was no sign of increased complications in centres with low experience (Figure 1). The major complication rate was 3% in low-volume centres and 8% in high-volume centres (p = ns). A previous report from 2016 showed better outcomes and fewer complications after LAAC of 30 patients [27]. Proper proctoring and optimization of standard operating procedures may have reduced the risk of early procedural complications in learning investigators.
Furthermore, literature suggests liver cirrhosis [28], female sex [29], and increased age combined with previous gastrointestinal bleeding [30] are predictive factors for procedural complications. These parameters were not associated with a worse prognosis in this analysis.
4.3. Limitations
While the major strengths of this study are the inclusion of all consecutive patients undergoing LAAC in a whole country, external evaluation of clinical data, and complete follow up regarding survival, it still represents a retrospective study with all forms of associated bias. Furthermore, as this cohort presents a high-risk cohort, the results may not be applicable to other patient cohorts with different risk profiles. Finally, this analysis focused on procedural complications and not on the overall long-term benefits of LAAC.
5. Conclusions
Despite the fact that the patient cohort undergoing LAAC in Austria resembled a high-risk cohort, the rate of major procedural complications was considerably low. Chronic dialysis and low haemoglobin were independent predictors of complications.
Author Contributions
Conceptualization, D.Z. and D.S.; Data curation, D.Z. and D.S.; Formal analysis, D.Z. and D.S.; Funding acquisition, D.Z. and D.S.; Investigation, D.Z., L.F., G.G.T., A.S., G.D.-K., G.S., H.G., R.K.B., M.R., M.P., P.V., B.L., C.S., K.S., F.H., S.M., F.B., M.M., W.T., N.V., K.A., A.Z. and D.S.; Methodology, D.Z. and D.S.; Project administration, D.Z. and D.S.; Resources, D.Z. and D.S.; Software, D.Z.; Supervision, D.Z., A.Z. and D.S.; Validation, D.Z. and D.S.; Visualization, D.Z. and D.S.; Writing—original draft, D.Z. and D.S.; Writing—review & editing, D.Z., L.F., G.G.T., A.S., G.D.-K., G.S., H.G., R.K.B., M.R., M.P., P.V., B.L., C.S., K.S., F.H., S.M., F.B., M.M., W.T., N.V., K.A., A.Z. and D.S. All authors have read and agreed to the published version of the manuscript.
Funding
The Austrian Left Atrial Appendage Closure Registry received funding in the form of an unrestricted grant from Boston Scientific. This work received no specific funding.
Institutional Review Board Statement
This analysis has been approved by the institutional review board of the Medical University of Graz (29-355 ex 16/17).
Informed Consent Statement
According to the decision of the institutional review board, patient consent was waived as no specific study-related procedures were performed and data collection was retrospective.
Data Availability Statement
Raw data are available upon reasonable request from the corresponding author.
Acknowledgments
We owe our sincere gratitude to all colleagues of the participating centres for their collaboration.
Conflicts of Interest
G.G.T: consulting fees from Abbott, Medtronic, Terumo, and Biotronik; D.Z.: consulting fees from Daiichi Sankyo, unrestricted grant from Boston Scientific; F.B.: grants from Boston Scientific and Abbott Laboratories, consulting fees from Boston Scientific, speaker honoraria and travel support from Daichii Sankyo. R.K.B.: proctor for Boston Scientific, speaker fees from Abbott, and travel support from Boston Scientific and Abbott. All remaining authors report no conflict of interest whatsoever.
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019, 140, e125. [Google Scholar] [CrossRef] [PubMed]
- Masjuan, J.; Salido, L.; DeFelipe, A.; Hernández-Antolín, R.; Fernández-Golfín, C.; Cruz-Culebras, A.; Matute, C.; Vera, R.; Pérez-Torre, P.; Zamorano, J.L. Oral anticoagulation and left atrial appendage closure: A new strategy for recurrent cardioembolic stroke. Eur. J. Neurol. 2019, 26, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.V.; Varosy, P.; Price, M.J.; Slotwiner, D.; Kusumoto, F.M.; Rammohan, C.; Kavinsky, C.J.; Turi, Z.G.; Akar, J.; Koutras, C.; et al. The NCDR Left Atrial Appendage Occlusion Registry. J. Am. Coll. Cardiol. 2020, 75, 1503–1518. [Google Scholar] [CrossRef]
- Glikson, M.; Wolff, R.; Hindricks, G.; Mandrola, J.; Camm, A.J.; Lip, G.Y.H.; Fauchier, L.; Betts, T.R.; Lewalter, T.; Saw, J.; et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion—An update. Europace 2020, 22, 184. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Holmes, D.; Doshi, S.K.; Neuzil, P.; Kar, S. Safety of percutaneous left atrial appendage closure: Results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation 2011, 123, 417–424. [Google Scholar] [CrossRef]
- Holmes, D.R., Jr.; Kar, S.; Price, M.J.; Whisenant, B.; Sievert, H.; Doshi, S.K.; Huber, K.; Reddy, V.Y. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J. Am. Coll. Cardiol. 2014, 64, 1–12. [Google Scholar] [CrossRef]
- Holmes, D.R.; Reddy, V.Y.; Turi, Z.G.; Doshi, S.K.; Sievert, H.; Buchbinder, M.; Mullin, C.M.; Sick, P.; Investigators, P.A. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: A randomised non-inferiority trial. Lancet 2009, 374, 534–542. [Google Scholar] [CrossRef]
- Osmancik, P.; Herman, D.; Neuzil, P.; Hala, P.; Taborsky, M.; Kala, P.; Poloczek, M.; Stasek, J.; Haman, L.; Branny, M.; et al. Left Atrial Appendage Closure Versus Direct Oral Anticoagulants in High-Risk Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 75, 3122–3135. [Google Scholar] [CrossRef]
- Osmancik, P.; Herman, D.; Neuzil, P.; Hala, P.; Taborsky, M.; Kala, P.; Poloczek, M.; Stasek, J.; Haman, L.; Branny, M.; et al. 4-Year Outcomes After Left Atrial Appendage Closure Versus Nonwarfarin Oral Anticoagulation for Atrial Fibrillation. J. Am. Coll. Cardiol. 2022, 79, 1–14. [Google Scholar] [CrossRef]
- Zweiker, D.; Sieghartsleitner, R.; Fiedler, L.; Toth, G.G.; Luha, O.; Stix, G.; Gabriel, H.; Vock, P.; Lileg, B.; Strouhal, A.; et al. Indications and Outcome in Patients Undergoing Left Atrial Appendage Closure-The Austrian LAAC Registry. J. Clin. Med. 2020, 9, 3274. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; World Health Organization: Geneva, Switzerland, 2011.
- Garot, P.; Iriart, X.; Aminian, A.; Kefer, J.; Freixa, X.; Cruz-Gonzalez, I.; Berti, S.; Rosseel, L.; Ibrahim, R.; Korsholm, K.; et al. Value of FEops HEARTguide patient-specific computational simulations in the planning of left atrial appendage closure with the Amplatzer Amulet closure device: Rationale and design of the PREDICT-LAA study. Open Heart 2020, 7, e001326. [Google Scholar] [CrossRef] [PubMed]
- Power, A. Stroke in dialysis and chronic kidney disease. Blood Purif 2013, 36, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Tokmakova, M.P.; Skali, H.; Kenchaiah, S.; Braunwald, E.; Rouleau, J.L.; Packer, M.; Chertow, G.M.; Moye, L.A.; Pfeffer, M.A.; Solomon, S.D. Chronic kidney disease, cardiovascular risk, and response to angiotensin-converting enzyme inhibition after myocardial infarction: The Survival And Ventricular Enlargement (SAVE) study. Circulation 2004, 110, 3667–3673. [Google Scholar] [CrossRef]
- Jamal, S.; Mughal, M.S.; Kichloo, A.; Edigin, E.; Khan, M.Z.; Minhas, A.M.K.; Ali, M.; Kanjwal, K. Left atrial appendage closure using WATCHMAN device in chronic kidney disease and end stage renal disease patients. Pacing Clin. Electrophysiol. 2022, 45, 866–873. [Google Scholar] [CrossRef]
- Benini Tapias, J.; Flores-Umanzor, E.; Cepas-Guillén, P.L.; Regueiro, A.; Sanchís, L.; Broseta, J.J.; Cases, A.; Freixa, X. Prognostic impact of the presence of chronic kidney disease on percutaneous left trial appendage closure for atrial fibrillation: A single center experience. Nefrologia (Engl. Ed.) 2022, 42, 290–300. [Google Scholar] [CrossRef]
- Dunn, A.N.; Huded, C.; Simpfendorfer, C.; Raymond, R.; Kapadia, S.; Tuzcu, E.M.; Ellis, S.G. End-stage renal disease as an independent risk factor for in-hospital mortality after coronary drug-eluting stenting: Understanding and modeling the risk. Catheter. Cardiovasc. Interv. 2021, 98, 246–254. [Google Scholar] [CrossRef]
- Schymik, G.; Bramlage, P.; Herzberger, V.; Bergmann, J.; Conzelmann, L.O.; Wurth, A.; Luik, A.; Schrofel, H.; Tzamalis, P. Impact of Dialysis on the Prognosis of Patients Undergoing Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2019, 123, 315–322. [Google Scholar] [CrossRef]
- Muñoz, M.; Laso-Morales, M.J.; Gómez-Ramírez, S.; Cadellas, M.; Núñez-Matas, M.J.; García-Erce, J.A. Pre-operative haemoglobin levels and iron status in a large multicentre cohort of patients undergoing major elective surgery. Anaesthesia 2017, 72, 826–834. [Google Scholar] [CrossRef]
- Mueller, M.M.; Van Remoortel, H.; Meybohm, P.; Aranko, K.; Aubron, C.; Burger, R.; Carson, J.L.; Cichutek, K.; De Buck, E.; Devine, D.; et al. Patient Blood Management: Recommendations From the 2018 Frankfurt Consensus Conference. JAMA 2019, 321, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Shavit, L.; Hitti, S.; Silberman, S.; Tauber, R.; Merin, O.; Lifschitz, M.; Slotki, I.; Bitran, D.; Fink, D. Preoperative hemoglobin and outcomes in patients with CKD undergoing cardiac surgery. Clin. J. Am. Soc. Nephrol. 2014, 9, 1536–1544. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ranucci, M.; Pavesi, M.; Pistuddi, V.; Baryshnikova, E. Preoperative Anemia Correction in Cardiac Surgery: A Propensity-Matched Study. J. Cardiothorac. Vasc. Anesth. 2021, 35, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Zhang, B.; Wang, J.; Pan, L.; Cheng, T.; Wang, Y.; Xiong, E. Comparison between Amplatzer and Watchman left atrial appendage closure devices for stroke prevention in atrial fibrillation: A systematic review and meta-analysis. Cardiology 2022, 147, 290–297. [Google Scholar] [CrossRef]
- Lakkireddy, D.; Thaler, D.; Ellis, C.R.; Swarup, V.; Sondergaard, L.; Carroll, J.; Gold, M.R.; Hermiller, J.; Diener, H.C.; Schmidt, B.; et al. Amplatzer amulet left atrial appendage occluder versus watchman™ device for stroke prophylaxis (amulet ide): A randomized controlled trial. Circulation 2021, 144, 1543–1552. [Google Scholar] [CrossRef]
- Ledwoch, J.; Krollmann, C.; Staubach, S.; Hug, M.; Strohm, H.; Mudra, H. Learning Curve Assessment for Percutaneous Left Atrial Appendage Closure With the WATCHMAN Occluder. J. Interv. Cardiol. 2016, 29, 393–399. [Google Scholar] [CrossRef]
- Thotamgari, S.R.; Sheth, A.R.; Patel, H.P.; Bretzman, J.; Ward, R.C.; Thakkar, S.; Patel, J.T.; Asirvatham, S.J.; Holmes, D.R., Jr.; Egbe, A.; et al. Liver cirrhosis is independently associated with increased in-hospital mortality in patients undergoing left atrial appendage occlusion device implantation. Heart Rhythm 2022, 19, 1392–1393. [Google Scholar] [CrossRef]
- Patel, N.; Ranka, S.; Hajra, A.; Bandyopadhyay, D.; Amgai, B.; Chakraborty, S.; Khalid, M.; Goyal, A.; Dalia, T.; Reddy, Y.M.; et al. Gender-Specific Outcomes after Percutaneous Left Atrial Appendage Closure—A Nationwide Readmission Database Analysis. J. Cardiovasc. Electrophysiol. 2022, 33, 430–436. [Google Scholar] [CrossRef]
- López-Mínguez, J.R.; Nogales-Asensio, J.M.; Infante De Oliveira, E.; Santos, L.; Ruiz-Salmerón, R.; Arzamendi-Aizpurua, D.; Costa, M.; Gutiérrez-García, H.; Fernández-Díaz, J.A.; Freixa, X.; et al. Major Bleeding Predictors in Patients with Left Atrial Appendage Closure: The Iberian Registry II. J. Clin. Med. 2020, 9, 2295. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).