Rupture Prediction for Microscopic Oocyte Images of Piezo Intracytoplasmic Sperm Injection by Principal Component Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Oversight
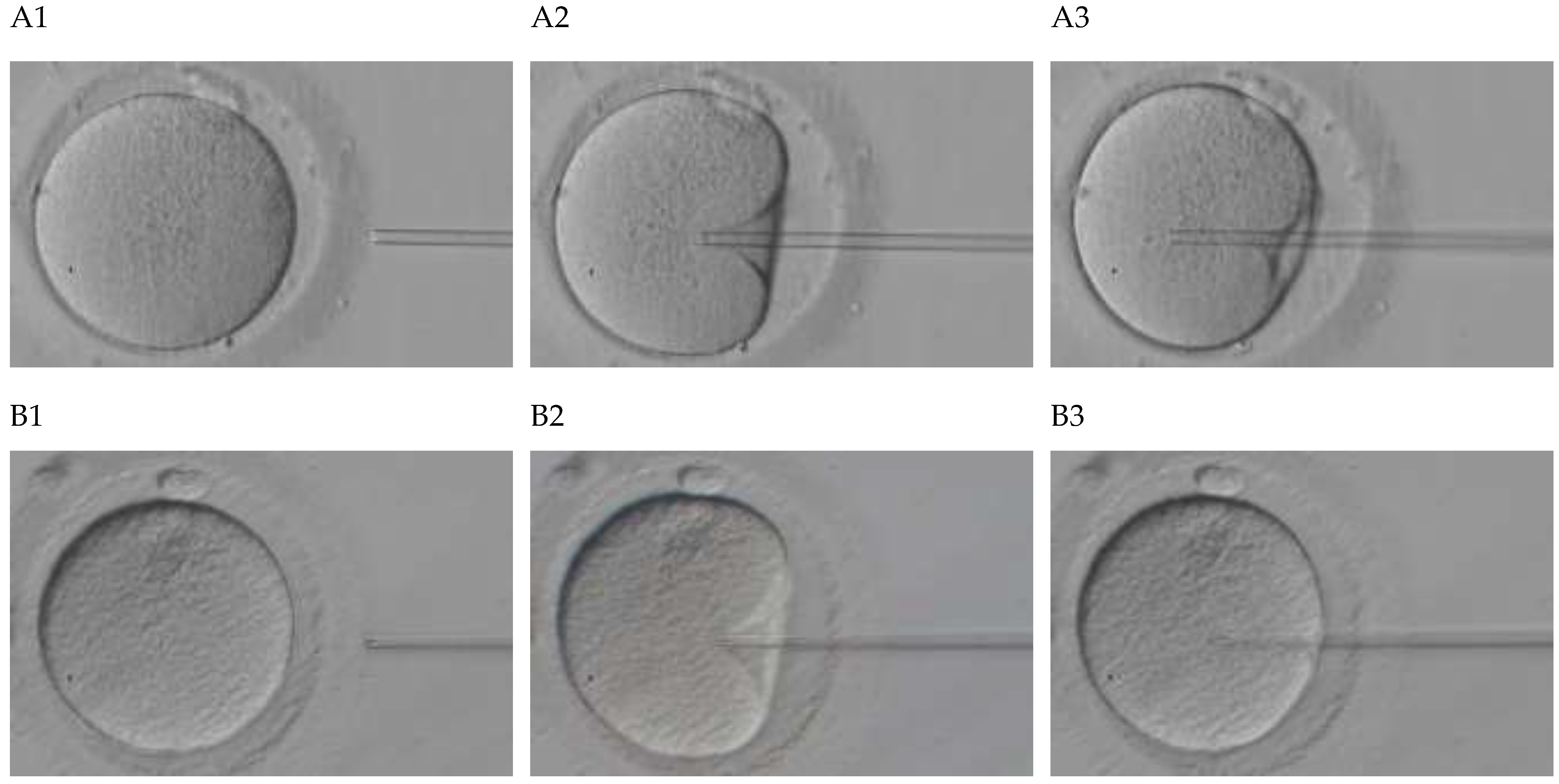
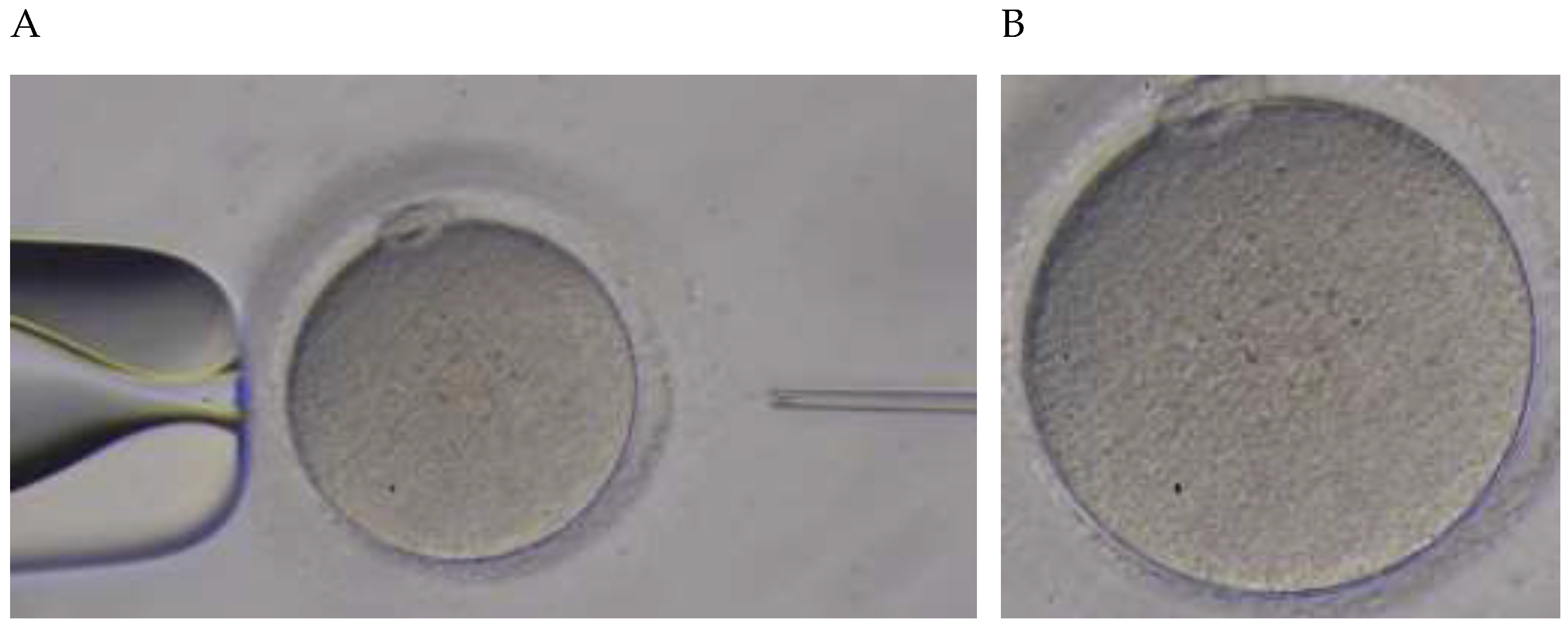
2.2. Procedure Recording Protocol
2.3. Feature Vectors
2.4. SVM
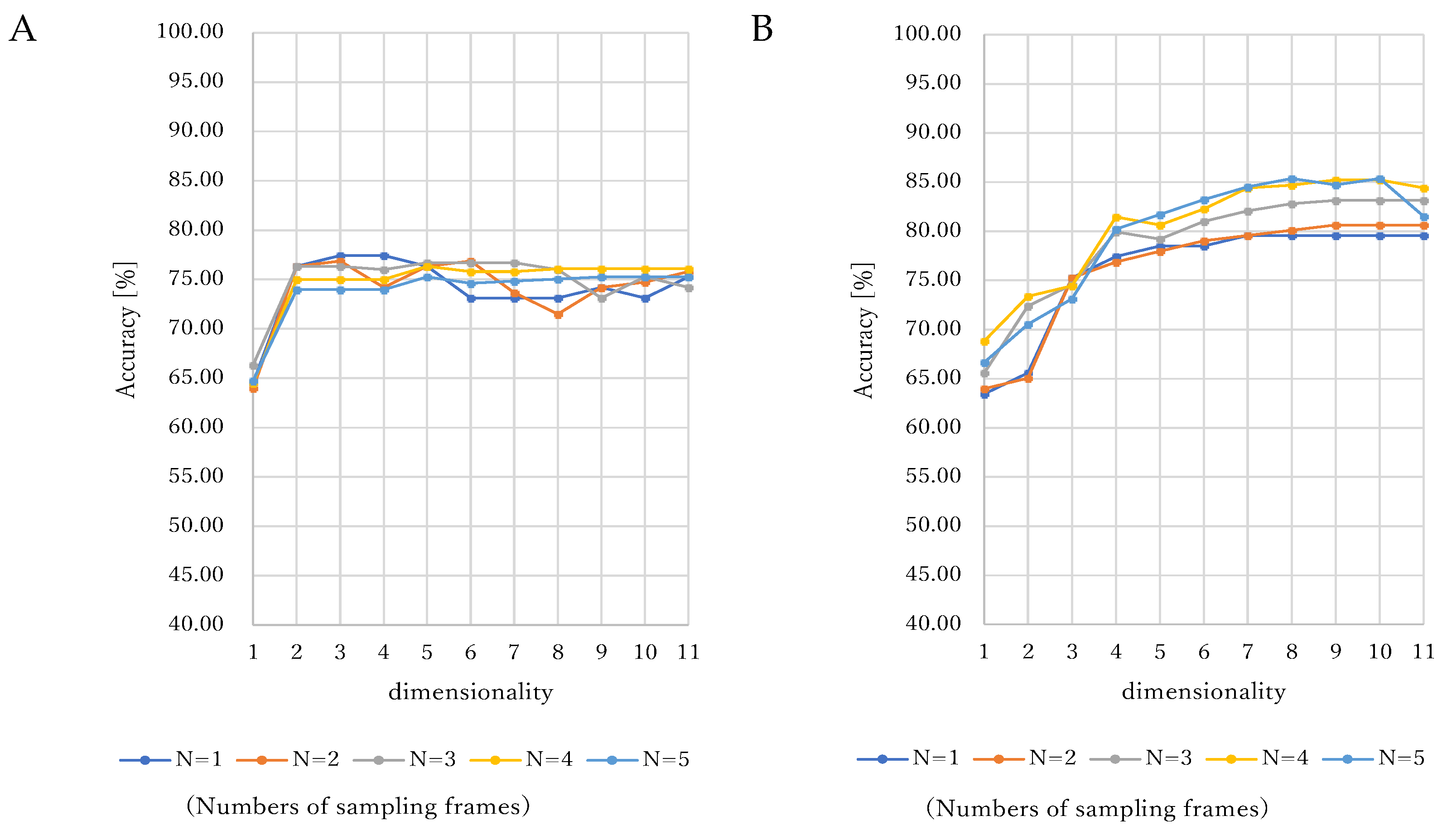
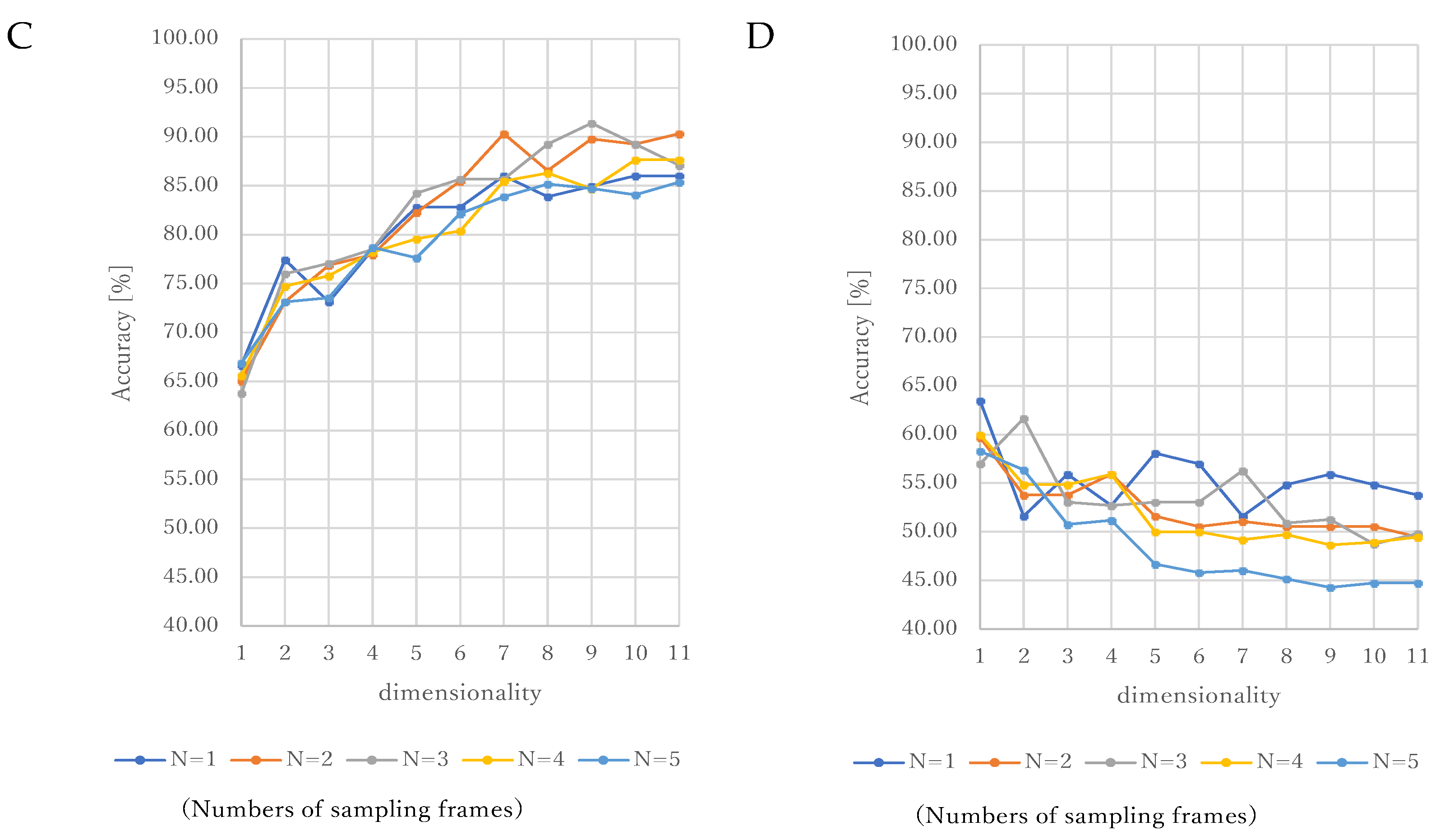
3. Results
4. Discussion
4.1. Consideration of Sampling Frames
4.2. Consideration of Dimensionality and Kernels in SVM
4.3. Sample Imbalance and Size
4.4. Study Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katagiri, Y.; Jwa, S.C.; Kuwahara, A.; Iwasa, T.; Ono, M.; Kato, K.; Kishi, H.; Kuwabara, Y.; Harada, M.; Hamatani, T.; et al. Assisted reproductive technology in Japan: A summary report for 2019 by the Ethics Committee of the Japan Society of Obstetrics and Gynecology. Reprod. Med. Biol. 2021, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chambers, G.M.; Dyer, S.; Zegers-Hochschild, F.; de Mouzon, J.; Ishihara, O.; Banker, M.; Mansour, R.; Kupka, M.S.; Adamson, G.D. International committee for monitoring assisted reproductive technologies world report: Assisted reproductive technology, 2014. Hum. Reprod. 2021, 36, 2921–2934. [Google Scholar] [CrossRef]
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992, 340, 17–18. [Google Scholar] [CrossRef]
- Huang, T.; Kimura, Y.; Yanagimachi, R. The use of piezo micromanipulation for intracytoplasmic sperm injection of human oocytes. J. Assist. Reprod. Genet. 1996, 13, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Yanagimachi, R. Intracytoplasmic sperm injection in the mouse. Biol. Reprod. 1995, 52, 709–720. [Google Scholar] [CrossRef]
- Yanagida, K.; Katayose, H.; Yazawa, H.; Kimura, Y.; Konnai, K.; Sato, A. The usefulness of a piezo-micromanipulator in intracytoplasmic sperm injection in humans. Hum. Reprod. 1999, 14, 448–453. [Google Scholar] [CrossRef]
- Palermo, G.D.; Alikani, M.; Bertoli, M.; Colombero, L.T.; Moy, F.; Cohen, J.; Rosenwaks, Z. Oolemma characteristics in relation to survival and fertilization patterns of oocytes treated by intracytoplasmic sperm injection. Hum. Reprod. 1996, 11, 172–176. [Google Scholar] [CrossRef]
- Iwayama, H.; Yamashita, M. Quantitative evaluation of intercellular local deformation of human oocytes during Piezo-assisted intracytoplasmic sperm injection using video-based motion analysis. F&S Sci. 2021, 2, 124–134. [Google Scholar]
- Rubino, P.; Vigano, P.; Luddi, A.; Piomboni, P. The ICSI procedure from past to future: A systematic review of the more controvercial aspects. Hum. Reprod. Update 2016, 22, 194–227. [Google Scholar] [CrossRef]
- Dana, H.B.; Christopher, M.B. Computer Vision; Prentice Hall: Hoboken, NJ, USA, 1982; ISBN 13:9780131653160. [Google Scholar]
- He, D.; Wang, L. Texture Unit, Texture Spectrum, And Texture Analysis. IEEE Trans. Geosci. Remote Sens. 1990, 28, 509–512. [Google Scholar]
- Ojala, T.; Pietikäinen, M.; Harwood, D. A comparative study of texture measures with classification based on feature distributions. Pattern Recognit. 1996, 29, 51–59. [Google Scholar] [CrossRef]
- Huang, D.; Shan, C.; Ardabilian, M.; Wang, Y.; Chen, L. Local binary patterns and its application to facial image analysis: A survey. IEEE Trans. Syst. Man Cybern. Part C 2011, 41, 765–781. [Google Scholar] [CrossRef]
- Basile, T.M.A.; Caponetti, L.; Castellano, G.; Sforza, G. A Texture-based image processing approach for the description of human oocyte cytoplasm. IEEE Trans. Instrum. Meas. 2010, 59, 2591–2601. [Google Scholar] [CrossRef]
- Wang, R.; Pan, W.; Jin, L.; Li, Y.; Geng, Y.; Gao, C.; Chen, G.; Wang, H.; Ma, D.; Liao, S. Artificial intelligence in reproductive medicine. Reproduction 2019, 158, R139–R154. [Google Scholar] [CrossRef]
- Kinishi, Y.; Maekawa, T.; Mizuta, S.; Ishikawa, T.; Hata, Y. Detection of optimal puncture position in ovum images for artificial insemination. In 2019 International Conference on Machine Learning and Cybernetics; IEEE: New York, NY, USA, 2019; pp. 140–144. [Google Scholar]
- Maekawa, T.; Mizuta, S.; Kinishi, Y.; Takahashi, C.; Matsubayashi, H.; Kitaya, K.; Takeuchi, T.; Hata, Y.; Ishikawa, T. Identification of the optimal puncture position in PIEZO-ICSI using image analysis: A pilot study. Fertil. Steril. 2019, 112, E279. [Google Scholar] [CrossRef]
- Mori, K.; Kitaya, K.; Ishikawa, T.; Hata, Y. Analysis of endometrium form by using LBP for female infertility. In 2018 International Conference on Machine Learning and Cybernetics; IEEE: New York, NY, USA, 2018; pp. 75–79. [Google Scholar]
- Morimoto, T.; Maekawa, T.; Mizuta, S.; Matsubayashi, H.; Takeuchi, T.; Hata, Y.; Ishikawa, T. Optimal puncture position for intracytoplasmic sperm injection can be detected by image analysis using Local Binary Pattern. Reprod. BioMed. Online 2022. [Google Scholar] [CrossRef]
- Pearson, K. On lines and planes of closest fit to systems of points in space. Philos. Mag. 1901, 2, 559–572. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Vapnik, V.; Lerner, A. Pattern recognition using generalized portrait method. Autom. Remote Control 1963, 24, 774–780. [Google Scholar]
- Tsochantaridis, I.; Joachims, T.; Hofmann, T.; Altun, Y. Large margin methods for structured and interdependent output variables. J. Mach. Learn. Res. 2005, 6, 1453–1484. [Google Scholar]
- Nakamura, S.; Yagi, N.; Kawamura, N.; Kashioka, H.; Hirata, M.; Maezawa, H.; Yanagida, T.; Hata, Y.; Sakai, Y. Relationship between singing experience and laryngeal movement obtained by DeepLabCut. In 5th IEEE International Conference on Cybernetics (CYBCONF); IEEE: New York, NY, USA, 2021; pp. 073–078. [Google Scholar]
- Inoue, K.; Yoshioka, M.; Yagi, N.; Nagami, S.; Oku, Y. Using machine learning and a combination of respiratory flow, laryngeal motion, and swallowing sounds to classify safe and unsafe swallowing. IEEE Trans. Biomed. Eng. 2018, 65, 2529–2541. [Google Scholar] [CrossRef] [PubMed]
- Ukai, K.; Rahman, R.; Yagi, N.; Hayashi, K.; Maruo, A.; Muratsu, H.; Kobashi, S. Detecting pelvic fracture on 3D-CT using deep convolutional neural networks with multi-orientated slab images. Sci. Rep. 2021, 11, 11716. [Google Scholar] [CrossRef] [PubMed]
- Kohavi, R. A study of cross-validation and bootstrap for accuracy estimation and model selection. In Proceedings of the 14th International Joint Conference on Artificial Intelligence; Morgan Kaufmann Publishers Inc.: San Francisco, CA, USA, 1995; Volume 2, pp. 1137–1143. [Google Scholar]
- Yagi, N.; Ishikawa, T.; Hata, Y. Stem cell quantity determination in artificial culture bone by ultrasonic testing. IEICE Trans. Fundam. Electron. Commun. Comput. Sci. 2014, 97, 913–922. [Google Scholar] [CrossRef]
- Cramér, H. Mathematical Methods of Statistics; Princeton University Press: Princeton, NJ, USA, 1999. [Google Scholar]
- William, C.G. The Chi-square Test of Goodness of Fit. Ann. Math. Stat. 1952, 23, 315–345. [Google Scholar]
- Power, D.M.W. Evaluation: From Precision, Recall and F-Measure to ROC, Informedness, Markedness & Correlation. Int. J. Mach. Learn. Technol. 2011, 2, 37–63. [Google Scholar]
| Sampling Frames | Dimensionality | Accuracy (%) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| 2 | 1 | 65.05 | 62.86 | 100.00 |
| 2 | 73.12 | 69.23 | 93.33 | |
| 3 | 76.88 | 73.76 | 86.67 | |
| 4 | 77.96 | 79.49 | 75.36 | |
| 5 | 82.26 | 84.07 | 79.45 | |
| 6 | 85.48 | 90.29 | 79.52 | |
| 7 | 90.32 | 89.66 | 91.43 | |
| 8 | 86.56 | 88.29 | 84.00 | |
| 9 | 89.78 | 90.99 | 88.00 | |
| 10 | 89.25 | 91.67 | 85.90 | |
| 11 | 90.32 | 91.82 | 88.16 | |
| 3 | 1 | 63.80 | 62.03 | 100.00 |
| 2 | 75.99 | 71.12 | 100.00 | |
| 3 | 77.06 | 73.06 | 91.67 | |
| 4 | 78.49 | 76.65 | 82.93 | |
| 5 | 84.23 | 83.06 | 86.46 | |
| 6 | 85.66 | 87.43 | 83.04 | |
| 7 | 85.66 | 88.82 | 81.36 | |
| 8 | 89.25 | 91.41 | 86.21 | |
| 9 | 91.40 | 91.23 | 91.67 | |
| 10 | 89.25 | 89.02 | 89.62 | |
| 11 | 87.10 | 89.57 | 83.62 |
| Sampling Frames | Mean and SD of Accuracy (Amount of Change; %) | |||||||
|---|---|---|---|---|---|---|---|---|
| Liner Kernel | Nonliner Kernel | |||||||
| RBF | Polynomial Function | Sigmoid Function | ||||||
| 1 | 74.00 ± 3.59 | ― | 76.05 ± 5.87 | ― | 80.74 ± 6.26 | ― | 55.43 ± 3.38 | ― |
| 2 | 74.05 ± 3.72 | (0.05) | 76.34 ± 6.10 | (0.29) | 82.45 ± 8.31 | (1.71) | 52.49 ± 3.06 | (−2.93) |
| 3 | 74.88 ± 3.06 | (0.83) | 78.82 ± 5.69 | (2.48) | 82.54 ± 8.08 | (0.08) | 53.41 ± 3.68 | (0.91) |
| 4 | 74.71 ± 3.42 | (−0.17) | 80.45 ± 5.66 | (1.63) | 80.55 ± 6.79 | (−1.99) | 51.96 ± 3.78 | (−1.45) |
| 5 | 73.84 ± 3.07 | (−0.87) | 79.73 ± 6.56 | (−0.72) | 79.57 ± 6.17 | (−0.98) | 48.54 ± 4.93 | (−3.41) |
| Dimensionality | Mean and SD of Accuracy (Amount of Change; %) | |||||||
|---|---|---|---|---|---|---|---|---|
| Linear Kernel | Nonlinear Kernel | |||||||
| RBF | Polynomial Function | Sigmoid Function | ||||||
| 1 | 64.81 ± 0.88 | ― | 65.70 ± 2.16 | ― | 65.60 ± 1.26 | ― | 59.67 ± 2.42 | ― |
| 2 | 75.60 ± 1.08 | (10.79) | 69.39 ± 3.86 | (3.70) | 74.87 ± 1.86 | (9.28) | 55.64 ± 3.77 | (−4.03) |
| 3 | 75.92 ± 1.41 | (0.32) | 74.53 ± 0.88 | (5.14) | 75.28 ± 1.85 | (0.41) | 53.66 ± 1.96 | (−1.98) |
| 4 | 75.32 ± 1.42 | (−0.61) | 79.18 ± 1.95 | (4.65) | 78.38 ± 0.29 | (3.09) | 53.68 ± 2.13 | (0.01) |
| 5 | 76.20 ± 0.54 | (0.89) | 79.61 ± 1.55 | (0.43) | 81.30 ± 2.65 | (2.92) | 51.88 ± 4.20 | (−1.80) |
| 6 | 75.43 ± 1.57 | (−0.77) | 80.80 ± 2.03 | (1.20) | 83.29 ± 2.26 | (2.00) | 51.28 ± 4.12 | (−0.60) |
| 7 | 74.82 ± 1.48 | (−0.60) | 82.03 ± 2.45 | (1.23) | 86.27 ± 2.41 | (2.98) | 50.84 ± 3.74 | (−0.44) |
| 8 | 74.35 ± 1.98 | (−0.48) | 82.51 ± 2.62 | (0.48) | 86.23 ± 2.00 | (−0.05) | 50.23 ± 3.45 | (−0.60) |
| 9 | 74.57 ± 1.13 | (0.22) | 82.66 ± 2.48 | (0.16) | 87.11 ± 3.23 | (0.88) | 50.13 ± 4.21 | (−0.10) |
| 10 | 74.89 ± 1.10 | (0.32) | 82.79 ± 2.63 | (0.13) | 87.25 ± 2.22 | (0.14) | 49.56 ± 3.65 | (−0.58) |
| Additionally | ||||||||
| 11 | 75.32 ± 0.72 | (0.43) | 81.86 ± 1.94 | (−0.94) | 87.29 ± 1.91 | (0.04) | 49.45 ± 3.20 | (−0.11) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yagi, N.; Tsuji, H.; Morimoto, T.; Maekawa, T.; Mizuta, S.; Ishikawa, T.; Hata, Y. Rupture Prediction for Microscopic Oocyte Images of Piezo Intracytoplasmic Sperm Injection by Principal Component Analysis. J. Clin. Med. 2022, 11, 6546. https://doi.org/10.3390/jcm11216546
Yagi N, Tsuji H, Morimoto T, Maekawa T, Mizuta S, Ishikawa T, Hata Y. Rupture Prediction for Microscopic Oocyte Images of Piezo Intracytoplasmic Sperm Injection by Principal Component Analysis. Journal of Clinical Medicine. 2022; 11(21):6546. https://doi.org/10.3390/jcm11216546
Chicago/Turabian StyleYagi, Naomi, Hyodo Tsuji, Takashi Morimoto, Tomohiro Maekawa, Shimpei Mizuta, Tomomoto Ishikawa, and Yutaka Hata. 2022. "Rupture Prediction for Microscopic Oocyte Images of Piezo Intracytoplasmic Sperm Injection by Principal Component Analysis" Journal of Clinical Medicine 11, no. 21: 6546. https://doi.org/10.3390/jcm11216546
APA StyleYagi, N., Tsuji, H., Morimoto, T., Maekawa, T., Mizuta, S., Ishikawa, T., & Hata, Y. (2022). Rupture Prediction for Microscopic Oocyte Images of Piezo Intracytoplasmic Sperm Injection by Principal Component Analysis. Journal of Clinical Medicine, 11(21), 6546. https://doi.org/10.3390/jcm11216546







