Not to Rush—Laboratory Parameters and Procedural Complications in Patients Undergoing Left Atrial Appendage Closure
Abstract
1. Introduction
2. Materials and Methods
2.1. Recruitment and Procedure
2.2. Data Collection
2.3. Endpoints
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Procedural Details and Outcome
- One patient had dislocation of the LAAC device into the left atrium two days after the procedure, requiring cardiac surgery. The patient aspirated in the postoperative phase, developed a severe pneumonia, and died due to respiratory failure at day 27 after LAAC.
- One patient developed massive throat bleeding, probably caused by the insertion of the transoesophageal probe, leading to tracheal obstruction on the day of the LAAC. Despite an emergency tracheotomy and resuscitation, the patient deteriorated and passed away in the operating theatre.
3.3. Predictors for Complications
3.4. Multivariate Analysis
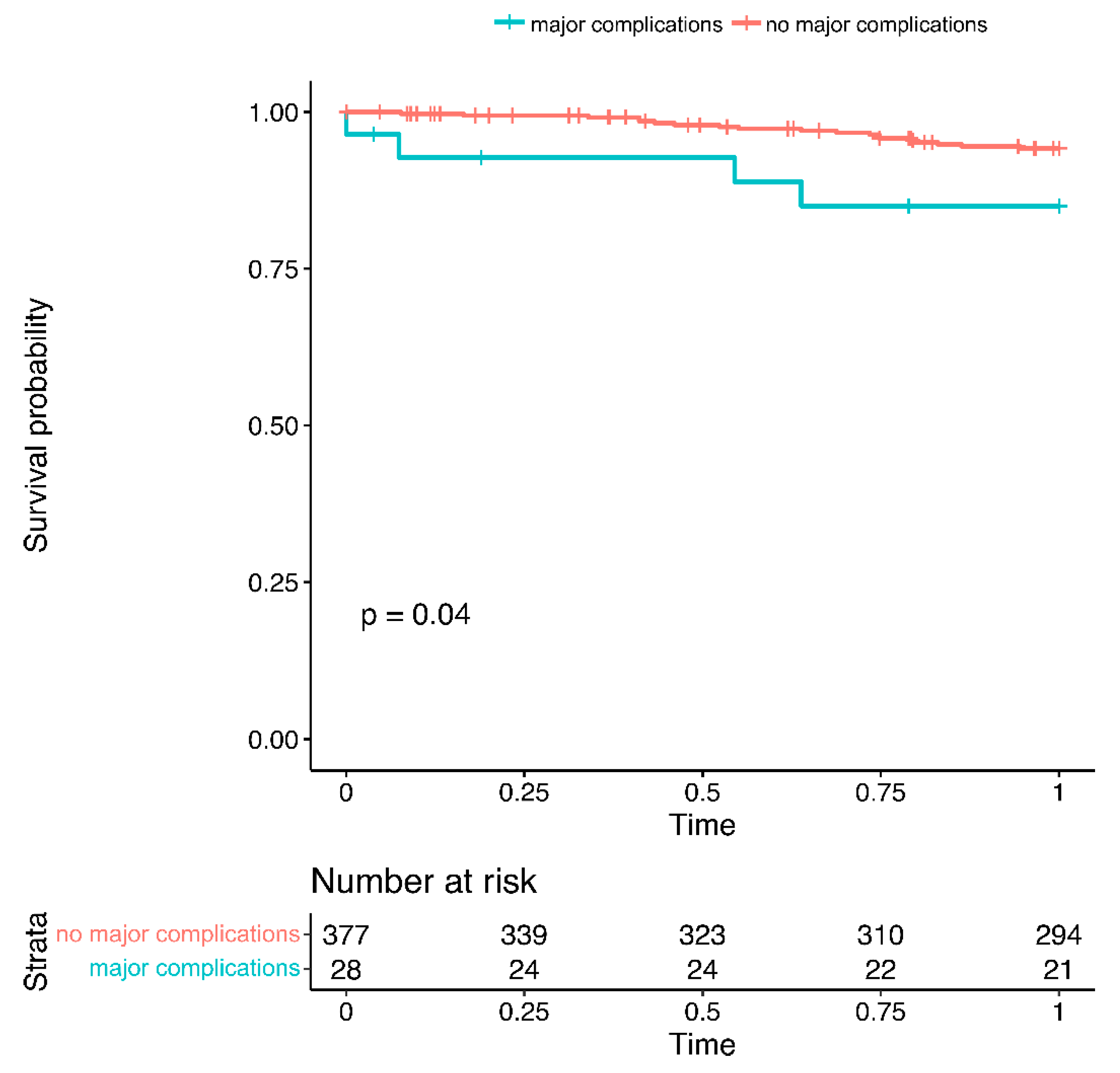
3.5. Complications and Long-Term Outcomes
4. Discussion
4.1. Types of Complications
4.2. Predictors for Complications
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019, 140, e125. [Google Scholar] [CrossRef] [PubMed]
- Masjuan, J.; Salido, L.; DeFelipe, A.; Hernández-Antolín, R.; Fernández-Golfín, C.; Cruz-Culebras, A.; Matute, C.; Vera, R.; Pérez-Torre, P.; Zamorano, J.L. Oral anticoagulation and left atrial appendage closure: A new strategy for recurrent cardioembolic stroke. Eur. J. Neurol. 2019, 26, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.V.; Varosy, P.; Price, M.J.; Slotwiner, D.; Kusumoto, F.M.; Rammohan, C.; Kavinsky, C.J.; Turi, Z.G.; Akar, J.; Koutras, C.; et al. The NCDR Left Atrial Appendage Occlusion Registry. J. Am. Coll. Cardiol. 2020, 75, 1503–1518. [Google Scholar] [CrossRef]
- Glikson, M.; Wolff, R.; Hindricks, G.; Mandrola, J.; Camm, A.J.; Lip, G.Y.H.; Fauchier, L.; Betts, T.R.; Lewalter, T.; Saw, J.; et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion—An update. Europace 2020, 22, 184. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Holmes, D.; Doshi, S.K.; Neuzil, P.; Kar, S. Safety of percutaneous left atrial appendage closure: Results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation 2011, 123, 417–424. [Google Scholar] [CrossRef]
- Holmes, D.R., Jr.; Kar, S.; Price, M.J.; Whisenant, B.; Sievert, H.; Doshi, S.K.; Huber, K.; Reddy, V.Y. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J. Am. Coll. Cardiol. 2014, 64, 1–12. [Google Scholar] [CrossRef]
- Holmes, D.R.; Reddy, V.Y.; Turi, Z.G.; Doshi, S.K.; Sievert, H.; Buchbinder, M.; Mullin, C.M.; Sick, P.; Investigators, P.A. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: A randomised non-inferiority trial. Lancet 2009, 374, 534–542. [Google Scholar] [CrossRef]
- Osmancik, P.; Herman, D.; Neuzil, P.; Hala, P.; Taborsky, M.; Kala, P.; Poloczek, M.; Stasek, J.; Haman, L.; Branny, M.; et al. Left Atrial Appendage Closure Versus Direct Oral Anticoagulants in High-Risk Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 75, 3122–3135. [Google Scholar] [CrossRef]
- Osmancik, P.; Herman, D.; Neuzil, P.; Hala, P.; Taborsky, M.; Kala, P.; Poloczek, M.; Stasek, J.; Haman, L.; Branny, M.; et al. 4-Year Outcomes After Left Atrial Appendage Closure Versus Nonwarfarin Oral Anticoagulation for Atrial Fibrillation. J. Am. Coll. Cardiol. 2022, 79, 1–14. [Google Scholar] [CrossRef]
- Zweiker, D.; Sieghartsleitner, R.; Fiedler, L.; Toth, G.G.; Luha, O.; Stix, G.; Gabriel, H.; Vock, P.; Lileg, B.; Strouhal, A.; et al. Indications and Outcome in Patients Undergoing Left Atrial Appendage Closure-The Austrian LAAC Registry. J. Clin. Med. 2020, 9, 3274. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; World Health Organization: Geneva, Switzerland, 2011.
- Garot, P.; Iriart, X.; Aminian, A.; Kefer, J.; Freixa, X.; Cruz-Gonzalez, I.; Berti, S.; Rosseel, L.; Ibrahim, R.; Korsholm, K.; et al. Value of FEops HEARTguide patient-specific computational simulations in the planning of left atrial appendage closure with the Amplatzer Amulet closure device: Rationale and design of the PREDICT-LAA study. Open Heart 2020, 7, e001326. [Google Scholar] [CrossRef] [PubMed]
- Power, A. Stroke in dialysis and chronic kidney disease. Blood Purif 2013, 36, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Tokmakova, M.P.; Skali, H.; Kenchaiah, S.; Braunwald, E.; Rouleau, J.L.; Packer, M.; Chertow, G.M.; Moye, L.A.; Pfeffer, M.A.; Solomon, S.D. Chronic kidney disease, cardiovascular risk, and response to angiotensin-converting enzyme inhibition after myocardial infarction: The Survival And Ventricular Enlargement (SAVE) study. Circulation 2004, 110, 3667–3673. [Google Scholar] [CrossRef]
- Jamal, S.; Mughal, M.S.; Kichloo, A.; Edigin, E.; Khan, M.Z.; Minhas, A.M.K.; Ali, M.; Kanjwal, K. Left atrial appendage closure using WATCHMAN device in chronic kidney disease and end stage renal disease patients. Pacing Clin. Electrophysiol. 2022, 45, 866–873. [Google Scholar] [CrossRef]
- Benini Tapias, J.; Flores-Umanzor, E.; Cepas-Guillén, P.L.; Regueiro, A.; Sanchís, L.; Broseta, J.J.; Cases, A.; Freixa, X. Prognostic impact of the presence of chronic kidney disease on percutaneous left trial appendage closure for atrial fibrillation: A single center experience. Nefrologia (Engl. Ed.) 2022, 42, 290–300. [Google Scholar] [CrossRef]
- Dunn, A.N.; Huded, C.; Simpfendorfer, C.; Raymond, R.; Kapadia, S.; Tuzcu, E.M.; Ellis, S.G. End-stage renal disease as an independent risk factor for in-hospital mortality after coronary drug-eluting stenting: Understanding and modeling the risk. Catheter. Cardiovasc. Interv. 2021, 98, 246–254. [Google Scholar] [CrossRef]
- Schymik, G.; Bramlage, P.; Herzberger, V.; Bergmann, J.; Conzelmann, L.O.; Wurth, A.; Luik, A.; Schrofel, H.; Tzamalis, P. Impact of Dialysis on the Prognosis of Patients Undergoing Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2019, 123, 315–322. [Google Scholar] [CrossRef]
- Muñoz, M.; Laso-Morales, M.J.; Gómez-Ramírez, S.; Cadellas, M.; Núñez-Matas, M.J.; García-Erce, J.A. Pre-operative haemoglobin levels and iron status in a large multicentre cohort of patients undergoing major elective surgery. Anaesthesia 2017, 72, 826–834. [Google Scholar] [CrossRef]
- Mueller, M.M.; Van Remoortel, H.; Meybohm, P.; Aranko, K.; Aubron, C.; Burger, R.; Carson, J.L.; Cichutek, K.; De Buck, E.; Devine, D.; et al. Patient Blood Management: Recommendations From the 2018 Frankfurt Consensus Conference. JAMA 2019, 321, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Shavit, L.; Hitti, S.; Silberman, S.; Tauber, R.; Merin, O.; Lifschitz, M.; Slotki, I.; Bitran, D.; Fink, D. Preoperative hemoglobin and outcomes in patients with CKD undergoing cardiac surgery. Clin. J. Am. Soc. Nephrol. 2014, 9, 1536–1544. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ranucci, M.; Pavesi, M.; Pistuddi, V.; Baryshnikova, E. Preoperative Anemia Correction in Cardiac Surgery: A Propensity-Matched Study. J. Cardiothorac. Vasc. Anesth. 2021, 35, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Zhang, B.; Wang, J.; Pan, L.; Cheng, T.; Wang, Y.; Xiong, E. Comparison between Amplatzer and Watchman left atrial appendage closure devices for stroke prevention in atrial fibrillation: A systematic review and meta-analysis. Cardiology 2022, 147, 290–297. [Google Scholar] [CrossRef]
- Lakkireddy, D.; Thaler, D.; Ellis, C.R.; Swarup, V.; Sondergaard, L.; Carroll, J.; Gold, M.R.; Hermiller, J.; Diener, H.C.; Schmidt, B.; et al. Amplatzer amulet left atrial appendage occluder versus watchman™ device for stroke prophylaxis (amulet ide): A randomized controlled trial. Circulation 2021, 144, 1543–1552. [Google Scholar] [CrossRef]
- Ledwoch, J.; Krollmann, C.; Staubach, S.; Hug, M.; Strohm, H.; Mudra, H. Learning Curve Assessment for Percutaneous Left Atrial Appendage Closure With the WATCHMAN Occluder. J. Interv. Cardiol. 2016, 29, 393–399. [Google Scholar] [CrossRef]
- Thotamgari, S.R.; Sheth, A.R.; Patel, H.P.; Bretzman, J.; Ward, R.C.; Thakkar, S.; Patel, J.T.; Asirvatham, S.J.; Holmes, D.R., Jr.; Egbe, A.; et al. Liver cirrhosis is independently associated with increased in-hospital mortality in patients undergoing left atrial appendage occlusion device implantation. Heart Rhythm 2022, 19, 1392–1393. [Google Scholar] [CrossRef]
- Patel, N.; Ranka, S.; Hajra, A.; Bandyopadhyay, D.; Amgai, B.; Chakraborty, S.; Khalid, M.; Goyal, A.; Dalia, T.; Reddy, Y.M.; et al. Gender-Specific Outcomes after Percutaneous Left Atrial Appendage Closure—A Nationwide Readmission Database Analysis. J. Cardiovasc. Electrophysiol. 2022, 33, 430–436. [Google Scholar] [CrossRef]
- López-Mínguez, J.R.; Nogales-Asensio, J.M.; Infante De Oliveira, E.; Santos, L.; Ruiz-Salmerón, R.; Arzamendi-Aizpurua, D.; Costa, M.; Gutiérrez-García, H.; Fernández-Díaz, J.A.; Freixa, X.; et al. Major Bleeding Predictors in Patients with Left Atrial Appendage Closure: The Iberian Registry II. J. Clin. Med. 2020, 9, 2295. [Google Scholar] [CrossRef]
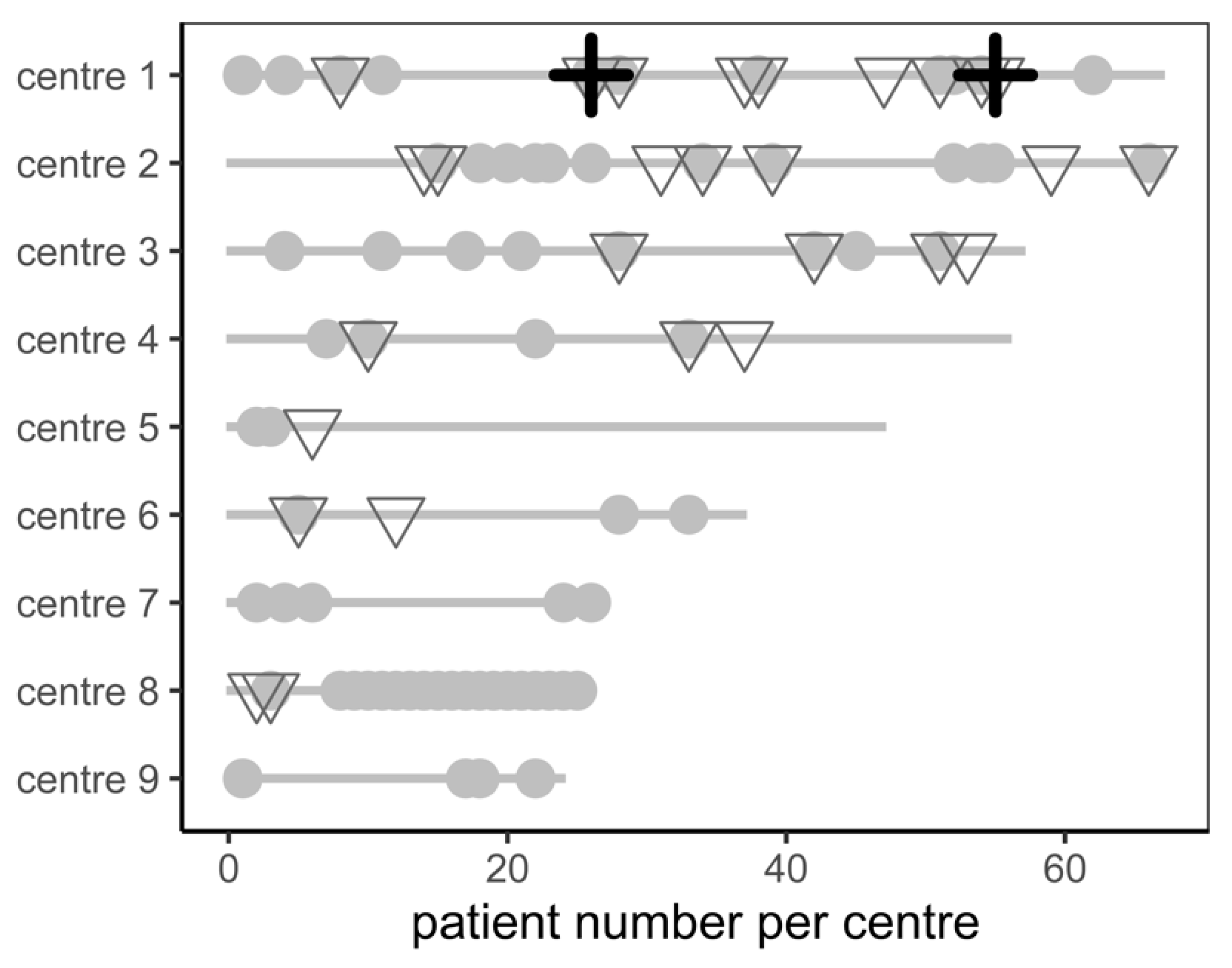
| Parameter | Total Population | Patients with Complications (n = 28) | Patients without Complications (n = 377) | p Value |
|---|---|---|---|---|
| Baseline Demographics | ||||
| female gender | 36.5% (n = 148) | 50% (n = 14) | 35.5% (n = 134) | 0.154 |
| age (years) | 75 (70–79) | 75.5 (72–79) | 75 (70–79) | 0.679 |
| body mass index (kg/m2) | 27 (24–30) | 29 (25–32) | 27 (24–30) | 0.424 |
| body surface area (m2) | 1.92 ± 0.21 | 1.88 ± 0.20 | 1.92 ± 0.21 | 0.316 |
| Comorbidities | ||||
| CHA2DS2-VASc score | 4 (3–5) | 5 (4–6) | 4 (3–5) | 0.408 |
| HAS-BLED score | 3 (2–4) | 3 (3–4) | 3 (2–4) | 0.797 |
| arterial hypertension | 88.4% (n = 358) | 89.3% (n = 25) | 88.3% (n = 333) | 1.000 |
| diabetes mellitus | 28.1% (n = 114) | 32.1% (n = 9) | 27.9% (n = 105) | 0.664 |
| congestive heart failure | 24% (n = 97) | 17.9% (n = 5) | 24.4% (n = 92) | 0.501 |
| stroke | 46.7% (n = 189) | 53.6% (n = 15) | 46.2% (n = 174) | 0.557 |
| ischaemic stroke | 25.2% (n = 102) | 32.1% (n = 9) | 24.7% (n = 93) | 0.373 |
| haemorrhagic stroke intracerebral bleeding subarachnoid bleeding subdural bleeding epidural bleeding | 25.9% (n = 105) 22% (n = 89) 4.2% (n = 17) 4.7% (n = 19) 1.2% (n = 5) | 25% (n = 7) 17.9% (n = 5) 10.7% (n = 3) 3.6% (n = 1) 0% (n = 0) | 26% (n = 98) 22.3% (n = 84) 3.7% (n = 14) 4.8% (n = 18) 1.3% (n = 5) | 1.000 0.813 0.105 1.000 1.000 |
| chronic kidney disease | 19.5% (n = 79) | 25% (n = 7) | 19.1% (n = 72) | 0.459 |
| chronic liver disease | 5.4% (n = 22) | 10.7% (n = 3) | 5% (n = 19) | 0.188 |
| anaemia | 38.0% (n = 154) | 32.1% (n = 9) | 38.5% (n = 145) | 0.552 |
| prior blood transfusion | 29.4% (n = 119) | 21.4% (n = 6) | 30% (n = 113) | 0.396 |
| coronary artery disease | 41.2% (n = 167) | 35.7% (n = 10) | 41.6% (n = 157) | 0.691 |
| cerebral artery disease | 13.8% (n = 56) | 17.9% (n = 5) | 13.5% (n = 51) | 0.568 |
| periphery artery disease | 9.6% (n = 39) | 7.1% (n = 2) | 9.8% (n = 37) | 1.000 |
| chronic obstructive pulmonary disease | 12.8% (n = 52) | 10.7% (n = 3) | 13% (n = 49) | 1.000 |
| chronic dialysis | 2.0% (n = 8) | 14.3% (n = 4) | 1.1% (n = 4) | 0.001 |
| paroxysmal AF | 34.1% (n = 138) | 46.4% (n = 13) | 33.2% (n = 125) | 0.155 |
| vascular malformation cerebral upper gastrointestinal tract lower gastrointestinal tract | 5.2% (n = 21) 4.2% (n = 17) 5.2% (n = 21) | 10.7% (n = 3) 10.7% (n = 3) 0.0% (n = 0) | 4.8% (n = 18) 3.7% (n = 14) 5.6% (n = 21) | 0.170 0.105 0.383 |
| prior acute coronary syndrome | 13.3% (n = 54) | 14.3% (n = 4) | 13.3% (n = 50) | 0.778 |
| prior pulmonary embolism | 1.7% (n = 7) | 0.0% (n = 0) | 1.9% (n = 7) | 1.000 |
| prior peripheral embolism | 2.0% (n = 8) | 0.0% (n = 0) | 2.1% (n = 8) | 1.000 |
| Echocardiography | ||||
| LVEF normal 35–50% <35% | 74.6% (n = 302) 18.0% (n = 73) 7.4% (n = 30) | 75.0% (n = 21) 21.4% (n = 6) 3.6% (n = 1) | 74.5% (n = 281) 17.8% (n = 67) 7.7% (n = 29) | 0.786 |
| severe aortic stenosis | 1.2% (n = 5) | 3.6% (n = 1) | 1.1% (n = 4) | 0.302 |
| severe mitral regurgitation | 6.2% (n = 25) | 14.3% (n = 4) | 5.6% (n = 21) | 0.084 |
| severe tricuspid regurgitation | 4.9% (n = 20) | 3.6% (n = 1) | 5% (n = 19) | 1.000 |
| Laboratory | ||||
| erythrocytes (T/L) | 4.3 ± 0.67 | 4.1 ± 0.84 | 4.32 ± 0.65 | 0.216 |
| haemoglobin (g/dL) | 12.5 (11.0–14.1) | 11.3 (10.4–12.9) | 12.7 (11.0–14.2) | 0.010 |
| haematocrit (%) | 38 (33–42) | 35 (32–38) | 38 (33–42) | 0.016 |
| platelets (G/L) | 219 (173–261) | 191 (173–253) | 219 (174–262.5) | 0.289 |
| NT-ProBNP (ng/L) | 912 (382–2153) | 1611 (410–2713) | 885 (384–2063) | 0.339 |
| creatinine (mg/dL) | 1.10 (0.90–1.41) | 1.10 (0.86–1.67) | 1.10 (0.9–1.4) | 0.892 |
| eGFR (ml/min/1.73 m2) | 75.0 (67.9–81.9) | 74.5 (63.6–82.3) | 75.1 (68.0–81.7) | 0.559 |
| ASAT (U/L) | 24 (20–30) | 23 (19–29) | 24 (20–30) | 0.745 |
| ALAT (U/L) | 20 (15–29) | 16 (13–26) | 20 (15–29) | 0.073 |
| INR | 1.1 (1–1.2) | 1.1 (1–1.11) | 1.1 (1–1.2) | 0.703 |
| aPTT (sec) | 34 (30–40) | 34 (32–37.25) | 34 (30–40) | 0.754 |
| albumine (g/L) | 41 (36–44) | 42 (32–44) | 41 (36–44) | 0.544 |
| total protein (g/L) | 70 (65–74) | 66 (58.5–72) | 71 (66–74) | 0.055 |
| total cholesterol (mg/dL) | 152 (123–189) | 156 (138–201) | 152 (123–189) | 0.511 |
| LDL (mg/dL) | 87 (65–113) | 94 (72–126) | 87 (65–112) | 0.358 |
| triglycerides (mg/dL) | 98 (73–144) | 103 (72–160) | 98 (74–143) | 0.657 |
| Indication for LAAC | ||||
| indication group bleeding other thromboembolism | 66.2% (n = 268) 24.2% (n = 98) 9.6% (n = 39) | 57.1% (n = 16) 28.6% (n = 8) 14.3% (n = 4) | 66.8% (n = 252) 23.9% (n = 90) 9.3% (n = 35) | 0.435 |
| primary indication for LAAC gastrointestinal bleeding intracranial Bleeding bleeding under OAC stroke other predisposition to bleeding other contraindication to OAC embolism despite OAC anaemia OAC intolerance patient preference requirement for triple therapy epistaxis | 29.1% (n = 118) 28.4% (n = 115) 6.9% (n = 28) 5.7% (n = 23) 4.7% (n = 19) 4.4% (n = 18) 4.2% (n = 17) 4.0% (n = 16) 3.7% (n = 15) 3.2% (n = 13) 2.0% (n = 8) 2.0% (n = 8) 1.7% (n = 7) | 21.4% (n = 6) 32.1% (n = 9) 3.6% (n = 1) 10.7% (n = 3) 0.0% (n = 0) 7.1% (n = 2) 7.1% (n = 2) 3.6% (n = 1) 3.6% (n = 1) 3.6% (n = 1) 7.1% (n = 2) 0.0% (n = 0) 0.0% (n = 0) | 29.7% (n = 112) 28.1% (n = 106) 7.2% (n = 27) 5.3% (n = 20) 5.0% (n = 19) 4.2% (n = 16) 4.0% (n = 15) 4.0% (n = 15) 3.7% (n = 14) 3.2% (n = 12) 1.6% (n = 6) 2.1% (n = 8) 1.9% (n = 7) | 0.588 |
| contraindication for OAC | 20.7% (n = 84) | 32.1% (n = 9) | 19.9% (n = 75) | 0.146 |
| device Amplatzer Watchman LAmbre | 56.3% (n = 228) 43.2% (n = 175) 0.5% (n = 2) | 67.9% (n = 19) 32.1% (n = 9) 0.0% (n = 0) | 55.4% (n = 209) 44% (n = 166) 0.5% (n = 2) | 0.342 |
| isolated procedure | 89.6% (n = 363) | 78.6% (n = 22) | 90.5% (n = 341) | 0.057 |
| Parameter | Total Population |
|---|---|
| fluoroscopy time (min) | 16 (11–23) |
| dose area product (µGym2) | 4430 (1574–8991) |
| contrast medium (mL) | 100 (68–139) |
| procedure duration (min) | 70 (53–98) |
| any complication | 19.3% (n = 78) |
| Major Complications | 6.9% (n = 28) |
| pericardial tamponade | 2.2% (n = 9) |
| access site complications requiring surgery requiring thrombin injection requiring angioseal | 3.2% (n = 11) 2.0% (n = 8) 0.7% (n = 3) 0.5% (n = 2) |
| stroke | 1.2% (n = 5) |
| death | 0.5% (n = 2) |
| heart surgery | 0.5% (n = 2) |
| interventional retrieval of dislocated LAAC device | 0.5% (n = 2) |
| cardiopulmonary resuscitation | 0.5% (n = 2) |
| device embolization | 0.2% (n = 1) |
| Minor Complications | 16.8% (n = 68) |
| admission to intensive care | 5.7% (n = 23) |
| shock | 5.7% (n = 23) |
| device not implanted | 3.2% (n = 13) |
| bleeding requiring transfusion | 2.5% (n = 10) |
| new pericardial effusion (no therapy) | 4.7% (n = 19) |
| iatrogenic atrial septum defect | 0.5% (n = 2) |
| prolonged hospital stay (>14 days) | 3.5% (n = 14) |
| Parameter | Bivariate Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| dialysis | 15.54 (3.49–69.50) | 0.001 | 13.0 (2.5–68.5) | 0.002 |
| haemoglobin (per g/dL) | 0.78 (0.63–0.94) | 0.010 | 0.80 (0.65–0.99) | 0.040 |
| age (per year) | 1.00 (0.96–1.05) | 0.679 | 0.42 (0.05–3.66) | 0.431 |
| LVEF < 35% | 0.44 (0.02–2.21) | 0.710 | 1.01 (0.95–1.06) | 0.847 |
| LVEF 35–50% | 1.26 (0.45–3.06) | 0.613 | 1.04 (0.38–2.82) | 0.945 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zweiker, D.; Fiedler, L.; Toth, G.G.; Strouhal, A.; Delle-Karth, G.; Stix, G.; Gabriel, H.; Binder, R.K.; Rammer, M.; Pfeffer, M.; et al. Not to Rush—Laboratory Parameters and Procedural Complications in Patients Undergoing Left Atrial Appendage Closure. J. Clin. Med. 2022, 11, 6548. https://doi.org/10.3390/jcm11216548
Zweiker D, Fiedler L, Toth GG, Strouhal A, Delle-Karth G, Stix G, Gabriel H, Binder RK, Rammer M, Pfeffer M, et al. Not to Rush—Laboratory Parameters and Procedural Complications in Patients Undergoing Left Atrial Appendage Closure. Journal of Clinical Medicine. 2022; 11(21):6548. https://doi.org/10.3390/jcm11216548
Chicago/Turabian StyleZweiker, David, Lukas Fiedler, Gabor G. Toth, Andreas Strouhal, Georg Delle-Karth, Guenter Stix, Harald Gabriel, Ronald K. Binder, Martin Rammer, Michael Pfeffer, and et al. 2022. "Not to Rush—Laboratory Parameters and Procedural Complications in Patients Undergoing Left Atrial Appendage Closure" Journal of Clinical Medicine 11, no. 21: 6548. https://doi.org/10.3390/jcm11216548
APA StyleZweiker, D., Fiedler, L., Toth, G. G., Strouhal, A., Delle-Karth, G., Stix, G., Gabriel, H., Binder, R. K., Rammer, M., Pfeffer, M., Vock, P., Lileg, B., Steinwender, C., Sihorsch, K., Hintringer, F., Mueller, S., Barbieri, F., Martinek, M., Tkalec, W., ... Scherr, D. (2022). Not to Rush—Laboratory Parameters and Procedural Complications in Patients Undergoing Left Atrial Appendage Closure. Journal of Clinical Medicine, 11(21), 6548. https://doi.org/10.3390/jcm11216548







