Abstract
Empathy has not been well studied in patients following ischemic stroke. We aimed to evaluate the relationships of multimodal neuroimaging parameters with the impairment of empathy in patients who had experienced subacute ischemic stroke. Patients who had experienced a first-event acute ischemic stroke were recruited, and we assessed their empathy using the Chinese version of the Empathy Quotient (EQ) 3 months after the index stroke. Multimodal magnetic resonance imaging (MRI) was conducted in all the participants to identify acute infarction and assess brain volumes, white matter integrity, and other preexisting abnormalities. We quantified the brain volumes of various subcortical structures, the ventricles, and cortical lobar atrophy. The microstructural integrity of the white matter was reflected in the mean fractional anisotropy (FA) and mean diffusivity (MD), and the regional mean values of FA and MD were quantified after mapping using the ICBM_DTI_81 Atlas. Twenty-three (56.1%) men and 18 (43.9%) women (mean age: 61.73 years, range: 41–77 years) were included. The median National Institutes of Health Stroke Scale (NIHSS) score at discharge was 1 (range: 0–4). On univariate analysis, the EQ was correlated with right cortical infarction (r = −0.39, p = 0.012), putamen volume (r = 0.382, p = 0.014), right putamen volume (r = 0.338, p = 0.031), and the FA value of the right sagittal stratum. EQ did not correlated with the MD value in any region of interest or pre-existing brain abnormalities. Multiple stepwise linear regression models were used to identify factors associated with EQ. After adjusting for age and the NIHSS score on admission, the frequency of right cortical infarcts negatively correlated with EQ (standardized β = −0.358, 95% confidence interval =−0.708 to −0.076, p = 0.016), and the putamen volume positively correlated with EQ (standardized β = 0.328, 95% confidence interval =0.044 to 0.676, p = 0.027). In conclusion, in patients who have experienced subacute ischemic stroke, right cortical infarction and a smaller putamen volume are associated with the impairment of empathy.
1. Introduction
Social cognition broadly refers to the cognitive processes used to encode and decode social information. It is also critical for people to understand themselves and others, as well as the norms and procedures of the social world [1]. One of the core concerns in social cognition is empathy [2]. Empathy refers to a person’s emotional response to the perceived situation of another [3]; it is an ability to understand the perspective of another person, and it provides an important foundation for relationships, communication, negotiations, and other social activities [4]. Empathy plays important roles in prosocial behavior, the inhibition of aggression, and moral reasoning, by permitting someone to understand another person’s psychological state and experience their emotions [5]. Several neurological and neuropsychiatric diseases disrupt empathy, such as autism, frontotemporal dementia, traumatic head injury, schizophrenia, and stroke [2].
Patients who experience stroke can suffer from a decline in empathy, which has clinical significance [6,7]. Patients who experience emotion recognition deficit may appear to have a variety of interpersonal difficulties, such as complaints of frustration in social relationships, feelings of social discomfort, desire to connect with others, feelings of social disconnection, and the use of controlling behaviors during normal living [6]. Yeh et al. found that, following stroke, the cognitive and emotional empathy of patients are commonly impaired, and emotional processing ability is also affected, leading to a poor outcome [8]. Another study showed that an impairment in social cognition, including empathy, persists in patients with good limb function over the long term following stroke, which leads to a restriction in participation in normal life [9]. Some previous studies have aimed to identify the brain regions engaged in empathy, and showed that specific regions are involved in emotional dysfunction. Leigh et al. found that an acute impairment of empathy is particularly associated with infarcts in the temporal pole and anterior insula [4]. A study that recruited patients following stroke showed that the right posterior superior temporal gyrus in the right hemisphere ventral stream is critical for the identification of emotion in speech [10]. This finding suggests that further assessment of impairment in patients who experience stroke and have damage to this area is required. Another study used functional magnetic resonance imaging (MRI) to show that the bilateral insula can be considered as forming a core network in empathy [11]. In addition, this study showed that cognitive–evaluative and affective–perceptual empathy can be distinguished at the level of regional activation. These findings are consistent with distinct regions being involved in the impairment of empathy. However, current knowledge of the emotional disorders that occur in patients who experience ischemic stroke is limited.
With the development of MRI methods and intelligent image quantification technology, we may be able to better understand the underlying pathogenesis of defects in empathy by combining studies of brain structure and white matter integrity. In the present study, we aimed to evaluate the relationship between the severity of defects in empathy and neuroimaging-derived parameters, including indices of brain structure and white matter integrity, in patients who had experienced subacute ischemic stroke.
2. Methods
2.1. Participants and Setting
The participants in the study were prospectively recruited at Division I, Department of Neurology, Dongguan People’s Hospital between 1 July 2021 and 30 December 2021. The inclusion criteria were as follows: (1) age > 18 years; (2) a first acute ischemic stroke occurring within the 7 days preceding admission; (3) a complete brain MRI examination was performed at the acute stage, involving T1-weighted (T1W), T2W, and diffusion-weighted imaging (DWI); (4) National Institute of Health stroke scale (NIHSS) score ≤ 15 on admission; and (5) a modified Rankin Scale (mRS) score ≤ 2 on discharge. The exclusion criteria were as follows: (1) a transient ischemic attack occurred; (2) there was a lack of complete MRI; (3) no infarction was detected on DWI; (4) the complication of hemorrhagic transformation occurred; (5) the patient died during hospitalization; (6) severe comorbidities were present (e.g., malignant tumors and, severe organ dysfunction); (7) there was a history of dementia or mental disorder, or obvious cognitive dysfunction or severe depression on admission, identified using medical records; and (8) the patient or their relative refused to provide written informed consent.
The study was performed in accordance with the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of Dongguan People’s Hospital. Written informed consent was obtained from all the participants.
2.2. Collection of Demographic Data
Demographic and clinical data were recorded. The ischemic stroke subtype was determined in accordance with the Trial of Org 10,172 in the Acute Stroke Treatment subtype system by the attending neurologist during hospitalization [12].
2.3. Follow-Up Examinations
All the participants were followed up for 3 months after the index stroke via a face-to-face interview. Empathy was assessed using the Chinese version of the Empathy Quotient (EQ) [13], which was translated from the original EQ and validated [13]. The EQ comprises 60 questions, which are divided into two types as follows: 40 questions regarding empathy and 20 filler items. The 20 filler items (items 2, 3, 5, 7, 9, 13, 16, 17, 20, 23, 24, 30, 31, 33, 40, 45, 47, 51, 53, and 56) are included to distract the participant from a relentless focus on empathy. An initial attempt to separate the items into purely affective and cognitive categories was abandoned because in most instances of empathy, the affective and cognitive components are both present and cannot be easily disentangled. To avoid a response bias, approximately half of the items were worded to produce a “disagree” response and half to produce an “agree” in individuals with intact empathy. For the questions used to assess empathy, “definitely agree” responses scored 2 points and “slightly agree” responses scored 1 point for the following items: 1, 6, 19, 22, 25, 26, 35, 36, 37, 38, 41, 42, 43, 44, 52, 54, 55, 57, 58, 59, and 60. Similarly, for questions aimed to elicit negative responses, “definitely disagree” responses scored 2 points and “slightly disagree” responses scored 1 point for the following items: 4, 8, 10, 11, 12, 14, 15, 18, 21, 27, 28,29, 32, 34, 39, 46, 48, 49, and 50. The total EQ score ranges between 0 and 80, with higher scores reflecting greater empathy [14].
To fully characterize the cognitive and mood statuses, in addition to empathy, we also assessed the quality of life using the stroke-specific quality of life scale [15], anxiety using the Hamilton Anxiety Rating Scale [16], depression using the Hamilton Depression Rating Scale [16], and cognition status using the Montreal Cognitive Assessment [17] at the follow-up examination. The NIHSS score, mRS score, recurrence of stroke and mortality rate during the follow-up were also recorded.
2.4. MRI Protocol and Analysis
All the participants underwent MRI scans using a 3.0-T scanner (Skyra; Siemens, Erlangen, Germany) on admission and at follow-up examinations. The MRI protocol included T1W, T2W, and DWI at the acute stage and three-dimensional T1W, T2W, and diffusion tensor imaging (DTI) at follow-up.
In MRI during the acute phase, DWI spin echo planar imaging was performed using the following parameters: repetition time (TR)/echo time (TE)/excitation = 2162/76/1, matrix = 128 × 128, field of view (FOV) = 230 mm, slice thickness/gap = 6 mm/1 mm, echo planar imaging factor = 47, and acquisition time = 25.9 s. Three orthogonally applied gradients were used, with b-values of 0 and 1000. Axial SE T1 TR/TE/excitation = 488/15/1, FOV = 230 mm, slice thickness/gap = 6 mm/1 mm, matrix = 256 × 256, and acquisition time = 1 min and 24.8 s and TSE T2 (TR/TE/excitation = 3992/110/2, turbo factor = 15, FOV = 230 mm, slice thickness/gap = 6 mm/1 mm, matrix = 512 × 512); and acquisition time = 1 min and 55.8 s images were also acquired.
For the multimodal MRI at follow-up, T1W was performed in the sagittal plane using a three-dimensional volumetric sequence and the following parameters: TR/TE/inversion time (TI) = 2300/2.27/900 ms, FOV = 250 × 235 mm2, matrix = 256 × 240, flip angle = 8°, slice thickness = 1 mm, and inter-slice spacing = 1 mm. DTI MRI was scanned using TR/TE = 9100/100 ms and, matrix = 224 × 224. The slice thickness was 2 mm, the inter-slice spacing was 2.6 mm, and the FOV was =285 × 285 mm2. Thirty diffusion gradient directions were realized using a b-value of 1000 s/mm2 and a non-DWI volume (b-value = 0 s/mm2; number of excitations = 2).
The magnetic resonance images were analyzed as follows. (1) Identification and localization of acute infarction: we visually assessed the site and volume of acute lesions in the DWI sequence. The locations of acute infarct were visually divided into cortical, subcortical, and infratentorial regions [18]. We also recorded unilateral acute cortical infarcts. (2) Brain volumetry: all the follow-up MRI scans were analyzed using AccuBrain® (BrainNow Medical Technology Limited, Shenzhen, China). AccuBrain is a fully automatic neuroanatomical volumetry tool that quantifies the brain volumes of various subcortical structures, ventricles, and cortical lobar atrophy within a clinically acceptable time. AccuBrain performs brain structure segmentation on the basis of a multi-atlas image registration scheme [19]. (3) White matter integrity: the microstructural integrity of the white matter was analyzed using DTI. The resampled data were used to compute the mean fractional anisotropy (FA) and mean diffusivity (MD), and the regional mean values of FA and MD were quantified after mapping using the ICBM_DTI_81 Atlas. Lower values of global FA and higher values of global MD reflected decreased WM microstructural integrity [20]. (4) Brain lesion quantification: we also assessed other brain abnormalities, such as the severity of defects in cortical cholinergic pathways according to Cholinergic Pathways Hyperintensities Scale [21], silent brain infarcts, enlarged perivascular spaces and the volumes of white matter hyperintensitiy. We classified silent brain infarction (SBI) lesions (historic stroke) according to their location, including in the brainstem, cerebellum, basal ganglia, thalamus, and lobes.
2.5. Statistical Analysis
Statistical analyses were performed using IBM SPSS for Windows (V 24.0, IBM Corp., Armonk, NY, USA). Descriptive data are presented as proportions, means or medians as appropriate. Univariate correlation analyses of variables with EQ were performed using Pearson’s (normally distributed data) or Spearman’s correlation (categorical or non-normally distributed data) analyses. Variables that were significantly associated with EQ (with p < 0.05) were then entered as independent variables into separate multiple linear regression analyses, using a backward stepwise selection strategy, with EQ as the dependent variable. The possibility of multicollinearity was tested for among the independent variables and was accepted when the correlation coefficient was ≥0.6. The significance level was set at 0.05 (two-sided).
3. Results
Two hundred and eighty-six patients who had experienced a first acute ischemic stroke were consecutively admitted during the study period. The selection of patients is shown in a flow chart (Figure 1). Forty-one patients were included in the final analysis. A comparison between the excluded and included patients showed no differences in age (59.9 ± 14.9 years vs. 61.7 ± 9 years, respectively, p = 0.464) or sex (men, 67.9% vs. 56.1%, respectively, p = 0.143), but there were significant differences in the NIHSS score on admission (4 (2–8) vs. 2 (0–3), respectively, p < 0.001) and the length of stay in hospital (12 (8–17) days vs. 6 (5–9) days, respectively, p < 0.001).
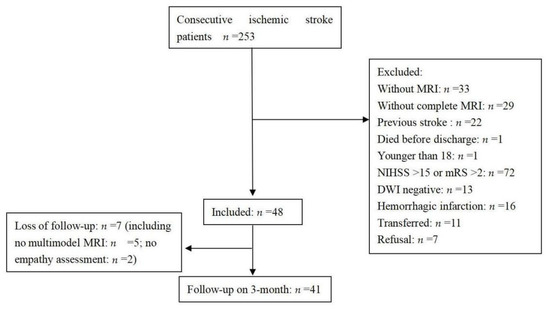
Figure 1.
Flow-chart of the study. NIHSS: National Institutes of Health Stroke Scale, DWI: Diffusio-weighted imaging, MRI: magnetic resonance imaging.
The baseline characteristics of the participants are shown in Table 1. The study sample consisted of 23 (56.1%) men and 18 (43.9%) women, with a mean age of 61.73 years (range, 41–77 years). The median NIHSS score at discharge was 1 (range: 0–4).

Table 1.
Results of the correlation analysis of the relationships between empathy and clinical characteristics.
The results of the other assessments at follow-up were as follows: SSQOL: 220.2 ± 21.6, HAMA: 3 (2–7), HAMD: 5 (2–10.5), MoCA: 22 (18.5–24.5), NIHSS: score: 0 (0–1), and mRS: 1 (0–1).
3.1. Univariate Correlates of EQ at Follow-Up
The univariate analyses are shown in Table 2, Table 3, Table 4, Table 5 and Table 6. It revealed that EQ was found to correlate with right cortical infarction (r = −0.39, p = 0.012), putamen volume (r = 0.382, p = 0.014), right putamen volume (r = 0.338, p = 0.031), and the FA value of the right sagittal stratum. EQ did not correlated with either the MD values of the regions of interest, or pre-existing brain abnormalities, including the location of SBI.

Table 2.
Results of correlation analysis for the relationships between empathy and structural magnetic resonance imaging-derived parameters.

Table 3.
Results of correlation analysis for the relationships between empathy and brain structure total volumes.

Table 4.
Results of the correlation analysis for the relationships between empathy and unilateral brain volumetry.

Table 5.
Results of correlation analysis for the relationships between empathy and brain atrophy.

Table 6.
Results of the correlation analysis for the relationships between empathy and white matter integrity.
3.2. Linear Regression Analysis of the EQ
The multiple stepwise linear regression models are shown in Table 7. Right frontal lobe infarct was not included in the model because it strongly correlated with right cortical infarction (r = 0.668). After adjustment for age and the NIHSS score on admission, the right putamen volume and FA of the right sagittal stratum did not significantly correlate with the EQ. The presence of a right cortical infarcts, significantly negatively correlated with the EQ, with a standardized β of −0.358 (95% confidence interval = −0.708 to −0.076, p = 0.016). Putamen volume was positively correlated with EQ, with a standardized β of 0.328 (95% confidence interval = 0.044 to 0.676, p = 0.027).

Table 7.
Results of linear regression analysis.
4. Discussions
By means of this observational study, we aimed to evaluate the relationships of biomarkers obtained by neuroimaging using multimodal MRI with the impairment of empathy in patients who had experienced subacute ischemic stroke. We assessed the empathy status of the patients 3 months after the index stroke. Brain structure volumetry of the various subcortical and ventricular structures and major lobar atrophy were quantified at follow-up using three-dimensional T1W images. In addition, neuroimaging-derived markers of white matter integrity, such as mean FA and mean MD, of the regions of interest defined in the ICBM_DTI_81 Atlas, were quantified. The principal findings were that the frequency of right cortical infarcts and the putamen volume significantly correlate with empathy in patients who have experienced subacute ischemic stroke.
In the present study, the frequency of right cortical infarcts, significantly negatively correlated with EQ (Figure 2), with a standardized β value of −0.358. This finding suggests that the presence of right cortical infarcts is associated with a lower EQ, indicating poorer empathy. Ischemic brain injury can lead to the impairment of empathy. Leigh et al. studied 27 patients with acute right hemisphere ischemic stroke and 24 neurologically intact inpatients using a test of affective empathy [4], and found that an acute impairment of affective empathy is associated with infarcts in the temporal pole and anterior insula. Another study showed that stroke involving the right posterior superior temporal gyrus in the right hemisphere ventral stream was critical for the identification of emotion in speech [10]. This finding suggests that patients who have experienced stroke and infarction in this area should be assessed for the impairment of emotion. Although these studies were inconclusive, they suggest that a network may play a major role in the processing of empathy. The present findings are consistent with the notion that the right cortical region may play an important role in the recognition of emotion.
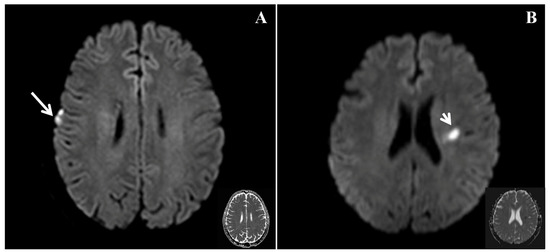
Figure 2.
Diffusio-weighted imaging (DWI) and the corresponding apparent diffusion coefficient (ADC) in patients with differing empathy statuses. (A) A patient with an EQ of 16 (poorer empathy) had an acute infarct involving the right frontal lobe marked by a long arrow (right cortical infarct) during the acute phase. (B) A patient with an EQ of 73 (better empathy) had an acute infarct in the left corona radiata marked by a short arrow (left subcortical infarct) during the acute phase.
In the present study, we also assessed the relationship between brain structure volumetry and empathy in patients who had experienced acute ischemic stroke. We found that the putamen volume significantly positively correlated with the EQ (Figure 3), with a standardized β value of 0.328. We also obtained data regarding putaminal volumes using quantitative images and present the corresponding relationships of EQ for each participant in the Supplemental materials. These findings show that a smaller putamen volume is associated with a more severe impairment of empathy. As a sequela of stroke may be related to the index stroke and/or SBIs, we assessed the relationship between EQ and the location of SBI (brainstem, cerebellum, basal ganglia, thalamus, or lobes), and found that the location of SBI was not significantly associated with EQ. This implies that a smaller putamen may be more affected both by a combination of stroke and a constitutional condition than by a single factor.
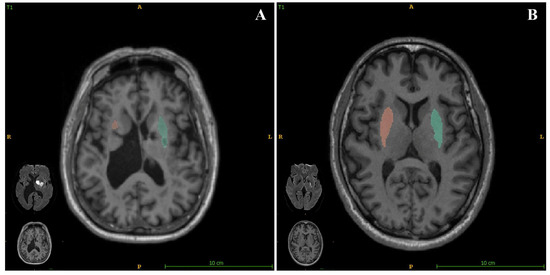
Figure 3.
AccuBrain® (BrainNow Research Institute, Shenzhen, China) was used to quantify of the volume of the putamen and corresponding diffusion-weighted imaging (DWI) in patients with differing empathy statuses. (A) Image of a patient with an EQ of 23 (poorer empathy), with a total putamen volume of 5.53 mL. (B) Image of a patient with EQ of 57 (better empathy) with a total putamen volume of 11.6 mL.
Some recent neuroimaging studies have identified a series of brain regions that are involved in empathy. A functional MRI study showed that stimuli are associated with greater activations of the caudate-putamen, paracingulate, anterior and posterior cingulate, and amygdala than that predicted by the theory of mind stimuli [22]. The authors of another study concluded that the dorsal anterior cingulate cortex-anterior mid-cingulate cortex-supplementary motor area and the bilateral insula can be considered to form a core network in empathy [23]. In additional, they concluded that cognitive evaluative and affective–perceptual empathy can be distinguished at the level of regional activation. The putamen is an important part of the lateral pathway of the cortical cholinergic pathway [24]. It is a subcortical structure that forms part of the dorsal striatum of the basal ganglia, and is conventionally thought to be associated with the reinforcement of learning and motor control, including speech articulation [25]. A study of macaque monkeys showed that deep and superficial cortical white matter neurons, peri-claustral white matter neurons, and the claustrum proper project to the putamen [26]. Moreover, Zhao et al. showed that the putamen may play an important role in instrumental learning processes, and that structural variations of associated brain regions are required for the successful acquisition of functional MRI neurofeedback-guided self-regulation [27]. The involvement of the unilateral putamen in various language components suggests that it may play a role in language [28]. A smaller putamen volume indicates the possibility of injury to neurons and projecting fibers, which may interfere with the processing of language during learning and the recognition of emotion, and thus be involved in empathy.
The present study had the following strengths. (1) We assessed the patients’ empathy after subacute ischemic stroke. (2) We used a comprehensive set of neuroimaging parameters obtained using a multimodal MRI protocol, including acute infarction, brain volumetry, white matter integrity, and other preexisting abnormalities. There were also some limitations to the study. First, the sample size was relatively small. Second, other social cognition domains were not evaluated, such as the theory of mind, perception and behavior. Third, the recruited patients had experienced relatively mild stroke, which may limit the generalizability of the current findings. Fourth, because the range of stroke subtypes was relatively limited, with only 5% of participants having atrial fibrillation, the representativeness of the sample may be insufficient to generalize the findings to all patients who experience stroke.
In conclusion, in patients with subacute ischemic stroke, right cortical infarction is negatively correlates with empathy, while the putamen volume is positively correlates with empathy. If these findings can be confirmed in a larger study, they should contribute to the understanding of the loss of empathy that occurs following stroke.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11154479/s1, Supplemental Data: Putaminal volumes of quantitative images and corresponding result of EQ for each patient
Author Contributions
J.-F.Q. wrote the manuscript. J.-F.Q., Y.-Q.Z., J.-F.L., H.-H.H., W.-Y.C., Z.-H.L., L.S., Y.-S.L. and L.Z. performed the study, collected and analyzed the data. Y.-K.C. designed the study, reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Dongguan Science and Technology of Social Development Program (20211800905182) and the Key Project of Guangdong Basic and Applied Basic Research Fund (Dongguan Joint Fund) [2020B1515120055].
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original data generated during the study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank Mark Cleasby for editing the language of a draft of this manuscript.
Conflicts of Interest
L.S. is the director of BrainNow Medical Technology Limited. Y.-S.L. and L.Z. are currently employed by BrainNow Medical Technology Limited. All other authors report no financial relationships with commercial interests.
References
- Beer, J.S.; Ochsner, K.N. Social cognition: A multi level analysis. Brain Res. 2006, 1079, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.D.; von Hippel, W.; Molenberghs, P.; Lee, T.; Sachdev, P.S. Clinical assessment of social cognitive function in neurological disorders. Nat. Rev. Neurol. 2016, 12, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.G.; Schweitzer, D.; Molenberghs, P.; Henry, J.D. A meta-analytic review of social cognitive function following stroke. Neurosci. Biobehav. Rev. 2019, 102, 400–416. [Google Scholar] [CrossRef] [PubMed]
- Leigh, R.; Oishi, K.; Hsu, J.; Lindquist, M.; Gottesman, R.F.; Jarso, S.; Crainiceanu, C.; Mori, S.; Hillis, A.E. Acute lesions that impair affective empathy. Brain 2013, 136, 2539–2549. [Google Scholar] [CrossRef] [PubMed]
- Shamay-Tsoory, S.G.; Tomer, R.; Goldsher, D.; Berger, B.D.; Aharon-Peretz, J. Impairment in cognitive and affective empathy in patients with brain lesions: Anatomical and cognitive correlates. J. Clin. Exp. Neuropsychol. 2004, 26, 1113–1127. [Google Scholar] [CrossRef] [PubMed]
- Yuvaraj, R.; Murugappan, M.; Norlinah, M.I.; Sundaraj, K.; Khairiyah, M. Review of emotion recognition in stroke patients. Dement. Geriatr. Cogn. Disord. 2013, 36, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zheng, L.; Zhang, W.; Zhu, L.; Li, J.; Wang, Q.; Dienes, Z.; Yang, Z. Empathic neural responses to others’ pain depend on monetary reward. Soc. Cogn. Affect Neurosci. 2012, 7, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Z.T.; Tsai, C.F. Impairment on theory of mind and empathy in patients with stroke. Psychiatry Clin. Neurosci. 2014, 68, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Nijsse, B.; Spikman, J.M.; Visser-Meily, J.M.; de Kort, P.L.; van Heugten, C.M. Social Cognition Impairments in the Long Term Post Stroke. Arch. Phys. Med. Rehabil. 2019, 100, 1300–1307. [Google Scholar] [CrossRef] [Green Version]
- Sheppard, S.M.; Keator, L.M.; Breining, B.L.; Wright, A.E.; Saxena, S.; Tippett, D.C.; Hillis, A.E. Right hemisphere ventral stream for emotional prosody identification: Evidence from acute stroke. Neurology 2020, 94, e1013–e1020. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Duncan, N.W.; de Greck, M.; Northoff, G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci. Biobehav. Rev. 2011, 35, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Neumann, D.L.; Cao, X.; Baron-Cohen, S.; Sun, X.; Cao, Y.; Yan, C.; Wang, Y.; Shao, L.; Shum, D.H.K. Validation of the Empathy Quotient in Mainland China. J. Pers. Assess. 2018, 100, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Wheelwright, S. The empathy quotient: An investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J. Autism. Dev. Disord. 2004, 34, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.S.; Weinberger, M.; Harris, L.E.; Clark, D.O.; Biller, J. Development of a stroke-specific quality of life scale. Stroke 1999, 30, 1362–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.Y. Depression and anxiety. In The Rating Scale for Psychiatry, 1st ed.; Zhang, M.Y., Ed.; Hunan Science and Technology Press: Changsha, China, 1998; pp. 30–32. [Google Scholar]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Chen, Y.; Luo, G.; Zhong, H.; Xiao, W.; Yin, H. Delirium in the Acute Phase of Ischemic Stroke: Incidence, Risk Factors, and Effects on Functional Outcome. J. Stroke Cerebrovasc. Dis. 2018, 27, 2641–2647. [Google Scholar] [CrossRef] [PubMed]
- Abrigo, J.; Shi, L.; Luo, Y.; Chen, Q.; Chu, W.C.W.; Mok, V.C.T. Alzheimer’s Disease Neuroimaging Initiative. Standardization of hippocampus volumetry using automated brain structure volumetry tool for an initial Alzheimer’s disease imaging biomarker. Acta Radiol. 2019, 60, 769–776. [Google Scholar] [CrossRef]
- Mori, S.; Oishi, K.; Jiang, H.; Jiang, L.; Li, X.; Akhter, K.; Hua, K.; Faria, A.V.; Mahmood, A.; Woods, R.; et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 2008, 40, 570–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, J.F.; Chen, Y.K.; Luo, G.P.; Zhao, J.H.; Zhong, H.H.; Yin, H.P. Severe Lesions Involving Cortical Cholinergic Pathways Predict Poorer Functional Outcome in Acute Ischemic Stroke. Stroke 2018, 49, 2983–2989. [Google Scholar] [CrossRef]
- Völlm, B.A.; Taylor, A.N.; Richardson, P.; Corcoran, R.; Stirling, J.; McKie, S.; Deakin, J.F.; Elliott, R. Neuronal correlates of theory of mind and empathy: A functional magnetic resonance imaging study in a nonverbal task. Neuroimage 2006, 29, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M. The neuronal mechanism underlying parkinsonism and dyskinesia: Differential roles of the putamen and caudate nucleus. Neurosci. Res. 1991, 12, 31–40. [Google Scholar] [CrossRef]
- Selden, N.R.; Gitelman, D.R.; Salamon-Murayama, N.; Parrish, T.B.; Mesulam, M.M. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain 1998, 121, 2249–2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abutalebi, J.; Della Rosa, P.A.; Green, D.W.; Hernandez, M.; Scifo, P.; Keim, R.; Cappa, S.F.; Costa, A. Bilingualism tunes the anterior cingulate cortex for conflict monitoring. Cereb. Cortex. 2012, 22, 2076–2086. [Google Scholar] [CrossRef] [PubMed]
- Borra, E.; Luppino, G.; Gerbella, M.; Rozzi, S.; Rockland, K.S. Projections to the putamen from neurons located in the white matter and the claustrum in the macaque. J. Comp. Neurol. 2020, 528, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yao, S.; Zweerings, J.; Zhou, X.; Zhou, F.; Kendrick, K.M.; Chen, H.; Mathiak, K.; Becker, B. Putamen volume predicts real-time fMRI neurofeedback learning success across paradigms and neurofeedback target regions. Hum. Brain Mapp. 2021, 42, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Viñas-Guasch, N.; Wu, Y.J. The role of the putamen in language: A meta-analytic connectivity modeling study. Brain Struct. Funct. 2017, 222, 3991–4004. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).