Patterns of Brain Sparing in a Fetal Growth Restriction Cohort
Abstract
:1. Introduction
- To determine how often each study vessel (MCA, ACA, PCA, and VA) had pulsatility index (PI) values below the 5th percentile in fetuses whose estimated fetal weights were below the 10th percentile and who were defined as having FGR or SGA using Delphi (ISUOG) criteria.
- To compare average cerebral vessel PI values in FGR and SGA fetuses with postnatal outcomes.
- To determine if any of the fetal cerebral vessels yielded additional useful information regarding perinatal outcome above that provided by the MCA as used in the CPR.
2. Methods
3. Results
4. Discussion
Principle Findings
- While fetuses defined by ISUOG as FGR were not associated with earlier deliveries than those defined as SGA (37.3 weeks vs. 37.8 weeks p = 0.186), they did have significantly lower average birthweights (2247 g vs. 2636 g, p < 0.0001) and lower birthweight percentiles (6.91% vs. 20.61% p < 0.0001). Although FGR fetuses were more frequently admitted to the NICU than SGA fetuses, this did not attain statistical significance.
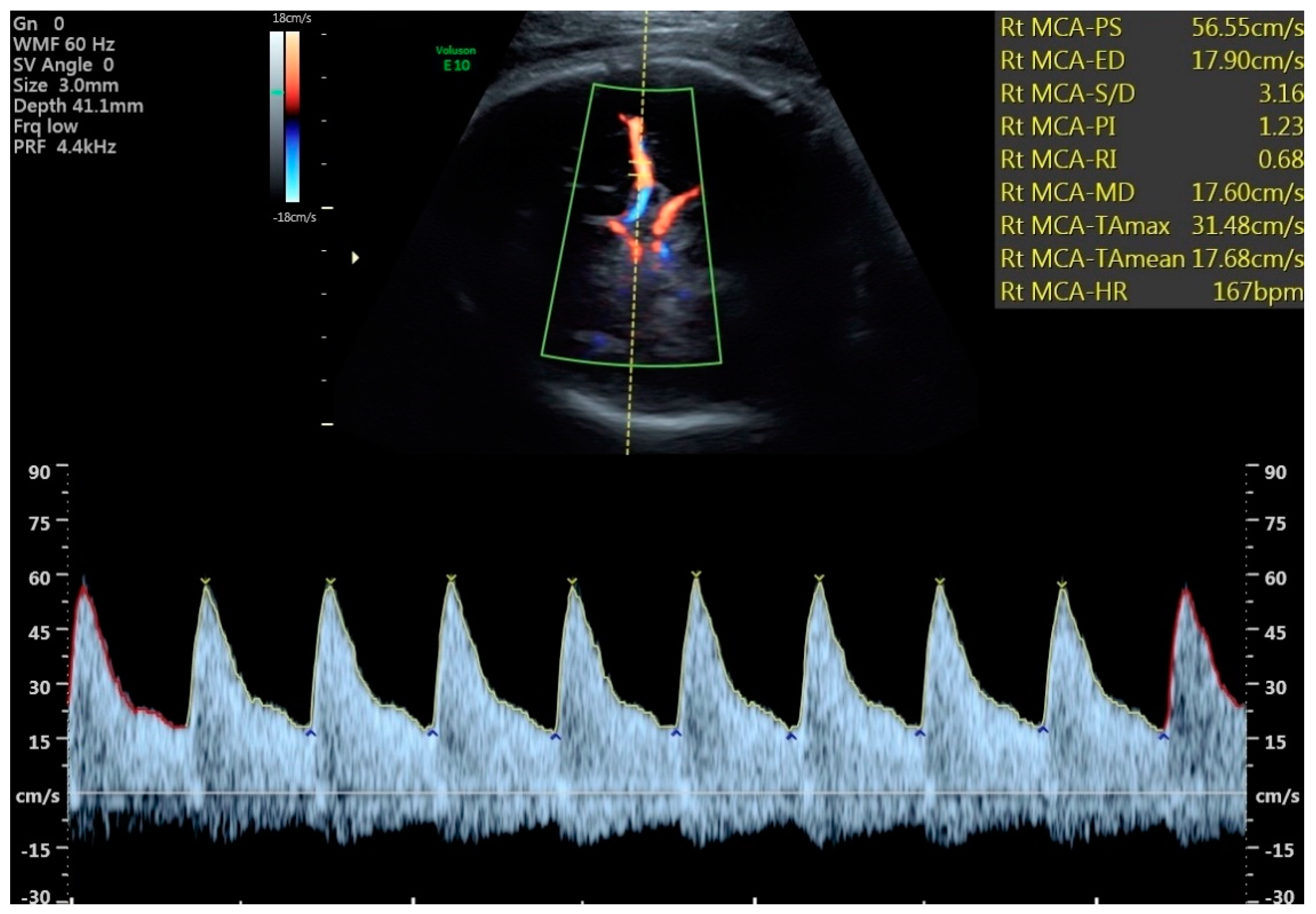
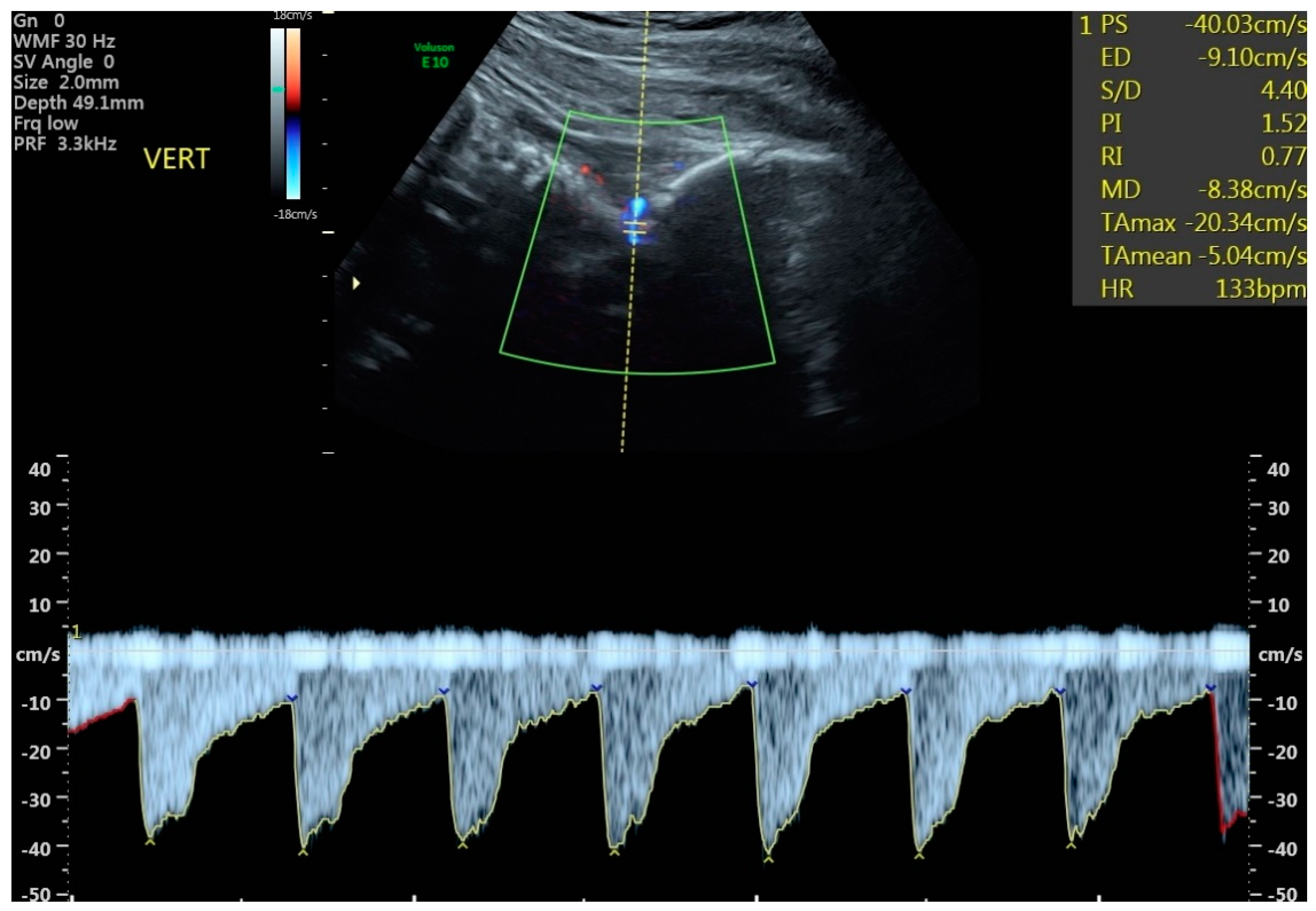
- The total numbers of abnormal PIs from the cerebral vessels studied were strikingly different between SGA and FGR (4 vs. 36, p = 0.055). However, the number of cerebral vessel PIs below the 5th centile was only significant between FGR and SGA groups for the MCA and VA (Table 3). All 8 fetuses in the study with abnormal VA PIs were FGR.
- Fetuses with an abnormal VA PI stood out as being different in birth metrics and in their distinct tendency to be linked with lower PIs in each vessel emanating from the circle of Willis, compared with those with normal VA PIs (Table 4).
- When comparing in utero cerebral Dopplers and neonatal outcome data between fetuses with abnormal VAs and the commonly used CPR, the VA was associated with lower average PIs from the companion vessels, earlier delivery, lower birthweight, higher rates of cesarean section, and more frequent admission to the NICU, suggesting a more specific measure for adverse perinatal outcome than MCA. However, birthweight centiles were not different between groups when corrected for gestational age (Table 4 and Table 5).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savchev, S.; Sanz-Cortes, M.; Cruz-Martinez, R.; Arranz, A.; Botet, F.; Gratacos, E.; Figueras, F. Neurodevelopmental outcome of full-term small-for-gestational-age infants with normal placental function. Ultrasound Obstet. Gynecol. 2013, 42, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Baschat, A.A. Neurodevelopment following fetal growth restriction and its relationship with antepartum parameters of placental dysfunction. Ultrasound Obstet. Gynecol. 2011, 37, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Clausen, C.F.; Morton, J.S.; Davidge, S.T. Effects of hypoxia-induced intrauterine growth restriction on cardiopulmonary structure and function during adulthood. Cardiovasc. Res. 2009, 81, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Crispi, F.; Miranda, J.; Gratacos, E. Long-term cardiovascular consequences of fetal growth restriction: Biology, clinical implications, and opportunities for prevention of adult disease. Am. J. Obstet. Gynecol. 2018, 218, S869–S879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hales, C.N.; Barker, D.J. The thrifty phenotype hypothesis. Br. Med. Bull. 2001, 60, 5–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Lees, C.C.; Stampalija, T.; Baschat, A.; da Silva Costa, F.; Ferrazzi, E.; Figueras, F.; Hecher, K.; Poon, L.C.; Salomon, L.J.; Unterscheider, J. ISUOG Practice Guidelines: Diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet. Gynecol. 2020, 56, 298–312. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 204: Fetal growth restriction. Obstet. Gynecol. 2019, 133, e97–e109. [Google Scholar] [CrossRef]
- Martins, J.G.; Biggio, J.R.; Abuhamad, A. Society for Maternal-Fetal Medicine (SMFM. Society for Maternal-Fetal Medicine Consult Series# 52: Diagnosis and management of fetal growth restriction: (replaces clinical guideline number 3, April 2012). Am. J. Obstet. Gynecol. 2020, 223, B2–B17. [Google Scholar] [PubMed]
- Giussani, D.A. The fetal brain sparing response to hypoxia: Physiological mechanisms. J. Physiol. 2016, 594, 1215–1230. [Google Scholar] [CrossRef] [Green Version]
- Figueroa-Diesel, H.; Hernandez-Andrade, E.; Acosta-Rojas, R.; Cabero, L.; Gratacos, E. Doppler changes in the main fetal brain arteries at different stages of hemodynamic adaptation in severe intrauterine growth restriction. Ultrasound Obstet. Gynecol. 2007, 30, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Oros, D.; Figueras, F.; Cruz-Martinez, R.; Padilla, N.; Meler, E.; Hernandez-Andrade, E.; Gratacos, E. Middle cerebral versus anterior cerebral Doppler for prediction of prenatal outcome and behavior in term small for gestational age fetuses with normal umbilical artery Dopplers. Ultrasound Obstet. Gynecol. 2010, 35, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Benavides-Serralde, J.A.; Hernandez-Andrade, E.; Cruz-Martinez, R.; Cruz-Lemini, M.; Scheier, M.; Figueras, F.; Mancilla, J.; Gratacos, E. Doppler evaluation of the posterior cerebral artery in normally grown and growth restricted fetuses. Prenat. Diagn. 2014, 34, 115–120. [Google Scholar] [CrossRef]
- Morales-Roselló, J.; Hervas Marin, D.; Perales Marin, A. The vertebral artery Doppler might be an alternative to the middle cerebral artery Doppler in the follow-up of the early onset growth-restricted fetus. Prenat. Diagn. 2014, 34, 109–114. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Committee Opinion No 700: Methods for Estimating the Due Date. Obstet. Gynecol. 2017, 129, e150–e154. [Google Scholar] [CrossRef] [PubMed]
- Hadlock, F.P.; Harrist, R.B.; Martinez-Poyer, J. In utero analysis of fetal growth: A sonographic weight standard. Radiology 1991, 181, 129–133. [Google Scholar] [CrossRef]
- Hadlock, F.P.; Harrist, R.B.; Sharman, R.S.; Deter, R.L.; Park, S.K. Estimation of fetal weight with the use of head, body, and femur measurements—A prospective study. Am. J. Obstet. Gynecol. 1985, 151, 333–337. [Google Scholar] [CrossRef]
- Ebbing, C.; Rasmussen, S.; Kiserud, T. Middle cerebral artery blood flow velocities and pulsatility index and the cerebroplacental pulsatility ratio: Longitudinal reference ranges and terms for serial measurements. Ultrasound Obstet. Gynecol. 2007, 30, 287–296. [Google Scholar] [CrossRef]
- DeVore, G.R. The importance of the cerebroplacental ratio in the evaluation of fetal well-being in SGA and AGA fetuses. Am. J. Obstet. Gynecol. 2015, 213, 5–15. [Google Scholar] [CrossRef]
- DeVore, G.R. Computing the Z score and centiles for cross-sectional analysis: A practical approach. J. Ultrasound Med. 2017, 36, 459–473. [Google Scholar] [CrossRef] [Green Version]
- Benavides-Serralde, J.A.; Hernandez-Andrade, E.; Figueroa-Diesel, H.; Oros, D.; Feria, L.A.; Scheier, M.; Figueras, F.; Gratacos, E. Reference values for Doppler parameters of the fetal anterior cerebral artery throughout gestation. Gynecol. Obstet. Investig. 2010, 69, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Andrade, E.; Figueroa-Diesel, H.; Jansson, T.; Rangel-Nava, H.; Gratacos, E. Changes in regional fetal cerebral blood flow perfusion in relation to hemodynamic deterioration in severely growth-restricted fetuses. Ultrasound Obstet. Gynecol. 2008, 32, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Dubiel, M.; Gunnarsson, G.O.; Gudmundsson, S. Blood redistribution in the fetal brain during chronic hypoxia. Ultrasound Obstet. Gynecol. 2002, 20, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Martinez, R.; Figueras, F.; Hernandez-Andrade, E.; Puerto, B.; Gratacós, E. Longitudinal brain perfusion changes in near-term small-for-gestational-age fetuses as measured by spectral Doppler indices or by fractional moving blood volume. Am. J. Obstet. Gynecol. 2010, 203, 42-e1. [Google Scholar] [CrossRef] [PubMed]
- Morales Roselló, J.; Hervás Marín, D.; Fillol Crespo, M.; Perales Marín, A. Doppler changes in the vertebral, middle cerebral, and umbilical arteries in fetuses delivered after 34 weeks: Relationship to severity of growth restriction. Prenat. Diagn. 2012, 32, 960–967. [Google Scholar] [CrossRef]
- Trudinger, B.J.; Stevens, D.; Connelly, A.; Hales, J.R.; Alexander, G.; Bradley, L.; Fawcett, A.; Thompson, R.S. Umbilical artery flow velocity waveforms and placental resistance: The effects of embolization of the umbilical circulation. Am. J. Obstet. Gynecol. 1987, 157, 1443–1448. [Google Scholar] [CrossRef]
- Ferrazzi, E.; Bozzo, M.; Rigano, S.; Bellotti, M.; Morabito, A.; Pardi, G.; Battaglia, F.C.; Galan, H.L. Temporal sequence of abnormal Doppler changes in the peripheral and central circulatory systems of the severely growth-restricted fetus. Ultrasound Obstet. Gynecol. 2002, 19, 140–146. [Google Scholar] [CrossRef]
- Hecher, K.; Bilardo, C.M.; Stigter, R.H.; Ville, Y.; Hackelöer, B.J.; Kok, H.J.; Senat, M.V.; Visser, G.H. Monitoring of fetuses with intrauterine growth restriction: A longitudinal study. Ultrasound Obstet. Gynecol. 2001, 18, 564–570. [Google Scholar] [CrossRef] [Green Version]
- Turan, O.M.; Turan, S.; Gungor, S.; Berg, C.; Moyano, D.; Gembruch, U.; Nicolaides, K.H.; Harman, C.R.; Baschat, A.A. Progression of Doppler abnormalities in intrauterine growth restriction. Ultrasound Obstet. Gynecol. 2008, 32, 160–167. [Google Scholar] [CrossRef]
- Hobbins, J.C.; Gumina, D.L.; Zaretsky, M.V.; Driver, C.; Wilcox, A.; DeVore, G.R. Size and shape of the four-chamber view of the fetal heart in fetuses with an estimated fetal weight less than the tenth centile. Am. J. Obstet. Gynecol. 2019, 221, 495.e1–495.e9. [Google Scholar] [CrossRef]
- DeVore, G.R.; Gumina, D.L.; Hobbins, J.C. Assessment of ventricular contractility in fetuses with an estimated fetal weight less than the tenth centile. Am. J. Obstet. Gynecol. 2019, 221, 498.e1–498.e22. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Martínez, R.; Figueras, F.; Hernandez-Andrade, E.; Oros, D.; Gratacos, E. Fetal brain Doppler to predict cesarean delivery for nonreassuring fetal status in term small-for-gestational-age fetuses. Obstet. Gynecol. 2011, 117, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Makhseed, M.; Jirous, J.; Ahmed, M.A.; Viswanathan, D.L. Middle cerebral artery to umbilical artery resistance index ratio in the prediction of neonatal outcome. Int. J. Gynaecol. Obstet. 2000, 71, 119–125. [Google Scholar] [CrossRef]
- Alfirevic, Z.; Stampalija, T.; Dowswell, T. Fetal umbilical Doppler ultrasound in high-risk pregnancies. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Eixarch, E.; Meler, E.; Iraola, A.; Illa, M.; Crispi, F.; Hernandez-Andrade, E.; Gratacos, E.; Figueras, F. Neurodevelopmental outcome in 2-year-old infants who were small-for-gestational age term fetuses with cerebral blood flow redistribution. Ultrasound Obstet. Gynecol. 2008, 32, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, K.H.; Wright, D.; Syngelaki, A.; Wright, A.; Akolekar, R. Fetal Medicine Foundation population weight charts. Ultrasound Obstet. Gynecol. 2018, 52, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales-Roselló, J.; Khalil, A.; Morlando, M.; Papageorghiou, A.; Bhide, A.; Thilaganathan, B. Changes in fetal Doppler indices as a marker of failure to reach growth potential at term. Ultrasound Obstet. Gynecol. 2014, 43, 303–310. [Google Scholar] [CrossRef]
| Early FGR: GA < 32 Weeks with No Congenital Abnormalities | Late FGR: GA ≥ 32 Weeks with No Congenital Abnormalities |
|---|---|
| AC or EFW less than 3rd centile or UA-AEDF | AC or EFW less than 3rd centile |
| Or AC or EFW < 10th centile combined with | Or at least two of the three: |
|
|
| Cerebral Dopplers between ISUOG Grouping | SGA Mean ± SEM (n = 30) | FGR Mean ± SEM (n = 51) | p Value |
|---|---|---|---|
| Mean MCA PI centile † | 41.36% ± 4.86% | 41.05% ± 4.46% | 0.787 |
| Mean ACA PI centile † | 37.80% ± 5.75% | 32.38% ± 4.26% | 0.377 |
| Mean PCA PI centile † | 44.00% ± 4.51% | 34.93% ± 4.25% | 0.104 |
| Mean UA PI centile † | 63.47% ± 3.70% | 77.41% ± 3.18% | 0.0008 *** |
| Clinical Variables | |||
| GA at Analysis (wks) † | 35.82 ± 0.27 | 35.94 ± 0.22 | 0.710 |
| GA at Delivery (wks) † | 37.84 ± 0.22 | 37.29 ± 0.23 | 0.186 |
| Birthweight (g) ‡ | 2636 ± 59.86 | 2247 ± 65.06 | <0.0001 *** |
| Cesarean Section Rate § | 4/26 (15.38%) | 10/37 (27.03%) | 0.362 |
| Fenton Birthweight (%) ‡ | 20.61% ± 2.88% | 6.91% ± 0.78% | <0.0001 *** |
| NICU Admission § | 4/22 (18.18%) | 13/36 (36.11%) | 0.234 |
| Number of Abnormal Dopplers in ISUOG Groups | SGA (n = 30) | FGR (n = 51) | p-Value |
|---|---|---|---|
| Total # of abnormal Dopplers † (1 pt each for MCA, ACA, PCA, VA; max: 4 pts/fetus) | 4 | 36 | 0.055 |
| # of fetuses with ≥1 abnormal cerebral Doppler ‡ | 4/30 | 15/51 | 0.113 |
| # of fetuses with MCA PI < 5th centile ‡ | 1/30 | 10/51 | 0.047 * |
| # of fetuses with ACA PI < 5th centile ‡ | 2/27 | 11/46 | 0.113 |
| # of fetuses with PCA PI < 5th centile ‡ | 1/29 | 7/46 | 0.141 |
| # of fetuses with VA PI < 5th centile ‡ | 0/26 | 8/43 | 0.021 * |
| Cerebral Dopplers in Fetuses with Normal and Abnormal VA | Pts w/Abnormal VA Dopplers Mean ± SEM (n = 8) | Pts w/Normal VA Dopplers Mean ± SEM (n = 61) | p-Value |
|---|---|---|---|
| Mean MCA PI %ile † | 9.96% ± 5.56% | 44.02% ± 3.81% | 0.0002 *** |
| Mean ACA PI %ile † | 3.41% ± 0.56% | 37.44% ± 3.62% | <0.0001 *** |
| Mean PCA PI %ile † | 7.72% ± 3.22% | 41.09% ± 3.35% | <0.0001 *** |
| Clinical Variables | |||
| GA at Analysis (wks) † | 34.64 ± 0.71 | 36.13 ± 0.17 | 0.023 * |
| GA at Delivery (wks) ‡ | 35.22 ± 0.63 | 37.89 ± 0.14 | 0.0052 ** |
| Birthweight (g) ‡ | 1712 ± 151.71 | 2500 ± 48.85 | <0.0001 *** |
| Cesarean Section Rate § | 5/7 (71.43%) | 8/49 (16.32%) | 0.005 ** |
| Fenton Birthweight (%) † | 4.64% ± 1.37% | 13.37% ± 1.80% | 0.036 * |
| Admission to NICU § | 5/7 (71.43%) | 11/45 (24.44%) | 0.023 * |
| Cerebral Dopplers in Fetuses with Normal and Abnormal CPR | Pts w/Abnormal CPR Dopplers Mean ± SEM (n = 14) | Pts w/Normal CPR Dopplers Mean ± SEM (n = 67) | p-Value |
|---|---|---|---|
| Mean MCA PI %ile † | 7.83% ± 2.14% | 48.14% ± 3.42% | <0.0001 *** |
| Mean ACA PI %ile † | 12.78% ± 3.29% | 39.06% ± 3.84% | 0.0011 ** |
| Mean PCA PI %ile † | 21.19% ± 6.16% | 42.05% ± 3.44% | 0.006 ** |
| # of VA PI < 5th centile § | 5/12 | 3/57 | 0.003 ** |
| Clinical Variables | |||
| GA at Analysis (wks) † | 35.68 ± 0.48 | 35.94 ± 0.18 | 0.350 |
| GA at Delivery (wks) † | 36.73 ± 0.52 | 37.69 ± 0.16 | 0.037 * |
| Birthweight (g) ‡ | 2009 ± 135.31 | 2492 ± 47.67 | 0.0001 *** |
| Cesarean Section Rate § | 4/11 (36.36%) | 10/52 (19.23%) | 0.243 |
| Fenton Birthweight (%) † | 3.93% ± 1.05% | 14.30% ± 1.71% | <0.0001 *** |
| Admission to NICU § | 5/12 (41.67%) | 12/46 (26.09%) | 0.307 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steller, J.G.; Gumina, D.; Driver, C.; Peek, E.; Galan, H.L.; Reeves, S.; Hobbins, J.C. Patterns of Brain Sparing in a Fetal Growth Restriction Cohort. J. Clin. Med. 2022, 11, 4480. https://doi.org/10.3390/jcm11154480
Steller JG, Gumina D, Driver C, Peek E, Galan HL, Reeves S, Hobbins JC. Patterns of Brain Sparing in a Fetal Growth Restriction Cohort. Journal of Clinical Medicine. 2022; 11(15):4480. https://doi.org/10.3390/jcm11154480
Chicago/Turabian StyleSteller, Jon G., Diane Gumina, Camille Driver, Emma Peek, Henry L. Galan, Shane Reeves, and John C. Hobbins. 2022. "Patterns of Brain Sparing in a Fetal Growth Restriction Cohort" Journal of Clinical Medicine 11, no. 15: 4480. https://doi.org/10.3390/jcm11154480
APA StyleSteller, J. G., Gumina, D., Driver, C., Peek, E., Galan, H. L., Reeves, S., & Hobbins, J. C. (2022). Patterns of Brain Sparing in a Fetal Growth Restriction Cohort. Journal of Clinical Medicine, 11(15), 4480. https://doi.org/10.3390/jcm11154480






