Abstract
Evidence suggests that factors associated with a family history of neurodegenerative disease (fhNDD) may influence outcomes following a concussion. However, the relevance of these findings in adolescent populations has not been fully explored. Therefore, the present study sought to evaluate the relationship between fhNDD and neurological outcomes following an adolescent concussion. Data from a local pediatric concussion clinic were used to compare adolescents with (n = 22) and without (n = 44) an fhNDD. Clinical symptom burden, emotional health, cardio-autonomic function, and cognitive performance were assessed at initial (~2 weeks) and follow-up (~5 weeks) post-injury evaluations. Cardio-autonomic function was assessed at rest and during isometric handgrip contraction (IHGC). Results indicated no significant group differences in emotional health or cognitive performance. Across evaluations, those with an fhNDD exhibited greater somatic symptom severity, alterations in HRV at rest, and early blunted cardio-autonomic reactivity during IHGC compared to those without an fhNDD. These findings suggest that positive fhNDD is negatively associated with clinical symptomology and cardio-autonomic functioning following an adolescent concussion. Further, these findings encourage clinicians to utilize a comprehensive neurological evaluation to monitor concussion recovery. Future studies should look into exploring the role of specific neurodegenerative processes and conditions on concussion outcomes in adolescents.
1. Introduction
A concussion is a form of mild traumatic brain injury (mTBI) induced by a direct blow to the head, neck, or body that is transmitted to cerebral tissues [1]. These biomechanical forces result in cascading alterations in neurometabolic function, which subsequently produce transient neurological impairment [2,3,4]. Post-concussive symptoms are often heuristically divided into three clinical domains: somatic (e.g., headache, dizziness, nausea), affective (e.g., depression, anxiety, irritability), and cognitive (e.g., attentional deficits, poor memory) [5,6]. Emerging research suggests that physiological deficits (e.g., cardio-autonomic dysfunction) present alongside the conventional concussion sequelae [7,8,9]. While post-concussive symptoms are expected to resolve within a few weeks, a subset of individuals (~15–20%) will remain symptomatic beyond the typical window of recovery [10,11]. Compared to adults, adolescents display an increased risk of both sustaining concussion and experiencing prolonged recovery thereafter [12,13]. These findings are particularly concerning as persisting deficits following concussion not only negatively affect the quality of life and academic achievement [14,15] but may leave adolescents susceptible to abnormal neurological development, which may present as hyperactivity or sustained cognitive impairment [16,17]. If adolescents are susceptible to future morbidity as a result of sustaining a concussion, researchers and clinicians need to identify premorbid factors that may be culpable in modulating neurological outcomes after injury.
Several factors associated with abnormal concussion recovery have been identified, including biological sex [18], history of concussion [19], and prior diagnoses of psychiatric or neurodevelopmental disorders [20,21]. In light of prominent research that has linked repetitive TBI to the onset of neurodegenerative disease (e.g., chronic traumatic encephalopathy, Alzheimer’s, Parkinson’s and dementia) in retired athletes [22,23], investigators have begun to examine how genetic precursors of neurodegenerative diseases might influence recovery trajectories following a concussion. Genetic polymorphisms associated with neurodegenerative diseases (i.e., APOE-ε4 allele, COMT Val/Met alleles) have been linked to both structural and functional neuronal abnormalities in healthy individuals [24,25,26]. Extensive research suggests individuals carrying these polymorphisms may be at risk for poorer outcomes following moderate/severe TBI [27,28]. It is postulated that particular gene variants may also predispose individuals to unfavorable neurogenic responses following concussion [29]. The APOE-ε4 allele has been shown to moderate post-concussion symptom reporting and neurocognitive performance in adult athletes and veterans across recovery milestones [30,31,32]. However, these findings have not been observed in studies following adolescent concussion [33]. While informative, current research focusing on APOE-ε4 allele-dependent gene expression has failed to integrate other heritable factors associated with neurodegenerative diseases.
An investigation of composite factors, such as an immediate family history of neurodegenerative disease (fhNDD), would allow researchers to acknowledge the influence of several modifiable risk factors. To date, only one study has investigated the influence of fhNDD on concussion outcomes [34]. However, this study exclusively examined college-aged males, thus, limiting the generalizability of its findings. Therefore, the present study sought to prospectively examine the influence of an immediate fhNDD (i.e., biological parent/grandparent) on adolescent concussion outcomes. In accordance with the most recent Consensus Statement on Concussion in Sport [1], we utilized a comprehensive assessment battery that assessed: symptom burden, emotional health, cardio-autonomic function, and cognitive performance following adolescent concussion at two remote time points after injury. We hypothesized that adolescents with a positive fhNDD would exhibit diminished cardio-autonomic regulation and poorer cognitive performance compared to adolescents without an fhNDD following concussion.
2. Experimental Section
2.1. Participants
This study is a retrospective analysis of data extracted from part of a larger study on the clinical evaluation of concussion. Four-hundred fifty adolescents, between the ages of 12–17 years old, suspected of having sustained a concussion recently, were evaluated at a local pediatric concussion clinic. Concussion diagnoses were confirmed by an attending physician (J.P.H.) in accordance with the guidelines established by the Consensus Statement on Concussion in Sport and the American Academy of Neurology [1,35]. Of the adolescents that were screened at the clinic for the presence of a concussion, those individuals that received a concussion diagnosis within 30 days of injury and returned for a follow-up post-acute evaluation were screened for eligibility in this research study (Figure 1). Participants with pre-existing neurological conditions such as seizure, psychiatric condition(s), developmental disorder(s), or those that were taking medications with known actions on neurological or cardiac function(s) were excluded from the present study.
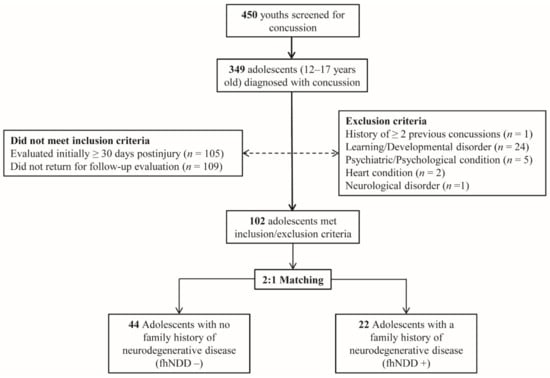
Figure 1.
Flow diagram of sampled participants. Dotted line indicates excluded participants. Solid line indicates included participants.
A comprehensive demographic/health-information survey given to parents/legal guardians were used to identify those with (fhNDD+) and without (fhNDD−) a family history of neurodegenerative disease. Family history of neurodegenerative disease was defined as having either a biological parent or grandparent with a medical diagnosis of Alzheimer’s disease, Parkinson’s disease, or non-Alzheimer’s dementia. The fhNDD− and fhNDD+ groups were matched 2:1 on key demographic and injury information, including age, sex, body mass index (BMI), race/ethnicity, concussion history, time since injury, and cause of injury. After triage, the resultant dataset included twenty-two adolescents with fhNDD+ and forty-four adolescents without fhNDD− for analyses.
2.2. Procedure
Following diagnosis and initial evaluation, concussed adolescents were instructed to return in 2–3 weeks for a follow-up evaluation. Data were de-identified prior to data collection and analyses. All study procedures were approved by the Health Sciences South Carolina institutional ethics review board (reference #: Pro00075286). At each visit, participants were asked to complete a comprehensive neurological assessment battery consisting of a self-reported clinical symptoms checklist, psychological questionnaires, assessments of cardio-autonomic function, and post-injury cognitive performance tasks. Figure 2 provides a visual representation of the testing battery at each evaluation.

Figure 2.
Visual representation of testing evaluation. Abbreviations: RPQ, Rivermead post-concussion symptoms questionnaire; BYI-2, Beck Youth Inventories—Second Edition; HRV, heart rate variability.
2.3. Measures
2.3.1. Clinical Symptoms
The Rivermead post-concussion symptoms questionnaire (RPQ) was used to assess self-reported symptom burden. The RPQ is a 16-item survey used to rate the current severity of symptoms compared to premorbid status following concussion [36]. Beyond total symptom burden, the RPQ provides valuable information regarding specific symptom clusters, across: somatic (e.g., headache, nausea, dizziness or photophobia), emotional (e.g., irritable, depressed, frustrated), and cognitive (e.g., forgetfulness, poor concentration, taking longer to think) symptoms [37]. Higher scores indicate a greater symptom burden.
2.3.2. Emotional Health
The depression subscale of Beck Youth Inventories—Second Edition (BYI-2) was used to evaluate emotional health following a concussion. The BYI-2 is a 20-item self-report questionnaire consisting of items that assess depressive traits such as sadness, pessimism, guilt, loss of pleasure, and fatigue in youth [38]. The BYI-2 has shown acceptable test-retest reliability (0.74–0.93) and convergent validity with other instruments used to assess depressive symptoms in youth [38]. T-scores were calculated from raw total scores using sex- and age-adjusted normative data. Higher raw and standardized scores indicate worse depressive symptoms.
2.3.3. Cardio-Autonomic Function
Heart rate variability (HRV) was used to index cardio-autonomic function. HRV quantifies the temporal beat-to-beat variations in heart rate that arise through the intrinsic interplay between parasympathetic and sympathetic nervous systems [39]. HRV recordings were collected through an EmWave Pro Plus infrared pulse plethysmograph ear sensor (HeartMath, Boulder Creek, CA, USA) in a temperature and light-controlled environment. Participants maintained a self-paced breathing rate in a seated position for the duration of a 5-min recording. Additionally, during 1-min recordings, participants maintained a resonant breathing frequency to further investigate vagal activity and respiratory sinus arrhythmia during rest and physical exertion (isometric handgrip contraction; IHGC) [40,41]. By investigating individual changes from rest to physical exertion, HRV reactivity to acute stressors can be quantified and identify any self-regulatory dysfunction [42]. Data reduction and computation of time-domain and frequency-domain parameters were conducted with Kubios HRV Standard, version 3.0.2 (Biosignal Analysis and Medical Imaging Group, Kuopio, Finland) in accordance with recommendations by the Task for Force of the European Society of Cardiology and North American Society of Pacing and Electrophysiology [43]. HRV data were visually inspected and corrected for artifacts, and a 10% Hanning window was applied to the corrected data.
Time-domain parameters included the standard deviation of NN intervals (SDNN) and root mean square of successive NN interval differences (RMSSD) [44]. SDNN is known to reflect total cardiac variability, while RMSSD primarily reflects vagal tone [44]. Frequency-domain parameters included low-frequency (LF; 0.04–0.15 Hz) and high-frequency (HF; 0.15–0.4 Hz) band power components derived via fast Fourier transformations [45]. LF and HF band power (msec2) were quantified and expressed in natural logarithm transformed units. LF band components are known to reflect baroreflex activity and total cardiac variability, while HF band components reflect respiratory vagal tone [45]. Frequency-domain parameters have not been validated for ultra-short-term recordings (<5 min); thus, they were not calculated for one-minute recordings [46].
2.3.4. Cognitive Performance
A modified CogState brain injury testing battery (CogState Ltd., Melbourne, Australia) was used to assess cognitive performance. The modified battery tests key domains of cognitive function, including working memory (one-back task; ONB), executive function (Groton maze learning test; GMLT), and visual memory recall (Groton maze delayed recall; GMR). Primary outcome measures included total errors (Groton maze learning and recall), reaction time (one back), and performance accuracy (one back). The selected tasks have shown acceptable validity and good reliability across various age-groups and clinical populations [47,48,49]. T-scores were calculated from raw test scores using age-adjusted normative data. Higher raw and standardized scores indicate poorer cognitive performance.
2.4. Data Analysis
A priori power analysis (G*Power 3.1) [50], with an alpha = 0.05 and power = 0.80, estimated a participant sample of 34 was sufficient to detect moderate effect sizes (ηp2 = 0.06). All statistical analyses were conducted using SPSS software version 27.0 (IBM Corporation, Armonk, NY, USA). To account for skewed distributions of RPQ and BYI-2 scores, a natural logarithm transformation was applied. HRV reactivity was quantified by calculating the change in HRV between 1-min assessments (∆HRV = IHGC HRV − resonant resting-state HRV). Independent samples t-tests were used for continuous data, and chi-squared tests were used for categorical data to compare group differences in demographic (age, sex, BMI, race/ethnicity) and injury characteristics (concussion history, time since injury, and cause of injury). Outcome measures were examined via a series of 2 (group: fhNDD+, fhNDD−) × 2 (time: initial evaluation, follow-up evaluation) repeated-measures analyses of variance (rmANOVA). Significant interactions were further decomposed via post hoc independent samples t-tests with Bonferroni correction for multiple comparisons. Levene’s tests were utilized to evaluate violations of equal variances and were corrected accordingly in the event of a violation. Partial eta squared (ηp2) values were calculated to estimate the magnitude of significant differences (0.01 = small, 0.06 = medium, 0.14 = large). Pearson’s correlations were used to examine the associations between outcome measures in both fhNDD+ and fhNDD− patients. A priori level of statistical significance was set to p < 0.05.
3. Results
Participant demographic and injury characteristic information can be found in Table 1. Demographic characteristics did not differ between fhNDD+ and fhNDD− participants (p ≥ 0.17). In terms of injury characteristics, there were no differences between fhNDD+ and fhNDD− participants across concussion history, cause of injury, or time from injury to initial/follow-up evaluations (p ≥ 0.64). Of the fhNDD+ group, 17 adolescents had a family history of Alzheimer’s disease, and 6 adolescents had a family history of Parkinson’s disease. Analyses did not reveal any differences in concussion outcomes between those with a family history of Alzheimer’s and Parkinson’s disease (p ≥ 0.05).

Table 1.
Participant demographic information and injury characteristics.
3.1. Clinical Symptoms
Descriptive statistics for self-reported symptom burden can be found in Table 2. A significant main effect of group was revealed for the RPQ somatic subdomain (F(1,64) = 4.503, p = 0.038, ηp2 = 0.066; Figure 3A), whereby those with an fhNDD+ reported more somatic symptoms than those without an fhNDD−. Repeated measures ANOVAs did not reveal any other main effects of group or group x time interactions for overall or subdomain RPQ scores (F(1,64) ≤ 3.927, p ≥ 0.052). However, significant main effects of time were observed for all RPQ measures (F(1,64) ≥ 37.795, p < 0.001; Table 2). Irrespective of fhNDD, symptom burden decreased from initial evaluation to follow-up evaluation.

Table 2.
Descriptive statistics for RPQ and Beck Youth Inventories—depression scale (BYI-D) scores at each evaluation.
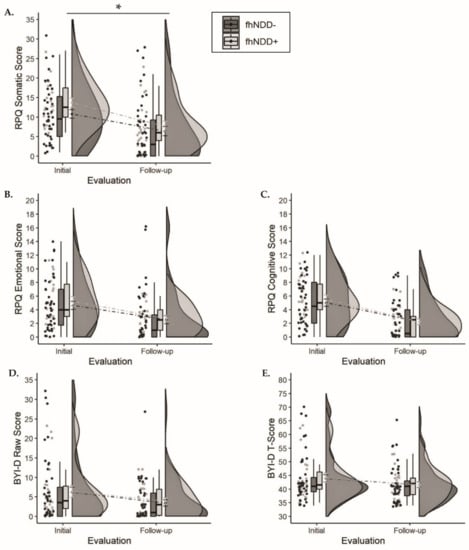
Figure 3.
Group comparisons of symptom questionnaires between those with a family history of neurodegenerative disease (fhNDD+; light gray) and without a family history of neurodegenerative disease (fhNDD−; dark gray). (A) RPQ somatic score. (B) RPQ emotional score. (C) RPQ cognitive score. (D) BYI-D raw score. (E) BYI-D t-score. * p < 0.05.
3.2. Emotional Health
Descriptive statistics for self-reported depressive symptoms can be found in Table 2. Repeated measures ANOVAs did not reveal any main effects of group or group × time interactions for Beck Youth Inventories—depression scale (BYI-D) raw scores or T-scores (F(1,64) ≤ 1.261, p ≥ 0.266; Figure 3D,E). Irrespective of fhNDD, BYI-D raw scores and T-scores decreased from initial evaluation to follow-up evaluation (F(1,64) ≥ 7.221, p < 0.01; Table 2).
3.3. Cardio-Autonomic Function
Descriptive statistics for HRV during the five-minute self-paced breathing assessment are displayed in Table 3. A significant main effect of group was observed for SDNN (F(1,64) = 9.081, p = 0.004, ηp2 = 0.124), RMSSD (F(1,64) = 4.929, p = 0.03, ηp2 = 0.072), LF power (F(1,64) = 10.176, p = 0.002, ηp2 = 0.137), and HF power (F(1,64) = 8.961, p = 0.004, ηp2 = 0.123). Group comparisons revealed that regardless of evaluation, the fhNDD+ group displayed significantly altered HRV compared to the fhNDD− group during a short-term self-paced breathing assessment (Figure 4). Repeated measures ANOVAs did not reveal any significant main effects of time or group x time interactions for HRV during self-paced breathing (F(1,64) ≤ 1.733, p ≥ 0.193). Pearson’s correlations revealed that lower SDNN (r = −0.513, p = 0.015), RMSSD (r = −0.590, p = 0.004), and HF power (r = −0.505, p = 0.017) at rest were correlated with greater somatic symptoms in concussed adolescents with an fhNDD+, but not in fhNDD− individuals during follow-up evaluation.

Table 3.
Descriptive statistics for heart rate variability (HRV) during the 5-min self-paced breathing assessment at each evaluation.
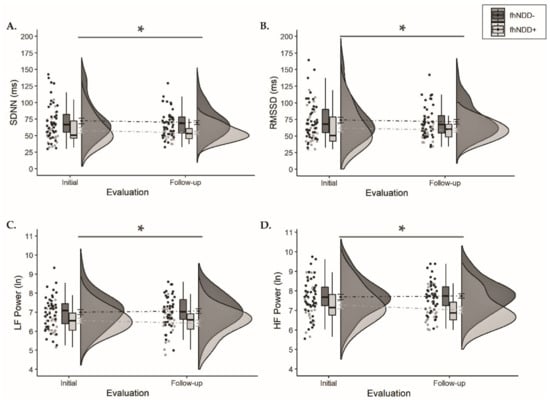
Figure 4.
Group comparisons of HRV between those with a family history of neurodegenerative disease (fhNDD+; light gray) and without a family history of neurodegenerative disease (fhNDD−; dark gray) during self-paced breathing/resting-state assessment at each evaluation. (A) SDNN. (B) RMSSD. (C) LF power (natural logarithmic units). (D) HF power (natural logarithmic units). * p < 0.05.
Descriptive statistics for HRV during one-minute resonant breathing assessment can be found in Table 4. A significant main effect of group was revealed for SDNN (F(1,64) = 9.598, p = 0.003, ηp2 = 0.130) and RMSSD (F(1,64) = 8.658, p = 0.005, ηp2 = 0.119) during resonant resting-state assessment. Similarly, a significant main effect of group was revealed for SDNN (F(1,64) = 9.019, p = 0.004, ηp2 = 0.124) and RMSSD (F(1,64) = 11.705, p = 0.001, ηp2 = 0.155) during the physical exertion assessment. Regardless of timepoint, the fhNDD+ group had lower HRV at resonant resting-state (Figure 5A,B) and during IHGC (Figure 5C,D) compared to the fhNDD− group. No significant main effects of time or group x time interactions were revealed for HRV during either resonant breathing assessment (F(1,64) ≤ 3.619, p’s ≥ 0.062). However, significant group x time interactions were revealed for ∆SDNN (F(1,64) = 11.002, p = 0.002, ηp2 = 0.147) and ∆RMSSD (F(1,64) = 12.032, p = 0.001, ηp2 = 0.158). Bonferroni corrected post hoc analyses revealed lesser SDNN (−2.00 ± 2.26 vs. −14.96 ± 2.75; p = 0.001) and RMSSD (1.72 ± 3.03 vs. −11.25 ± 3.09; p = 0.01) change in response to IHGC in the fhNDD+ group compared to the fhNDD− group at initial evaluation (Figure 5E,F). Main effects for group and time were not revealed for measures of HRV reactivity (F(1,64) ≤ 0.504, p ≥ 0.48; Table 4). Symptoms were not correlated to HRV metrics during IHGC in concussed individuals.

Table 4.
Descriptive statistics for HRV during the 1-min resonant breathing assessments at each evaluation.
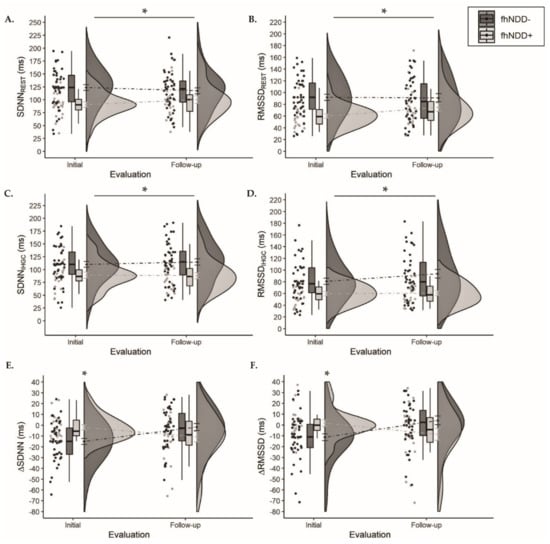
Figure 5.
Group comparisons of HRV between those with a family history of neurodegenerative disease (fhNDD+; light gray) and without a family history of neurodegenerative disease (fhNDD−; dark gray) during resonant breathing assessments at each evaluation. (A) SDNN at rest. (B) RMSSD at rest. (C) SDNN during isometric handgrip contraction (IHGC). (D) RMSSD during isometric handgrip contraction (IHGC). (E) Change in SDNN from rest to IHGC. (F) Change in RMSSD from rest to IHGC.* p < 0.05.
3.4. Cognitive Performance
Descriptive statistics for CogState measures can be found in Table 5. Repeated measures ANOVAs did not reveal any main effects of group for raw CogState measures or T-scores (F(1,64) ≤ 2.133, p ≥ 0.149). Main effects of time were observed for GMLT errors (F(1,64) ≥ 22.225, p < 0.001), GMR errors T-score (F(1,64) = 4.115, p = 0.047), ONB reaction time (F(1,64) ≥ 13.447, p < 0.001), and ONB accuracy (F(1,64) > 11.609, p < 0.001), whereby cognitive performance improved from initial evaluation to follow-up evaluation. No significant group x time interactions were observed for any cognitive measures (F(1,64) ≤ 3.388, p ≥ 0.070; Figure 6).

Table 5.
Descriptive statistics for CogState measures at each evaluation.
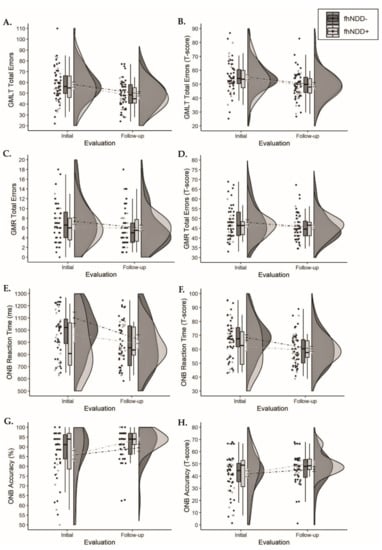
Figure 6.
Group comparisons of cognitive performance between those with a family history of neurodegenerative disease (fhNDD+; light gray) and without a family history of neurodegenerative disease (fhNDD−; dark gray). (A) GMLT total errors. (B) GMLT total errors t-score. (C) GMR total errors. (D) GMR total errors t-scores. (E) ONB reaction time (ms). (F) ONB reaction time t-score. (G) ONB accuracy (%). (H) ONB accuracy t-score.
4. Discussion
The aim of the present study was to evaluate the relationship between immediate fhNDD and adolescent concussion outcomes by assessing clinical symptoms, mental health, cardio-autonomic function, and post-injury cognitive performance. Our findings indicated that fhNDD+ adolescents report greater somatic symptom severity than fhNDD− adolescents following a concussion. With regard to cardio-autonomic function, fhNDD+ adolescents displayed diminished HRV at rest and during IHGC compared to fhNDD− across evaluations. Further, fhNDD+ adolescents exhibited blunted HRV reactivity compared to fhNDD− adolescents during the initial evaluation, as indicated by a lesser change in SDNN and RMSSD from rest to IHGC. However, an fhNDD did not influence mental health or cognition following an adolescent concussion.
Although clinical management of concussion commonly relies upon subjective symptom reporting, the understanding of factors that contribute to the experience of these symptoms is tentative and incomplete. The present findings suggest that a positive fhNDD may impact symptom reporting following a concussion, as fhNDD+ adolescents reported greater somatic symptom severity compared to their counterparts. Our results align with prior findings that demonstrate individuals with potential genetic factors associated with an fhNDD report a greater prevalence of posttraumatic headache and physical symptoms following concussion [30,51]. Interestingly, greater somatic symptom severity was associated with lower HRV at rest in fhNDD+ individuals during follow-up evaluation. This suggests that differences in HRV and post-injury somatic symptoms in fhNDD+ adolescents could be linked through a “psycho-somatic” origin and not from actual pathology.
Autonomic dysfunction is commonly experienced in individuals with neurodegenerative disease [52,53,54] and adolescents males with a family history of neurodegenerative disease [55]. In general, lower resting HRV is associated with worse health outcomes across neurological populations [56,57] and may be indicative of maladaptive self-regulation in response to environmental demands [58,59]. Research has shown cardio-autonomic impairment following concussion may manifest as sympathetic hyperarousal (i.e., decreased HRV) at rest [60,61] or as an atypical HRV response to physiological stressors [62,63,64]. Accordingly, we observed an attenuated HRV during both rest and IHGC in fhNDD+ adolescents compared to fhNDD− adolescents across evaluation time points. The Vagal Tank theory assumes a relative degree of vagal withdrawal (e.g., decrease in HRV) is needed to support metabolically demanding tasks, such as physical exertion [42]. Preliminary evidence suggests that individuals may demonstrate blunted cardio-autonomic reactivity following concussion [65,66]. Importantly, we observed that fhNDD+ adolescents show minimal to no change in HRV in response to IHGC compared to fhNDD− adolescents during the subacute phase of recovery (<30 days of injury). Together, these findings suggest that an fhNDD may partially account for the heterogeneity of cardio-autonomic dysfunction observed following an adolescent concussion. These results also support the use of HRV in clinical practice as a metric of concussion recovery.
Research suggests that reduced brain “reserve” may contribute to prolonged recovery trajectories following concussion [67,68]. Two concepts of “reserve”, neural and cognitive reserve, have been proposed by researchers to account for individual differences in concussion outcome despite similarities in insult and/or neurodegeneration [69,70]. Neural reserve, or the “passive” model of the reserve, refers to the ability of anatomical brain structures to withstand relative neuronal loss without functional consequence. Cognitive reserve, or the “active” model of the reserve, refers to the functional capacity of neural networks to compensate for neurodegeneration and/or neuronal loss. Diminished neural resilience in both structure and function have been exhibited in those with an fhNDD [71,72,73]. Accordingly, heritable factors associated with neurodegenerative diseases have been linked to impaired neurocognitive recovery following concussion [34,74,75]. Contrary to our hypotheses and prior evidence, fhNDD+ adolescents did not display greater neurocognitive deficits compared to fhNDD− adolescents following a concussion. However, current literature has primarily examined these subtle relations in older individuals. Advancing age is associated with a progressive loss of compensatory neural reserve [76,77]. Therefore, the younger age of our sample may explain why differences in cognitive performance were not observed between groups. Furthermore, the cognitive tasks selected may not be sensitive enough to capture group differences between fhNDD+ and fhNDD− adolescents. Future studies should incorporate higher-level cognitive tasks for a more in-depth investigation into the effect of an fhNDD following an adolescent concussion.
To date, this study is the first to prospectively examine the influence of an immediate fhNDD on concussion outcome among adolescents. Our results reflect studies in other neurological populations, which suggest that non-modifiable risk factors associated with neurodegenerative diseases may predispose individuals to adverse outcomes following neurological trauma [78,79]. Although it is plausible that a particular genotype (family history of Alzheimer’s disease or Parkinson’s disease) may contribute to the current findings, it is difficult to determine the role of genetic variability among genes that have previously been associated with neurodegenerative diseases as genotyping was not part of the data collection efforts. While these neurodegenerative diseases may share similar pathological characteristics, the genetic interactions are notably distinct and complex in heritability and onset [80,81]. This is an important point as many researchers have sought to examine particular gene variants in regard to concussion recovery. Future studies should explore polygenic profiles rather than a specific gene to elucidate the relationship between fhNDD and concussion outcome. From a clinical perspective, it is also more feasible to administer a simple questionnaire than conducting patient genotyping.
Further, the present findings underscore the importance of a comprehensive concussion assessment as recommend by the most recent Consensus Statement on Concussion in Sport, including cardio-autonomic assessment [1]. Even so, this study does not examine potential group differences in vestibular function, balance, anxiety, and sleep disturbances, which are commonly affected following an adolescent concussion. Future research should seek to further explore the effect of fhNDD on these domains of function. Although the two groups had comparable cognitive performance, fhNDD+ adolescents exhibited increased somatic symptoms and diminished cardio-autonomic function following a concussion. Somatic symptoms following concussion can affect a patient’s day-to-day functioning and quality of life. Additionally, even subtle deficits in cardio-autonomic function may contribute to exercise intolerance, postural hypotension, fatigue, and persistent posttraumatic headache [82,83,84]. Therefore, the current findings indicate that greater somatic symptom severity and cardio-autonomic dysfunction appear to be related in fhNDD+ adolescents through an undefined mechanism. More specifically, additional research is needed to explain why somatic symptoms were associated with resting-state HRV but not IHGC at the respective evaluations. Beyond postponing return to activity, these symptoms can negatively affect a child’s quality of life [85]. Therefore, the authors recommend that clinicians incorporate pre-evaluation screening questions into their practice to identify adolescents with an fhNDD, as they may be at greater risk to experience a more adverse outcome following concussion compared to others with no fhNDD.
Though the present study contributes novel findings to extant knowledge regarding concussion, it is not without its limitations. First, our sample size was not large enough to detect a small but potentially meaningful difference between groups. Therefore, we cannot determine if subtle group differences, perhaps in cognition, are present between fhNDD+ and fhNDD− adolescents. Additionally, we cannot determine to what effect concussive injury is responsible for the observed findings as pre-injury baseline measurements were not obtained. Furthermore, we cannot rule out differences in other components of mental health, such as anxiety, as mental health evaluation was limited to depressive symptoms. Lastly, the true fhNDD+ sample is likely underestimated. We examined adolescents; thus, it is reasonable to assume that parents or grandparents may be younger than the typical age (50–80 years old) in which many neurodegenerative diseases are diagnosed.
5. Conclusions
In summary, the present study is the first to examine the influence of an immediate fhNDD on adolescent concussion outcomes. Our findings provide evidence that concussed adolescents with an fhNDD may exhibit greater somatic symptomology and cardio-autonomic dysfunction relative to those without an fhNDD. Thus, our findings provide an impetus for clinicians to include screening for fhNDD and use of HRV in their clinical assessment to gain a greater understanding of pathophysiological differences between patients. HRV is feasible for use in clinical settings as it is non-invasive, cost-effective, and can be used under various conditions (e.g., rest, exercise, cognition). Furthermore, measuring HRV does not require the level of training or space of other psychophysiological measures and can be collected from simple ear clips or 3-lead ECGs. Together our findings suggest that screening for fhNDD and using HRV may be simple and effective ways for clinicians to identify patients who are likely to experience persisting symptoms as well as pathophysiology following a concussion. Doing so may help clinicians to preemptively change management strategies to maximize positive outcomes. However, more research is needed to determine if the same results are observed in adults and geriatric populations before broadly implementing HRV for patients across the lifespan.
Author Contributions
Conceptualization, C.A.C., J.J.M.K., and R.D.M.; methodology, C.A.C., J.J.M.K., A.T.H.; formal analysis, C.A.C.; investigation, J.J.M.K., C.A.C., and A.T.H.; resources, R.D.M.; data curation, C.A.C.; writing—original draft preparation, C.A.C.; writing—review and editing, R.D.M., J.J.M.K., M.F.L. and A.T.H.; visualization, A.T.H. and C.A.C.; supervision, R.D.M. and J.P.H.; project administration, J.J.M.K., J.P.H., and R.D.M.; funding acquisition, R.D.M. and J.P.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Health Sciences South Carolina Institutional Ethics Review Board (Reference #: Pro00075286; Date of approval: 02/19/2018).
Informed Consent Statement
Patient consent was waived as our comprehensive concussion evaluation subserves the standard of clinical care.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- McCrory, P.; Meeuwisse, W.; Dvorak, J.; Aubry, M.; Bailes, J.; Broglio, S.; Cantu, R.C.; Cassidy, D.; Echemendia, R.J.; Castellani, R.J.; et al. Consensus Statement on Concussion in Sport—The 5th International Conference on Concussion in Sport Held in Berlin, October 2016. Br. J. Sports Med. 2017, 51, 838–847. [Google Scholar] [CrossRef]
- Romeu-Mejia, R.; Giza, C.C.; Goldman, J.T. Concussion Pathophysiology and Injury Biomechanics. Curr. Rev. Musculoskelet. Med. 2019, 12, 105–116. [Google Scholar] [CrossRef]
- Wang, Y.; Nencka, A.S.; Meier, T.B.; Guskiewicz, K.; Mihalik, J.P.; Alison Brooks, M.; Saykin, A.J.; Koch, K.M.; Wu, Y.-C.; Nelson, L.D.; et al. Cerebral Blood Flow in Acute Concussion: Preliminary ASL Findings from the NCAA-DoD CARE Consortium. Brain Imaging Behav. 2019, 13, 1375–1385. [Google Scholar] [CrossRef]
- Champagne, A.; Coverdale, N.; Fernandez-Ruiz, J.; Mark, C.; Cook, D. Compromised Resting Cerebral Metabolism after Sport-Related Concussion: A Calibrated MRI Study. Brain Imaging Behav. 2020, 1–14. [Google Scholar] [CrossRef]
- Giza, C.C.; Hovda, D.A. The New Neurometabolic Cascade of Concussion. Neurosurgery 2014, 75, S24–S33. [Google Scholar] [CrossRef]
- Polinder, S.; Cnossen, M.C.; Real, R.G.L.; Covic, A.; Gorbunova, A.; Voormolen, D.C.; Master, C.L.; Haagsma, J.A.; Diaz-Arrastia, R.; von Steinbuechel, N. A Multidimensional Approach to Post-Concussion Symptoms in Mild Traumatic Brain Injury. Front. Neurol. 2018, 9, 1113. [Google Scholar] [CrossRef]
- Balestrini, C.S.; Moir, M.E.; Abbott, K.C.; Johnson, M.; Fischer, L.K.; Fraser, D.D.; Shoemaker, J.K. Autonomic Dysregulation in Adolescent Concussion: Characterization and Temporal Resolution of Neurological Outcomes. FASEB J. 2017, 31, 863.3. [Google Scholar] [CrossRef]
- Hutchison, M.G.; Mainwaring, L.; Senthinathan, A.; Churchill, N.; Thomas, S.; Richards, D. Psychological and Physiological Markers of Stress in Concussed Athletes Across Recovery Milestones. J. Head Trauma Rehabil. 2017, 32, E38–E48. [Google Scholar] [CrossRef]
- Dobson, J.L.; Yarbrough, M.B.; Perez, J.; Evans, K.; Buckley, T. Sport-Related Concussion Induces Transient Cardiovascular Autonomic Dysfunction. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2017, 312, R575–R584. [Google Scholar] [CrossRef]
- Ryan, L.M.; Warden, D.L. Post Concussion Syndrome. Int. Rev. Psychiatry 2003, 15, 310–316. [Google Scholar] [CrossRef]
- Grubenhoff, J.A.; Deakyne, S.J.; Brou, L.; Bajaj, L.; Comstock, R.D.; Kirkwood, M.W. Acute Concussion Symptom Severity and Delayed Symptom Resolution. Pediatrics 2014, 134, 54–62. [Google Scholar] [CrossRef]
- Makdissi, M.; Cantu, R.C.; Johnston, K.M.; McCrory, P.; Meeuwisse, W.H. The Difficult Concussion Patient: What Is the Best Approach to Investigation and Management of Persistent (>10 Days) Postconcussive Symptoms? Br. J. Sports Med. 2013, 47, 308–313. [Google Scholar] [CrossRef]
- Coronado, V.G.; Haileyesus, T.; Cheng, T.A.; Bell, J.M.; Haarbauer-Krupa, J.; Lionbarger, M.R.; Flores-Herrera, J.; McGuire, L.C.; Gilchrist, J. Trends in Sports- and Recreation- Related Traumatic Brain Injuries Treated in US Emergency Departments: The National Electronic Injury Surveillance System-All Injury Program (NEISS-AIP) 2001–2012. J. Head Trauma Rehabil. 2015, 30, 185–197. [Google Scholar] [CrossRef]
- Kirkwood, M.W. Pediatric Sport-Related Concussion: A Review of the Clinical Management of an Oft-Neglected Population. Pediatrics 2006, 117, 1359–1371. [Google Scholar] [CrossRef]
- Rozbacher, A.; Selci, E.; Leiter, J.; Ellis, M.; Russell, K. The Effect of Concussion or Mild Traumatic Brain Injury on Academic Outcomes: A Systematic Review. J. Neurotrauma 2017, 34, 2195–2203. [Google Scholar] [CrossRef]
- Iverson, G.L.; Gardner, A.J.; Terry, D.P.; Ponsford, J.L.; Sills, A.K.; Broshek, D.K.; Solomon, G.S. Predictors of Clinical Recovery from Concussion: A Systematic Review. Br. J. Sports Med. 2017, 51, 941–948. [Google Scholar] [CrossRef]
- Daneshvar, D.H.; Riley, D.O.; Nowinski, C.J.; McKee, A.C.; Stern, R.A.; Cantu, R.C. Long Term Consequences: Effects on Normal Development Profile after Concussion. Phys. Med. Rehabil. Clin. N. Am. 2011, 22, 683–700. [Google Scholar] [CrossRef]
- Baker, J.G.; Leddy, J.J.; Darling, S.R.; Shucard, J.; Makdissi, M.; Willer, B.S. Gender Differences in Recovery From Sports-Related Concussion in Adolescents. Clin. Pediatr. (Phila.) 2016, 55, 771–775. [Google Scholar] [CrossRef]
- Guskiewicz, K.M.; McCrea, M.; Marshall, S.W.; Cantu, R.C.; Randolph, C.; Barr, W.; Onate, J.A.; Kelly, J.P. Cumulative Effects Associated with Recurrent Concussion in Collegiate Football Players: The NCAA Concussion Study. JAMA J. Am. Med. Assoc. 2003, 290, 2549–2555. [Google Scholar] [CrossRef]
- Guerriero, R.M.; Kuemmerle, K.; Pepin, M.J.; Taylor, A.M.; Wolff, R.; Meehan, W.P. The Association Between Premorbid Conditions in School-Aged Children With Prolonged Concussion Recovery. J. Child Neurol. 2018, 33, 168–173. [Google Scholar] [CrossRef]
- Aggarwal, S.S.; Ott, S.D.; Padhye, N.S.; Schulz, P.E. Sex, Race, ADHD, and Prior Concussions as Predictors of Concussion Recovery in Adolescents. Brain Inj. 2020, 34, 811–819. [Google Scholar] [CrossRef]
- McKee, A.C.; Cairns, N.J.; Dickson, D.W.; Folkerth, R.D.; Keene, C.D.; Litvan, I.; Perl, D.P.; Stein, T.D.; Vonsattel, J.-P.; Stewart, W.; et al. The First NINDS/NIBIB Consensus Meeting to Define Neuropathological Criteria for the Diagnosis of Chronic Traumatic Encephalopathy. Acta Neuropathol. (Berl.) 2016, 131, 75–86. [Google Scholar] [CrossRef]
- Gardner, R.C.; Yaffe, K. Epidemiology of Mild Traumatic Brain Injury and Neurodegenerative Disease. Mol. Cell. Neurosci. 2015, 66, 75–80. [Google Scholar] [CrossRef]
- Bendlin, B.B.; Ries, M.L.; Canu, E.; Sodhi, A.; Lazar, M.; Alexander, A.L.; Carlsson, C.M.; Sager, M.A.; Asthana, S.; Johnson, S.C. White Matter Is Altered with Parental Family History of Alzheimer’s Disease. Alzheimers Dement. 2010, 6, 394–403. [Google Scholar] [CrossRef]
- Verfaillie, S.C.J.; Binette, A.P.; Vachon-Presseau, E.; Tabrizi, S.; Savard, M.; Bellec, P.; Ossenkoppele, R.; Scheltens, P.; van der Flier, W.M.; Breitner, J.C.S.; et al. Subjective Cognitive Decline Is Associated With Altered Default Mode Network Connectivity in Individuals With a Family History of Alzheimer’s Disease. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 463–472. [Google Scholar] [CrossRef]
- Mosconi, L.; Brys, M.; Switalski, R.; Mistur, R.; Glodzik, L.; Pirraglia, E.; Tsui, W.; De Santi, S.; de Leon, M.J. Maternal Family History of Alzheimer’s Disease Predisposes to Reduced Brain Glucose Metabolism. Proc. Natl. Acad. Sci. USA 2007, 104, 19067–19072. [Google Scholar] [CrossRef]
- Ariza, M.; Pueyo, R.; del M Matarín, M.; Junqué, C.; Mataró, M.; Clemente, I.; Moral, P.; Poca, M.A.; Garnacho, Á.; Sahuquillo, J. Influence of APOE Polymorphism on Cognitive and Behavioural Outcome in Moderate and Severe Traumatic Brain Injury. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1191–1193. [Google Scholar] [CrossRef]
- McFadyen, C.A.; Zeiler, F.A.; Newcombe, V.; Synnot, A.; Steyerberg, E.; Gruen, R.L.; Rosand, J.; Palotie, A.; Maas, A.I.R.; Menon, D.K. Apolipoprotein E4 Polymorphism and Outcomes from Traumatic Brain Injury: A Living Systematic Review and Meta-Analysis. J. Neurotrauma 2019. [Google Scholar] [CrossRef]
- Finnoff, J.; Jelsing, E.; Smith, J. Biomarkers, Genetics, and Risk Factors for Concussion. PM&R 2011, 3, S452–S459. [Google Scholar] [CrossRef]
- Merritt, V.C.; Arnett, P.A. Apolipoprotein E (APOE) Ε4 Allele Is Associated with Increased Symptom Reporting Following Sports Concussion. J. Int. Neuropsychol. Soc. 2016, 22, 89–94. [Google Scholar] [CrossRef]
- Merritt, V.C.; Rabinowitz, A.R.; Arnett, P.A. The Influence of the Apolipoprotein E (APOE) Gene on Subacute Post-Concussion Neurocognitive Performance in College Athletes. Arch. Clin. Neuropsychol. 2018, 33, 36–46. [Google Scholar] [CrossRef]
- Merritt, V.C.; Lapira, K.M.; Clark, A.L.; Sorg, S.F.; Werhane, M.L.; Jak, A.J.; Bondi, M.W.; Schiehser, D.M.; Delano-Wood, L. APOE-Ε4 Genotype Is Associated with Elevated Post-Concussion Symptoms in Military Veterans with a Remote History of Mild Traumatic Brain Injury. Arch. Clin. Neuropsychol. 2019, 34, 706–712. [Google Scholar] [CrossRef]
- Moran, L.M.; Taylor, H.G.; Ganesalingam, K.; Gastier-Foster, J.M.; Frick, J.; Bangert, B.; Dietrich, A.; Nuss, K.E.; Rusin, J.; Wright, M.; et al. Apolipoprotein E4 as a Predictor of Outcomes in Pediatric Mild Traumatic Brain Injury. J. Neurotrauma 2009, 26, 1489–1495. [Google Scholar] [CrossRef]
- Harrison, A.; McAllister, T.; McCrea, M.; Broglio, S.P.; Moore, R.D. Recovery Profiles Following Concussion Among Male Student-Athletes and Service Cadets with a Family History of Neurodegenerative Disease: Data from the NCAA-DOD CARE Consortium. J. Neurotrauma 2020. [Google Scholar] [CrossRef]
- Giza, C.C.; Kutcher, J.S.; Ashwal, S.; Barth, J.; Getchius, T.S.D.; Gioia, G.A.; Gronseth, G.S.; Guskiewicz, K.; Mandel, S.; Manley, G.; et al. Summary of Evidence-Based Guideline Update: Evaluation and Management of Concussion in Sports: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2013, 80, 2250–2257. [Google Scholar] [CrossRef]
- King, N.S.; Crawford, S.; Wenden, F.J.; Moss, N.E.G.; Wade, D.T. The Rivermead Post Concussion Symptoms Questionnaire: A Measure of Symptoms Commonly Experienced after Head Injury and Its Reliability. J. Neurol. 1995, 242, 587–592. [Google Scholar] [CrossRef]
- Potter, S.; Leigh, E.; Wade, D.; Fleminger, S. The Rivermead Post Concussion Symptoms Questionnaire. J. Neurol. 2006, 253, 1603–1614. [Google Scholar] [CrossRef]
- Beck, J.S.; Beck, A.T.; Jolly, J.B. Beck Youth Inventories of Emotional & Social Impairment: Depression Inventory for Youth, Anxiety Inventory for Youth, Anger Inventory for Youth, Disruptive Behavior for Youth, Self-Concept Inventory for Youth: Manual; Psychological Corporation: New York, NY, USA, 2001. [Google Scholar]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A Healthy Heart Is Not a Metronome: An Integrative Review of the Heart’s Anatomy and Heart Rate Variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef]
- Jerath, R.; Edry, J.W.; Barnes, V.A.; Jerath, V. Physiology of Long Pranayamic Breathing: Neural Respiratory Elements May Provide a Mechanism That Explains How Slow Deep Breathing Shifts the Autonomic Nervous System. Med. Hypotheses 2006, 67, 566–571. [Google Scholar] [CrossRef]
- Vaschillo, E.; Vaschillo, B. Characteristics of Resonance in Heart Rate Variability Stimulated by Biofeedback. Appl. Psychophysiol. Biofeedback 2006, 31, 129–142. [Google Scholar] [CrossRef]
- Laborde, S.; Mosley, E.; Mertgen, A. Vagal Tank Theory: The Three Rs of Cardiac Vagal Control Functioning—Resting, Reactivity, and Recovery. Front. Neurosci. 2018, 12, 458. [Google Scholar] [CrossRef]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research—Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Heathers, J.A.J. Everything Hertz: Methodological Issues in Short-Term Frequency-Domain HRV. Front. Physiol. 2014, 5, 177. [Google Scholar] [CrossRef]
- Shaffer, F.; Shearman, S.; Meehan, Z.M. The Promise of Ultra-Short-Term (UST) Heart Rate Variability Measurements. Biofeedback 2016, 44, 229–233. [Google Scholar] [CrossRef]
- Louey, A.G.; Cromer, J.A.; Schembri, A.J.; Darby, D.G.; Maruff, P.; Makdissi, M.; Mccrory, P. Detecting Cognitive Impairment After Concussion: Sensitivity of Change From Baseline and Normative Data Methods Using the CogSport/Axon Cognitive Test Battery. Arch. Clin. Neuropsychol. 2014, 29, 432–441. [Google Scholar] [CrossRef]
- Maruff, P.; Thomas, E.; Cysique, L.; Brew, B.; Collie, A.; Snyder, P.; Pietrzak, R.H. Validity of the CogState Brief Battery: Relationship to Standardized Tests and Sensitivity to Cognitive Impairment in Mild Traumatic Brain Injury, Schizophrenia, and AIDS Dementia Complex. Arch. Clin. Neuropsychol. 2009, 24, 165–178. [Google Scholar] [CrossRef]
- Dingwall, M.K.M.; Lewis, M.S.; Maruff, P.; Cairney, S. Reliability of Repeated Cognitive Testing in Healthy Indigenous Australian Adolescents. Aust. Psychol. 2009, 44, 224–234. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical Power Analyses Using G*Power 3.1: Tests for Correlation and Regression Analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Merritt, V.C.; Ukueberuwa, D.M.; Arnett, P.A. Relationship between the Apolipoprotein E Gene and Headache Following Sports-Related Concussion. J. Clin. Exp. Neuropsychol. 2016, 38, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Allan, L.M.; Ballard, C.G.; Allen, J.; Murray, A.; Davidson, A.W.; McKeith, I.G.; Kenny, R.A. Autonomic Dysfunction in Dementia. J. Neurol. Neurosurg. Psychiatry 2007, 78, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Femminella, G.D.; Rengo, G.; Komici, K.; Iacotucci, P.; Petraglia, L.; Pagano, G.; de Lucia, C.; Canonico, V.; Bonaduce, D.; Leosco, D.; et al. Autonomic Dysfunction in Alzheimer’s Disease: Tools for Assessment and Review of the Literature. J. Alzheimers Dis. 2014, 42, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.-A.; Kaufmann, H. Autonomic Disorders Predicting Parkinson’s Disease. Parkinsonism Relat. Disord. 2014, 20, S94–S98. [Google Scholar] [CrossRef]
- Ravaja, N.; Räikkönen, K.; Lyytinen, H.; Lehtimäki, T.; Keltikangas-Järvinen, L. Apolipoprotein E Phenotypes and Cardiovascular Responses to Experimentally Induced Mental Stress in Adolescent Boys. J. Behav. Med. 1997, 20, 571–587. [Google Scholar] [CrossRef]
- Zhao, M.; Guan, L.; Collet, J.-P.; Wang, Y. Relationship between Ischemic Stroke Locations, Etiology Subtypes, Neurological Outcomes, and Autonomic Cardiac Function. Neurol. Res. 2020, 42, 630–639. [Google Scholar] [CrossRef]
- Sykora, M.; Czosnyka, M.; Liu, X.; Donnelly, J.; Nasr, N.; Diedler, J.; Okoroafor, F.; Hutchinson, P.; Menon, D.; Smielewski, P. Autonomic Impairment in Severe Traumatic Brain Injury: A Multimodal Neuromonitoring Study. Crit. Care Med. 2016, 44, 1173–1181. [Google Scholar] [CrossRef]
- Hansen, A.L.; Johnsen, B.H.; Thayer, J.F. Vagal Influence on Working Memory and Attention. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2003, 48, 263–274. [Google Scholar] [CrossRef]
- Thayer, J.F.; Hansen, A.L.; Saus-Rose, E.; Johnsen, B.H. Heart Rate Variability, Prefrontal Neural Function, and Cognitive Performance: The Neurovisceral Integration Perspective on Self-Regulation, Adaptation, and Health. Ann. Behav. Med. 2009, 37, 141–153. [Google Scholar] [CrossRef]
- Purkayastha, S.; Williams, B.; Murphy, M.; Lyng, S.; Sabo, T.; Bell, K.R. Reduced Heart Rate Variability and Lower Cerebral Blood Flow Associated with Poor Cognition during Recovery Following Concussion. Auton. Neurosci. 2019, 220, 102548. [Google Scholar] [CrossRef]
- Senthinathan, A.; Mainwaring, L.M.; Hutchison, M.M. Heart Rate Variability of Athletes Across Concussion Recovery Milestones: A Preliminary Study. Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 2017, 27, 288–295. [Google Scholar] [CrossRef] [PubMed]
- LaFountaine, M.F.; Heffernan, K.S.; Gossett, J.D.; Bauman, W.A.; Meersman, R.E.D. Transient Suppression of Heart Rate Complexity in Concussed Athletes. Auton. Neurosci. Basic Clin. 2009, 148, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Gall, B.; Parkhouse, W.; Goodman, D. Heart Rate Variability of Recently Concussed Athletes at Rest and Exercise. Med. Sci. Sports Exerc. 2004, 36, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Abaji, J.P.; Curnier, D.; Moore, R.D.; Ellemberg, D. Persisting Effects of Concussion on Heart Rate Variability during Physical Exertion. J. Neurotrauma 2016, 33, 811–817. [Google Scholar] [CrossRef]
- Huang, M.; Frantz, J.; Moralez, G.; Sabo, T.; Davis, P.F.; Davis, S.L.; Bell, K.R.; Purkayastha, S. Reduced Resting and Increased Elevation of Heart Rate Variability With Cognitive Task Performance in Concussed Athletes. J. Head Trauma Rehabil. 2019, 34, 45–51. [Google Scholar] [CrossRef]
- Haider, M.N.; Johnson, B.D.; Horn, E.C.; Leddy, J.J.; Wilber, C.G.; Reed, E.L.; O’Leary, M.; Bloomfield, A.; Decezaro, L.L.; Willer, B.S. Blunted Cardiac Parasympathetic Activation in Student Athletes With a Remote History of Concussion: A Pilot Study. Front. Neurol. 2020, 11, 1156. [Google Scholar] [CrossRef]
- Contribution of Brain or Biological Reserve and Cognitive or Neural Reserve to Outcome after TBI: A Meta-Analysis (Prior to 2015). Neurosci. Biobehav. Rev. 2015, 55, 573–593. [CrossRef]
- Fay, T.B.; Yeates, K.O.; Taylor, H.G.; Bangert, B.; Dietrich, A.; Nuss, K.E.; Rusin, J.; Wright, M. Cognitive Reserve as a Moderator of Postconcussive Symptoms in Children with Complicated and Uncomplicated Mild Traumatic Brain Injury. J. Int. Neuropsychol. Soc. JINS 2010, 16, 94–105. [Google Scholar] [CrossRef]
- Stern, Y. Cognitive Reserve. Neuropsychologia 2009, 47, 2015–2028. [Google Scholar] [CrossRef]
- Barulli, D.; Stern, Y. Efficiency, Capacity, Compensation, Maintenance, Plasticity: Emerging Concepts in Cognitive Reserve. Trends Cogn. Sci. 2013, 17, 502–509. [Google Scholar] [CrossRef]
- Merritt, V.C.; Guty, E.; Riegler, K.; Brewer, M.; Fink, S.; Echemendia, R.J.; Arnett, P.A. A-27 APOE & BDNF Polymorphisms Interact to Affect Memory Performance at Baseline in Adolescent Athletes. Arch. Clin. Neuropsychol. 2020, 35, 623. [Google Scholar] [CrossRef]
- Steffener, J.; Stern, Y. Exploring the Neural Basis of Cognitive Reserve in Aging. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2012, 1822, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Pietzuch, M.; King, A.E.; Ward, D.D.; Vickers, J.C. The Influence of Genetic Factors and Cognitive Reserve on Structural and Functional Resting-State Brain Networks in Aging and Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 30. [Google Scholar] [CrossRef]
- Yue, J.K.; Robinson, C.K.; Burke, J.F.; Winkler, E.A.; Deng, H.; Cnossen, M.C.; Lingsma, H.F.; Ferguson, A.R.; McAllister, T.W.; Rosand, J.; et al. Apolipoprotein E Epsilon 4 (APOE-Ε4) Genotype Is Associated with Decreased 6-Month Verbal Memory Performance after Mild Traumatic Brain Injury. Brain Behav. 2017, 7, e00791. [Google Scholar] [CrossRef] [PubMed]
- Merritt, V.C.; Clark, A.L.; Sorg, S.F.; Evangelista, N.D.; Werhane, M.L.; Bondi, M.W.; Schiehser, D.M.; Delano-Wood, L. Apolipoprotein E (APOE) Ε4 Genotype Is Associated with Reduced Neuropsychological Performance in Military Veterans with a History of Mild Traumatic Brain Injury. J. Clin. Exp. Neuropsychol. 2018, 40, 1050–1061. [Google Scholar] [CrossRef]
- Cramer, S.C.; Sur, M.; Dobkin, B.H.; O’Brien, C.; Sanger, T.D.; Trojanowski, J.Q.; Rumsey, J.M.; Hicks, R.; Cameron, J.; Chen, D.; et al. Harnessing Neuroplasticity for Clinical Applications. Brain 2011, 134, 1591–1609. [Google Scholar] [CrossRef]
- Steffener, J.; Reuben, A.; Rakitin, B.C.; Stern, Y. Supporting Performance in the Face of Age-Related Neural Changes: Testing Mechanistic Roles of Cognitive Reserve. Brain Imaging Behav. 2011, 5, 212–221. [Google Scholar] [CrossRef]
- Cramer, S.C.; Procaccio, V. Correlation between Genetic Polymorphisms and Stroke Recovery: Analysis of the GAIN Americas and GAIN International Studies. Eur. J. Neurol. 2012, 19, 718–724. [Google Scholar] [CrossRef]
- Ponsford, J.; McLaren, A.; Schönberger, M.; Burke, R.; Rudzki, D.; Olver, J.; Ponsford, M. The Association between Apolipoprotein E and Traumatic Brain Injury Severity and Functional Outcome in a Rehabilitation Sample. J. Neurotrauma 2011, 28, 1683–1692. [Google Scholar] [CrossRef]
- Arneson, D.; Zhang, Y.; Yang, X.; Narayanan, M. Shared Mechanisms among Neurodegenerative Diseases: From Genetic Factors to Gene Networks. J. Genet. 2018, 97, 795–806. [Google Scholar] [CrossRef]
- Itoh, Y.; Voskuhl, R.R. Cell Specificity Dictates Similarities in Gene Expression in Multiple Sclerosis, Parkinson’s Disease, and Alzheimer’s Disease. PLoS ONE 2017, 12, e0181349. [Google Scholar] [CrossRef] [PubMed]
- Howard, L.; Dumkrieger, G.; Chong, C.D.; Ross, K.; Berisha, V.; Schwedt, T.J. Symptoms of Autonomic Dysfunction Among Those With Persistent Posttraumatic Headache Attributed to Mild Traumatic Brain Injury: A Comparison to Migraine and Healthy Controls. Headache J. Head Face Pain 2018, 58, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, K.F.; Graham, J.; Leddy, J.J.; Devinney-Boymel, L.; Willer, B.S. Exercise Intolerance in Individuals With Postconcussion Syndrome. J. Athl. Train. 2013, 48, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Karas, B.; Grubb, B.P.; Boehm, K.; Kip, K. The Postural Orthostatic Tachycardia Syndrome: A Potentially Treatable Cause of Chronic Fatigue, Exercise Intolerance, and Cognitive Impairment in Adolescents. Pacing Clin. Electrophysiol. 2000, 23, 344–351. [Google Scholar] [CrossRef]
- Russell, K.; Selci, E.; Chu, S.; Fineblit, S.; Ritchie, L.; Ellis, M.J. Longitudinal Assessment of Health-Related Quality of Life Following Adolescent Sports- Related Concussion. J. Neurotrauma 2017, 34, 2147–2153. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).