Abstract
Background: The association between physical activity (PA) and arterial stiffness is particularly important in children with congenital heart disease (CHD) who are at risk for arterial stiffening. The aim of this study was to examine the association between objectively measured PA and arterial stiffness in children and adolescents with CHD. Methods: In 387 children and adolescents with various CHD (12.2 ± 3.3 years; 162 girls) moderate-to-vigorous PA (MVPA) was assessed with the “Garmin vivofit jr.” for 7 consecutive days. Arterial stiffness parameters including pulse wave velocity (PWV) and central systolic blood pressure (cSBP) were non-invasively assessed by oscillometric measurement via Mobil-O-Graph®. Results: MVPA was not associated with PWV (ß = −0.025, p = 0.446) and cSBP (ß = −0.020, p = 0.552) in children with CHD after adjusting for age, sex, BMI z-score, peripheral systolic blood pressure, heart rate and hypertensive agents. Children with CHD were remarkably active with 80% of the study population reaching the WHO recommendation of average 60 min of MVPA per day. Arterial stiffness did not differ between low-active and high-active CHD group after adjusting for age, sex, BMI z-score, peripheral systolic blood pressure, heart rate and hypertensive agents (PWV: F = 0.530, p = 0.467; cSBP: F = 0.843, p = 0.359). Conclusion: In this active cohort, no association between PA and arterial stiffness was found. Longer exposure to the respective risk factors of physical inactivity might be necessary to determine an impact of PA on the vascular system.
1. Introduction
Children with congenital heart disease (CHD) are at high cardiovascular risk as a consequence of their congenital condition. Hence, risk stratification and the identification of modifiable risk factors is particularly important [1]. Pulse wave velocity (PWV) and central systolic blood pressure (cSBP) are surrogate measures of arterial stiffness [2] and strong predictors for cardiovascular and all-cause morbidity and mortality [3,4,5]. Measures of arterial stiffness were shown to be increased already in children with CHD, predisposing this patient cohort to premature heart failure [6,7]. Physical activity (PA) is a clinically important modifiable risk factor for preventing arterial stiffening of the vessels and for determining risk of cardiovascular disease in children with CHD. The importance of PA in children and adolescents with CHD has been emphasized frequently in the recent years [8,9], yet the actual level of PA in children with CHD remains uncertain as the reported PA levels vary considerably between different studies [10,11,12,13].
In healthy children, the interplay of arterial stiffness and PA has provided contradictory findings [14,15,16,17,18,19]. Studies investigating the effect of PA on arterial stiffness in children and adolescents with CHD are rare. One study reported of increased arterial stiffness in low-active children with CHD compared to high-active children with CHD and healthy controls [20]. Another study reported of an inverse association between high levels of MVPA and lower aortic PWV in children with CHD [21]. This association is particularly important in the way of preventing cardiovascular disease in this patient cohort who showed reduced elasticity of the vascular system already in childhood [6,7]. Therefore, this study aimed to analyze the association between objectively measured PA and arterial stiffness in a large cohort of children and adolescents with CHD.
2. Materials and Methods
2.1. Study Participants
In a cross-sectional design, objectively measured PA and surrogates of arterial stiffness were assessed in 387 children and adolescents with various CHD (12.2 ± 3.3 years; 162 girls). Study participants were recruited during their routine outpatient visit at the German Heart Center Munich from March 2016 to January 2021. Study participants were free of acute infections or any neurologic diseases and without restriction to sports and exercise. Various CHDs were categorized into five major subgroups. Detailed information on the characteristics of the study population is given in Table 1. Part of the data have already been published in a cross-sectional comparison of PA levels with healthy controls [10].

Table 1.
Population characteristic of children with congenital heart disease.
2.2. Assessment of Arterial Stiffness
Aortic PWV and cSBP was obtained by a single measurement recording brachial oscillometric blood pressure waves using an automated Mobil-o-Graph® device (I.E.M, Stolberg, Germany) as previously described [22,23]. HMS software version 4.7 with C1 calibration was used. The Mobil-o-Graph® device is non-invasive and has shown good validity and reliability in several studies and is regularly used in the pediatric population [24,25,26,27]. PWV and cSBP were calculated by a proprietary algorithm of the device [28,29]. Measurements were performed at the left upper arm in a supine position after 5 minutes of rest with cuff size adjusted for individual arm circumference
2.3. Objective Assessment of Physical Activity
PA was objectively assessed with the “Garmin vivofit jr.” device (Garmin Ltd., Olathe, KS, USA), which children wore on their wrist for 7 consecutive days. The “Garmin vivofit jr.” is a wearable that tracks every single minute of moderate-to-vigorous PA (MVPA) and steps throughout the day. The physical activity wearable was shown to be accurate is assessing MVPA and step count [30,31].Participants and their guardians were asked to transfer the data from the device to a report sheet at the end of each day. Overall, 329 children (85%) provided complete and valid data for objective PA on 7 consecutive days. Data of at least 3 weekdays and 1 weekend day were considered the minimum to calculate a weekly average and were available for another 58 children (15%). According to the WHO guidelines on PA and sedentary behavior published 2020, children and adolescents should do at least an average of 60 min per day of MVPA across the week [32].
2.4. Data Analysis
Descriptive data of the children with CHD are shown in mean values, standard deviations (mean ± SD) and total numbers (%) if appropriate. MVPA and steps were analyzed for every single day and computed to weekly averages for statistical purposes. The association between PA, in the form of MVPA and step count, and surrogates of arterial stiffness was analyzed using multivariate linear regression analyses adjusted for age, sex, z-score of the body mass index (BMI), peripheral systolic blood pressure (pSBP) heart rate and intake of hypertensive agents (yes/no) as in our previous analysis on this topic [22,23]. The activity level of CHD subgroups was compared to the WHO recommendation of average 60 min per day with one-sample t-test. The study population was categorized as high-active and low-active based on whether children met the WHO recommendations [32]. In addition, 10,000 steps on a daily average were identified as a cut-off for low-active and high-active CHD-groups. Differences in anthropometric data were analyzed with chi-square test and t-test for unpaired samples. General linear models adjusted for age, sex, z-score BMI, pSBP, heart rate and intake of hypertensive agents were calculated to detect differences between the high-active and low-active CHD group in PWV and cSBP. All analyses were performed with RStudio (version 1.3.1093) and SPSS (V25.0, IBM Corporation) with the level of significance set to two-sided p-values < 0.050 for all tests.
3. Results
Daily MVPA was not associated with PWV (ß = −0.025, p = 0.446) and cSBP (ß = −0.020, p = 0.552) after adjusting for age, sex, BMI z-score, pSBP, heart rate and hypertensive agents in 387 children with CHD, (Table 2). This multivariate model explained 74.8% of the variance of PWV and of cSBP. There were no associations between daily step count and surrogates of arterial stiffness. Separate analysis for patients with and without antihypertensive treatment can be found in Supplementary Table S1.

Table 2.
Association between surrogates of arterial stiffness and MVPA adjusted for age, sex, BMI z-score, peripheral systolic blood pressure, heart rate and hypertensive agents.
Overall, children with CHD were remarkably active with a weekly average of 83.3 ± 28.1 min MVPA per day. In total, 80% of the study population (309 participants) accumulated at least 60 min of MVPA per day on a weekly average and thus met the WHO recommendations [32], (Table 1 and Figure 1). Physical activity level did not significantly differ in the CHD severity groups (p = 0.313, Supplementary Table S2).
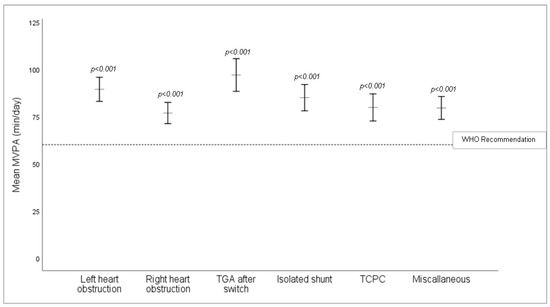
Figure 1.
Mean values of MVPA (min/day) between CHD subgroups corrected for age, sex and BMI z-score. All CHD subgroups significantly exceeded the minimum 60 min MVPA per day recommended by the WHO. TCPC = total cavopulmonary connection, TGA = transposition of the great arteries.
PWV z-Scores based on age, body height and sex were calculated according to Elemenhorst et al. 2015 [28], with an overall PWV z-score of 0.098 ± 1.76 m/s in our study population.
Comparing the high-active and low-active group, both groups did not differ in regard to surrogates of arterial stiffness after adjusting for age, sex, BMI z-score, pSBP, heart rate and hypertensive agents for PWV (high-active: 4.55 ± 0.4 m/s vs. low-active: 4.70 ± 0.4 m/s, F = 0.53, p = 0.467) and cSBP (high-active: 98.9 ± 11.0 mmHg vs. low-active: 101.1 ± 9.9 mmHg, F = 0.843, p = 0.359), (Table 3). The results remain unchanged when evaluating 10,000 steps on a daily average as a cut-off for low-active and high-active CHD-groups.

Table 3.
Population characteristic of high-active and low-active children with congenital heart disease.
4. Discussion
The main finding of the present study is that objectively measured PA was not related to arterial stiffness measures in a largely active cohort of children with CHD.
In healthy children, the relationship between PA and arterial stiffness remains unclear [14], as only half of the included studies in a recently published review reported of improved vascular function in physically active healthy children. In particular, the type of vascular measurement and the type of PA assessment has a considerable influence on reported outcomes [19].
Compared to healthy children, several diagnostic subgroups of patients with CHD show increased arterial stiffness already in the childhood [6,7]. Arterial stiffening in children with CHD has anatomic, histologic, and surgical reasons. Underlying factors are great arterial medial abnormalities of smooth muscle, elastic fibers, collagen and ground substance, impairing the natural buffering function of the vessels in children with CHD [33]. Additionally, surgical scars and resulting fibrotic tissue, implanted patches or conduits as well as pharmacological treatment foster arterial stiffness [2,34]. Due to the pre-existing pathology in children with CHD, the influence of PA becomes particularly important and sufficient evidence is lacking.
In contrast to our results, a study by Boyes and colleagues [20] with a rather small sample size of 17 children reported of increased arterial stiffness in low-active children with CHD compared to high-active children with CHD based on objectively measured daily step-counts. However, this study did not take confounders into account even though several influencing factors on arterial stiffness are well known [3], especially age, obesity and hypertension [35]. When examining the association between PA and arterial stiffness it is therefore important to consider these confounders, as otherwise the impact of PA on arterial stiffness will be biased. Furthermore, our larger sample size might contribute to a clearer picture on the association.
Lopez and colleagues [21] reported of an inverse association between MVPA and aortic PWV after adjusting for sex, age and BMI in 104 children with CHD at a mean age of 12 years. In contrast to our study their patient cohort was rather inactive with an average of 46.7 ± 20.0 min MVPA per day and only 25% of the cohort meeting the WHO recommendation, which markedly differs from the 80% in our patient cohort [21]. In our specialized tertiary center children are encouraged to be physically active during their follow-up appointments which may result in our cohort being more active than the average CHD population. Previous studies investigating PA levels in children with CHD reported rather conflicting results, as some studies showed reduced PA [11,12]. Other studies found high PA levels in children with CHD [10] with no significant differences to healthy peers [10,13]. Overall, the disease-awareness of our patients and their families who attend the regular follow-up appointments is considerably high. In combination with the high activity level, this might have been the most explanatory factor for our results. However, these findings cannot be generalized as our findings largely depend on a large high-active and small low-active CHD group. Further studies are needed to explore and understand the association of arterial stiffness and PA in high-active and low-active children with CHD.
Limitation
As the exposure to risk factors is relatively short in studies with children it is often difficult to show correlations. In addition, when sampling patients from 8 to 18 years of age the time period is just 10 years. This narrow time window and the lack of exposure indicates the necessity for a huge sample size or a huge effect to yield significant results.
As our institution is a specialized tertiary center, complex CHD might be overrepresented in this study population. Combined with the already mention encouragement of PA in our institution the novelty and excitement of wearing the “Garmin vivofit jr.” device might have additionally increased the PA level during the measurement period [36]. Therefore, PA levels in our study cohort might be over-represented compared to reality. However, compared to recent research on PA level of children with CHD in our institution, the measured PA level seems rather normal [10].
A prior study has shown a systematic underestimation of the PWV in high PWV values with the Mobil-O-Graph® device [29], which might have influenced our results even in this young cohort. The algorithm used by the Mobil-O-Graph® yields only estimates of the PWV, whereby estimates of PWV were shown to be strongly dependent on age and systolic BP and less on the waveform characteristics [29,37]. Mobil-O-Graph® is nevertheless considered as a good and valid method for estimating PWV as it is easy to perform, operator-independent and reliable [29]. In children and adolescents, oscillometric noninvasive estimations using Mobil-O-Graph® were shown to be effective for evaluating cSBP in this age group [27].
5. Conclusions
No association between PA and arterial stiffness was found in this active cohort of children with CHD. The influence of PA on arterial stiffness in low-active children and patients with a longer exposure to respective risk factors needs further investigation. Nevertheless, PA promotion is indicated in patients with CHD, as its benefits are unquestionable.
Supplementary Materials
The following tables are available online at https://www.mdpi.com/article/10.3390/jcm10153266/s1, Table S1: Association between surrogates of arterial stiffness and MVPA adjusted for age, sex, BMI z-score, peripheral systolic blood pressure and heart rate, separated for patients treated with antihypertensive drugs and untreated patients; Table S2: Physical activity level based on MVPA of different CHD severities after adjusting for age, sex and BMI z-score.
Author Contributions
Conceptualization, J.M.; methodology, J.M. and L.W.; software, J.M. and L.W.; validation, J.M.; formal analysis, L.W. and J.M.; investigation, L.W. and L.B.; resources, J.M. and P.E.; data curation, J.M.; writing—original draft preparation, L.W.; writing—review and editing, J.M., L.B., P.E. and R.O.-F.; visualization, L.W.; supervision, J.M.; project administration, J.M.; funding acquisition, J.M. and P.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by an unrestricted grant from the “Stiftung KinderHerz Deutschland gGmbH.”
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the local ethical board of the Technical University of Munich (project number: 314/14).
Informed Consent Statement
Informed consent was obtained from all subjects and their guardians involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bigras, J.-L. Cardiovascular Risk Factors in Patients with Congenital Heart Disease. Can. J. Cardiol. 2020, 36, 1458–1466. [Google Scholar] [CrossRef]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H.; et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef] [Green Version]
- Laurent, S.; Boutouyrie, P.; Asmar, R.; Gautier, I.; Laloux, B.; Guize, L.; Ducimetiere, P.; Benetos, A. Aortic Stiffness Is an Independent Predictor of All-Cause and Cardiovascular Mortality in Hypertensive Patients. Hypertension 2001, 37, 1236–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEniery, C.M.; Yasmin; McDonnell, B.; Munnery, M.; Wallace, S.M.; Rowe, C.V.; Cockcroft, J.R.; Wilkinson, I.B. Central pressure: Variability and impact of cardiovascular risk factors: The Anglo-Cardiff Collaborative Trial II. Hypertension 2008, 51, 1476–1482. [Google Scholar] [CrossRef] [Green Version]
- Sharman, J.E.; Marwick, T.H.; Gilroy, D.; Otahal, P.; Abhayaratna, W.P.; Stowasser, M. Randomized trial of guiding hypertension management using central aortic blood pressure compared with best-practice care: Principal findings of the BP GUIDE study. Hypertension 2013, 62, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Häcker, A.-L.; Reiner, B.; Oberhoffer, R.; Hager, A.; Ewert, P.; Müller, J. Increased arterial stiffness in children with congenital heart disease. Eur. J. Prev. Cardiol. 2018, 25, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Ewert, P.; Hager, A. Increased aortic blood pressure augmentation in patients with congenital heart defects—A cross-sectional study in 1125 patients and 322 controls. Int. J. Cardiol. 2015, 184, 225–229. [Google Scholar] [CrossRef]
- Longmuir, P.E.; Brothers, J.A.; De Ferranti, S.D.; Hayman, L.L.; Van Hare, G.F.; Matherne, G.P.; Davis, C.K.; Joy, E.A.; McCrindle, B.W. Promotion of physical activity for children and adults with congenital heart disease: A scientific statement from the American Heart Association. Circulation 2013, 127, 2147–2159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takken, T.; Giardini, A.; Reybrouck, T.; Gewillig, M.; Hövels-Gürich, H.; Longmuir, P.; McCrindle, B.; Paridon, S.; Hager, A. Recommendations for physical activity, recreation sport, and exercise training in paediatric patients with congenital heart disease: A report from the Exercise, Basic & Translational Research Section of the European Association of Cardiovascular Prevention and Rehabilitation, the European Congenital Heart and Lung Exercise Group, and the Association for European Paediatric Cardiology. Eur. J. Prev. Cardiol. 2012, 19, 1034–1065. [Google Scholar] [CrossRef]
- Brudy, L.; Hock, J.; Häcker, A.-L.; Meyer, M.; Oberhoffer, R.; Hager, A.; Ewert, P.; Müller, J. Children with Congenital Heart Disease Are Active but Need to Keep Moving: A Cross-Sectional Study Using Wrist-Worn Physical Activity Trackers. J. Pediatr. 2020, 217, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Moola, F.; McCrindle, B.W.; Longmuir, P.E. Physical activity participation in youth with surgically corrected congenital heart disease: Devising guidelines so Johnny can participate. Paediatr. Child Health 2009, 14, 167–170. [Google Scholar] [CrossRef] [Green Version]
- Siaplaouras, J.; Niessner, C.; Helm, P.C.; Jahn, A.; Flemming, M.; Urschitz, M.S.; Sticker, E.; Abdul-Khaliq, H.; Bauer, U.M.; Apitz, C. Physical Activity Among Children with Congenital Heart Defects in Germany: A Nationwide Survey. Front. Pediatr. 2020, 8. [Google Scholar] [CrossRef]
- Ewalt, L.A.; Danduran, M.J.; Strath, S.J.; Moerchen, V.; Swartz, A.M. Objectively assessed physical activity and sedentary behaviour does not differ between children and adolescents with and without a congenital heart defect: A pilot examination. Cardiol. Young 2011, 22, 34–41. [Google Scholar] [CrossRef]
- Böhm, B.; Oberhoffer, R. Vascular health determinants in children. Cardiovasc. Diagn. Ther. 2019, 9, S269–S280. [Google Scholar] [CrossRef] [PubMed]
- Schack-Nielsen, L.; Mølgaard, C.; Larsen, D.; Martyn, C.; Michaelsen, K.F. Arterial stiffness in 10-year-old children: Current and early determinants. Br. J. Nutr. 2005, 94, 1004–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakuragi, S.; Abhayaratna, K.; Gravenmaker, K.J.; O’Reilly, C.; Srikusalanukul, W.; Budge, M.M.; Telford, R.D.; Abhayaratna, W.P. Influence of adiposity and physical activity on arterial stiffness in healthy children: The lifestyle of our kids study. Hypertension 2009, 53, 611–616. [Google Scholar] [CrossRef] [Green Version]
- Heil, L.; Oberhoffer, R.; Böhm, B. Association Between Physical Activity Intensity Levels and Arterial Stiffness in Healthy Children. J. Phys. Act. Health 2020, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Khadilkar, A.V.; Chiplonkar, S.A.; Khadilkar, V.V.; Kinare, A.S.; Pandit, D.S. Arterial stiffness in obese children: Role of adiposity and physical activity. Indian J. Endocrinol. Metab. 2014, 18, 70–76. [Google Scholar] [CrossRef]
- Baumgartner, L.; Weberruß, H.; Oberhoffer-Fritz, R.; Schulz, T. Vascular Structure and Function in Children and Adolescents: What Impact Do Physical Activity, Health-Related Physical Fitness, and Exercise Have? Front. Pediatr. 2020, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Boyes, N.G.; Stickland, M.K.; Fusnik, S.; Hogeweide, E.; Fries, J.T.; Haykowsky, M.J.; Baril, C.L.; Runalls, S.; Kakadekar, A.; Pharis, S.; et al. Physical activity modulates arterial stiffness in children with congenital heart disease: A CHAMPS cohort study. Congenit. Heart Dis. 2018, 13, 578–583. [Google Scholar] [CrossRef]
- Lopez, J.R.; Voss, C.; Kuan, M.T.; Hemphill, N.M.; Sandor, G.G.; Harris, K.C. Physical activity is associated with better vascular function in children and adolescents with congenital heart disease. Can. J. Cardiol. 2020, 36, 1474–1481. [Google Scholar] [CrossRef]
- Hock, J.; Häcker, A.-L.; Reiner, B.; Oberhoffer, R.; Hager, A.; Ewert, P.; Müller, J. Functional outcome in contemporary children and young adults with tetralogy of Fallot after repair. Arch. Dis. Child. 2018, 104, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Häcker, A.-L.; Reiner, B.; Oberhoffer, R.; Hager, A.; Ewert, P.; Müller, J. Functional outcomes in children with anatomically repaired transposition of the great arteries with regard to congenital ventricular septal defect and coronary pattern. Arch. Dis. Child. 2019, 104, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Hametner, B.; Wassertheurer, S.; Kropf, J.; Mayer, C.; Eber, B.; Weber, T. Oscillometric estimation of aortic pulse wave velocity: Comparison with intra-aortic catheter measurements. Blood Press. Monit. 2013, 18, 173–176. [Google Scholar] [CrossRef]
- Weiss, W.; Gohlisch, C.; Zidek, W.; Van Der Giet, M. Oscillometric Estimation of Central Blood Pressure: Validation of the Mobil-O-Graph® in Comparison to the Sphygmocor® Device. J. Hypertens. 2011, 29, e1–e2. [Google Scholar] [CrossRef]
- Sharman, J.E.; Avolio, A.P.; Baulmann, J.; Benetos, A.; Blacher, J.; Blizzard, C.L.; Boutouyrie, P.; Chen, C.-H.; Chowienczyk, P.; Cockcroft, J.R.; et al. Validation of non-invasive central blood pressure devices: ARTERY Society task force consensus statement on protocol standardization. Eur. Heart J. 2017, 38, 2805–2812. [Google Scholar] [CrossRef]
- Shiraishi, M.; Murakami, T.; Higashi, K. The accuracy of central blood pressure obtained by oscillometric noninvasive method using Mobil-O-Graph in children and adolescents. J. Hypertens. 2020, 38, 813–820. [Google Scholar] [CrossRef]
- Elmenhorst, J.; Hulpke-Wette, M.; Barta, C.; Pozza, R.D.; Springer, S.; Oberhoffer, R. Percentiles for central blood pressure and pulse wave velocity in children and adolescents recorded with an oscillometric device. Atherosclerosis 2015, 238, 9–16. [Google Scholar] [CrossRef]
- Salvi, P.; Scalise, F.; Rovina, M.; Moretti, F.; Salvi, L.; Grillo, A.; Gao, L.; Baldi, C.; Faini, A.; Furlanis, G.; et al. Noninvasive estimation of aortic stiffness through different approaches: Comparison with intra-aortic recordings. Hypertension 2019, 74, 117–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsubheen, S.A.; George, A.M.; Baker, A.; Rohr, L.E.; Basset, F.A. Accuracy of the vivofit activity tracker. J. Med. Eng. Technol. 2016, 40, 298–306. [Google Scholar] [CrossRef]
- Price, K.; Bird, S.R.; Lythgo, N.; Raj, I.S.; Wong, J.Y.; Lynch, C. Validation of the Fitbit One, Garmin Vivofit and Jawbone UP activity tracker in estimation of energy expenditure during treadmill walking and running. J. Med. Eng. Technol. 2017, 41, 208–215. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Niwa, K.; Perloff, J.K.; Bhuta, S.M.; Laks, H.; Drinkwater, D.C.; Child, J.S.; Miner, P.D. Structural abnormalities of great arterial walls in congenital heart disease: Light and electron microscopic analyses. Circulation 2001, 103, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Tateno, S.; Kawasoe, Y.; Niwa, K. Aortic surgery is one of the risk factors for enhancement of pressure wave reflection in adult patients with congenital heart disease. Int. J. Cardiol. 2014, 175, 451–454. [Google Scholar] [CrossRef]
- Giannattasio, C.; Mancia, G. Arterial distensibility in humans. Modulating mechanisms, alterations in diseases and effects of treatment. J. Hypertens. 2002, 20, 1889–1899. [Google Scholar] [CrossRef] [PubMed]
- Lubans, D.R.; Morgan, P.J.; Callister, R.; Collins, C.E. Effects of Integrating Pedometers, Parental Materials, and E-mail Support Within an Extracurricular School Sport Intervention. J. Adolesc. Health 2009, 44, 176–183. [Google Scholar] [CrossRef]
- Schwartz, J.E.; Feig, P.U.; Izzo, J.L., Jr. Pulse wave velocities derived from cuff ambulatory pulse wave analysis: Effects of age and systolic blood pressure. Hypertension 2019, 74, 111–116. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).