Abstract
In recent years, there has been a significant increase in the incidence of Crohn’s disease. Despite significant medical progress, the treatment options available today do not meet the needs of all patients. Recent reports indicate that external environmental factors, including diet, are key in the pathomechanism of the disease. It was proven that the so-called Western dietary pattern is associated with an increased risk of disease. In the pediatric population, exclusive enteral nutrition is the only nutritional therapy option recommended today with proven high efficacy in inducing remission. Recent publications that indicate at least comparable efficacy and significantly better tolerability of a specialised elimination diet, the Crohn’s Disease Exclusion Diet (CDED), provide the basis for a change in recommendations. This article discusses the mechanism of action, principles of use, and scientific evidence evaluating the efficacy of CDED in the treatment of children with Crohn’s disease.
1. Introduction
The incidence of Crohn’s disease (CD), a chronic inflammatory disease of the gastrointestinal tract, has increased dramatically over the past two decades [1]. Increasingly, this problem affects children—according to current data, approximately 10% of patients have a diagnosis before the age of 17 [2]. Despite significant progress in the field of pharmacological treatment, its effectiveness is still unsatisfactory—at present, we do not have a drug that would act not only on the effects but also on the causes of the disorders. CD remains an incurable condition that is difficult to control and has a risk of recurrence and complications. The progressive nature of the disease, resistance/loss of response to subsequent drugs, the need for surgical treatment, or the use of steroids and immunosuppressive drugs characterised by a high profile of side effects, further worsen the prognosis [3,4,5].
Recent data suggest that the development of chronic inflammation in CD may be related to specific external environmental factors. One of the key factors that can negatively impact the delicate immune balance between the microbiome and the intestinal mucosa is diet [6,7,8]. This concept is supported by the results of studies in cellular and animal models as well as epidemiological studies, which indicate a positive correlation between the so-called “Western” dietary pattern and increased risk of CD [6,9,10]. These reports gave rise to a growing interest in nutritional therapy as an alternative therapeutic option to pharmacological treatment.
The primary goal of treatment for CD is to achieve deep remission, i.e., clinical, biochemical and histopathological remission [11,12]. In the pediatric population, it was proved that exclusive enteral nutrition (EEN) is more effective in inducing deep remission than systemic steroids [13,14]. In addition, this form of treatment has a beneficial effect on the nutritional status and bone mineral density, often impaired by the disease, and is free of side effects. For these reasons, EEN is recommended today as the first-line treatment for active CD in children [5,15,16]. However, nutritional therapy, which consists of a complete exclusion of the natural diet and feeding solely on a specialised preparation (enteral diet) for 6–8 weeks, is difficult in practical application and lacks the concept of long-term management to maintain the therapeutic effect [17,18].
The mechanism of action of EEN is to exclude ingredients from the diet that negatively affect the homeostasis between the microbiota, the intestinal mucosa and the immune system [6,7]. The lack of a more affordable option for nutritional therapy has, until recently, been a barrier to large-scale use of this treatment. Previously published data on the therapeutic efficacy of specialised elimination diets (e.g., specific carbohydrate diet or autoimmune protocol diet) were not groundbreaking and did not indicate that EEN could be replaced by a diet composed of appropriately selected natural products [16,19]. However, in 2019, results of a multicenter randomised trial were published, indicating comparable efficacy of EEN and a specific elimination diet developed for patients with CD, the CDED, in inducing remission in children with active CD. Importantly, therapy tolerance was significantly higher in the CDED group than in the EEN group [7]. The results of the study and regularly published summaries of subsequent analyses give hope that this modern method of nutritional treatment will soon find its place in official recommendations, replacing or providing an alternative to EEN. The purpose of this article is to discuss the mechanism of action and protocol of the CDED diet as well as the results of scientific reports supporting its efficacy in the treatment of CD in the pediatric population.
2. Diet and the Pathogenesis of Crohn’s Disease
The etiology of CD is unknown. Currently, interactions between environmental factors and the intestinal microflora and immune system (intestinal barrier) in individuals with a genetic predisposition to develop the disease are considered the most likely pathomechanism (Figure 1) [20]. The critical importance of environmental factors is confirmed by epidemiological data, including incidence rates around the world. Interesting information was provided by observations among people who moved from regions with low incidence to countries with high incidence—it was shown that the offspring of immigrants had the same risk of CD as children coming from families living in regions with high incidence for many generations [21]. One of the best-studied environmental factors in the context of CD pathogenesis is diet. Recent data suggest that as a result of specific dietary factors, the delicate immune balance between the microbiota and the intestinal mucosa is disrupted, leading to chronic inflammation. This hypothesis is supported by research findings that show a correlation between the so-called Western (industrialised) dietary pattern and increased risk of CD [6,9,10].
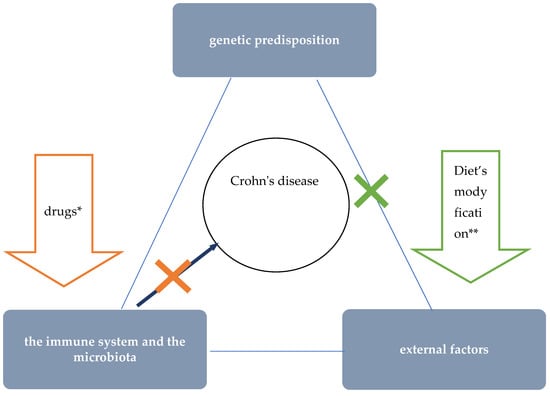
Figure 1.
The role of nutritional therapy in the treatment of Crohn’s disease. * steroids, immunosuppressants, and biologics’ ** such as EEN.
Epidemiological studies proved the influence of the Western diet on an increased incidence of CD and, conversely, a lower incidence among those following a Mediterranean diet [6,10]. One of the features typical of the Western dietary pattern is the high consumption of processed foods. Meanwhile, a number of adverse consequences resulting from exposure to a variety of food additives were demonstrated in studies in cellular and animal models [6]. For example, the destructive effects of emulsifiers (i.e., carboxymethylcellulose and polysorbate-80) on the mucus layer that protects intestinal epithelial cells was proved [22,23,24]. Due to their gelling and thickening properties, these additives are commonly used in meat and dairy products, among other things. Another problem specific to the Western diet is low dietary fibre intake. Particularly relevant to the pathogenesis of CD may be a low supply of food that provides substrates for the production of short-chain fatty acids (SCFAs), especially water-soluble dietary fibre and resistant starch [8]. Short-chain fatty acids, which are a metabolic product of the bacteria residing in the intestines, play an important role in maintaining normal intestinal barrier and immune system function in a number of ways, including:
- by influencing the activity of immune cells and their migration to the site of inflammation, they exhibit anti-inflammatory effects;
- by nourishing colonocytes, providing them with a primary source of energy;
- by reducing the pH in the intestine, positively influencing the composition of the intestinal microbiota (stimulating the growth of beneficial strains of bacteria and inhibiting the growth of pathogenic bacteria).
On the other hand, in the absence of sources for SCFA production, the mucus layer that protects intestinal epithelial cells can be used by bacteria as a medium, allowing their translocation through the intestinal mucosa [8,25].
Other features of the Western diet with potential negative effects on CD development include high consumption of red/processed meat, animal fat, and wheat (Figure 2) [6]. For example, studies in animal models proved, among other things, the effect of a diet high in fat and sugars on adverse changes in the composition of the intestinal microbiota and on impaired expression of short-chain fatty acid receptors (GPR43). GPR43 are involved in mechanisms regulating SCFA-mediated immune responses [26].
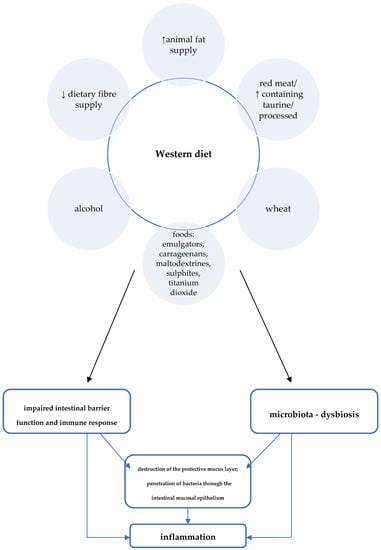
Figure 2.
Western diet and the pathogenesis of Crohn’s disease [6].
A summary of Western dietary factors considered as particularly important in the pathomechanism of inflammation in CD is shown in Figure 2.
3. Why Are the Currently Recommended Nutritional Treatments in the Pediatric Population Not Fully Satisfying for Us?
The achievement of deep remission with healing of the intestinal mucosa has a fundamental impact on long-term treatment outcomes and is an essential goal of CD treatment today [12]. In the pediatric population, the comparable efficacy of EEN and glucocorticosteroids in the treatment of active CD, with a significantly more favorable effect of nutritional therapy on intestinal mucosal healing, was sufficiently proved in high-quality scientific studies [5,27]. Moreover, unlike steroid therapy, nutritional treatment has a positive effect on nutritional status and is devoid of side effects [15]. Therefore, exclusive enteral nutrition is now recommended as the first-line treatment for the active luminal CD in children [16,28]. The implementation of EEN into daily clinical practice enabled a significant reduction in the use of corticosteroids in the pediatric population. However, the therapy, which requires complete exclusion of the natural diet and feeding solely on a specialised formula (enteral diet) for 6–8 weeks, is difficult to implement in practice—it requires high motivation from the patient and parents, and in some cases, it requires the use of a nasogastric tube [17,18]. In addition, this idea of nutritional treatment lacks a management strategy to maintain remission and the rate of disease re-exacerbation after returning to the habitual diet is high [18,28,29].
EEN is a safe, effective, and so far, the only causal treatment concept that achieves clinical and endoscopic remission in the majority of treated children (Figure 1) [5,15,16,27,28]. The previously mentioned limitations of EEN are an obstacle to the large-scale application of this therapeutic method. Until recently, data on the possibility of application of different elimination diets have not been promising and no nutritional treatment concept other than EEN has found its way into the official recommendations of major scientific societies so far [17]. However, over the past few years, further evidence has emerged for the effectiveness of a specialised elimination diet developed for CDED, in treating children with active CD [7,30,31]. These reports are very promising and offer hope for replacing EEN, which is cumbersome to apply, with this innovative method of nutritional treatment.
4. CDED—Protocol and Mechanism of Action
The CDED diet is a new generation of nutritional therapy—in its initial stages, aimed at inducing remission, it involves a combination of partial enteral nutrition (PEN) with selected natural diet products. The primary mechanism of action is to exclude or limit exposure to dietary factors with potentially deleterious effects on the pathogenesis and course of CD (Table 1).

Table 1.
Major food groups eliminated in the Crohn’s Disease Exclusion Diet.
The direct effect of the elimination diet on restoring eubiosis, normal intestinal barrier function, and immune response, causes the quenching of inflammation and healing of the intestinal mucosa (Figure 3).
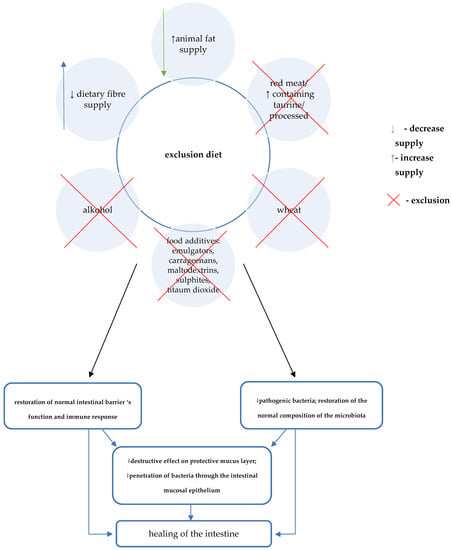
The auxiliary mechanism of CDED involves consideration of the supply of specific components that may provide additional benefits [7,30,31]. A key role in this regard is attributed to the effect of increasing the production of short-chain fatty acids. Therefore, foods that are mandatory in CDED include selected foods rich in water-soluble fibre (e.g., pectin) as well as resistant starch (Table 2) [6,7,30,31].

Table 2.
Foods mandatory/allowed during the induction phase of CDED (stages 1 and 2).
In the first phase, due to the greatest restrictions regarding the allowed foods, 50% of daily energy demand must be satisfied by enteral diet. Phase 2 is the time of reintroduction of some foods that had to be eliminated during the first 6 weeks. Due to the increasing variety of foods that are allowed, the recommended % of energy provided with the formula is reduced to 25% of the demand. Satisfying some nutritional needs with the use of a complete enteral diet is aimed at preventing deficiencies and maintaining/improving the nutritional status in the period of the greatest restrictions, i.e., in the first 2 phases of the diet. The principles of formula selection are the same as the guidelines for exclusive enteral nutrition in children. Standard polymeric, normocaloric (1 kcal/1 mL) diets are recommended. In justified cases, that is in patients with food intolerances, allergy to cow’s milk proteins or insufficient tolerance of the polymeric preparation, preparations containing hydrolysed protein should be recommended.
Foods that can be consumed in the first two phases of the CDED are divided into foods that are:
- –
- mandatory, i.e., recommended for daily consumption. Their role is to provide adequate nutritional value of the diet as well as substrates for the production of SCFA;
- –
- neutral, which are supposed to add variety to the daily menu, but do not necessarily have to be consumed.
- –
- forbidden.
Table 2 presents the summary of mandatory and neutral products that can be consumed during phases 1 and 2 of the diet. The quantity of enteral feeding and particular supplementing products should be determined individually, in accordance with the state of nutrition stemming from sex, age and activity of the disease as well as energy and nutrient demand.
In phase 3 (maintenance), patients should function according to the principle of controlled exposure to dietary components with potentially negative effects on pathogenesis and the course of the disease. Therefore, for five days a week, they should compose their meals based on products allowed in phase 2 as well as selected additional products. During these days, especially in the case of patients who did not normalise the parameters of their nutritional status despite good treatment effects, it is recommended that supplementation with formula be continued. In addition, on selected consecutive days it is possible to eat two meals composed of products that are not recommended for daily consumption. Patients should still avoid particularly harmful foods, mainly highly processed products, i.e., processed meats, frozen, ready-to-eat foods and sweetened beverages. Table 3 presents a summary of foods/meals that can be consumed additionally during the maintenance phase of CDED.

Table 3.
Foods allowed in the maintenance phase of CDED.
5. Effectiveness of Crohn’s Disease Exclusion Diet in Studies
CDED was developed in 2010 by Professor Arie Levine (Wolfson Medical Center, Tel Aviv). The first reports—results of a retrospective analysis of treatment results of CDED followed for 12 weeks by a group of 47 children and young adults with active CD—were published in 2014 [30]. After the end of the first phase of CDED, i.e., after six weeks, clinical remission was achieved in 33/47 patients (70.2%) in the study group. Intestinal mucosa healed in 11 (70%) out of 15 patients who underwent endoscopic examination before the beginning of the diet and after following it for six weeks. A publication confirming high efficacy of CDED in a group of 21 patients who lost response to biological treatment was published in 2017 [31]. After 6 weeks, 62% of patients were in clinical remission, and in 38% and 43% of patients, respectively, researchers observed normalisation or a decrease in the parameters of inflammatory state. In 2019, Gastroenterology published groundbreaking results of a multicentre randomised study, indicating that the effectiveness of CDED and standard treatment (EEN) in terms of the induction of clinical remission in children with active CD (80% vs. 73.5% in the sixth week of treatment, respectively) was comparable, and that the treatment of significantly better tolerated in the intervention group [7]. However, after 12 weeks of observation, 76.2% of children treated with CDED remained in steroid-free remission, compared to only 45.1% of patients in the EEN group. In addition, patients treated with the new exclusion diet showed better results with regard to reducing intestinal permeability. After 3 weeks of nutritional treatment the intestinal permeability lowered in the CDED group while increased in the EEN group. What is more, both in the CDED and EEN groups the correction of dysbiosis was observed at week 6 (decreased proteo-bacteria), however, the reduction in proteobacteria was maintained at 12 weeks only in the CDED group, while a major rebound of this tax was observed in EEN group at the same time point. Unlike in EEN, the beneficial effect of CDED on the reduction of the intestinal permeability and on the favorable and persistent modification of microbiota composition may be of key importance for long-term maintenance of treatment effects. In the past year, researchers published a presentation of a case series, suggesting that the range of indications for CDED may potentially be broader [32]. The paper confirmed high effectiveness of CDED, applied not only as monotherapy, but also within the framework of a therapy combined with pharmacological treatment and as a salvage treatment in patients resistant to pharmacological treatment. Further studies are underway or at the stage of data analysis, including a publication presenting the results of a randomised study evaluating the efficacy of CDED compared to standard treatment in adult patients.
6. The ModuLife Project and Application
In 2019, the ModuLife software, developed by the creators of CDED in collaboration with Nestle, was made globally available for use. The primary goal of the project is to train CDED specialists and popularise the method among patients. The application for patients contains a recipe/meal database and provides a lot of help in everyday diet. Access to the platform can only be granted by a CDED expert. The training for specialists is available at: https://modulifexpert.com/ (accessed on 6 July 2021). Apart from lectures, it includes auxiliary materials as well as regularly held seminars that supplement the latest knowledge on CDED [33].
7. Summary
As of today, EEN is the sole method of nutritional treatment with proven efficacy in inducing remission in children with active Crohn’s disease that is recommended in the official guidelines. This form of dietary treatment, however, has significant limitations that have a negative impact on the possibility of its wide use and long-term maintenance of its positive effects. It seems that the groundbreaking reports concerning CDED, a modern method of dietary treatment, which indicates that it is at least comparable with EEN in terms of its effectiveness in inducing remission and that is much better tolerated by patients, CDED will be included in the latest guidelines of scientific societies.
Author Contributions
Conceptualization, M.M. and J.K.; methodology, M.M.; software, M.M.; validation, M.M., J.K.; formal analysis, M.M.; investigation, M.M.; resources, M.M.; data curation, M.M.; writing—original draft preparation, M.M.; writing—review and editing, M.M.; visualization, M.M.; supervision, J.K.; project administration, J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Ghione, S.; Sarter, H.; Fumery, M.; Armengol-Debeir, A.; Savoye, G.; Ley, D.; Spyckerelle, C.; Pariente, B.; Peyrin-Biroulet, L.; Turck, D.; et al. Dramatic increase in incidence of ulcerative colitis and Crohn’s disease [1988–2011]: A population-based study of French adolescents. Am. J. Gastroenterol. 2018, 113, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Olen, O.; Askling, J.; Sachs, M.C.; Frumento, P.; Neovius, M.; Smedby, K.E.; Ekbom, A.; Malmborg, P.; Ludvigsson, J.F. Increased mortality of patients with childhood-onset inflammatory bowel diseases, compared with the general population. Gastroenterology 2019, 156, 614–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joosse, M.E.; Aardoom, M.A.; Kemos, P.; Turner, D.; Wilson, D.C.; Koletzko, S.; Martin-de-Carpi, J.; Fagerbelg, U.L.; Spray, C.; Tzivinikos, C.; et al. Malignancy and mortality in paediatric-onset inflammatory bowel disease: A 3-year prospective, multinational study from the paediatric IBD Porto group of ESPGHAN. Aliment. Pharmacol. Ther. 2018, 48, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Grover, Z.; Lewindon, P. Two-year outcomes after exclusive enteral nutrition induction are superior to corticosteroids in pediatric Crohn’s disease treated early with thiopurines. Dig. Dis. Sci. 2015, 60, 3069–3074. [Google Scholar] [CrossRef]
- Levine, A.; Boneh, R.S.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738. [Google Scholar] [CrossRef]
- Levine, A.; Wine, E.; Assa, A.; Sigall Boneh, R.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440–450. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.D.; Abreu, M.T. Diet as a Trigger or Therapy for Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 398–414. [Google Scholar] [CrossRef]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Khalili, H.; Hakansson, N.; Chan, S.S.; Chen, Y.; Lochhead, P.; Ludvigsson, J.F.; Chan, A.T.; Hart, A.R.; Olén, O.; Wolk, W. Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn’sdisease: Results from two large prospective cohort studies. Gut 2020, 69, 1637–1644. [Google Scholar] [CrossRef]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, K.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohns Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Maconi, G.; Armuzzi, A. Beyond remission and mucosal healing in Crohn’s disease. Exploring the deep with cross sectional imaging. Dig. Liver Dis. 2017, 49, 457–458. [Google Scholar] [CrossRef]
- Borrelli, O.; Cordischi, L.; Cirulli, M.; Paganelli, M.; Labalestra, V.; Uccini, S.; Russo, P.M.; Cucchiara, S. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: A randomized controlled open-label trial. Clin. Gastroenterol. Hepatol. 2006, 4, 744–753. [Google Scholar] [CrossRef]
- Pigneur, B.; Lepage, P.; Mondot, S.; Schmitz, J.; Goulet, O.; Dore, J.; Romuelle, F. Mucosal healing and bacterial composition in response to enteral nutrition vs steroid-based induction therapy-a randomised prospective clinical trial in children with Crohn’s disease. J. Crohns Colitis 2019, 13, 846–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, A.; Turner, D.; Gik, T.P.; Dias, J.A.; Veres, G.; Shaoul, R.; Staiano, A.; Escher, J.; Kolho, K.L.; Paerregaard, A.; et al. Comparison of outcomes parameters for induction of remission in new onset pediatric Crohn’s disease: Evaluation of the porto IBD group growth relapse and outcomes with therapy (GROWTH CD) study. Inflamm. Bowel Dis. 2014, 20, 278–285. [Google Scholar] [CrossRef] [PubMed]
- van Rheenen, P.F.; Aloi, M.; Assa, A.; Bronsky, J.; Escher, J.C.; Fagerberg, U.L.; Gasparetto, M.; Gerasimidis, K.; Griffiths, A.; Henderson, P.; et al. The Medical Management of Paediatric Crohn’s Disease: An ECCO-ESPGHAN Guideline Update. J. Crohns Colitis 2020, 15, 171–194. [Google Scholar] [CrossRef]
- Van Limbergen, J.; Haskett, J.; Griffiths, A.M.; Critch, J.; Huynh, H.; Ahmed, N.; deBruyn, J.C.; Issenman, R.; El-Matary, W.; Walters, T.D.; et al. Toward enteral nutrition for the treatment of pediatric Crohn disease in Canada: A workshop to identify barriers and enablers. Can. J. Gastroenterol. Hepatol. 2015, 29, 351–356. [Google Scholar] [CrossRef]
- Lawley, M.; Wu, J.W.; Navas-Lopez, V.M.; Huynh, Q.H.; Carroll, M.W.; Chen, M.; Medvedev, P.; Day, A.S.; Hussey, S.; Sigall-Boneh, R.; et al. Global variation in use of enteral nutrition for pediatric Crohn disease. J. Pediatr. Gastroenterol. Nutr. 2018, 67, e22–e29. [Google Scholar] [CrossRef]
- Miele, E.; Shamir, R.; Aloi, M.; Assa, A.; Braegger, C.; Bronsky, J.; de Ridder, L.; Escher, J.C.; Hojsak, I.; Kolaček, S.; et al. Nutrition in Paediatric Inflammatory Bowel Disease: A Position Paper on Behalf of The Porto IBD Group of ESPGHAN. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 687–708. [Google Scholar] [CrossRef] [Green Version]
- Svolos, V.; Hansen, R.; Nichols, B.; Quince, C.; Ijaz, U.Z.; Papadopoulou, R.T.; Edwards, C.A.; Watson, D.; Alghamdi, A.; Brejnrod, A.; et al. Treatment of active Crohn’s disease with an ordinary food-based diet that replicates exclusive enteral nutrition. Gastroenterology 2019, 156, 1354–1367. [Google Scholar] [CrossRef] [Green Version]
- Charlebois, A.; Rosenfeld, G.; Bressler, B. The impact of dietary interventions on the symptoms of inflammatory bowel disease: A systematic review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1370–1378. [Google Scholar] [CrossRef]
- Roberts, C.L.; Rushworth, S.L.; Richman, E.; Rhodes, J.M. Hypothesis: Increased consumption of emulsifiers as an explanation for the rising incidence of Crohn’s disease. J. Crohns Colitis 2013, 7, 338–341. [Google Scholar] [CrossRef] [Green Version]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chassaing, B.; Van de Wiele, T.; De Bodt, J.; Marzorati, M.; Gewirtz, A.T. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut 2017, 66, 1414–1427. [Google Scholar] [CrossRef]
- Czajkowska, A.; Szponar, B. Krótkołańcuchowe kwasy tłuszczowe (SCFA) jako produkty metabolizmu bakterii jelitowych oraz ich znaczenie dla organizmu gospodarza. Postepy. Hig. Med. Dosw. 2018, 72, 131–142. [Google Scholar] [CrossRef]
- Agus, A.; Denizot, J.; Thévenot, J.; Martinez-Medina, M.; Massier, S.; Sauvanet, P.; Bernalier-Donadille, A.; Denis, S.; Hofman, P. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive, E. coli infection and intestinal inflammation. Sci. Rep. 2016, 6, 19032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swaminath, A.; Feathers, A.; Ananthakrishnan, A.; Falzon, L.; Ferry, S.L. Systematic Review with Meta-Analysis: Enteral Nutrition Therapy for the Induction of Remission in Pediatric Crohn’s Disease. Aliment. Pharmacol. Ther. 2017, 46, 645–656. [Google Scholar] [CrossRef] [Green Version]
- Matuszczyk, M.; Gawecka, A.; Grzybowska-Chlebowczyk, U.; Książyk, J.; Lebensztejn, D.; Popińska, K.; Romanowska, H.; Sładek, M.; Socha, P.; Szlagatys-Sidorkiewicz, A.; et al. Polskie wytyczne leczenia żywieniowego w nieswoistych chorobach zapalnych jelit u dzieci. Wytyczne Polskiego Towarzystwa Gastroenterologii, Hepatologii i Żywienia Dzieci oraz Polskiego Towarzystwa Żywienia Klinicznego Dzieci. Stand. Med. Pediatr. 2017, 14, 195–226. [Google Scholar]
- Nakar, I.; Focht, G.; Church, P.; Church, P.; Walters, T.D.; Abitbol, G.; Anupindi, S.; Berteloot, L.; Hulst, J.M.; Ruemmele, F.; et al. The association of mucosal healing (MH), transmural healing (TH) and calprotectin in paediatric Crohn’s disease: A report from the ImageKids study. Clin. Gastroenterol. Hepatol. 2018, 16, 1089–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigall-Boneh, R.; Pfeffer-Gik, T.; Segal, I.; Zangen, T.; Boaz, M.; Levine, A. Partial enteral nutrition with a Crohn’s Disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Sigall Boneh, R.; Sarbagili Shabat, C.; Yanai, H.; Chermesh, I.; Avraham, S.B.; Mona Boaz, M.; Levine, A. Dietary therapy with the Crohn’s Disease exclusion diet is a successful strategy for induction of remission in children and adults failing biological therapy. J. Crohns Colitis 2017, 11, 1205–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, A.; El-Matary, W.; Van Limbergen, J. A Case-Based Approach to New Directions in Dietary Therapy of Crohn’s Disease: Food for Thought. Nutrients 2020, 12, 880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://modulifexpert.com (accessed on 6 July 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).