The Association of Post-Concussion and Post-Traumatic Stress Disorder Symptoms with Health-Related Quality of Life, Health Care Use and Return-to-Work after Mild Traumatic Brain Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Measures
2.2.1. Sociodemographic Data
2.2.2. Medical History
2.2.3. Injury Characteristics
2.2.4. Functional Outcome at Six Months Post-TBI
2.2.5. Post-Concussion Symptoms at Six Months Post-TBI
2.2.6. Post-Traumatic Stress Symptoms at Six Months Post-TBI
2.2.7. Health Care Utilization
2.2.8. Return to Work at Six Months Post-TBI
2.2.9. Health Related Quality of Life at Six Months Post-TBI
2.3. Ethical Approval
2.4. Statistical Analysis
3. Results
3.1. Patient Characterstics
3.2. Prevalence of Post-Concussion and Post-Traumatic Stress Disorder Symptoms
3.3. Differences in HRQoL, Health Care Utilization, and Return to Work among the Severe PC and PTSD Groups
4. Discussion
4.1. Strengths and Limitations
4.2. Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
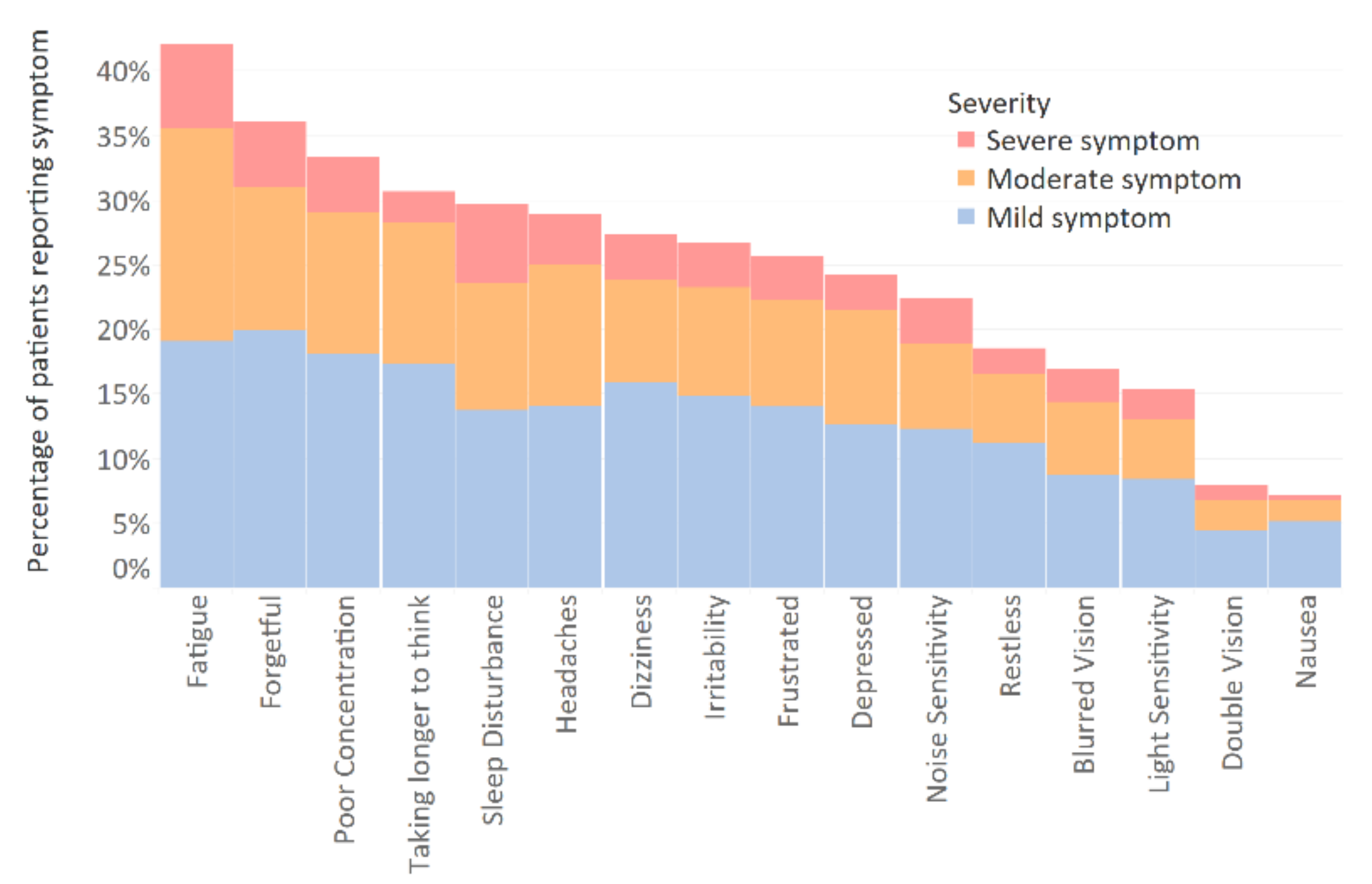
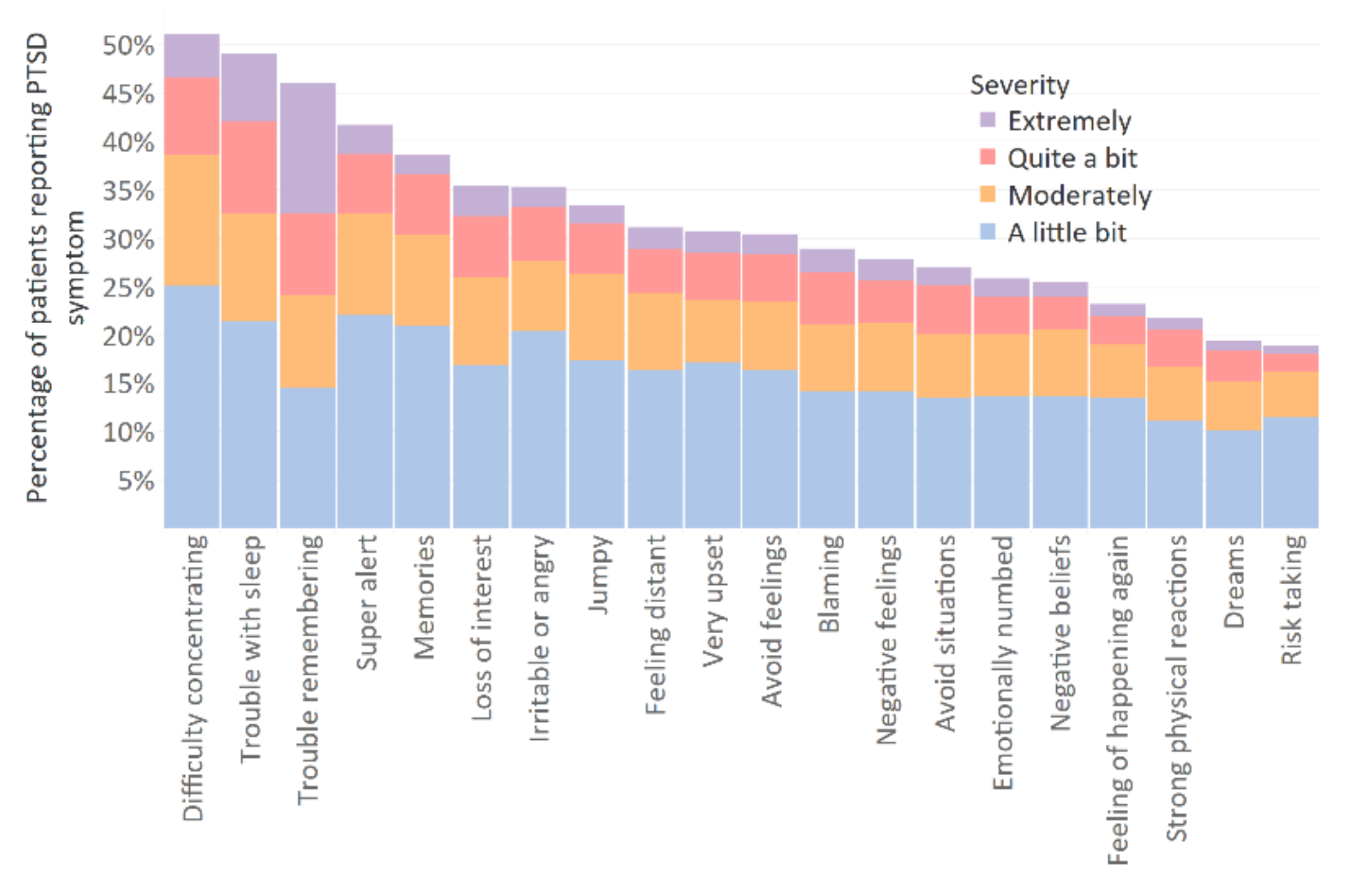

Appendix B
| SF-12 PCS | SF-12 MCS | QOLIBRI-OS Total Score | ||||
|---|---|---|---|---|---|---|
| B (95%CI) | p-Value | B (95%CI) | p-Value | B (95%CI) | p-Value | |
| Unadjusted | ||||||
| No/mild symptoms (ref PC symptoms) | 8.77 (7.49; 10.05) | <0.001 | 10.77 (9.59; 11.96) | <0.001 | 22.28 (20.01; 24.54) | <0.001 |
| Severe PC and PTSD symptoms (ref PC symptoms) | −0.94 (−3.03; 1.14) | 0.374 | −9.64 (−11.56; −7.71) | <0.001 | −11.13 (−14.83; −7.42) | <0.001 |
| PTSD symptoms (ref PC symptoms) | 7.12 (3.38; 10.86) | <0.001 | −4.37 (−7.83; −0.91) | 0.013 | −2.33 (−8.90; 4.24) | 0.487 |
| Adjusted 1 | ||||||
| No/mild symptoms (ref PC symptoms) | 7.24 (6.04; 8.43) | 0.005 | 10.03 (8.83; 11.23) | <0.001 | 20.58 (18.31; 22.84) | <0.001 |
| Severe PC and PTSD symptoms (ref PC symptoms) | −2.73 (−4.64; −0.83) | 0.001 | −9.37 (−11.28; −7.46) | <0.001 | −11.87 (−15.49; −8.24) | <0.001 |
| PTSD symptoms (ref PC symptoms) | 5.99 (2.57; 9.41) | <0.001 | −3.66 (−7.10; −0.22) | 0.037 | −1.60 (−8.02; −4.82) | 0.625 |
| Hospital Ward Admission | ICU Admission | Inpatient Rehabilitation | Ongoing Outpatient Rehabilitation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | CI (95%) | p-Value | OR | CI (95%) | p-Value | OR | CI (95%) | p-Value | OR | CI (95%) | p-Value | |
| Unadjusted | ||||||||||||
| No/mild symptoms (ref PC symptoms) | 0.70 | 0.52–0.94 | 0.019 | 0.44 | 0.33–0.58 | <0.001 | 0.58 | 0.41–0.84 | 0.003 | 0.31 | 0.21–0.45 | <0.001 |
| Severe PC and PTSD symptoms (ref PC symptoms) | 0.71 | 0.45–1.12 | 0.141 | 0.52 | 0.32–0.84 | 0.007 | 0.76 | −0.42–1.37 | 0.358 | 1.07 | 0.63–1.84 | 0.799 |
| PTSD symptoms (ref PC symptoms) | 1.29 | 0.51–3.30 | 0.595 | 0.62 | 0.27–1.46 | 0.275 | 0.99 | 0.36–2.72 | 0.985 | 0.33 | 0.08–1.42 | 0.136 |
| Adjusted 1 | ||||||||||||
| No/mild symptoms (ref PC symptoms) | 1.00 | 0.70–1.41 | 0.986 | 0.54 | 0.36–0.81 | 0.003 | 0.95 | 0.62–1.45 | 0.818 | 0.39 | 0.26–0.60 | <0.001 |
| Severe PC and PTSD symptoms (ref PC symptoms) | 0.95 | 0.55–1.63 | 0.843 | 0.42 | 0.20–0.84 | 0.015 | 1.25 | 0.62–2.52 | 0.538 | 1.39 | 0.77–2.49 | 0.272 |
| PTSD symptoms (ref PC symptoms) | 2.04 | 0.71–5.80 | 0.184 | 0.60 | 0.17–2.12 | 0.428 | 1.97 | 0.61–6.41 | 0.259 | 0.37 | 0.07–1.88 | 0.231 |
| No/Mild Symptoms | Severe PC Symptoms, No PTSD | PTSD, No Severe PC Symptoms | Severe PC and PTSD Symptoms | |||||
|---|---|---|---|---|---|---|---|---|
| Rehabilitation at 6 Months | No Rehab Care | Ongoing | No Rehab Care | Ongoing | No Rehab Care | Ongoing | No Rehab Care | Ongoing |
| SF-12 PCS | 49.3 (9.1) | 43.3 (10.6) | 40.6 (11.4) | 37.8 (9.8) | 47.9 (10.5) | 39.2 (11.4) | 39.9 (10.5) | 36.0 (11.4) |
| SF-12 MCS | 52.2 (8.7) | 50.1 (8.5) | 41.6 (10.4) | 40.4 (8.9) | 36.5 (8.6) | 42.8 (14.7) | 31.3 (9.6) | 32.7 (9.9) |
| QoLIBRI-OS | 77.4 (16.6) | 71.8 (15.1) | 55.4 (18.9) | 50.9 (17.0) | 52.7 (21.4) | 50.0 (29.7) | 41.3 (20.5) | 45.0 (24.9) |
| No/Mild Symptoms | Severe PC Symptoms, No PTSD | PTSD, No Severe PC Symptoms | Severe PC and PTSD Symptoms | |||||
|---|---|---|---|---|---|---|---|---|
| N | 540 (47.8%) | 138 (48.6%) | 16 (55.2%) | 66 (53.2%) | ||||
| Ongoing rehabilitation at 6 months | No | Yes | No | Yes | No | Yes | No | Yes |
| ISS, mean (SD) | 12.2 | 20.3 | 15.0 | 27.1 | 13.2 | 35.0 | 13.5 | 18.2 |
| (9.6) | (12.0) | (11.5) | (17.1) | (10.8) | (33.9) | (12.4) | (13.5) | |
| Unadjusted | Adjusted 1 | |||||
|---|---|---|---|---|---|---|
| OR | CI (95%) | p-Value | OR | CI (95%) | p-Value | |
| No/mild symptoms (ref PC symptoms) | 0.17 | 0.11–0.26 | <0.001 | 0.21 | 0.14–0.34 | <0.001 |
| Severe PC and PTSD symptoms (ref PC symptoms) | 0.51 | 0.18–1.50 | 0.223 | 0.49 | 0.15–1.63 | 0.246 |
| PTSD symptoms (ref PC symptoms) | 0.60 | 0.32–1.10 | 0.099 | 0.82 | 0.42–1.63 | 0.575 |
| No/Mild Symptoms | Severe PC Symptoms, no PTSD | PTSD, No Severe PC Symptoms | Severe PC and PTSD Symptoms | |||||
|---|---|---|---|---|---|---|---|---|
| 540 (47.8%) | 138 (48.6%) | 16 (55.2%) | >66 (53.2%) | |||||
| Return to work | Full RTW | No RTW | Full RTW | No RTW | Full RTW | No RTW | Full RTW | No RTW |
| SF-12 PCS | 52.8 (6.3) | 43.1 (11.0) | 48.2 (7.9) | 38.6 (9.2) | 54.6 (4.3) | 38.9 (8.4) | 43.3 (10.8) | 36.3 (11.4) |
| SF-12 MCS | 52.7 (7.7) | 51.0 (10.0) | 41.0 (10.4) | 41.7 (9.5) | 37.2 (36.6) | 34.9 (7.2) | 31.9 (9.7) | 32.3 (9.1) |
| QoLIBRI-OS | 82.1 (14.3) | 70.5 (16.4) | 63.4 (17.5) | 50.7 (18.2) | 58.3 (20.3) | 46.5 (20.8) | 46.9 (16.4) | 45.5 (25.0) |
| No Severe PC/PTSD Symptoms | Severe PC Symptoms, No PTSD | PTSD, No Severe PC Symptoms | Severe PC and PTSD Symptoms | |||||
|---|---|---|---|---|---|---|---|---|
| N | 540 (47.8%) | 138 (48.6%) | 16 (55.2%) | 66 (53.2%) | ||||
| Return to work | Full RTW | No RTW | Full RTW | No RTW | Full RTW | No RTW | Full RTW | No RTW |
| ISS, mean (SD) | 11.3 | 22.1 | 13.8 | 23.7 | 19.5 | 14.5 | 11.3 | 15.7 |
| (8.8) | (15.0) | (9.4) | (17.0) | (19.0) | (8.7) | (10.0) | (13.6) | |
References
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef]
- Menon, D.K.; Schwab, K.; Wright, D.; Maas, A.I. Position Statement: Definition of Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2010, 91, 1637–1640. [Google Scholar] [CrossRef] [PubMed]
- Van der Naalt, J.; Timmerman, M.E.; de Koning, M.E.; van der Horn, H.J.; Scheenen, M.E.; Jacobs, B.; Hageman, G.; Yilmaz, T.; Roks, G.; Spikman, J.M. Early predictors of outcome after mild traumatic brain injury (UPFRONT): An observational cohort study. Lancet Neurol. 2017, 16, 532–540. [Google Scholar] [CrossRef]
- Konrad, C.; Geburek, A.J.; Rist, F.; Blumenroth, H.; Fischer, B.; Husstedt, I.; Arolt, V.; Schiffbauer, H.; Lohmann, H. Long-term cognitive and emotional consequences of mild traumatic brain injury. Psychol. Med. 2010, 41, 1197–1211. [Google Scholar] [CrossRef]
- Smith-Seemiller, L.; Fow, N.R.; Kant, R.; Franzen, M.D. Presence of post-concussion syndrome symptoms in patients with chronic pain vs. mild traumatic brain injury. Brain Inj. 2003, 17, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.A.; Harvey, A.G. Postconcussive Symptoms and Posttraumatic Stress Disorder after Mild Traumatic Brain Injury. J. Nerv. Ment. Dis. 1999, 187, 302–305. [Google Scholar] [CrossRef]
- Karr, J.E.; Iverson, G.L.; Berghem, K.; Kotilainen, A.-K.; Terry, D.P.; Luoto, T.M. Complicated mild traumatic brain injury in older adults: Post-concussion symptoms and functional outcome at one week post injury. Brain Inj. 2020, 34, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Voormolen, D.C.; Haagsma, J.A.; Polinder, S.; Maas, A.I.; Steyerberg, E.W.; Vuleković, P.; Sewalt, C.A.; Gravesteijn, B.Y.; Covic, A.; Andelic, N.; et al. Post-Concussion Symptoms in Complicated vs. Uncomplicated Mild Traumatic Brain Injury Patients at Three and Six Months Post-Injury: Results from the CENTER-TBI Study. J. Clin. Med. 2019, 8, 1921. [Google Scholar] [CrossRef]
- McMillan, T.M.; Williams, W.H.; Bryant, R. Post-traumatic stress disorder and traumatic brain injury: A review of causal mechanisms, assessment, and treatment. Neuropsychol. Rehabil. 2003, 13, 149–164. [Google Scholar] [CrossRef]
- Van Praag, D.L.; Cnossen, M.C.; Polinder, S.; Wilson, L.; Maas, A.I. Post-Traumatic Stress Disorder after Civilian Traumatic Brain Injury: A Systematic Review and Meta-Analysis of Prevalence Rates. J. Neurotrauma 2019, 36, 3220–3232. [Google Scholar] [CrossRef]
- Asmundson, G.J.; Frombach, I.; McQuaid, J.; Pedrelli, P.; Lenox, R.; Stein, M.B. Dimensionality of posttraumatic stress symptoms: A confirmatory factor analysis of DSM-IV symptom clusters and other symptom models. Behav. Res. Ther. 2000, 38, 203–214. [Google Scholar] [CrossRef]
- Friedman, M.J.; Resick, P.A.; Bryant, R.A.; Brewin, C.R. Considering PTSD for DSM. Depress. Anxiety 2011, 28, 750–769. [Google Scholar] [CrossRef]
- Lagarde, E.; Salmi, L.-R.; Holm, L.W.; Contrand, B.; Masson, F.; Ribéreau-Gayon, R.; Laborey, M.; Cassidy, J.D. Association of Symptoms Following Mild Traumatic Brain Injury with Posttraumatic Stress Disorder vs. Postconcussion Syndrome. JAMA Psychiatry 2014, 71, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Polinder, S.; Cnossen, M.C.; Real, R.G.L.; Covic, A.; Gorbunova, A.; Voormolen, D.C.; Master, C.; Haagsma, J.A.; Diaz-Arrastia, R.; Von Steinbuechel, N. A Multidimensional Approach to Post-concussion Symptoms in Mild Traumatic Brain Injury. Front. Neurol. 2018, 9, 1113. [Google Scholar] [CrossRef]
- Bryant, R.A. Posttraumatic stress disorder and traumatic brain injury: Can they co-exist? Clin. Psych. Rev. 2001, 21, 931–948. [Google Scholar] [CrossRef]
- Haagsma, J.A.; Scholten, A.C.; Andriessen, T.M.; Vos, P.E.; Van Beeck, E.F.; Polinder, S. Impact of Depression and Post-Traumatic Stress Disorder on Functional Outcome and Health-Related Quality of Life of Patients with Mild Traumatic Brain Injury. J. Neurotrauma 2015, 32, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Voormolen, D.C.; Polinder, S.; von Steinbuechel, N.; Vos, P.E.; Cnossen, M.C.; Haagsma, J.A. The association between post-concussion symptoms and health-related quality of life in patients with mild traumatic brain injury. Injury 2019, 50, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Stulemeijer, M.; Van Der Werf, S.; Borm, G.F.; Vos, P.E. Early prediction of favourable recovery 6 months after mild traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 2008, 79, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Emanuelson, I.; Holmkvist, E.A.; Björklund, R.; Stålhammar, D. Quality of life and post-concussion symptoms in adults after mild traumatic brain injury: A population-based study in western Sweden. Acta Neurol. Scand. 2003, 108, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Scholten, A.; Haagsma, J.; Andriessen, T.; Vos, P.; Steyerberg, E.; van Beeck, E.; Polinder, S. Health-related quality of life after mild, moderate and severe traumatic brain injury: Patterns and predictors of suboptimal functioning during the first year after injury. Injury 2015, 46, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Stålnacke, B.-M. Community integration, social support and life satisfaction in relation to symptoms 3 years after mild traumatic brain injury. Brain Inj. 2007, 21, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.I.; Menon, D.K.; Steyerberg, E.W.; Citerio, G.; Lecky, F.; Manley, G.T.; Hill, S.; Legrand, V.; Sorgner, A. Collaborative European NeuroTrauma effectiveness research in traumatic brain injury (CENTER-TBI) a prospective longitudinal observational study. Neurosurgery 2015, 76, 67–80. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Wiegers, E.; Sewalt, C.; Buki, A.; Citerio, G.; De Keyser, V.; Ercole, A.; Kunzmann, K.; Lanyon, L.; Lecky, F.; et al. Case-mix, care pathways, and outcomes in patients with traumatic brain injury in CENTER-TBI: A European prospective, multicentre, longitudinal, cohort study. Lancet Neurol. 2019, 18, 923–934. [Google Scholar] [CrossRef]
- King, N.S.; Crawford, S.; Wenden, F.J.; Moss, N.E.G.; Wade, D.T. The Rivermead Post Concussion Symptoms Questionnaire: A measure of symptoms commonly experienced after head injury and its reliability. J. Neurol. 1995, 242, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Blevins, C.A.; Weathers, F.W.; Davis, M.T.; Witte, T.K.; Domino, J.L. The PTSD Checklist for DSM-5 (PCL-5). Available online: www.ptsd.va.gov (accessed on 1 March 2021).
- Gennarelli, T.A.; Wodzin, E. Abbreviated Injury Scale 2005: Update 2008; Russ Reeder: Redwood City, CA, USA, 2008. [Google Scholar]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness: A practical scale. Lancet 1974, 304, 81–84. [Google Scholar] [CrossRef]
- Thompson, C.; Davies, P.; Herrmann, L.; Summers, M.; Potter, S. Approaches to establishing validated cut-off scores on the Rivermead post-concussion symptoms questionnaire (RPQ). In Brain Injury; Taylor & Francis, Inc.: Philadelphia, PA, USA, 2016. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Bovin, M.J.; Marx, B.P.; Weathers, F.W.; Gallagher, M.W.; Rodriguez, P.; Schnurr, P.P.; Keane, T. Psychometric properties of the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders—Fifth Edition (PCL-5) in veterans. Psychol. Assess. 2016, 28, 1379–1391. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.; Marsden-Loftus, I.; Koskinen, S.; Bakx, W.; Bullinger, M.; Formisano, R.; Maas, A.; Neugebauer, E.; Powell, J.; Sarajuuri, J.; et al. Interpreting quality of life after brain injury scores: Cross-walk with the short form. J. Neurotrauma 2017, 34, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Von Steinbuechel, N.; Wilson, L.; Gibbons, H.; Muehlan, H.; Schmidt, H.; Schmidt, S.; Sasse, N.; Koskinen, S.; Sarajuuri, J.; Hoefer, S.; et al. QOLIBRI Overall Scale: A brief index of health-related quality of life after traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 2012, 83, 1041–1047. [Google Scholar] [CrossRef]
- Cohen, J. Set Correlation and Contingency Tables. Appl. Psychol. Meas. 1988, 12, 425–434. [Google Scholar] [CrossRef]
- Polinder, S.; Haagsma, J.A.; Van Klaveren, D.; Steyerberg, E.W.; Van Beeck, E.F. Health-related quality of life after TBI: A systematic review of study design, instruments, measurement properties, and outcome. Popul. Heal. Metr. 2015, 13, 1–12. [Google Scholar] [CrossRef]
- Mols, F.; Pelle, A.J.; Kupper, N. Normative data of the SF-12 health survey with validation using postmyocardial infarction patients in the Dutch population. Qual. Life Res. 2009, 18, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.T.; Munoz, F. References Values and Validation of the Spanish Version of the SF-12. Barranquilla, Colombia, 2012. J. Psychol. Psychother. 2012, 5, 2161–2487. [Google Scholar] [CrossRef]
- Arango-Lasprilla, J.C.; Zeldovich, M.; Olabarrieta-Landa, L.; Forslund, M.V.; Núñez-Fernández, S.; Von Steinbuechel, N.; Howe, E.I.; Røe, C.; Andelic, N.; CENTER-TBI Participants and Investigators. Early Predictors of Employment Status One Year Post Injury in Individuals with Traumatic Brain Injury in Europe. J. Clin. Med. 2020, 9, 2007. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, A.; Fountain, R.; Alfred, D.; Combs, D. Quality of Life and Adaptation for Traumatic Brain Injury Survivors: Assessment of the Disability Centrality Model. J. Rehabil. 2015, 81, 3. [Google Scholar]
- Mahar, C.; Fraser, K. Barriers to Successful Community Reintegration Following Acquired Brain Injury (ABI). Int. J. Disabil. Manag. 2011, 6, 49–67. [Google Scholar] [CrossRef]
- Silverberg, N.D.; Iverson, G.L. Etiology of the post-concussion syndrome: Physiogenesis and psychogenesis revisited. NeuroRehabilitation 2011, 29, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Snell, D.L.; Macleod, A.D.S.; Anderson, T. Post-Concussion Syndrome after a Mild Traumatic Brain Injury: A Minefield for Clinical Practice. J. Behav. Brain Sci. 2016, 6, 227–232. [Google Scholar] [CrossRef]
- Carroll, L.J.; Cassidy, J.D.; Cancelliere, C.; Côté, P.; Hincapié, C.A.; Kristman, V.L.; Holm, L.W.; Borg, J.; Boussard, C.N.-D.; Hartvigsen, J. Systematic Review of the Prognosis After Mild Traumatic Brain Injury in Adults: Cognitive, Psychiatric, and Mortality Outcomes: Results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch. Phys. Med. Rehabil. 2014, 95, S152–S173. [Google Scholar] [CrossRef]
- Silver, J.M. Effort, exaggeration and malingering after concussion: Figure. J. Neurol. Neurosurg. Psychiatry 2012, 83, 836–841. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Praag, D.L.G.; Fardzadeh, H.E.; Covic, A.; Maas, A.I.R.; Von Steinbüchel, N. Preliminary validation of the Dutch version of the Posttraumatic stress disorder checklist for DSM-5 (PCL-5) after traumatic brain injury in a civilian population. PLoS ONE 2020, 15, e0231857. [Google Scholar] [CrossRef]
- Ashbaugh, A.R.; Houle-Johnson, S.; Herbert, C.; El-Hage, W.; Brunet, A. Psychometric Validation of the English and French Versions of the Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5). PLoS ONE 2016, 11, e0161645. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, H.C.; Morgan, G.A.; Leech, N.L.; Gliner, J.A.; Vaske, J.J.; Harmon, R.J. Measures of Clinical Significance. J. Am. Acad. Child Adolesc. Psychiatry 2003, 42, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cohen, P.; Chen, S. How Big Is a Big Odds Ratio? Interpreting the Magnitudes of Odds Ratios in Epidemiological Studies. Commun. Stat. Simul. Comput. 2010, 39, 860–864. [Google Scholar] [CrossRef]
- Bell, K.R.; Hoffman, J.M.; Temkin, N.R.; Powell, J.M.; Fraser, R.T.; Esselman, P.C.; Barber, J.K.; Dikmen, S. The effect of telephone counselling on reducing post-traumatic symptoms after mild traumatic brain injury: A randomised trial. J. Neurol. Neurosurg. Psychiatry 2008, 79, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Al Sayegh, A.; Sandford, D.; Carson, A.J. Psychological approaches to treatment of postconcussion syndrome: A systematic review. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
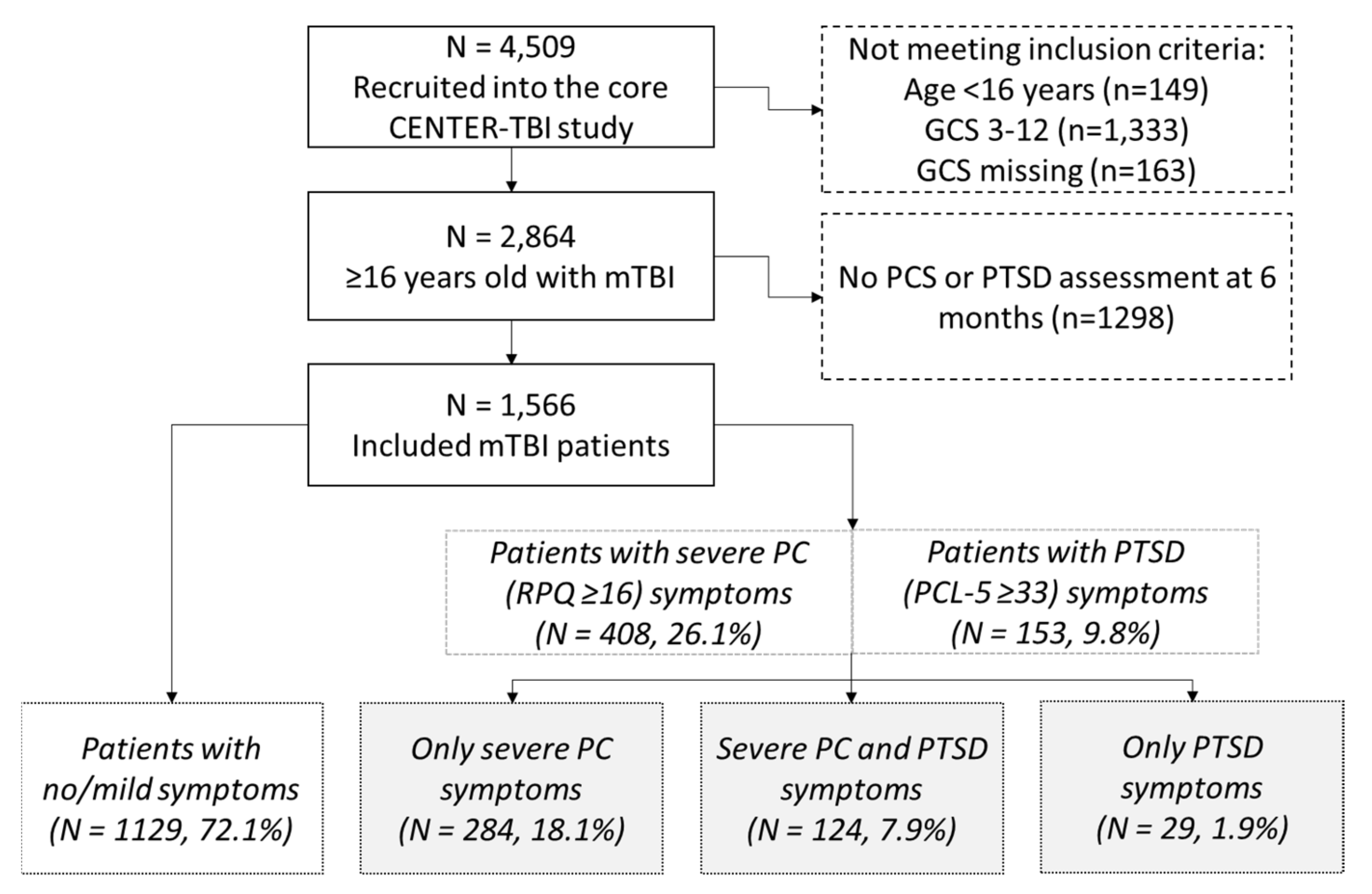
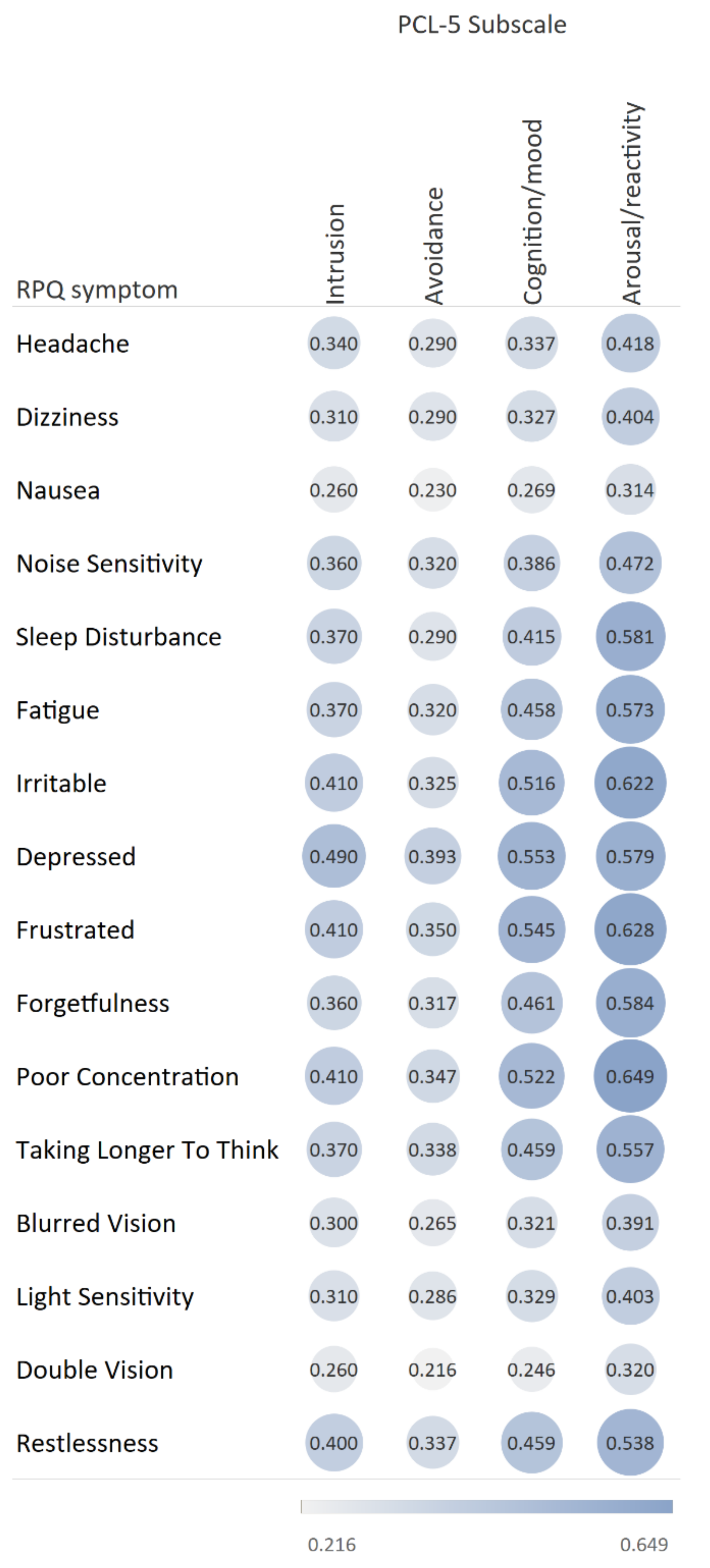
| Variable | Total Population | No/Mild Symptoms 1 | Severe PC Symptoms, no PTSD 2 | Severe PC and PTSD Symptoms 3 | PTSD, no Severe PC Symptoms 4 | p-Value |
|---|---|---|---|---|---|---|
| N | 1566 | 1129 | 284 | 124 | 29 | |
| Age, median (IQR) | 53.0 (35.0–66.0) | 53.0 (34.0–67.0) | 56.0 (41.5–66.8) | 49.0 (32.0–59.8) | 47.0 (30.5–59.5) | 0.001 |
| Sex, male | 993 (63.4%) | 752 (66.6%) | 145 (51.1%) | 77 (62.1%) | 19 (65.5%) | <0.001 |
| Highest level of education | 0.017 | |||||
| Primary | 202 (12.9%) | 138 (12.2%) | 39 (13.7%) | 15 (12.1%) | 10 (34.5%) | |
| Secondary | 452 (28.9%) | 324 (28.7%) | 81 (28.5%) | 41 (33.1%) | 6 (20.7%) | |
| Post-high school training | 291 (18.6%) | 199 (17.6%) | 60 (21.1%) | 28 (22.6%) | 4 (13.8%) | |
| College/University | 473 (30.2%) | 363 (32.2%) | 73 (25.7%) | 30 (24.2%) | 7 (24.1%) | |
| NA | 148 (9.5%) | 105 (9.3%) | 31 (10.9%) | 10 (8.1%) | 2 (6.9%) | |
| Baseline employment | <0.001 | |||||
| Full-time employed | 626 (40.0%) | 460 (40.7%) | 108 (38.0%) | 51 (41.1%) | 7 (24.1%) | |
| Part-time employed | 195 (12.5%) | 124 (11.0%) | 42 (14.8%) | 20 (16.1%) | 9 (31.0%) | |
| Unemployed | 91 (5.8%) | 51 (4.5%) | 21 (7.4%) | 16 (12.9%) | 3 (10.3%) | |
| Student | 139 (8.9%) | 108 (9.6%) | 19 (6.7%) | 9 (7.3%) | 3 (10.3%) | |
| Homemaker | 22 (1.4%) | 8 (0.7%) | 10 (3.5%) | 2 (1.6%) | 2 (6.9%) | |
| Retired | 406 (25.9%) | 315 (27.9%) | 70 (24.6%) | 17 (13.7%) | 4 (13.8%) | |
| NA | 87 (5.6%) | 63 (5.6%) | 14 (4.9%) | 9 (7.3%) | 1 (3.4%) | |
| Care pathway | <0.001 | |||||
| ER | 555 (28.4%) | 346 (30.6%) | 58 (20.4%) | 33 (26.6%) | 7 (24.1%) | |
| Hospital ward | 752 (48.0%) | 556 (49.2%) | 121 (42.6%) | 61 (49.2%) | 14 (48.3%) | |
| ICU | 370 (23.6%) | 227 (20.1%) | 105 (37.0%) | 30 (24.2%) | 8 (27.6%) | |
| Pre-injury psychiatric condition | <0.001 | |||||
| Yes | 190 (12.1%) | 100 (8.9%) | 49 (17.3%) | 32 (25.8%) | 9 (31.0%) | |
| No | 1369 (87.4%) | 1025 (90.8%) | 233 (82.0%) | 91 (73.4%) | 20 (69.0%) | |
| NA | 7 (0.4%) | 4 (0.4%) | 2 (0.7%) | 1 (0.8%) | 0 (0.0%) | |
| ISS, median (IQR) | 10.0 (5.0–18.0) | 9.0 (5.0–17.0) | 13.0 (9.0–25.0) | 9.0 (4.0–18.0) | 11.0 (7.0–17.5) | <0.001 |
| 6-month outcomes | ||||||
| Full recovery (GOSE = 8) | 745 (47.6%) | 679 (60.1%) | 38 (13.4%) | 20 (16.1%) | 8 (27.6%) | <0.001 |
| RPQ total score, median (IQR) | 6.0 (0.0–16.0) | 2.0 (0.0–7.0) | 25.0 (19.0–29.8) | 34.0 (24.0–42.0) | 8.0 (2.5–13.0) | <0.001 |
| PCL-5 total score, median (IQR) | 7.0 (2.0–16.0) | 4.0 (1.0–9.0) | 16.3 (10.0–23.0) | 45.5 (37.0–53.0) | 42.5 (35.0–47.5) | <0.001 |
| HRQoL Measure | No/Mild Symptoms 1 | Severe PC Symptoms, No PTSD 2 | Severe PC and PTSD Symptoms 3 | PTSD, No Severe PC Symptoms 4 |
|---|---|---|---|---|
| Participants | n = 1129 | n = 284 | n = 124 | n = 29 |
| SF-12 PCS | 48.9 * (9.3) | 40.2 (11.1) | 39.2 * (10.7) | 47.3 * (10.6) |
| SF-12 MCS | 52.0 * (8.7) | 41.3 (10.2) | 31.6 * (9.6) | 36.9 * (8.9) |
| QoLIBRI-OS | 77.1 * (16.6) | 54.5 (18.6) | 42.3 * (21.5) | 52.6 (21.3) |
| Health Care Utilization | No/Mild Symptoms 1 | Severe PC Symptoms, No PTSD 2 | Severe PC and PTSD Symptoms 3 | PTSD, No Severe PC Symptoms 4 |
|---|---|---|---|---|
| Participants | n = 1129 | n = 284 | n = 124 | n = 29 |
| In-hospital | ||||
| Admission at hospital ward | 67.6% | 74.8% | 67.7% | 79.3% |
| Hospital length of stay in days 5 | 5.7 (10.6) | 6.8 (9.1) | 8.6 (11.5) | 7.3 (8.6) |
| Admission at ICU | 21.0% * | 37.9% | 24.2% * | 27.6% |
| ICU length of stay in days 5 | 5.0 (7.4) | 4.9 (7.6) | 8.7 (10.9) | 5.6 (6.4) |
| Inpatient rehabilitation | 10.9% | 17.4% | 13.7% | 17.2% |
| Outpatient rehabilitation | 15.7% * | 31.2% | 30.9% | 24.1% |
| Ongoing after six months 6 | 6.5% | 18.4% | 19.5% | 6.9% |
| Return to Work | Total Population | No/Mild Symptoms 1 | Severe PC Symptoms, No PTSD 2 | Severe PC and PTSD Symptoms 3 | PTSD, No Severe PC Symptoms 4 |
|---|---|---|---|---|---|
| Participants | n = 1566 | n = 1129 | n = 284 | n = 124 | n = 29 |
| Pre-TBI employed participants 5 | n = 760 (48.5%) | n = 540 (47.8%) | n = 138 (48.6%) | n = 66 (53.2%) | n = 16 (55.2%) |
| Return to work at full level | 74.3% | 83.2% * | 46.1% | 59.0% | 62.5% |
| Return to work at reduced level/hours | 9.5% | 8.0% | 17.2% | 8.2% | 0.0% |
| No return to work | 16.2% | 8.8% | 36.7% | 32.8% | 37.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Vlegel, M.; Polinder, S.; Mikolic, A.; Kaplan, R.; von Steinbuechel, N.; Plass, A.M.; Zeldovich, M.; van Praag, D.; Bockhop, F.; Cunitz, K.; et al. The Association of Post-Concussion and Post-Traumatic Stress Disorder Symptoms with Health-Related Quality of Life, Health Care Use and Return-to-Work after Mild Traumatic Brain Injury. J. Clin. Med. 2021, 10, 2473. https://doi.org/10.3390/jcm10112473
van der Vlegel M, Polinder S, Mikolic A, Kaplan R, von Steinbuechel N, Plass AM, Zeldovich M, van Praag D, Bockhop F, Cunitz K, et al. The Association of Post-Concussion and Post-Traumatic Stress Disorder Symptoms with Health-Related Quality of Life, Health Care Use and Return-to-Work after Mild Traumatic Brain Injury. Journal of Clinical Medicine. 2021; 10(11):2473. https://doi.org/10.3390/jcm10112473
Chicago/Turabian Stylevan der Vlegel, Marjolein, Suzanne Polinder, Ana Mikolic, Rana Kaplan, Nicole von Steinbuechel, Anne Marie Plass, Marina Zeldovich, Dominique van Praag, Fabian Bockhop, Katrin Cunitz, and et al. 2021. "The Association of Post-Concussion and Post-Traumatic Stress Disorder Symptoms with Health-Related Quality of Life, Health Care Use and Return-to-Work after Mild Traumatic Brain Injury" Journal of Clinical Medicine 10, no. 11: 2473. https://doi.org/10.3390/jcm10112473
APA Stylevan der Vlegel, M., Polinder, S., Mikolic, A., Kaplan, R., von Steinbuechel, N., Plass, A. M., Zeldovich, M., van Praag, D., Bockhop, F., Cunitz, K., Mueller, I., Haagsma, J. A., & The CENTER-TBI Participants and Investigators. (2021). The Association of Post-Concussion and Post-Traumatic Stress Disorder Symptoms with Health-Related Quality of Life, Health Care Use and Return-to-Work after Mild Traumatic Brain Injury. Journal of Clinical Medicine, 10(11), 2473. https://doi.org/10.3390/jcm10112473








