The Role of Premorbid IQ and Age of Onset as Useful Predictors of Clinical, Functional Outcomes, and Recovery of Individuals with a First Episode of Psychosis
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Procedures
2.2.1. Definition of Premorbid IQ and Age of Onset Subgroups
2.2.2. Clinical Assessment
2.3. Statistical Analyses
3. Results
3.1. Sociodemographic and Clinical Characteristics
3.2. Pairwise Comparison of Clinical Symptoms, Functioning, Symptom Remission, and Clinical Recovery Rates
3.3. Clinical and Functioning Changes over Time
3.4. Predictive Value of pIQ and Age of Onset at Baseline over General Functioning at Two-Year Follow-Up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Austin, S.F.; Mors, O.; Budtz-Jørgensen, E.; Secher, R.G.; Hjorthøj, C.R.; Bertelsen, M.; Jeppesen, P.; Petersen, L.; Thorup, A.; Nordentoft, M. Long-term trajectories of positive and negative symptoms in first episode psychosis: A 10 year follow-up study in the OPUS cohort. Schizophr. Res. 2015, 168, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Martinuzzi, E.; Barbosa, S.; Daoudlarian, D.; Ali, W.B.H.; Gilet, C.; Fillatre, L.; Khalfallah, O.; Troudet, R.; Jamain, S.; Fond, G.; et al. Correction: Stratification and prediction of remission in first-episode psychosis patients: The OPTiMiSE cohort study. Transl. Psychiatry 2019, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Carbon, M.; Correll, C.U. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin. Neurosci. 2014, 16, 505–524. [Google Scholar] [PubMed]
- Fusar-Poli, P.; McGorry, P.D.; Kane, J.M. Improving outcomes of first-episode psychosis: An overview. World Psychiatry 2017, 16, 251–265. [Google Scholar] [CrossRef]
- Millan, M.J.; Andrieux, A.; Bartzokis, G.; Cadenhead, K.; Dazzan, P.; Fusar-Poli, P.; Gallinat, J.; Giedd, J.; Grayson, D.R.; Heinrichs, M.; et al. Altering the course of schizophrenia: Progress and perspectives. Nat. Rev. Drug Discov. 2016, 15, 485–515. [Google Scholar] [CrossRef]
- van Os, J.; Kenis, G.; Rutten, B.P.F. The environment and schizophrenia. Nature 2010, 468, 203–212. [Google Scholar] [CrossRef]
- Velthorst, E.; Fett, A.J.; Reichenberg, A.; Perlman, G.; van Os, J.; Bromet, E.J.; Kotov, R. The 20-Year Longitudinal Trajectories of Social Functioning in Individuals With Psychotic Disorders. Am. J. Psychiatry 2017, 174, 1075–1085. [Google Scholar] [CrossRef]
- Cassidy, C.M.; Norman, R.; Manchanda, R.; Schmitz, N.; Malla, A. Testing Definitions of Symptom Remission in First-Episode Psychosis for Prediction of Functional Outcome at 2 Years. Schizophr. Bull. 2010, 36, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.; Jody, D.; Geisler, S.; Alvir, J.; Loebel, A.; Szymanski, S.; Woerner, M.; Borenstein, M. Time course and biologic correlates of treatment response in first-episode schizophrenia. Arch. Gen. Psychiatry 1993, 50, 369–376. [Google Scholar] [CrossRef]
- Tohen, M.; Strakowski, S.M.; Zarate, C.; Hennen, J.; Stoll, A.L.; Suppes, T.; Faedda, G.L.; Cohen, B.M.; Gebre-Medhin, P.; Baldessarini, R.J. The McLean–Harvard first-episode project: 6-month symptomatic and functional outcome in affective and nonaffective psychosis. Biol. Psychiatry 2000, 48, 467–476. [Google Scholar] [CrossRef]
- Menezes, N.M.; Arenovich, T.; Zipursky, R.B. A systematic review of longitudinal outcome studies of first-episode psychosis. Psychol. Med. 2006, 36, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- González-Blanch, C.; Perez-Iglesias, R.; Pardo-García, G.; Rodríguez-Sánchez, J.M.; Martínez-García, O.; Vázquez-Barquero, J.L.; Crespo-Facorro, B. Prognostic value of cognitive functioning for global functional recovery in first-episode schizophrenia. Psychol. Med. 2010, 40, 935–944. [Google Scholar] [CrossRef]
- Klærke, L.R.; Baandrup, L.; Fagerlund, B.; Ebdrup, B.H.; Pantelis, C.; Glenthøj, B.Y.; Nielsen, M. Ø Diagnostic stability and long-term symptomatic and functional outcomes in first-episode antipsychotic-naïve patients with schizophrenia. Eur. Psychiatry 2019, 62, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Subramaniam, M.; Abdin, E.; Poon, L.Y.; Chong, S.A. Symptomatic and functional remission in patients with first-episode psychosis. Acta Psychiatr. Scand. 2012, 126, 282–289. [Google Scholar] [CrossRef]
- Schrank, B.; Slade, M. Recovery in psychiatry. Psychiatr. Bull. 2007, 31, 321–325. [Google Scholar] [CrossRef]
- Lally, J.; Ajnakina, O.; Stubbs, B.; Cullinane, M.; Murphy, K.C.; Gaughran, F.; Murray, R.M. Remission and recovery from first-episode psychosis in adults: A systematic review and meta-analysis of long-term outcome studies. Eur. Psychiatry 2017, 41, S819. [Google Scholar] [CrossRef]
- DeLisi, L.E. The significance of age of onset for schizophrenia. Schizophr. Bull. 1992, 18, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.; Liu, Y. Effects of age of onset on clinical characteristics in schizophrenia spectrum disorders. BMC Psychiatry 2010, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- McClellan, J.; Stock, S. Practice parameter for the assessment and treatment of children and adolescents with schizophrenia. J. Am. Acad. Child Adolesc. Psychiatry 2013, 52, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.L.; Li, A.W.; Leung, C.; Chang, W.; Chan, S.K.; Lee, E.H.; Chen, E.Y. Comparing illness presentation, treatment and functioning between patients with adolescent- and adult-onset psychosis. Psychiatry Res. 2014, 220, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Veru, F.; Jordan, G.; Joober, R.; Malla, A.; Iyer, S. Adolescent vs. adult onset of a first episode psychosis: Impact on remission of positive and negative symptoms. Schizophr. Res. 2016, 174, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Zammit, S.; Allebeck, P.; David, A.S.; Dalman, C.; Hemmingsson, T.; Lundberg, I.; Lewis, G. A Longitudinal Study of Premorbid IQ Score and Risk of Developing Schizophrenia, Bipolar Disorder, Severe Depression, and Other Nonaffective Psychoses. Arch. Gen. Psychiatry 2004, 61, 354–360. [Google Scholar] [CrossRef]
- Khandaker, G.M.; Barnett, J.H.; White, I.R.; Jones, P.B. A quantitative meta-analysis of population-based studies of premorbid intelligence and schizophrenia. Schizophr. Res. 2011, 132, 220–227. [Google Scholar] [CrossRef]
- Leeson, V.C.; Sharma, P.; Harrison, M.; Ron, M.A.; Barnes, T.R.E.; Joyce, E.M. IQ Trajectory, Cognitive Reserve, and Clinical Outcome Following a First Episode of Psychosis: A 3-Year Longitudinal Study. Schizophr. Bull. 2011, 37, 768–777. [Google Scholar] [CrossRef]
- Wells, R.; Swaminathan, V.; Sundram, S.; Weinberg, D.; Bruggemann, J.; Jacomb, I.; Cropley, V.; Lenroot, R.; Pereira, A.M.; Zalesky, A.; et al. The impact of premorbid and current intellect in schizophrenia: Cognitive, symptom, and functional outcomes. NPJ Schizophr. 2015, 1, 15043. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Ho, N.F.; Sum, M.Y.; Collinson, S.L.; Sim, K. Impact of duration of untreated psychosis and premorbid intelligence on cognitive functioning in patients with first-episode schizophrenia. Schizophr. Res. 2016, 175, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Ballageer, T.; Malla, A.; Manchanda, R.; Takhar, J.; Haricharan, R. Is Adolescent-Onset First-Episode Psychosis Different from Adult Onset? J. Am. Acad. Child Adolesc. Psychiatry 2005, 44, 782–789. [Google Scholar] [CrossRef]
- Joyce, E.M.; Hutton, S.B.; Mutsatsa, S.H.; Barnes, T.R.E. Cognitive heterogeneity in first-episode schizophrenia. Br. J. Psychiatry 2005, 187, 516–522. [Google Scholar] [CrossRef]
- Schimmelmann, B.G.; Conus, P.; Cotton, S.; McGorry, P.D.; Lambert, M. Pre-treatment, baseline, and outcome differences between early-onset and adult-onset psychosis in an epidemiological cohort of 636 first-episode patients. Schizophr. Res. 2007, 95, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Puig, O.; Baeza, I.; De La Serna, E.; Cabrera, B.; Mezquida, G.; Bioque, M.; Lobo, A.; González-Pinto, A.; Parellada, M.; Corripio, I.; et al. Persistent Negative Symptoms in First-Episode Psychosis. J. Clin. Psychiatry 2017, 78, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Immonen, J.; Jääskeläinen, E.; Korpela, H.; Miettunen, J. Age at onset and the outcomes of schizophrenia: A systematic review and meta-analysis. Early Interv. Psychiatry 2017, 11, 453–460. [Google Scholar] [CrossRef]
- Johnstone, E.C.; Owens, D.G.; Bydder, G.M.; Colter, N.; Crow, T.J.; Frith, C.D. The spectrum of structural brain changes in schizophrenia: Age of onset as a predictor of cognitive and clinical impairments and their cerebral correlates. Psychol. Med. 1989, 19, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Rajji, T.K.; Ismail, Z.; Mulsant, B.H. Age at onset and cognition in schizophrenia: Meta-analysis. Br. J. Psychiatry 2009, 195, 286–293. [Google Scholar] [CrossRef]
- Weickert, T.W.; Goldberg, T.E.; Gold, J.M.; Bigelow, L.B.; Egan, M.F.; Weinberger, D.R. Cognitive Impairments in Patients with Schizophrenia Displaying Preserved and Compromised Intellect. Arch. Gen. Psychiatry 2000, 57, 907–913. [Google Scholar] [CrossRef]
- Aylward, E.; Walker, E.; Bettes, B. Intelligence in schizophrenia: Meta-analysis of the research. Schizophr. Bull. 1984, 10, 430–459. [Google Scholar] [CrossRef] [PubMed]
- Trotta, A.; Murray, R.M.; MacCabe, J.H. Do premorbid and post-onset cognitive functioning differ between schizophrenia and bipolar disorder? A systematic review and meta-analysis. Psychol. Med. 2015, 45, 381–394. [Google Scholar] [CrossRef]
- Woodberry, K.A.; Giuliano, A.J.; Seidman, L.J. Premorbid IQ in Schizophrenia: A Meta-Analytic Review. Am. J. Psychiatry 2008, 165, 579–587. [Google Scholar] [CrossRef]
- Baeza, I.; de la Serna, E.; Amoretti, S.; Cuesta, M.C.; Díaz-Caneja, C.M.; Mezquida, G.; Lobo, A.; González-Pinto, A.; Corripio, I.; Vieta, E.; et al. Premorbid characteristics as predictors of early onset vs. adult onset in patients with a first-episode of psychosis. J. Clin. Psychiatry. in press.
- Esterberg, M.L.; Trotman, H.D.; Holtzman, C.; Compton, M.T.; Walker, E.F. The impact of a family history of psychosis on age-at-onset and positive and negative symptoms of schizophrenia: A meta-analysis. Schizophr. Res. 2010, 120, 121–130. [Google Scholar] [CrossRef]
- Musket, C.W.; Kuo, S.S.; Rupert, P.E.; Almasy, L.; Gur, R.C.; Prasad, K.; Wood, J.; Roalf, D.R.; Gur, R.E.; Nimgaonkar, V.L.; et al. Why does age of onset predict clinical severity in schizophrenia? A multiplex extended pedigree study. Am. J. Med Genet. Part B Neuropsychiatr. Genet. 2020, 183, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Groom, M.J.; Jackson, G.M.; Calton, T.G.; Andrews, H.K.; Bates, A.T.; Liddle, P.F.; Hollis, C. Cognitive deficits in early-onset schizophrenia spectrum patients and their non-psychotic siblings: A comparison with ADHD. Schizophr. Res. 2007, 99, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.J.; Kim, N.; Park, T.; Oh, S.; Jeon, H.O.; Yoon, S.C.; Lee, Y.; Lee, W.K.; Ha, K.; Kim, J.; et al. Cognitive profiles of healthy siblings of schizophrenia patients: Application of the cognitive domains of the MATRICS consensus battery. World J. Biol. Psychiatry 2009, 10, 452–460. [Google Scholar] [CrossRef]
- Sheffield, J.M.; Karcher, N.R.; Barch, D.M. Cognitive Deficits in Psychotic Disorders: A Lifespan Perspective. Neuropsychol. Rev. 2018, 28, 509–533. [Google Scholar] [CrossRef]
- Castro-Fornieles, J.; Parellada, M.; Gonzalez-Pinto, A.; Moreno, D.; Graell, M.; Baeza, I.; Otero, S.; Soutullo, C.A.; Crespo-Facorro, B.; Ruiz-Sancho, A.; et al. The child and adolescent first-episode psychosis study (CAFEPS): Design and baseline results. Schizophr. Res. 2007, 91, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.; Bioque, M.; Parellada, M.; Saiz Ruiz, J.; Cuesta, M.J.; Llerena, A.; Sanjuán, J.; Castro-Fornieles, J.; Arango, C.; Cabrera, B. Assessing clinical and functional outcomes in a gene-environment interaction study in first episode of psychosis (PEPs). Rev. Psiquiatr. Salud Ment. 2013, 6, 4–16. [Google Scholar] [CrossRef]
- Bernardo, M.; Bioque, M.; Cabrera, B.; Lobo, A.; González-Pinto, A.; Pina, L.; Corripio, I.; Sanjuán, J.; Mané, A.; Castro-Fornieles, J.; et al. Modelling gene-environment interaction in first episodes of psychosis. Schizophr. Res. 2017, 189, 181–189. [Google Scholar] [CrossRef]
- Bernardo, M.; Cabrera, B.; Arango, C.; Bioque, M.; Castro-Fornieles, J.; Jesús Cuesta, M.; Lafuente, A.; Parellada, M.; Saiz-Ruiz, J.; Vieta, E. One decade of the first episodes project (PEPs): Advancing towards a precision psychiatry Una década del proyecto de primeros episodios (PEPs): Avanzando hacia una psiquiatría de precisión. Rev. Psiquiatr. Salud Ment. 2019, 12, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D.; Pando, A.C.; dela Cruz López, M.V. Escala de Inteligencia de Wechsler para Niños-Revisada; TEA: Madrid, Spain, 2001. [Google Scholar]
- Wechsler, D. (Ed.) Wechsler Intelligence Scale for Children-IV (WISCIV); Psychological Corporation: San Antonio, TX, USA, 2003. [Google Scholar]
- Wechsler, D. Wechsler Adult Intelligence Scale III; TEA Ediciones: Madrid, Spain, 1999. [Google Scholar]
- Ayesa-Arriola, R.; Setién-Suero, E.; Neergaard, K.D.; Belzunces, À.A.; Contreras, F.; van Haren, N.E.M.; Crespo-Facorro, B. Premorbid IQ subgroups in first episode non affective psychosis patients: Long-term sex differences in function and neurocognition. Schizophr. Res. 2018, 197, 370–377. [Google Scholar] [CrossRef]
- Eberhard, J.; Riley, F.; Levander, S. Premorbid IQ and schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2003, 253, 84–88. [Google Scholar] [CrossRef]
- de Oliveira, M.O.; Nitrini, R.; Yassuda, M.S.; Brucki, S.M.D. Vocabulary Is an Appropriate Measure of Premorbid Intelligence in a Sample with Heterogeneous Educational Level in Brazil. Behav. Neurol. 2014, 2014, 875960. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, A.S. Intelligent Testing with the WISC-III; John Wiley & Sons: New York, NY, USA, 1994. [Google Scholar]
- Ott, S.L.; Spinelli, S.; Rock, D.; Roberts, S.; Amminger, G.P.; Erlenmeyer-Kimling, L. The New York high-risk project: Social and general intelligence in children at risk for schizophrenia. Schizophr. Res. 1998, 31, 1–11. [Google Scholar] [CrossRef]
- Lyman Howard, B. Test Scores and what They Mean; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1971. [Google Scholar]
- Wieland, J.; Zitman, F.G. It is time to bring borderline intellectual functioning back into the main fold of classification systems. BJPsych Bull. 2016, 40, 204–206. [Google Scholar] [CrossRef]
- Ruiz, J.C.; Soler, M.J.; Fuentes, I.; Tomás, P. Intellectual functioning and memory deficits in schizophrenia. Compr. Psychiatry 2007, 48, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Fraguas, D.; del Rey-Mejías, Á.; Moreno, C.; Castro-Fornieles, J.; Graell, M.; Otero, S.; Gonzalez-Pinto, A.; Moreno, D.; Baeza, I.; Martínez-Cengotitabengoa, M.; et al. Duration of untreated psychosis predicts functional and clinical outcome in children and adolescents with first-episode psychosis: A 2-year longitudinal study. Schizophr. Res. 2013, 152, 130–138. [Google Scholar] [CrossRef]
- American Psychiatric Association. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- First, M.; Spitzer, R.; Giboon, M.; Williams, J. Entrevista Clínica Estructurada para los Trastornos del Eje I del DSM-IV; Masson: Barcelona, Spain, 1999. [Google Scholar]
- Kaufman, J.; Birmaher, B.; Brent, D.; Rao, U.; Flynn, C.; Moreci, P.; Williamson, D.; Ryan, N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 980–988. [Google Scholar] [CrossRef]
- Endicott, J.; Spitzer, R.L.; Fleiss, J.L.; Cohen, J. The Global Assessment Scale: A Procedure for Measuring Overall Severity of Psychiatric Disturbance. Arch. Gen. Psychiatry 1976, 33, 766–771. [Google Scholar] [CrossRef]
- Shaffer, D.; Gould, M.S.; Brasic, J.; Ambrosini, P.; Fisher, P.; Bird, H.; Aluwahlia, S. A children’s global assessment scale (CGAS). Arch. Gen. Psychiatry 1983, 40, 1228–1231. [Google Scholar] [CrossRef] [PubMed]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Peralta, V.; Cuesta, M.J. Validación de la escala de los síndromes positivo-negativo (PANSS) [Validation of the positive-negative syndrome scale (PANSS)]. Actas Luso Españolas Neurol. Psiquiatr. 1994, 22, 171–177. [Google Scholar]
- Andreasen, N.C.; Carpenter, W.T.; Kane, J.M.; Lasser, R.A.; Marder, S.R.; Weinberger, D.R. Remission in schizophrenia: Proposed criteria and rationale for consensus. Am. J. Psychiatry 2005, 162, 441–449. [Google Scholar] [CrossRef]
- Liberman, R.P.; Kopelowicz, A.; Ventura, J.; Gutkind, D. Operational criteria and factors related to recovery from schizophrenia. Int. Rev. Psychiatry 2009, 14, 256–272. [Google Scholar] [CrossRef]
- Gardner, D.M.; Murphy, A.L.; O’Donnell, H.; Centorrino, F.; Baldessarini, R.J. International Consensus Study of Antipsychotic Dosing. Am. J. Psychiatry 2010, 167, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, Y. A Sharper Bonferroni Procedure for Multiple Tests of Significance. Biometrika 1988, 75, 800–802. [Google Scholar] [CrossRef]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 398. [Google Scholar]
- Buchanan, R.W.; Kirkpatrick, B.; Heinrichs, D.W.; Carpenter, W.T.J. Clinical correlates of the deficit syndrome of schizophrenia. Am. J. Psychiatry 1990, 147, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Parellada, M.; Gomez-Vallejo, S.; Burdeus, M.; Arango, C. Developmental Differences Between Schizophrenia and Bipolar Disorder. Schizophr. Bull. 2017, 43, 1176–1189. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, S.; Mucci, A.; Bitter, I.; Libiger, J.; Bucci, P.; Fleischhacker, W.W.; Kahn, R.S.; Eufest Study Group. Persistent negative symptoms in first episode patients with schizophrenia: Results from the European First Episode Schizophrenia Trial. Eur. Neuropsychopharmacol. 2013, 23, 196–204. [Google Scholar] [CrossRef]
- Parellada, M.; Fraguas, D.; Bombín, I.; Otero, S.; Castro-Fornieles, J.; Baeza, I.; Gonzalez-Pinto, A.; Graell, M.; Soutullo, C.; Paya, B.; et al. Insight correlates in child- and adolescent-onset first episodes of psychosis: Results from the CAFEPS study. Psychol. Med. 2009, 39, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- Parellada, M.; Boada, L.; Fraguas, D.; Reig, S.; Castro-Fornieles, J.; Moreno, D.; Gonzalez-Pinto, A.; Otero, S.; Rapado-Castro, M.; Graell, M. Trait and state attributes of insight in first episodes of early-onset schizophrenia and other psychoses: A 2-year longitudinal study. Schizophr. Bull. 2011, 37, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Abdin, E.; Chong, S.A.; Vaingankar, J.A.; Peh, C.X.; Poon, L.Y.; Rao, S.; Verma, S.; Subramaniam, M. Trajectories of positive, negative and general psychopathology symptoms in first episode psychosis and their relationship with functioning over a 2-year follow-up period. PloS One 2017, 12, e0187141. [Google Scholar] [CrossRef]
- Morgan, C.; Lappin, J.; Heslin, M.; Donoghue, K.; Lomas, B.; Reininghaus, U.; Onyejiaka, A.; Croudace, T.; Jones, P.B.; Murray, R.M.; et al. Reappraising the long-term course and outcome of psychotic disorders: The AESOP-10 study. Psychol. Med. 2014, 44, 2713–2726. [Google Scholar] [CrossRef]
- Arango, C.; Fraguas, D.; Parellada, M. Differential neurodevelopmental trajectories in patients with early-onset bipolar and schizophrenia disorders. Schizophr. Bull. 2014, 40, S138–S146. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Caneja, C.M.; Pina-Camacho, L.; Rodríguez-Quiroga, A.; Fraguas, D.; Parellada, M.; Arango, C. Predictors of outcome in early-onset psychosis: A systematic review. NPJ Schizophr. 2015, 1, 14005. [Google Scholar] [CrossRef]
- González-Blanch, C.; Álvarez-Jiménez, M.; Rodríguez-Sánchez, J.; Pérez-Iglesias, R.; Vázquez-Barquero, J.; Crespo-Facorro, B. Cognitive functioning in the early course of first-episode schizophrenia spectrum disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2006, 256, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Lahera, G.; Pérez-Fuster, V.; Gálvez, J.L.; Martínez, M.; Sánchez, P.; Roca, M. Is it possible to achieve functional recovery in schizophrenia? A qualitative and quantitative analysis of psychiatrist´s opinion. Actas Esp. Psiquiatr. 2016, 44, 97–106. [Google Scholar] [PubMed]
- Slade, M.; Adams, N.; O’Hagan, M. Recovery: Past progress and future challenges. Int. Rev. Psychiatry 2012, 24, 1–4. [Google Scholar] [CrossRef]
- Suvisaari, J.; Mantere, O.; Keinänen, J.; Mäntylä, T.; Rikandi, E.; Lindgren, M.; Kieseppä, T.; Raij, T.T. Is It Possible to Predict the Future in First-Episode Psychosis? Front. Psychiatry 2018, 9, 580. [Google Scholar] [CrossRef]
- Barnett, J.H.; Salmond, C.H.; Jones, P.B.; Sahakian, B.J. Cognitive reserve in neuropsychiatry. Psychol. Med. 2006, 36, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Amoretti, S.; Cabrera, B.; Torrent, C.; Mezquida, G.; Lobo, A.; González-Pinto, A.; Parellada, M.; Corripio, I.; Vieta, E.; Serna, E.; et al. Cognitive reserve as an outcome predictor: First-episode affective versus non-affective psychosis. Acta Psychiatr. Scand. 2018, 138, 441–455. [Google Scholar] [CrossRef]
- Amoretti, S.; Bernardo, M.; Bonnin, C.M.; Bioque, M.; Cabrera, B.; Mezquida, G.; Solé, B.; Vieta, E.; Torrent, C. The impact of cognitive reserve in the outcome of first-episode psychoses: 2-year follow-up study. Eur. Neuropsychopharmacol. 2016, 26, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Amoretti, S.; Rosa, A.R.; Mezquida, G.; Cabrera, B.; Ribeiro, M.; Molina, M.; Bioque, M.; Lobo, A.; González-Pinto, A.; Fraguas, D.; et al. The impact of cognitive reserve, cognition and clinical symptoms on psychosocial functioning in first-episode psychoses. Psychol. Med. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- González-Ortega, I.; González-Pinto, A.; Alberich, S.; Echeburúa, E.; Bernardo, M.; Cabrera, B.; Amoretti, S.; Lobo, A.; Arango, C.; Corripio, I.; et al. Influence of social cognition as a mediator between cognitive reserve and psychosocial functioning in patients with first episode psychosis. Psychol. Med. 2020, 50, 270–271. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.R.; Sánchez-Moreno, J.; Martínez-Aran, A.; Salamero, M.; Torrent, C.; Reinares, M.; Comes, M.; Colom, F.; Van Riel, W.; Luis Ayuso-Mateos, J.; et al. Validity and reliability of the Functioning Assessment Short Test (FAST) in bipolar disorder. Clin Pract and Epidemiol Ment Health. 2007, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- González-Ortega, I.; Rosa, A.; Alberich, S.; Barbeito, S.; Vega, P.; Echeburúa, E.; Vieta, E.; González-Pinto, A. Validation and Use of the Functioning Assessment Short Test in First Psychotic Episodes. J. Nerv. Ment. Dis. 2010, 198, 836–840. [Google Scholar] [CrossRef]
- Ang, M.S.; Rekhi, G.; Lee, J. Validation of the Brief Negative Symptom Scale and its association with functioning. Schizophr. Res. 2019, 208, 97–104. [Google Scholar] [CrossRef]
- Kring, A.M.; Gur, R.E.; Blanchard, J.J.; Horan, W.P.; Reise, S.P. The Clinical Assessment Interview for Negative Symptoms (CAINS): Final Development and Validation. Am. J. Psychiatry 2013, 170, 165–172. [Google Scholar] [CrossRef] [PubMed]
| 1 | 2 | 3 | 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical Ratings | Whole FEP Sample | Early Onset | Adult Onset | Early Onset | Adult Onset | ||||
| Low pIQ | Average pIQ | ||||||||
| mean (SD) [95% IC] | N = 255 | N = 41 | N = 30 | N = 70 | N = 114 | Statistic | Significant Post-Hoc Comparison | ||
| F/χ2 (d.f.) | Sig. (p) | Pair Comparisons | p | ||||||
| Age of symptoms onset | 21.31 (6.03) [20.56–22.05] | 15.9 (1.78) [15.34–16.47] | 23.31 (4.60) [21.60–25.03] | 16.18 (1.55) [15.81–16.55] | 25.87 (4.96) [24.95–26.80] | ||||
| Baseline age | 21.66 (6.06) [20.91–22.41] | 16.17 (1.77) [15.61–16.73] | 23.80 (4.69) [22.05–25.56] | 16.48 (1.59) [16.10–16.86] | 26.25 (4.93) [25.34–27.17] | ||||
| Estimated premorbid IQ | 91.65 (15.31) [89.76–93.54] | 71.59 (7.19) [69.31–73.86] | 74.83 (6.22) [72.51–77.16] | 97.36 (11.51) [94.61–100.1] | 99.78 (10.51) [97.83–101.73] | ||||
| Sex N (%) Female | 83 (32.5) | 14 (34.1) | 16 (53.33) | 23 (32.9) | 30 (26.3) | 7.97 | 0.047 b | 2 > 4 | 0.005 |
| Parental SES–N (%) | 13.76 (12) | 0.32b | |||||||
| High | 48 (19.0) | 7 (17.1) | 4 (13.3) | 11 (16.2) | 26 (22.8) | ||||
| Medium High | 31 (12.3) | 6 (14.6) | 1 (3.3) | 9 (13.2) | 15 (13.2) | ||||
| Medium | 62 (24.5) | 4 (9.8) | 9 (30.0) | 19 (27.9) | 30 (26.3) | ||||
| Medium Low | 79 (31.2) | 17 (41.5) | 11 (36.7) | 18 (26.5) | 33 (28.9) | ||||
| Low | 33 (13.0) | 7 (17.1) | 5 (16.7) | 11 (16.2) | 10 (8.8) | ||||
| DUP, mean (SD) [range] | 129.79 (124.61) [114.30–145.28] | 95.32 (109.49) [60–76–129.87] | 180.69 (114.44) [137.15–224.21] | 111.14 (11.31) [84.40–137.89] | 140.72 (135.30) [115.39–166.06] | 3.57 (3) | 0.015 a | 2 > 1 | 0.027 |
| PAS Infancy | 0.73 (0.18) [0.71–0.75] | 0.67 (0.2) [0.61–0.74] | 0.69 (0.22) [0.60–0.78] | 0.72 (0.19) [0.68–0.77] | 0.77 (0.15) [0.73–0.79] | 3.14 (3) | 0.026 a | 1 < 4 | 0.04 |
| Baseline AP main daily dose | 522.30 (401.08) [181.08–246.67] | 505.37 (527.58) [338.84–671.88] | 681.42 (421.14) [518.12–844.72] | 348.47 (192.72) [302.17–394.76] | 596.46 (404.87) [520.30–672.62] | 7.65 (3) | <0.001 a | 3 < 2 3 < 4 | 0.001 <0.001 |
| Two-year AP main daily dose | 0.73 (0.18) [0.71–0.75] | 280.58 (291.81) [184.66–376.50] | 298.86 (330.56) [176.01–301.07] | 238.54 (252.34) [176.01–301.07] | 154.69 (221.36) [113.44–195.95] | 4.17 (3) | 0.007 a | 4 < 2 | 0.04 |
| Baseline diagnosis N (%) | 22.58 (6) | 0.001 b | ASD 1 > ASD 2 ASD 1 > ASD 4 ASD 3 > ASD 2 ASD 3 > ASD 4 OPs 3 < OPs 2 OPs 3 < OPs 4 | 0.004 0.02 <0.001 <0.001 0.001 0.002 | |||||
| Schizophrenia Spectrum Disorders (SSD) | 150 (58.8%) | 25 (61.0%) | 17 (56.7%) | 45 (64.3%) | 63 (55.3%) | ||||
| Affective Spectrum Disorders (ASD) | 51 (20%) | 11 (26.8%) | 2 (6.7%) | 20 (28.6%) | 18 (15.8%) | ||||
| Other Psychoses (OPs) | 54 (21.2%) | 5 (12.2%) | 11 (36.7%) | 5 (7.1%) | 33 (28.9%) | ||||
| Clinical Ratings | Whole FEP Sample | 1 | 2 | 3 | 4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Early Onset | Adult Onset | Early Onset | Adult Onset | |||||||
| Low pIQ | Average pIQ | |||||||||
| N= 255 | N= 41 | N= 30 | N= 70 | N= 114 | Test Statistic | Significant post-hoc Comparison | ||||
| F/χ2 (d.f.) | Sig. (p) | Pair Comparisons | p | d/V | ||||||
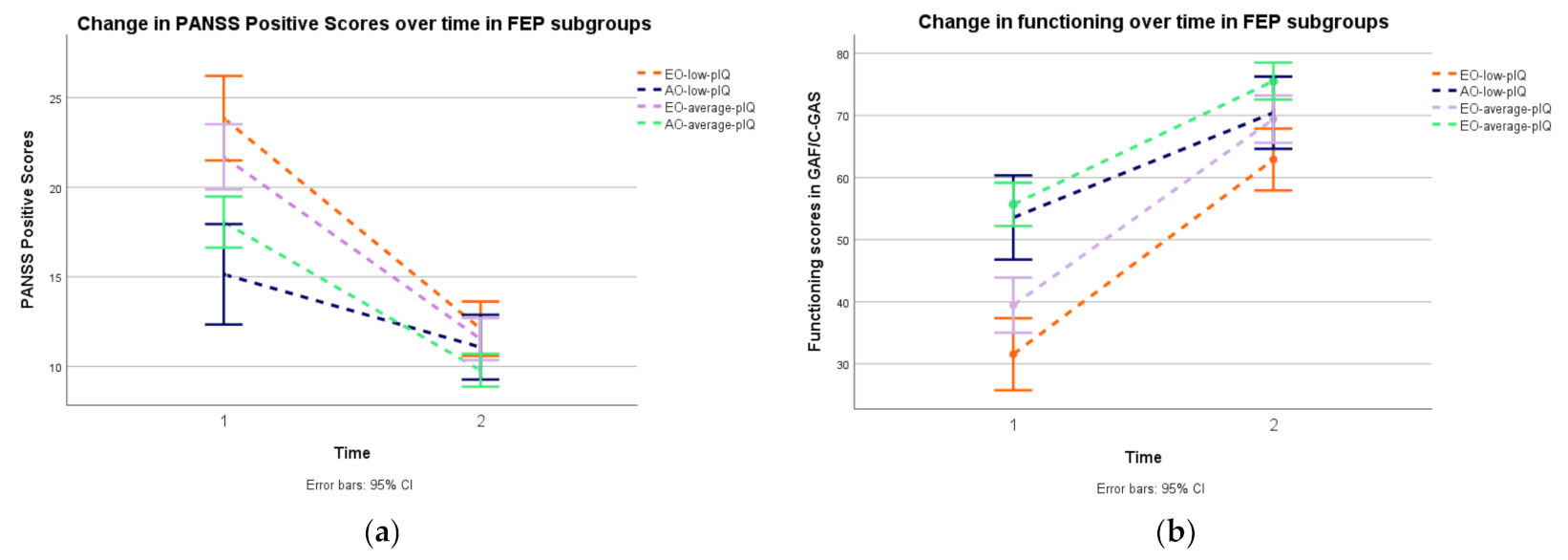
| PANSS Positive Symptoms baseline mean (SD) [95% CI] | 19.54 (8.12) [18.09–20.46] | 23.85 (6.57) [21.77–25.93] | 15.23 (7.18) [12.55–17.91] | 21.60 (7.69) [19.76–23.43] | 17.86 (8.14) [16.34–19.37] | 10.96 (3) | <0.001 a | 1 > 2 | <0.001 | 1.21 |
| 1 > 4 | <0.001 | 0.78 | ||||||||
| 3 > 2 | 0.001 | 0.29 | ||||||||
| 3> 4 | 0.009 | 0.51 | ||||||||
| PANSS Positive Symptomsmean at two years (SD) [95% CI] | 10.79 (5.00) [9.78–11.11] | 12.10 (6.28) [10.11–14.08] | 11.07 (5.56) [8.95–13.18] | 11.52 (5.47) [10.20–12.83] | 9.79 (3.73) [9.08–10.48] | 3.38 (3) | 0.03 a | n.s. | ||
| PANSS Negative Symptoms baselinemean (SD) [95% CI] | 18.67 (8.52) [17.03–19.31] | 20.63 (10.72) [17.24–24.01] | 19.03 (9.05) [15.65–22.41] | 19.03 (8.84) [16.92–21.13] | 17.65 (7.15) [16.32–18.97] | 1.44 (3) | 0.26 a | n.s. | ||
| PANSS Negative Symptomsmean at two years (SD) [95% CI] | 14.73 (6.84) [13.02–14.89] | 17.49 (8.18) [14.90–20.07] | 15.62 (6.82) [13.02–18.21] | 14.84 (7.29) [13.08–16.59] | 13.42 (5.69) [12.35–14.48] | 4.18 (3) | 0.01 a | 1 > 4 | 0.005 | 0.59 |
| PANSS General Symptoms baselinemean (SD) [95% CI] | 39.09 (13.24) [36.63–40.28] | 44.83 (14.90) [40.12–49.53] | 35.10 (13.10) [30.20–39.99] | 41.03 (13.41) [37.83–44.22] | 36.89 (11.76) [34.70–39.06] | 5.27 (3) | 0.002 a | 1 > 2 1 > 4 | 0.01 0.005 | 0.60 0.61 |
| PANSS General Symptoms mean at two years (SD) [95% CI] | 43.04 (18.45) [40.17–45.31] | 37.02 (19.15) [30.97–43.07] | 53.45 (19.25) [46.12–60.77] | 34.07 (15.81) [30.30–37.84] | 48.16 (16.44) [45.08–51.23] | 15.21 (3) | <0.001 a | 1 < 2 | 0.001 | 0.93 |
| 1 < 4 | 0.003 | 1.27 | ||||||||
| 3 < 2 | <0.001 | 0.67 | ||||||||
| 3 < 4 | <0.001 | 0.92 | ||||||||
| PANSS Total baselinemean (SD) [95% CI] | 77.30 (25.17) [72.43–79.40] | 89.32 (26.45) [80.96–97.66] | 69.37 (26.07) [59.63–79.10] | 81.66 (24.77) [75.74–87.56] | 72.39 (22.86) [68.15–76.63] | 6.63 (3) | <0.001 a | 1 > 2 1 > 4 | 0.005 0.001 | 0.68 0.71 |
| PANSS Totalmean at two years (SD) [95% CI] | 51.83 (19.06) [47.56–52.79] | 57.98 (23.04) [50.70–65.24] | 53.45 (19.25) [46.12–60.77] | 53.16 (19.94) [48.36–57.95] | 48.34 (16.19) [45.31–51.37] | 3.17 (3) | 0.035 a | 1 > 4 | 0.026 | 0.5 |
| GAF baselinemean (SD) [95% CI] | 47.10 (21.08) [46.49–52.52] | 31.56 (17.26) [26.11–37.01] | 53.57 (18.52) [46.65–60.48] | 39.46 (19.46) [34.81–44.09] | 55.68 (19.11) [52.13–59.22] | 22.19(3) | <0.001 a | 1 < 2 | <0.001 | 1.17 |
| 1 < 4 | <0.001 | 1.29 | ||||||||
| 3 < 2 | 0.004 | 0.72 | ||||||||
| 3 < 4 | <0.001 | 0.88 | ||||||||
| GAFmean at two years (SD) [95% CI] | 71.22 (16.67) [70.85–75.53] | 62.90 (18.68) [57.01–68.79] | 70.43 (14.03) [65.19–75.67] | 69.40 (19.32) [64.79–74.01] | 75.53 (13.31) [73.05–77.99] | 6.51 (3) | <0.001 a | 1 < 4 | <0.001 | 0.82 |
| Good functioning at two years N (%) | 135 (52.9) | 14 (34.1) | 14 (46.7) | 35 (50) | 72 (63.2) | 11.31 (3) | 0.01 b | 1 < 4 | 0.001 | 0.26 |
| Symptom remission at two years N (%) | 181 (71) | 25 (61) | 19 (65.5) | 47 (67.1) | 90 (81.1) | 8.45(3) | 0.03 b | 1 < 4 | 0.01 | 0.21 0.16 |
| 3 < 4 | 0.03 | |||||||||
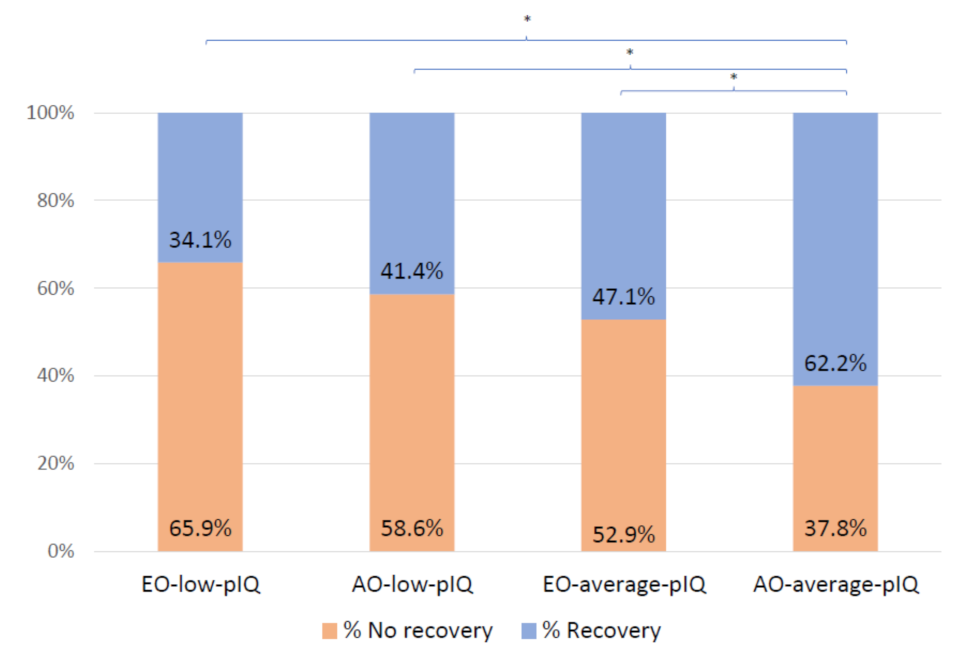
| Recovery at two years N (%) | 128 (51) | 14 (34.1) | 12 (41.4) | 33 (47.1) | 69 (62.2) | 11.69(3) | 0.009 b | 1 < 4 | 0.002 | 0.25 0.17 0.14 |
| 2 < 4 | 0.04 | |||||||||
| 3 < 4 | 0.04 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina-García, M.; Fraguas, D.; del Rey-Mejías, Á.; Mezquida, G.; Sánchez-Torres, A.M.; Amoretti, S.; Lobo, A.; González-Pinto, A.; Andreu-Bernabeu, Á.; Corripio, I.; et al. The Role of Premorbid IQ and Age of Onset as Useful Predictors of Clinical, Functional Outcomes, and Recovery of Individuals with a First Episode of Psychosis. J. Clin. Med. 2021, 10, 2474. https://doi.org/10.3390/jcm10112474
Molina-García M, Fraguas D, del Rey-Mejías Á, Mezquida G, Sánchez-Torres AM, Amoretti S, Lobo A, González-Pinto A, Andreu-Bernabeu Á, Corripio I, et al. The Role of Premorbid IQ and Age of Onset as Useful Predictors of Clinical, Functional Outcomes, and Recovery of Individuals with a First Episode of Psychosis. Journal of Clinical Medicine. 2021; 10(11):2474. https://doi.org/10.3390/jcm10112474
Chicago/Turabian StyleMolina-García, Mariola, David Fraguas, Ángel del Rey-Mejías, Gisela Mezquida, Ana M. Sánchez-Torres, Silvia Amoretti, Antonio Lobo, Ana González-Pinto, Álvaro Andreu-Bernabeu, Iluminada Corripio, and et al. 2021. "The Role of Premorbid IQ and Age of Onset as Useful Predictors of Clinical, Functional Outcomes, and Recovery of Individuals with a First Episode of Psychosis" Journal of Clinical Medicine 10, no. 11: 2474. https://doi.org/10.3390/jcm10112474
APA StyleMolina-García, M., Fraguas, D., del Rey-Mejías, Á., Mezquida, G., Sánchez-Torres, A. M., Amoretti, S., Lobo, A., González-Pinto, A., Andreu-Bernabeu, Á., Corripio, I., Vieta, E., Baeza, I., Mané, A., Cuesta, M., de la Serna, E., Payá, B., Zorrilla, I., Arango, C., Bernardo, M., ... Parellada, M. (2021). The Role of Premorbid IQ and Age of Onset as Useful Predictors of Clinical, Functional Outcomes, and Recovery of Individuals with a First Episode of Psychosis. Journal of Clinical Medicine, 10(11), 2474. https://doi.org/10.3390/jcm10112474







