Advanced Materials-Based Nanofiltration Membranes for Efficient Removal of Organic Micropollutants in Water and Wastewater Treatment
Abstract
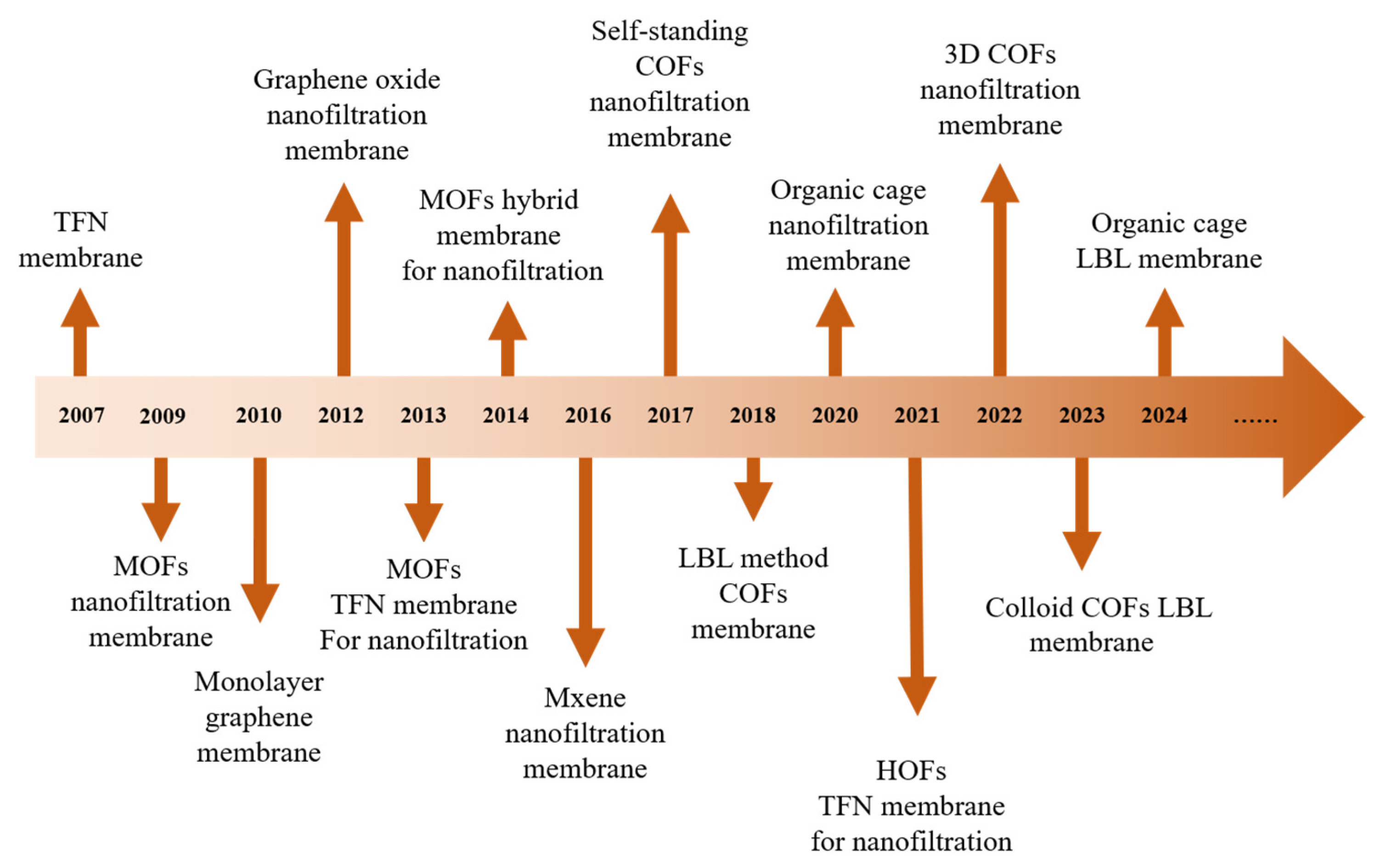
1. Introduction
2. OMPs in Water and Wastewater
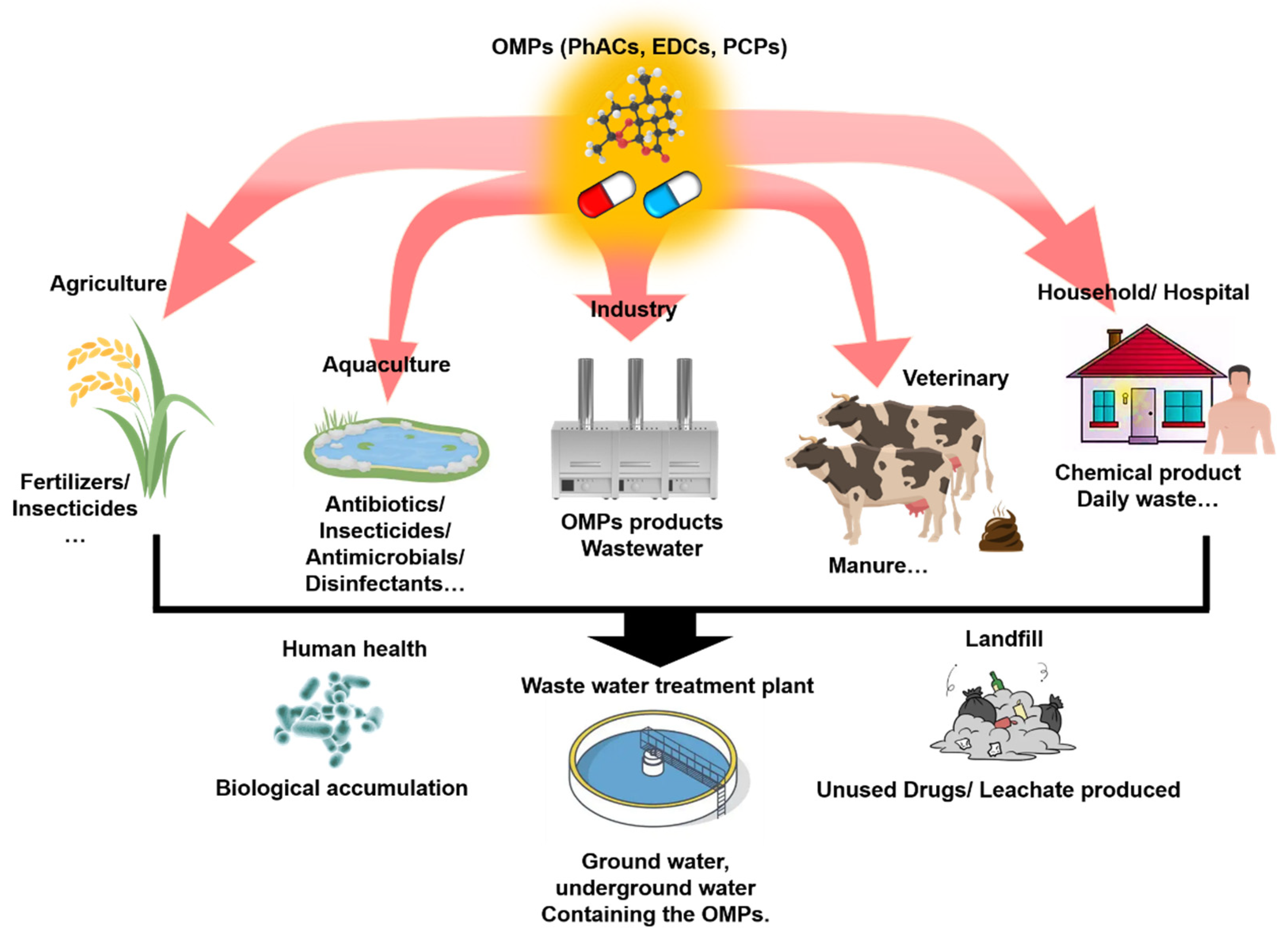
2.1. The Source of OMPs
2.2. The Risk of OMPs
2.3. Treatment Technologies for OMPs
3. Key Properties of NF Membranes for Separation
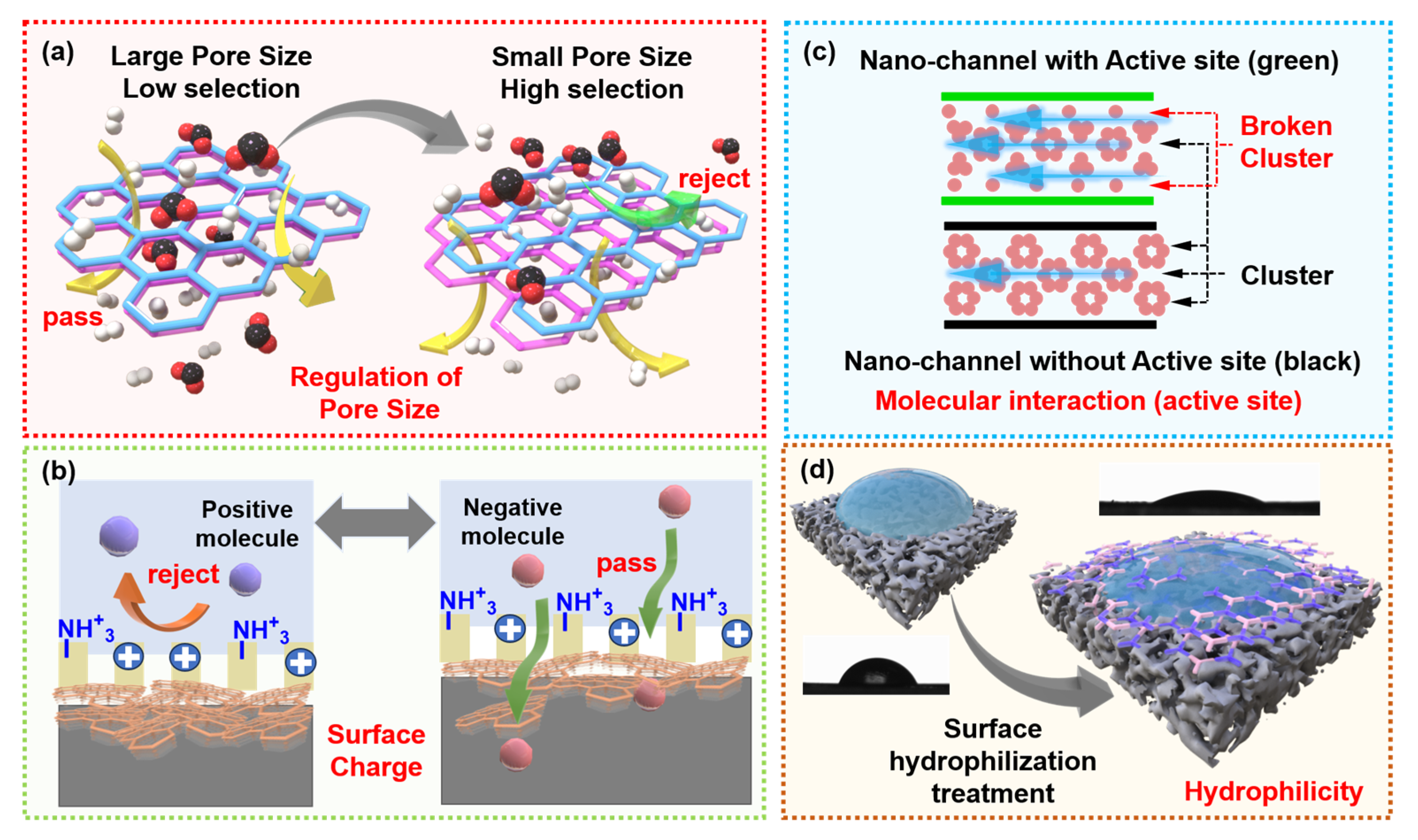
3.1. Pore Size
3.2. Surface Charge
3.3. Molecular Interaction
3.4. Hydrophilicity
4. NF Membrane with Advanced Materials for Removal of OMs
4.1. Organic Framework
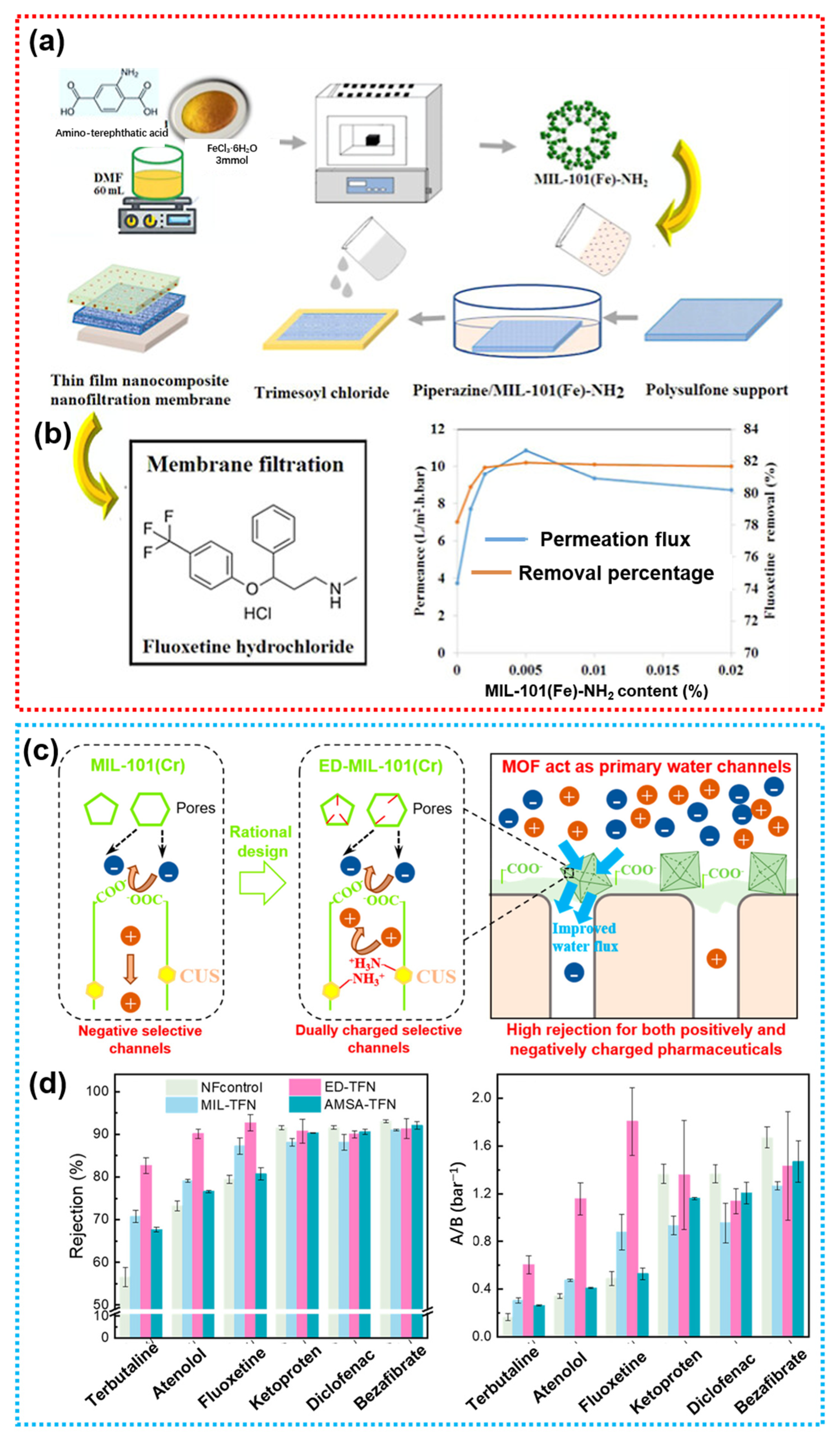
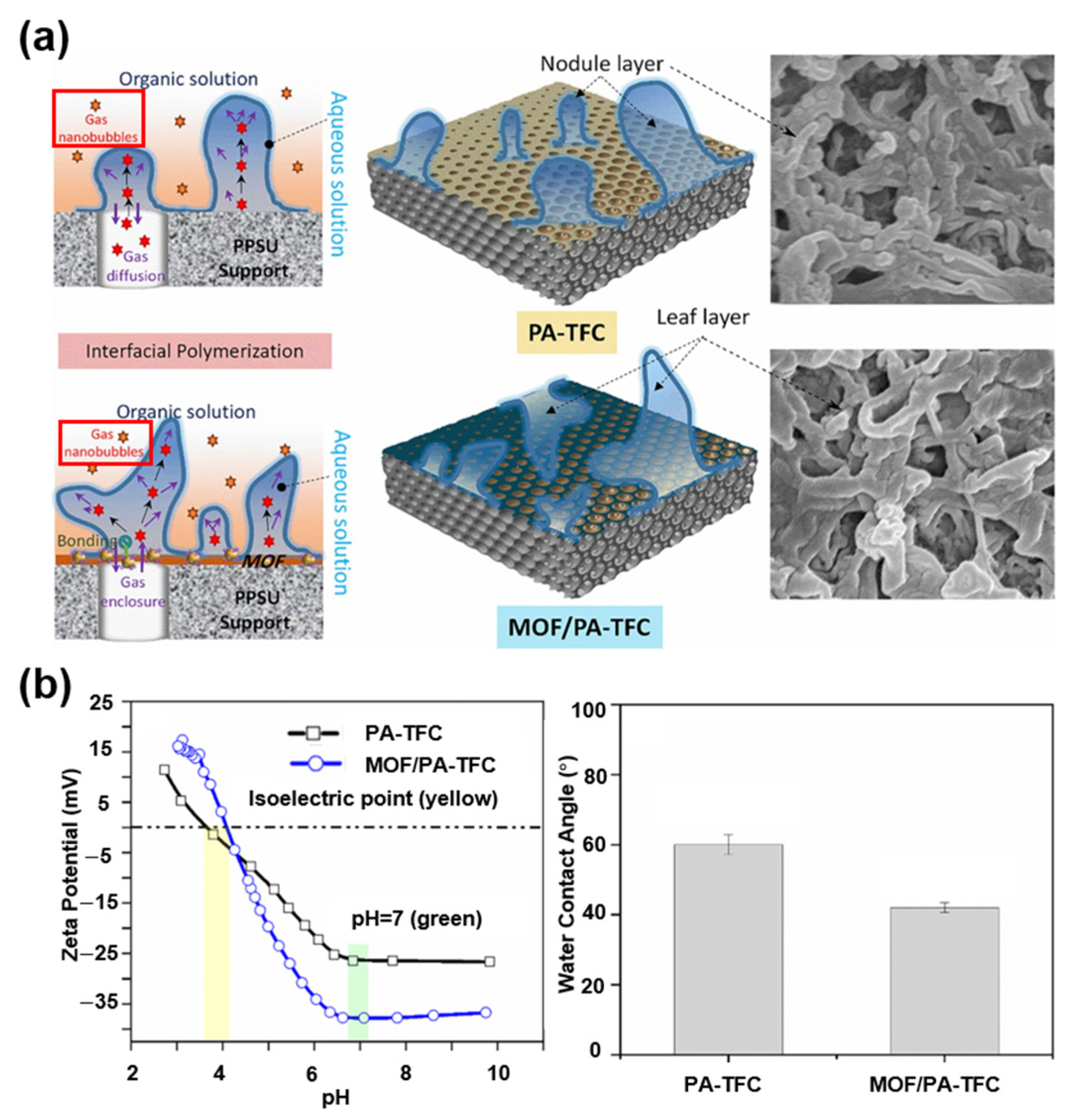
4.1.1. MOFs
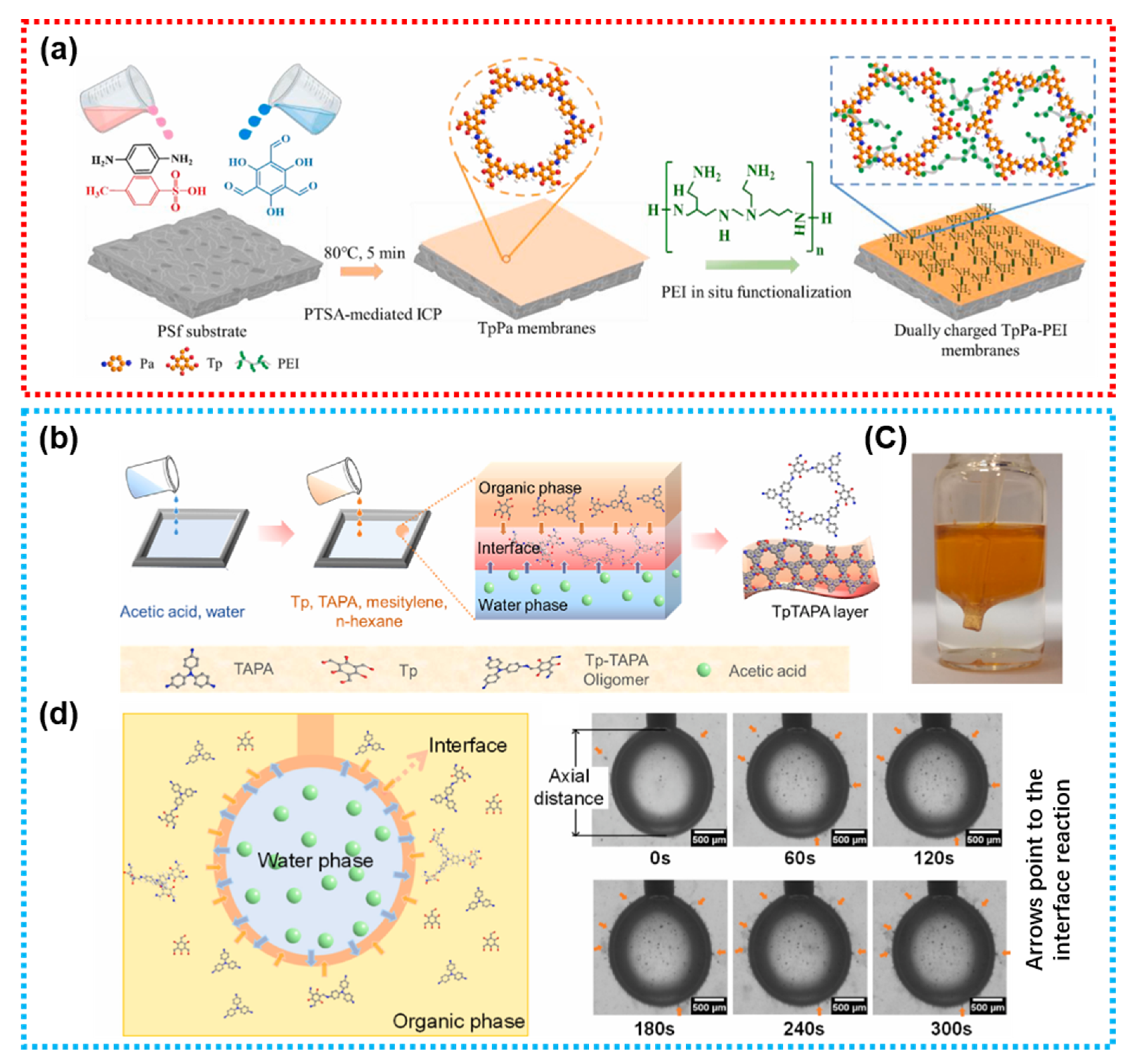
4.1.2. COFs
4.1.3. POFs and HOFs
4.2. Inorganic Laminar Materials
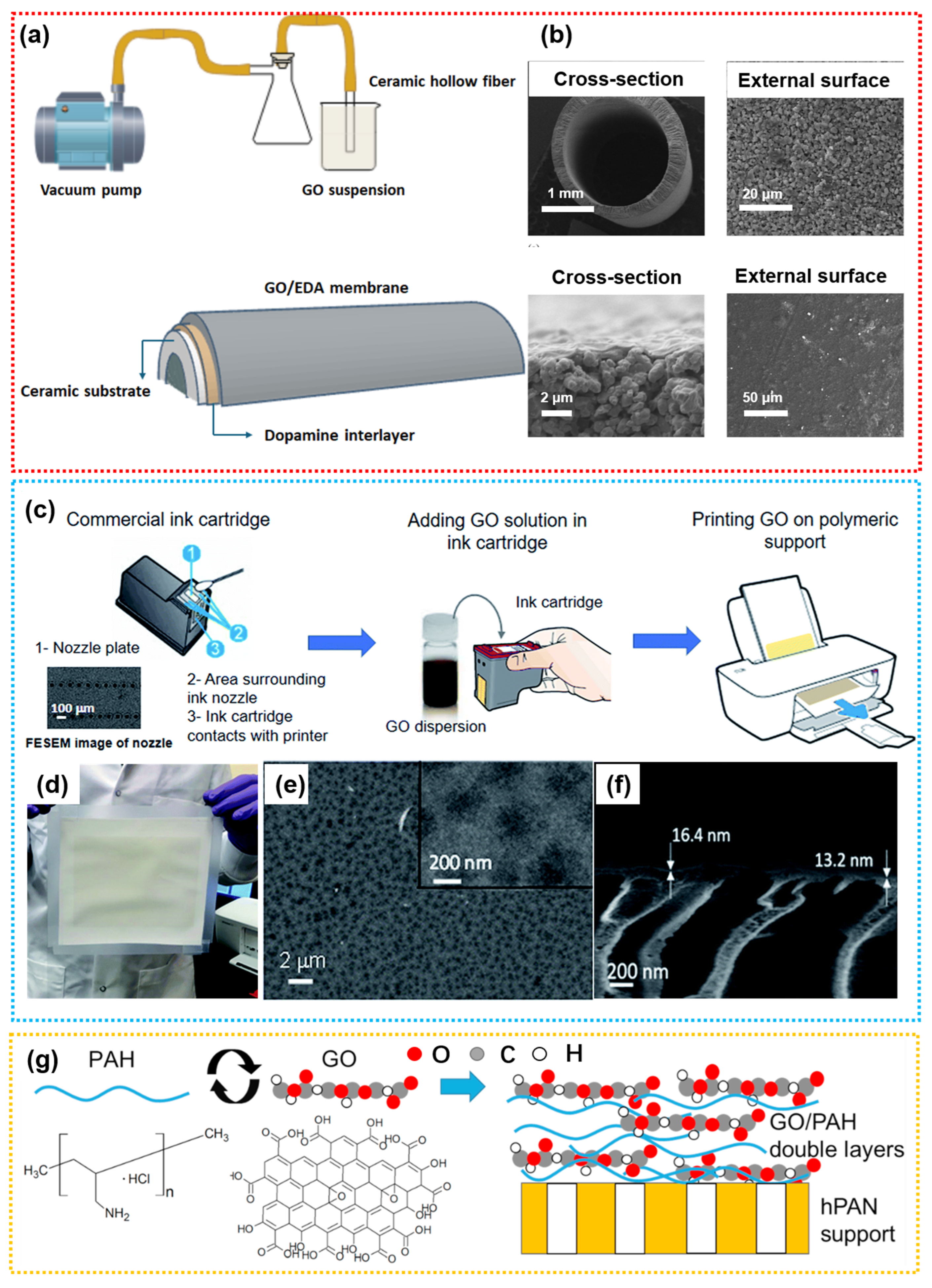
4.2.1. GO
4.2.2. MXene/MoS2
4.3. Hybrid Materials and Environmentally Friendly Materials
5. Challenges and Future Perspectives
5.1. Scalable Fabrication Process
5.2. Environmental Sustainability Goals
5.3. Complex Separation System
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PhACs | pharmaceutically active compounds |
| PCPs | personal care products |
| EDCs | endocrine disrupting compounds |
| OMPs | organic micropollutants |
| NF | nanofiltration |
| MOFs | metal–organic frameworks |
| COFs | covalent organic frameworks |
| GO | graphene oxide |
| MWCO | molecular weight cut-off |
| AOPs | advanced oxidation processes |
| RO | reverse osmosis |
| FO | forward osmosis |
| ISA | integrally skinned asymmetric |
| TFC | membrane: thin-film composite membrane |
| TFN | membrane: thin-film nanocomposite membrane |
| NIPS | non-solvent-induced phase separation |
| LbL | layer by layer |
| PEI | polyethyleneimine |
| PVDF | poly (vinylidene fluoride) |
| BSA | bovine serum albumin |
| GA | gallic acid |
| PEG | polyethylene glycol |
| PSf | polysulfone |
| DABA | 3,5-diaminobenzoic acid |
| HOFs | hydrogen-bonded organic frameworks |
| POFs | porous organic frameworks |
| PA | polyamide |
| ED | ethylenediamine |
| PDA | polydopamine |
| V/P-D | vacuum/pressure-driven |
| NPs | nanoparticles |
| IP | interface polymerization |
| Tp | 1,3,5-triformylphloroglucinol |
| Pa | p-phenylenediamine |
| ICP | interfacial catalytic polymerization |
| PTSA | p-toluenesulfonic acid |
| EDA | ethylenediamine |
| β-CD-EDA | β-cyclodextrin-functionalized EDA |
| ROS | reactive oxygen species |
| CQD | carbon quantum dot |
| POCs | porous organic cages |
| PES | polyethersulfone |
References
- Taheran, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y.; Zhang, T.C.; Valéro, J.R. Membrane processes for removal of pharmaceutically active compounds (PhACs) from water and wastewaters. Sci. Total Environ. 2016, 547, 60–77. [Google Scholar] [CrossRef] [PubMed]
- Pothitou, P.; Voutsa, D. Endocrine disrupting compounds in municipal and industrial wastewater treatment plants in Northern Greece. Chemosphere 2008, 73, 1716–1723. [Google Scholar] [CrossRef] [PubMed]
- Khoo, Y.S.; Goh, P.S.; Lau, W.J.; Ismail, A.F.; Abdullah, M.S.; Ghazali, N.H.M.; Yahaya, N.K.E.M.; Hashim, N.; Othman, A.R.; Mohammed, A.; et al. Removal of emerging organic micropollutants via modified-reverse osmosis/nanofiltration membranes: A review. Chemosphere 2022, 305, 135151. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Gao, C.; Ma, W.; He, Y.; Wu, J.; Luo, K.; Ouyang, D.; Lin, Z.; Cai, Z. High-throughput screening of bisphenols using magnetic covalent organic frameworks as a SELDI-TOF-MS probe. Microchim. Acta 2020, 187, 370. [Google Scholar] [CrossRef]
- Majumder, A.; Gupta, B.; Gupta, A.K. Pharmaceutically active compounds in aqueous environment: A status, toxicity and insights of remediation. Environ. Res. 2019, 176, 108542. [Google Scholar] [CrossRef]
- Roschangar, F.; Sheldon, R.A.; Senanayake, C.H. Overcoming barriers to green chemistry in the pharmaceutical industry-the Green Aspiration Level concept. Green Chem. 2015, 17, 752–768. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Xue, J.; Zhao, Y.; Taylor, A.A.; Zenobio, J.E.; Sun, Y.; Han, Z.; Salawu, O.A.; Zhu, Y. Abundance, fate, and effects of pharmaceuticals and personal care products in aquatic environments. J. Hazard. Mater. 2022, 424, 127284. [Google Scholar] [CrossRef]
- de Andrade, J.R.; Oliveira, M.F.; da Silva, M.G.; Vieira, M.G. Adsorption of Pharmaceuticals from Water and Wastewater Using Nonconventional Low-Cost Materials: A Review. Ind. Eng. Chem. Res. 2018, 57, 3103–3127. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Anil, A.G.; Kapoor, D.; Khasnabis, S.; Shekar, S.; Pavithra, N.; Samuel, J.; Subramanian, S.; Singh, J.; et al. Adsorption and detoxification of pharmaceutical compounds from wastewater using nanomaterials: A review on mechanism, kinetics, valorization and circular economy. J. Environ. Manag. 2021, 300, 113569. [Google Scholar] [CrossRef]
- Pal, P. Treatment and Disposal of Pharmaceutical Wastewater: Toward the Sustainable Strategy. Sep. Purif. Rev. 2018, 47, 179–198. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, H.; Wang, Q.; Li, Y. Review of the Mechanisms of Single-Atom-Based Advanced Oxidation Processes for the Degradation of Persistent Organic Pollutants in Water Environments. ACS EST Water 2024, 4, 4708–4720. [Google Scholar] [CrossRef]
- Simiyu, R.; Apollo, S.; Muriithi, G. A Review of Municipal Wastewater Disinfection using Advanced Oxidation Processes. J. Chem. Soc. Pak. 2024, 46, 600–616. [Google Scholar] [CrossRef]
- Emadikhiav, A.; Mafigholami, R.; Davood, A.; Mahvi, A.; Salimi, L. A review on hazards and treatment methods of released antibiotics in hospitals wastewater during the COVID-19 pandemic. Environ. Monit. Assess. 2024, 196, 820. [Google Scholar] [CrossRef]
- García-Ávila, F.; Zambrano-Jaramillo, A.; Velecela-Garay, C.; Coronel-Sánchez, K.; Valdiviezo-Gonzales, L. Effectiveness of membrane technologies in removing emerging contaminants from wastewater: Reverse Osmosis and Nanofiltration. Water Cycle 2025, 6, 357–373. [Google Scholar] [CrossRef]
- Al Kholif, M.; Hermana, J.; Bilad, M.R.; Chao, H.P. Comprehensive updates on recent advances, fouling mechanisms, and future perspectives of nanofiltration. J. Water Process Eng. 2024, 63, 105565. [Google Scholar] [CrossRef]
- Yadav, D.; Karki, S.; Ingole, P.G. Current advances and opportunities in the development of nanofiltration (NF) membranes in the area of wastewater treatment, water desalination, biotechnological and pharmaceutical applications. J. Environ. Chem. Eng. 2022, 10, 108109. [Google Scholar] [CrossRef]
- Khanzada, N.K.; Farid, M.U.; Kharraz, J.A.; Choi, J.; Tang, C.Y.; Nghiem, L.D.; Jang, A.; An, A.K. Removal of organic micropollutants using advanced membrane-based water and wastewater treatment: A review. J. Membr. Sci. 2020, 598, 117672. [Google Scholar] [CrossRef]
- Yang, L.; Xiao, X.; Shen, S.; Lama, J.; Hu, M.; Jia, F.; Han, Z.; Qu, H.; Huang, L.; Wang, Y.; et al. Recent Advances in Graphene Oxide Membranes for Nanofiltration. ACS Appl. Nano Mater. 2021, 5, 3121–3145. [Google Scholar] [CrossRef]
- Luo, Z.; Tu, Y.; Li, H.; Qiu, B.; Liu, Y.; Yang, Z. Endocrine-disrupting compounds in the Xiangjiang River of China: Spatio-temporal distribution, source apportionment, and risk assessment. Ecotoxicol. Environ. Saf. 2019, 167, 476–484. [Google Scholar] [CrossRef]
- Tan, R.; Liu, R.; Li, B.; Liu, X.; Li, Z. Typical Endocrine Disrupting Compounds in Rivers of Northeast China: Occurrence, Partitioning, and Risk Assessment. Arch. Environ. Contam. Toxicol. 2018, 75, 213–223. [Google Scholar] [CrossRef]
- Chen, Z.; Lian, J.Z.; Zhu, H.; Zhang, J.; Zhang, Y.; Xiang, X.; Huang, D.; Tjokro, K.; Barbarossa, V.; Cucurachi, S.; et al. Application of Life Cycle Assessment in the pharmaceutical industry: A critical review. J. Clean. Prod. 2024, 459, 142550. [Google Scholar] [CrossRef]
- Quddus, F.; Shah, A.; Iftikhar, F.J.; Shah, N.S.; Haleem, A. Environmentally Benign Nanoparticles for the Photocatalytic Degradation of Pharmaceutical Drugs. Catalysts 2023, 13, 511. [Google Scholar] [CrossRef]
- Vogel, M.; Kakani, P.; Chandra, A.; Conti, R.M. Medicare price negotiation and pharmaceutical innovation following the Inflation Reduction Act. Nat. Biotechnol. 2024, 42, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Gadipelly, C.; Pérez-González, A.; Yadav, G.D.; Ortiz, I.; Ibáñez, R.; Rathod, V.K.; Marathe, K.V. Pharmaceutical Industry Wastewater: Review of the Technologies for Water Treatment and Reuse. Ind. Eng. Chem. Res. 2014, 53, 11571–11592. [Google Scholar] [CrossRef]
- Boonhat, H.; Guo, Y.L.; Chan, C.C.; Lin, R.T. Estimates of the global burden of cancer-related deaths attributable to residential exposure to petrochemical industrial complexes from 2020 to 2040. Environ. Pollut. 2024, 350, 123955. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Wang, L.; Kuang, M.; Wang, S.; Yang, J. Toward carbon neutrality: Selective conversion of waste plastics into value-added chemicals. Matter 2023, 6, 3322–3347. [Google Scholar] [CrossRef]
- Sable, D.A.; Vadagaonkar, K.S.; Kapdi, A.R.; Bhanage, B.M. Carbon dioxide based methodologies for the synthesis of fine chemicals. Org. Biomol. Chem. 2021, 19, 5725–5757. [Google Scholar] [CrossRef]
- Kar, S.; Sanderson, H.; Roy, K.; Benfenati, E.; Leszczynski, J. Green Chemistry in the Synthesis of Pharmaceuticals. Chem. Rev. 2022, 122, 3637–3710. [Google Scholar] [CrossRef]
- Boxall, A.B.; Rudd, M.A.; Brooks, B.W.; Caldwell, D.J.; Choi, K.; Hickmann, S.; Innes, E.; Ostapyk, K.; Staveley, J.P.; Verslycke, T.; et al. Pharmaceuticals and Personal Care Products in the Environment: What Are the Big Questions? Environ. Health Perspect. 2012, 120, 1221–1229. [Google Scholar] [CrossRef]
- Nowak, K.; Jabłońska, E.; Ratajczak-Wrona, W. Controversy around parabens: Alternative strategies for preservative use in cosmetics and personal care products. Environ. Res. 2021, 198, 110488. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, Y.; Lu, Y.; Johnson, A.C.; Sarvajayakesavalu, S.; Liu, Z.; Su, C.; Zhang, Y.; Juergens, M.D.; Jin, X. The relative risk and its distribution of endocrine disrupting chemicals, pharmaceuticals and personal care products to freshwater organisms in the Bohai Rim, China. Sci. Total Environ. 2017, 590, 633–642. [Google Scholar] [CrossRef]
- Boyer, E.W. Management of Opioid Analgesic Overdose. N. Engl. J. Med. 2012, 367, 146–155. [Google Scholar] [CrossRef]
- Clarke, G.L.; Bhattacherjee, A.; Tague, S.E.; Hasan, W.; Smith, P.G. β-Adrenoceptor Blockers Increase Cardiac Sympathetic Innervation by Inhibiting Autoreceptor Suppression of Axon Growth. J. Neurosci. 2010, 30, 12446–12454. [Google Scholar] [CrossRef] [PubMed]
- Haro, N.K.; Del Vecchio, P.; Marcilio, N.R.; Féris, L.A. Removal of atenolol by adsorption-Study of kinetics and equilibrium. J. Clean. Prod. 2017, 154, 214–219. [Google Scholar] [CrossRef]
- Ghauch, A.; Tuqan, A.M.; Kibbi, N. Ibuprofen removal by heated persulfate in aqueous solution: A kinetics study. Chem. Eng. J. 2012, 197, 483–492. [Google Scholar] [CrossRef]
- Vieira, W.T.; de Farias, M.B.; Spaolonzi, M.P.; da Silva, M.G.C.; Vieira, M.G.A. Endocrine-disrupting compounds: Occurrence, detection methods, effects and promising treatment pathways-A critical review. J. Environ. Chem. Eng. 2021, 9, 104558. [Google Scholar] [CrossRef]
- van der Meer, T.P.; Artacho-Cordón, F.; Swaab, D.F.; Struik, D.; Makris, K.C.; Wolffenbuttel, B.H.; Frederiksen, H.; Frederiksen, J.V. Distribution of Non-Persistent Endocrine Disruptors in Two Different Regions of the Human Brain. Int. J. Environ. Res. Public Health 2017, 14, 1059. [Google Scholar] [CrossRef]
- Charnock, C.; Finsrud, T. Combining esters of para-hydroxy benzoic acid (parabens) to achieve increased antimicrobial activity. J. Clin. Pharm. Ther. 2007, 32, 567–572. [Google Scholar] [CrossRef]
- Kizhedath, A.; Wilkinson, S.; Glassey, J. Assessment of hepatotoxicity and dermal toxicity of butyl paraben and methyl paraben using HepG2 and HDFn in vitro models. Toxicol. Vitr. 2019, 55, 108–115. [Google Scholar] [CrossRef]
- Nemes, D.; Kovács, R.; Nagy, F.; Mező, M.; Poczok, N.; Ujhelyi, Z.; Pető, Á.; Fehér, P.; Fenyvesi, F.; Váradi, J.; et al. Interaction between Different Pharmaceutical Excipients in Liquid Dosage Forms Assessment of Cytotoxicity and Antimicrobial Activity. Molecules 2018, 23, 1827. [Google Scholar] [CrossRef]
- Flasiński, M.; Kowal, S.; Broniatowski, M.; Wydro, P. Influence of Parabens on Bacteria and Fungi Cellular Membranes: Studies in Model Two-Dimensional Lipid Systems. J. Phys. Chem. B 2018, 122, 2332–2340. [Google Scholar] [CrossRef]
- Nagar, Y.; Thakur, R.S.; Parveen, T. Toxicity assessment of parabens in in caenorhabditis elegans. Chemosphere 2020, 246, 125730. [Google Scholar] [CrossRef]
- Darbre, P.D.; Aljarrah, A.; Miller, W.R.; Coldham, N.G.; Sauer, M.J.; Pope, G.S. Concentrations of parabens in human breast tumours. J. Appl. Toxicol. Int. J. 2004, 24, 5–13. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. The presence of pharmaceuticals in the environment due to human use—Present knowledge and future challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J. Hazard. Mater. 2017, 323, 274–298. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Khedri, M.; Didandeh, M.; Taheri, M.; Ghasemy, E.; Maleki RShon, H.K.; Razmjou, A. Removal of pharmaceutical pollutants from wastewater using 2D covalent organic frameworks (COFs): An in silico engineering study. Ind. Eng. Chem. Res. 2022, 61, 8809–8820. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman Jr, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- von Gunten, U. Oxidation Processes in Water Treatment: Are We on Track? Environ. Sci. Technol. 2018, 52, 5062–5075. [Google Scholar] [CrossRef]
- Sravan, J.S.; Matsakas, L.; Sarkar, O. Advances in Biological Wastewater Treatment Processes: Focus on Low-Carbon Energy and Resource Recovery in Biorefinery Context. Bioengineering 2024, 11, 281. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E. Nanofiltration membranes for food and pharmaceutical industries. Emergent Mater. 2021, 5, 1329–1343. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, S.; Kang, Y.; Zhao, W.; Xia, Y.; Yang, J.; Wang, H.; Zhang, X. Manipulating interfacial polymerization for polymeric nanofilms of composite separation membranes. Prog. Polym. Sci. 2021, 122, 101450. [Google Scholar] [CrossRef]
- Freger, V.; Ramon, G.Z. Polyamide desalination membranes: Formation, structure, and properties. Prog. Polym. Sci. 2021, 122, 101451. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, P.F.; Li, X.; Gan, B.; Wang, L.; Song, X.; Park, H.-D.; Tang, C.Y. A Critical Review on Thin-Film Nanocomposite Membranes with Interlayered Structure: Mechanisms, Recent Developments, and Environmental Applications. Environ. Sci. Technol. 2020, 54, 15563–15583. [Google Scholar] [CrossRef]
- Li, B.; Japip, S.; Chuang, T.S. Molecularly tunable thin-film nanocomposite membranes with enhanced molecular sieving for organic solvent forward osmosis. Nat. Commun. 2020, 11, 1198. [Google Scholar] [CrossRef]
- Neuwald, I.J.; Hübner, D.; Wiegand, H.L.; Valkov, V.; Borchers, U.; Nödler, K.; Scheurer, M.; Hale, S.E.; Arp, H.P.H.; Zahn, D. Occurrence, Distribution, and Environmental Behavior of Persistent, Mobile, and Toxic (PMT) and Very Persistent and Very Mobile (vPvM) Substances in the Sources of German Drinking Water. Environ. Sci. Technol. 2022, 56, 10857–10867. [Google Scholar] [CrossRef]
- Naim, R.; Ismail, A.F. Effect of polymer concentration on the structure and performance of PEI hollow fiber membrane contactor for CO2 stripping. J. Hazard. Mater. 2013, 250, 354–361. [Google Scholar] [CrossRef]
- Li, Z.; Ren, J.; Fane, A.G.; Li, D.F.; Wong, F.S. Influence of solvent on the structure and performance of cellulose acetate membranes. J. Membr. Sci. 2006, 279, 601–607. [Google Scholar] [CrossRef]
- Wang, Z.G.; Xu, Z.K.; Wan, L.S. Modulation the morphologies and performance of polyacrylonitrile-based asymmetric membranes containing reactive groups: Effect of non-solvents in the dope solution. J. Membr. Sci. 2006, 278, 447–456. [Google Scholar] [CrossRef]
- Shafie, N.A.; Seman, M.N.A.; Saufi, S.M.; Mohammad, A.W. Influence of Polyethersulfone substrate properties on the performance of thin film composite forward osmosis membrane: Effect of additive concentration, polymer concentration and casting thickness. Chem. Eng. Res. Des. 2023, 200, 186–201. [Google Scholar] [CrossRef]
- Liu, Z.; Xiang, J.; Hu, X.; Cheng, P.; Zhang, L.; Du, W.; Wang, S.; Tang, N. Effects of coagulation-bath conditions on polyphenylsulfone ultrafiltration membranes. Chin. J. Chem. Eng. 2021, 34, 332–340. [Google Scholar] [CrossRef]
- Xu, J.; Tang, Y.; Wang, Y.; Shan, B.; Yu, L.; Gao, C. Effect of coagulation bath conditions on the morphology and performance of PSf membrane blended with a capsaicin-mimic copolymer. J. Membr. Sci. 2014, 455, 121–130. [Google Scholar] [CrossRef]
- Cheng, Y.; Ying, Y.; Japip, S.; Jiang, S.D.; Chung, T.S.; Zhang, S.; Zhao, D. Advanced Porous Materials in Mixed Matrix Membranes. Adv. Mater. 2018, 30, 1870355. [Google Scholar] [CrossRef]
- Raaijmakers, M.J.T.; Benes, N.E. Current trends in interfacial polymerization chemistry. Prog. Polym. Sci. 2016, 63, 86–142. [Google Scholar] [CrossRef]
- Zhang, F.; Fan, J.; Wang, S. Interfacial Polymerization: From Chemistry to Functional Materials. Angew. Chem. Int. Ed. 2020, 59, 21840–21856. [Google Scholar] [CrossRef]
- Sorribas, S.; Gorgojo, P.; Téllez, C.; Coronas, J.; Livingston, A.G. High Flux Thin Film Nanocomposite Membranes Based on Metal-Organic Frameworks for Organic Solvent Nanofiltration. J. Am. Chem. Soc. 2013, 135, 15201–15208. [Google Scholar] [CrossRef]
- Colburn, A.S.; Meeks, N.; Weinman, S.T.; Bhattacharyya, D. High Total Dissolved Solids Water Treatment by Charged Nanofiltration Membranes Relating to Power Plant Applications. Ind. Eng. Chem. Res. 2016, 55, 4089–4097. [Google Scholar] [CrossRef]
- Manikandan, S.; Subbaiya, R.; Saravanan, M.; Ponraj, M.; Selvam, M.; Pugazhendhi, A. A critical review of advanced nanotechnology and hybrid membrane based water recycling, reuse, and wastewater treatment processes. Chemosphere 2022, 289, 132867. [Google Scholar] [CrossRef]
- Pendergast, M.M.; Hoek, E.M.V. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef]
- Zhao, Y.; Tong, T.; Wang, X.; Lin, S.; Reid, E.M.; Chen, Y. Differentiating Solutes with Precise Nanofiltration for Next Generation Environmental Separations: A Review. Environ. Sci. Technol. 2021, 55, 1359–1376. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiao, K.; Wang, X. Role of coexistence of negative and positive membrane surface charges in electrostatic effect for salt rejection by nanofiltration. Desalination 2018, 444, 75–83. [Google Scholar] [CrossRef]
- Khorram, M.; Chianeh, F.N.; Shamsodin, M. Preparation and characterization of a novel polyethersulfone nanofiltration membrane modified with BiO nanoparticles for enhanced separation performance and antifouling properties. J. Ind. Eng. Chem. 2022, 114, 456–474. [Google Scholar] [CrossRef]
- Omidvar, M.; Soltanieh, M.; Mousavi, S.M.; Saljoughi, E.; Moarefian, A.; Saffaran, H. Preparation of hydrophilic nanofiltration membranes for removal of pharmaceuticals from water. J. Environ. Health Sci. Eng. 2015, 13, 42. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, C.; Tan, L.; Zhou, W.; Shen, H.; Lu, C.; Dong, L. Enhancement of compatibility between covalent organic framework and polyamide membrane via an interfacial bridging method: Toward highly efficient water purification. J. Membr. Sci. 2022, 656, 120590. [Google Scholar] [CrossRef]
- Xu, G.R.; Wang, S.H.; Zhao, H.L.; Wu, S.B.; Xu, J.M.; Li, L.; Liu, X.Y. Layer-by-layer (LBL) assembly technology as promising strategy for tailoring pressure-driven desalination membranes. J. Membr. Sci. 2015, 493, 428–443. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Lan, Q.Q.; Wang, Y. Gradient nanoporous phenolics as substrates for high-flux nanofiltration membranes by layer-by-layer assembly of polyelectrolytes. Chin. J. Chem. Eng. 2020, 28, 114–121. [Google Scholar] [CrossRef]
- Zhan, X.; Tan, Z.A.; Domercq, B.; An, Z.; Zhang, X.; Barlow, S.; Li, Y.; Zhu, D.; Kippelen, B.; Marder, S.R. A high-mobility electron-transport polymer with broad absorption and its use in field-effect transistors and all-polymer solar cells. J. Am. Chem. Soc. 2007, 129, 7246–7247. [Google Scholar] [CrossRef]
- Lin, Y.L.; Tsai, C.C.; Zheng, N.Y. Improving organic and biological fouling resistance and removal of pharmaceutical and personal care products through nanofiltration by using in situ radical graft polymerization. Sci. Total Environ. 2018, 635, 543–550. [Google Scholar] [CrossRef]
- Kamrani, M.; Akbari, A.; Lehi, A.Y. Chitosan-modified acrylic nanofiltration membrane for efficient removal of pharmaceutical compounds. J. Environ. Chem. Eng. 2018, 6, 583–587. [Google Scholar] [CrossRef]
- Ouyang, Z.; Huang, Z.; Tang, X.; Xiong, C.; Tang, M.; Lu, Y. A dually charged nanofiltration membrane by pH-responsive polydopamine for pharmaceuticals and personal care products removal. Sep. Purif. Technol. 2019, 211, 90–97. [Google Scholar] [CrossRef]
- Song, J.; Xu, D.; Luo, X.; Han, Y.; Ding, J.; Zhu, X.; Yang, L.; Li, G.; Liang, H. In-situ assembled amino-quinone network of nanofiltration membrane for simultaneously enhanced trace organic contaminants separation and antifouling properties. J. Membr. Sci. 2022, 661, 120891. [Google Scholar] [CrossRef]
- Li, Y.; Su, Y.; Zhao, X.; Zhang, R.; Zhao, J.; Fan, X.; Jiang, Z. Surface fluorination of polyamide nanofiltration membrane for enhanced antifouling property. J. Membr. Sci. 2014, 455, 15–23. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Z. A loose nano-filtration membrane prepared by coating HPAN UF membrane with modified PEI for dye reuse and desalination. J. Membr. Sci. 2017, 524, 214–224. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, C.; Yang, T.; Zhou, W.; Chen, Y.; Wang, L.; Lu, C.; Dong, L. Imparting Outstanding Dispersibility to Nanoscaled 2D COFs for Constructing Organic Solvent Forward Osmosis Membranes. Small 2023, 19, 2300456. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, J.; Li, H. Chiral Covalent Organic Framework Packed Nanochannel Membrane for Enantioseparation. Angew. Chem. 2022, 61, e202204012. [Google Scholar] [CrossRef]
- Yuan, S.; Li, X.; Zhu, J.; Zhang, G.; Van Puyvelde, P.; Van der Bruggen, B. Covalent organic frameworks for membrane separation. Chem. Soc. Rev. 2019, 10, 2665–2681. [Google Scholar] [CrossRef]
- Khatoon, N.; Ali, N.; Yang, H.; Jun, W. Review of MOFs and their applications in the preparation of loose nanofiltration membranes for dye and salt fractionation. Desalination Water Treat. 2024, 317, 100092. [Google Scholar] [CrossRef]
- Li, X.; Lin, W.; Sharma, V.; Gorecki, R.; Ghosh, M.; Moosa BAAristizabal, S.; Hong, S.; Khashab, N.M.; Nunes, S.P. Polycage membranes for precise molecular separation and catalysis. Nat. Commun. 2023, 14, 3112. [Google Scholar] [CrossRef]
- Zhang, H.; Wan, Y.; Luo, J.; Darling, S.B. Drawing on membrane photocatalysis for fouling mitigation. ACS Appl. Mater. Interfaces 2021, 13, 14844–14865. [Google Scholar] [CrossRef]
- Zhang, K.; He, Z.; Gupta, K.M.; Jiang, J. Computational design of 2D functional covalent-organic framework membranes for water desalination. Environ. Sci. Water Res. Technol. 2017, 3, 735–743. [Google Scholar] [CrossRef]
- Chen, L.; Du, J.; Zhou, W.; Shen, H.; Tan, L.; Zhou, C.; Dong, L. Microwave-Assisted Solvothermal Synthesis of Covalent Organic Frameworks (COFs) with Stable Superhydrophobicity for Oil/Water Separation. Chem. Asian J. 2020, 15, 3421–3427. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, J.; Cheng, X.; Qiu, Q.; Ma, G.; Jiang, C.; Pan, J. Colloidal 2D Covalent Organic Framework-Tailored Nanofiltration Membranes for Precise Molecular Sieving. ACS Appl. Mater. Interfaces 2023, 15, 53924–53934. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Z.; Yin, C.; Zhang, X.; Long, J.; Zhang, Z.; Wang, Y. Design of Three-Dimensional Covalent Organic Framework Membranes for Fast and Robust Organic Solvent Nanofiltration. Angew. Chem. Int. Ed. 2022, 61, e202207559. [Google Scholar] [CrossRef]
- Jiang, X.T.; Yin, Q.; Liu, B.T.; Chen, J.Y.; Wang, R.; Liu, T.F. Porous hydrogen-bonded organic framework membranes for high-performance molecular separation. Nanoscale Adv. 2021, 3, 3441–3446. [Google Scholar] [CrossRef]
- Zhai, Z.; Jiang, C.; Zhao, N.; Dong, W.; Li, P.; Sun, H.; Niu, Q.J. Polyarylate membrane constructed from porous organic cage for high-performance organic solvent nanofiltration. J. Membr. Sci. 2020, 595, 117505. [Google Scholar] [CrossRef]
- Shinde, D.B.; Sheng, G.; Li, X.; Ostwal, M.; Emwas, A.H.; Huang, K.W.; Lai, Z. Crystalline 2D Covalent Organic Framework Membranes for High-Flux Organic Solvent Nanofiltration. J. Am. Chem. Soc. 2018, 140, 14342–14349. [Google Scholar] [CrossRef]
- Kandambeth, S.; Biswal, B.P.; Chaudhari, H.D.; Rout, K.C.; Mitra, S.; Karak, S.; Das, A.; Mukherjee, R.; Kharul, U.K.; Banerjee, R. Selective Molecular Sieving in Self-Standing Porous Covalent-Organic-Framework Membranes. Adv. Mater. 2017, 29, 1603945. [Google Scholar] [CrossRef]
- Wu, X.; Hao, L.; Zhang, J.; Zhang, X.; Wang, J.; Liu, J. Polymer-TiCT composite membranes to overcome the trade-off in solvent resistant nanofiltration for alcohol-based system. J. Membr. Sci. 2016, 515, 175–188. [Google Scholar] [CrossRef]
- Zhang, R.; Ji, S.; Wang, N.; Wang, L.; Zhang, G.; Li, J.R. Coordination-Driven In Situ Self-Assembly Strategy for the Preparation of Metal-Organic-Framework Hybrid Membranes. Angew. Chem. Int. Ed. 2014, 53, 9775–9779. [Google Scholar] [CrossRef]
- Wang, N.; Ji, S.; Zhang, G.; Li, J.; Wang, L. Self-assembly of graphene oxide and polyelectrolyte complex nanohybrid membranes for nanofiltration and pervaporation. Chem. Eng. J. 2012, 213, 318–329. [Google Scholar] [CrossRef]
- He, K.T.; Koepke, J.C.; Barraza-Lopez, S.; Lyding, J.W. Separation-Dependent Electronic Transparency of Monolayer Graphene Membranes on III-V Semiconductor Substrates. Nano Lett. 2010, 10, 3446–3452. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Maes, M.; Cano-Odena, A.; Alaerts, L.; De Vos, D.E.; Vankelecom, I.F. Solvent resistant nanofiltration (SRNF) membranes based on metal-organic frameworks. J. Membr. Sci. 2009, 344, 190–198. [Google Scholar] [CrossRef]
- Guan, X.; Li, H.; Ma, Y.; Xue, M.; Fang, Q.; Yan, Y.; Valtchev, V.; Qiu, S. Chemically stable polyarylether-based covalent organic frameworks. Nat. Chem. 2019, 11, 587–594. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, M.; Wang, H.; Han, C.; Pan, F.; Sun, J. Covalent Organic Framework for Rechargeable Batteries: Mechanisms and Properties of Ionic Conduction. Adv. Energy Mater. 2022, 12, 2200057. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, Z.; Hussein, I.; Wang, Z.; Zhao, S. Tunable Ionic Sieving Membrane via Reactive Layer-By-Layer Assembly of Porous Organic Cages. Adv. Funct. Mater. 2024, 34, 2315750. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, C.; Feng, F.; Jia, Y.; Dong, L.; Zhang, S. Lewis acid-driven interlayer shifting in sub-nm 2D covalent organic framework membranes for hydrogen purification. AIChE J. 2025, 71, e18835. [Google Scholar] [CrossRef]
- Li, J.R.; Sculley, J.; Zhou, H.C. Metal-organic frameworks for separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef]
- Rad, L.R.; Anbia, M.; Vatanpour, V. MIL-101(Fe)- and MIL-101(Fe)-NH2-Loaded Thin Film Nanofiltration Membranes for the Removal of Fluoxetine Hydrochloride from Pharmaceutical Wastewater. Ind. Eng. Chem. Res. 2025, 64, 3154–3167. [Google Scholar] [CrossRef]
- Abdi, S.; Nasiri, M. Enhanced performance of thin film nanocomposite (TFN) membranes by incorporating hydrophilic MOF-808 into the organic phase: Towards eliminating non-steroidal anti-inflammatory drugs (NSAIDs) from aqueous solutions. Desalination 2025, 601, 118590. [Google Scholar] [CrossRef]
- Dai, R.; Wang, X.; Tang, C.Y.; Wang, Z. Dually Charged MOF-Based Thin-Film Nanocomposite Nanofiltration Membrane for Enhanced Removal of Charged Pharmaceutically Active Compounds. Environ. Sci. Technol. 2020, 54, 7619–7628. [Google Scholar] [CrossRef] [PubMed]
- Hajheidari, M.; Homayoonfal, M. Dual stimuli pH-responsive nanofiltration membranes with tunable permeability and selectivity during separation of antitumor drug doxorubicin. J. Environ. Chem. Eng. 2023, 11, 110469. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Mishra, U. A sustainable approach for the removal of pharmaceutical contaminants from effluent using polyamide thin-film composite membranes integrated with Zn-based metal organic frameworks. Environ. Sci. Pollut. Res. 2023, 30, 110104–110118. [Google Scholar] [CrossRef] [PubMed]
- Banjerdteerakul, K.; Peng, H.; Li, K. COF-based nanofiltration membrane for effective treatment of wastewater containing pharmaceutical residues. J. Membr. Sci. 2023, 6811, 121780. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, S.; Chi, M.; Wang, Y.; Van Eygen, G.; Zhao, R.; Zhang, X.; Li, G.; Volodine, A.; Hu, S.; et al. Efficient capture of endocrine-disrupting compounds by a high-performance nanofiltration membrane for wastewater treatment. Water Res. 2022, 227, 119322. [Google Scholar] [CrossRef]
- Yue, L.P.; Kong, F.X.; Wang, Y.; Chen, J.F.; Zhou, A.G. Crystalline covalent organic framework membrane with tailored chargeability for efficient pharmaceutical rejection by in-situ functionalization of polyethylene-imine. J. Membr. Sci. 2025, 716, 123486. [Google Scholar] [CrossRef]
- Zhao, S.; Di, N.; Lei, R.; Wang, J.; Wang, Z. Triphenylamine-based COFs composite membrane fabricated through oligomer-triggered interfacial polymerization. J. Membr. Sci. 2023, 672, 121424. [Google Scholar] [CrossRef]
- Kong, F.X.; Zhang, S.Y.; Zhang, D.W.; Xia, P.; Chen, J.F. The influence of the preheated PSf substrate on COF-LZU1 layer formation and the performance for salt and pharmaceutical rejection. Desalination 2024, 579, 117514. [Google Scholar] [CrossRef]
- Liu, D.; Tian, C.; Shan, M.; Zhu, J.; Zhang, Y. Interface synthesis of flexible benzimidazole-linked polymer molecular-sieving membranes with superior antimicrobial activity. J. Membr. Sci. 2022, 648, 120344. [Google Scholar] [CrossRef]
- Guo, B.Y.; Jiang, S.D.; Tang, M.J.; Li, K.; Sun, S.; Chen, P.Y.; Zhang, S. MoS2 Membranes for Organic Solvent Nanofiltration: Stability and Structural Control. J. Phys. Chem. Lett. 2019, 10, 4609–4617. [Google Scholar] [CrossRef]
- Tong, Z.; Guo, H.; Liu, X.; Zhang, B. Organic Solvent Forward Osmosis of Graphene Oxide-Based Membranes for Enrichment of Target Products. Ind. Eng. Chem. Res. 2020, 59, 19012–19019. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Van der Bruggen, B. An MXene-based membrane for molecular separation. Environ. Sci. Nano 2020, 7, 1289–1304. [Google Scholar] [CrossRef]
- Tandel, A.M.; Guo, W.; Bye, K.; Huang, L.; Galizia, M.; Lin, H. Designing organic solvent separation membranes: Polymers, porous structures, 2D materials, and their combinations. Mater. Adv. 2021, 2, 4574–4603. [Google Scholar] [CrossRef]
- Macha, M.; Marion, S.; Nandigana, V.V.; Radenovic, A. 2D materials as an emerging platform for nanopore-based power generation. Nat. Rev. Mater. 2019, 4, 588–605. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, L.; Deng, H.; Liu, Q.; You, X.; Yuan, J.; Jiang, Z.; Zhang, S. Ultrathin Membranes for Separations: A New Era Driven by Advanced Nanotechnology. Adv. Mater. 2022, 34, e2108457. [Google Scholar] [CrossRef]
- Han, J.L.; Xia, X.; Haider, M.R.; Jiang, W.L.; Tao, Y.; Liu, M.J.; Wang, H.C.; Ding, Y.C.; Hou, Y.N.; Cheng, H.Y.; et al. Functional graphene oxide membrane preparation for organics/inorganic salts mixture separation aiming at advanced treatment of refractory wastewater. Sci. Total Environ. 2018, 628, 261–270. [Google Scholar] [CrossRef]
- Chu, K.H.; Fathizadeh, M.; Yu, M.; Flora, J.R.; Jang, A.; Jang, M.; Park, C.M.; Yoo, S.S.; Her, N.; Yoon, Y. Evaluation of removal mechanisms in a graphene oxide-coated ceramic ultrafiltration membrane for retention of natural organic matter, pharmaceuticals, and inorganic salts. ACS Appl. Mater. Interfaces 2017, 9, 40369–40377. [Google Scholar] [CrossRef]
- Hidalgo, A.M.; Gómez, M.; Murcia, M.D.; León, G.; Miguel, B.; Gago, I.; Martínez, P.M. Ibuprofen Removal by Graphene Oxide and Reduced Graphene Oxide Coated Polysulfone Nanofiltration Membranes. Membranes 2022, 12, 562. [Google Scholar] [CrossRef]
- Cardoso, A.M.D.J.M.; Cardoso, V.L.; Reis, M.H.M. Graphene oxide membranes coated on porous ceramic hollow fibers for the removal of pharmaceutical contaminants from wastewater. Open Ceram. 2025, 21, 100750. [Google Scholar] [CrossRef]
- Fathizadeh, M.; Tien, H.N.; Khivantsev, K.; Chen, J.T.; Yu, M. Printing ultrathin graphene oxide nanofiltration membranes for water purification. J. Mater. Chem. A 2017, 5, 20860–20866. [Google Scholar] [CrossRef]
- Oh, Y.; Armstrong, D.L.; Finnerty, C.; Zheng, S.; Hu, M.; Torrents, A.; Mi, B. Understanding the pH-responsive behavior of graphene oxide membrane in removing ions and organic micropollulants. J. Membr. Sci. 2017, 541, 235–243. [Google Scholar] [CrossRef]
- Yadav, D.; Gohain, M.B.; Bora, M.; Sarma, S.S.; Karki, S.; Kumar, D.; Ingole, P.G. Greener synthesis of thin-film nanocomposite membranes with varied nanofillers for enhanced organic micropollutant removal. Sep. Purif. Technol. 2024, 335, 126125. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Zheng, N.-Y.; Chen, Y.S. Enhancing hydrogen peroxide Tolerance and Separation Performance through the Modification of the Polyamide Layer of a Thin-Film Composite Nanofiltration Membrane by Using Graphene Oxide. Membranes 2021, 11, 592. [Google Scholar] [CrossRef]
- Abraham, J.; Vasu, K.S.; Williams, C.D.; Gopinadhan, K.; Su, Y.; Cherian, C.T.; Dix, J.; Prestat, E.; Haigh, S.J.; Grigorieva, I.V.; et al. Tunable sieving of ions using graphene oxide membranes. Nat. Nanotechnol. 2017, 12, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.K.; Carbone, P.; Wang, F.C.; Kravets, V.G.; Su, Y.; Grigorieva, I.V.; Wu, H.A.; Geim, A.K.; Nair, R.R. Precise and Ultrafast Molecular Sieving Through Graphene Oxide Membranes. Science 2014, 343, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.X.; Liu, Q.; Dong, L.Q.; Zhang, T.; Wei, Y.B.; Chen, J.F.; Wang, Y.; Guo, C.-M. Rejection of pharmaceuticals by graphene oxide membranes: Role of crosslinker and rejection mechanism. J. Membr. Sci. 2020, 612, 118338. [Google Scholar] [CrossRef]
- Kong, F.X.; Liu, Q.; You, L.; Lu, P.; Liu, T.; Sun, G.D.; Wang, Y.; Chen, J.-F. Facile preparation of dopamine mediated graphene oxide composite membranes with enhanced stability for nanofiltration: Structure, performance and stability. Desalination 2022, 534, 115778. [Google Scholar] [CrossRef]
- Tan, R.; Shao, H.; Wan, Z.; Li, Y.; Gu, J.; Jia, R.; Hong, Z.; Ji, Z.; Zhang, S.; Li, X.; et al. De novo contrived three-in-one oriented graft molecule for high-performance GO nanofiltration membranes with ultra-low friction 2D nanochannels. J. Membr. Sci. 2024, 709, 123151. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef]
- Arshad, F.; Ali, M.S.; Ali, L.; Zou, L. Catalytic MnO/MXene/chitosan nanocomposite membrane in removing caffeine from wastewater. Sep. Purif. Technol. 2025, 361, 131504. [Google Scholar] [CrossRef]
- Karahan, H.E.; Goh, K.; Zhang, C.; Yang, E.; Yıldırım, C.; Chuah, C.Y.; Ahunbay, M.G.; Lee, J.; Tantekin-Ersolmaz, B.; Bae, T.-H. MXene Materials for Designing Advanced Separation Membranes. MXene Materials for Designing Advanced Separation Membranes. Adv. Mater. 2020, 32, 1906697. [Google Scholar] [CrossRef]
- Zu, P.; Xing, X.; Wan, H.; Yan, G.; Zhang, G. Preparation of larger MXene layers and research progress in the field of gas adsorption and separation. Sep. Purif. Technol. 2023, 327, 125010. [Google Scholar] [CrossRef]
- Li, Z.K.; Wei, Y.; Gao, X.; Ding, L.; Lu, Z.; Deng JYang, X.; Caro, J.; Wang, H. Antibiotics Separation with MXene Membranes Based on Regularly Stacked High-Aspect-Ratio Nanosheets. Angew. Chem. Int. Ed. 2020, 59, 9751–9756. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yi, F.; Li, R.H.; Min, X.; Qin, H.; Cheng, S.Q.; Liu, Y. Inorganic-Organic Hybrid Membrane Based on Pillararene-Intercalated MXene Nanosheets for Efficient Water Purification. Angew. Chem. Int. Ed. 2022, 61, e202200482. [Google Scholar] [CrossRef] [PubMed]
- Irani-nezhad, M.H.; Khataee, A.; Vatanpour, V.; Arefi-Oskoui, S.; Orooji, Y. Surface modification of nanofiltration membrane using 2D-black mica@graphene oxide nanocomposite with improved performance in water treatment. Sep. Purif. Technol. 2025, 354, 129133. [Google Scholar] [CrossRef]
- Oikawa, M.; Takeuchi, H.; Koide, R.; Yoshizawa, N.; Wang, Z.M.; Koura, S. Achieving overall rejection of pharmaceuticals and personal care products of different polarities by controlling interlayer charging environment of graphene oxide membrane using carbon quantum dots. Chem. Eng. J. 2023, 472, 144811. [Google Scholar] [CrossRef]
- Fang, S.Y.; Zhang, P.; Gong, J.L.; Tang, L.; Zeng, G.M.; Song, B.; Cao, W.-C.; Li, J.; Ye, J. Construction of highly water-stable metal-organic framework UiO-66 thin-film composite membrane for dyes and antibiotics separation. Chem. Eng. J. 2020, 385, 123400. [Google Scholar] [CrossRef]
- Jillani, S.M.S.; Waheed, A.; Baig, U.; Aljundi, I.H. Fabrication of highly efficient nanofiltration membranes decorated with praseodymium based triamino-functionalized MCM-41 for desalination and micropollutants removal. Arab. J. Chem. 2024, 17, 105450. [Google Scholar] [CrossRef]
- Mahato, P.; Arshad, F.; Ali, M.S.; Perera, C.S.; Zou, L. Removal of pharmaceutical compounds by chitosan nanocomposite membranes with catalytic additives from wastewater. Desalination 2025, 602, 118635. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Mukherjee, D.; Deb, N.; Swarnakar, S.; Banerjee, S. Application of green synthesized ZnO nanoparticle coated ceramic ultrafiltration membrane for remediation of pharmaceutical components from synthetic water: Reusability assay of treated water on seed germination. J. Environ. Chem. Eng. 2020, 8, 103803. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H.; Cui, J.; Yang, Z.; Hou, R.; Ju, Y.; Lu, X.; Chen, F. Covalent organic framework-immobilized wood membrane for efficient, durable, and high-flux nanofiltration. J. Clean. Prod. 2023, 384, 135595. [Google Scholar] [CrossRef]
- Basu, S.; Balakrishnan, M. Polyamide thin film composite membranes containing ZIF-8 for the separation of pharmaceutical compounds from aqueous streams. Sep. Purif. Technol. 2017, 179, 118–125. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Liu, Y.L.; Wang, X.M.; Huang, X.; Xie, Y.F. Impacts of Metal-Organic Frameworks on Structure and Performance of Polyamide Thin-Film Nanocomposite Membranes. ACS Appl. Mater. Interfaces 2019, 11, 13724–13734. [Google Scholar] [CrossRef] [PubMed]
- Paseta, L.; Antoran, D.; Coronas, J.; Tellez, C. 110th Anniversary: Polyamide/Metal–Organic Framework Bilayered Thin Film Composite Membranes for the Removal of Pharmaceutical Compounds from Water. Ind. Eng. Chem. Res. 2019, 58, 4222–4230. [Google Scholar] [CrossRef]
- Chen, Q.-C.; Yu, B.; Deng, H.Y. High flux and antifouling nanofiltration membrane modified by Ag@UiO-66-NH2 and its application for biphenol a removal. Adv. Polym. Technol. 2022, 2022, 4197365. [Google Scholar] [CrossRef]
- Matin, A.; Baig, N.; Anand, D.; Ahmad, I.; Sajid, M.; Nawaz, M.S. Thin-film nanocomposite membranes for efficient removal of emerging pharmaceutical organic contaminants from water. Environ. Res. 2023, 237, 116905. [Google Scholar] [CrossRef]
- Dai, R.; Guo, H.; Tang, C.Y.; Chen, M.; Li, J.; Wang, Z. Hydrophilic Selective Nanochannels Created by Metal Organic Frameworks in Nanofiltration Membranes Enhance Rejection of Hydrophobic Endocrine-Disrupting Compounds. Environ. Sci. Technol. 2019, 53, 13776–13783. [Google Scholar] [CrossRef]
- Baig, N.; Matin, A. Incorporating functionalized graphene oxide into diethylene triamine-based nanofiltration membranes can improve the removal of emerging organic micropollutants. J. Colloid Interface Sci. 2024, 676, 657–669. [Google Scholar] [CrossRef]
- Wang, J.; Li, N.; Zhao, Y.; Xia, S. Graphene oxide modified semi-aromatic polyamide thin film composite membranes for PPCPs removal. Desalin. Water Treat. 2017, 66, 166–175. [Google Scholar] [CrossRef]
- Dai, R.; Han, H.; Wang, T.L. Enhanced removal of hydrophobic endocrine disrupting compounds from wastewater by nanofiltration membranes intercalated with hydrophilic MoS2 nanosheets: Role of surface properties and internal nanochannels. J. Membr. Sci. 2021, 628, 119267. [Google Scholar] [CrossRef]
- Dai, R.; Han, H.; Wang, T.; Li, X.; Wang, Z. Enhanced Removal of Endocrine-Disrupting Compounds from Wastewater Using Reverse Osmosis Membrane with Titania Nanotube-Constructed Nanochannels. Membranes 2022, 12, 958. [Google Scholar]
- Rakhshan, N.; Pakizeh, M. Removal of triazines from water using a novel OA modified SiO2/PA/PSf nanocomposite membrane. Sep. Purif. Technol. 2015, 147, 245–256. [Google Scholar] [CrossRef]
- Guo, H.; Deng, Y.; Yao, Z.; Yang, Z.; Wang, J.; Lin, C.; Zhang, T.; Zhu, B.; Tang, C.Y. A highly selective surface coating for enhanced membrane rejection of endocrine disrupting compounds: Mechanistic insights and implications. Water Res. 2017, 121, 197–203. [Google Scholar] [CrossRef]
- Fang, C.; Garcia-Rodriguez, O.; Shang, C.; Imbrogno, J.; Swenson, T.M.; Lefebvre, O.; Zhang, S. An omniphobic membrane with macro-corrugation for the treatment of real pharmaceutical wastewater via membrane distillation. J. Membr. Sci. 2023, 676, 121582. [Google Scholar] [CrossRef]
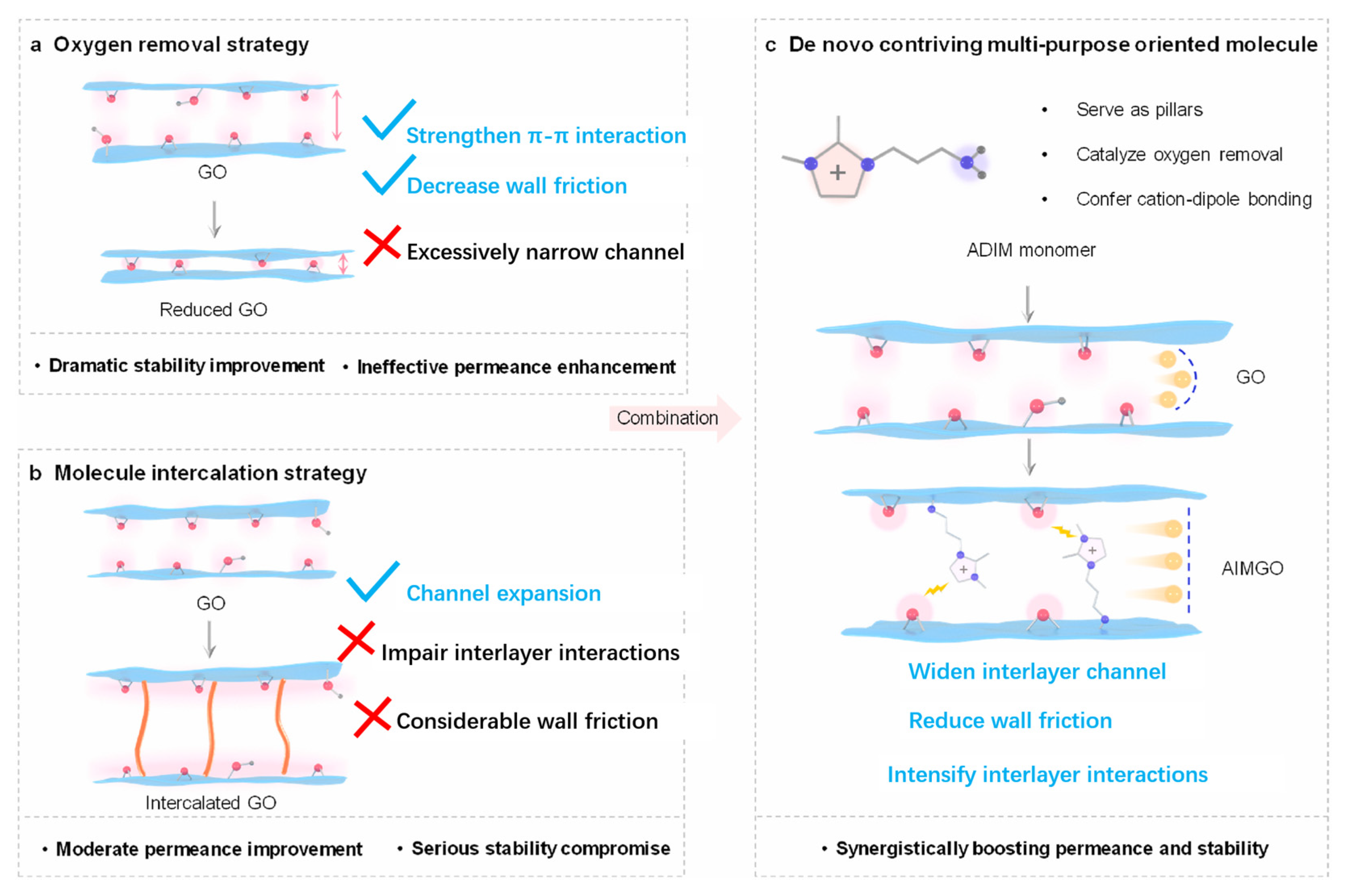
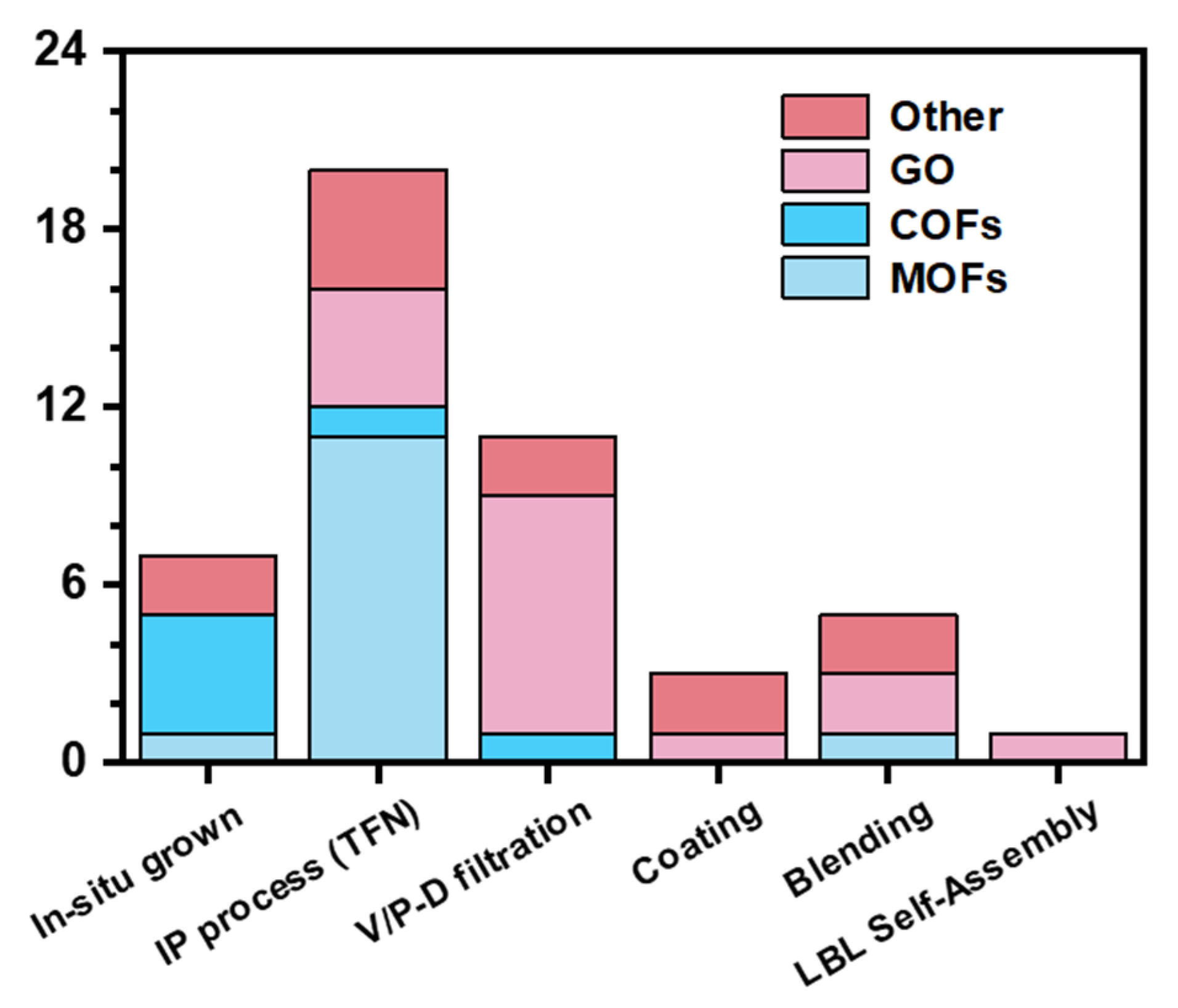
| OMPs | Sub-Class | Chemicals |
|---|---|---|
| PhACs | Antibiotics | Amoxicillin, Cephalexin, Rifampicin, Bacitracin, Azithromycin, Erythromycin, Tetracycline, Penicillin, Ampicillin Sodium, Thiamphenicol, Oxytetracycline, Chlortetracycline, Clarithromycin, Roxithromycin, Tylosin, Ciprofloxacin, Norfloxacin, Levofloxacin, Lincomycin |
| Analgesics/ Antipyretics/ Anti-inflammatory | Paracetamol, Acetaminophen, Ibuprofen, Naproxen, Diclofenac, Mefenamic acid, Aspirin, Ketoprofen, Indometacin, Clofibric acid, Bezafibrate, Fenoprofen, Ethenzamide, Antipyrine, Isopropylantipyrine | |
| Insecticides | DEET, Crotamiton, Atrazine, Propazine, Prometryn | |
| Antiepileptic | Carbamazepine, Primidone | |
| β-receptor blockers/Agonist | Atenolol, Propranolol, Salbutamol, Metoprolol, Terbutaline | |
| Antipsychotic/Antidepressants | Fluoxetine, Sulpiride, Amitriptyline, Sertraline, Paroxetine, Nortriptyline, Doxorubicin, Omeprazole, Diltiazem | |
| PCPs | Preservatives (Parabens) | Methylparaben, Propylparaben, Ethylparaben, Benzylparaben |
| Antimicrobials/Disinfectants | Triclosan, Triclocarban | |
| Surfactants/Dyes/Miscellaneous | Congo red, Methyl blue, Rhodamine B, Calcein, Coomassie brilliant blue, Sodium 2-biphenylate, DEET | |
| EDCs | Hormones | 17α-Ethynylestradiol |
| Hormone-like/Metabolic regulator | Bisphenol A, Bisphenol AF, Sodium 2-biphenylate, Metformin, Gemfibrozil | |
| Others | Loperamide HCl, Caffeine, Omeprazole, Dipyridamole, Iodixanol, Berberine chloride | |
| Industry Segment (e.g.) | Annual Product Tonnage (Each) | E-Factor (kg Waste/ kg Product) | Total Annual Waste Tonnage | Synthetic Steps | Years of Development |
|---|---|---|---|---|---|
| Petrochemicals (solvents, detergents) [25] | 5 × 109 | 0.1–5 | 109 | - | 110+ |
| Bulk chemicals (plastics, polymers) [26] | 5 × 108 | 1–5 | 2 × 109 | 1–2 | 20–60 |
| Fine chemicals (coatings, electronic parts, pharmaceutical raw materials) [27] | 106–107 | 5–50 | 50 × 108 | 3–4 | 14–17 |
| Pharmaceuticals (antibiotics, drugs, vaccines) [28] | 104–105 | 50–100 | 107 | 5+ | 13–15 |
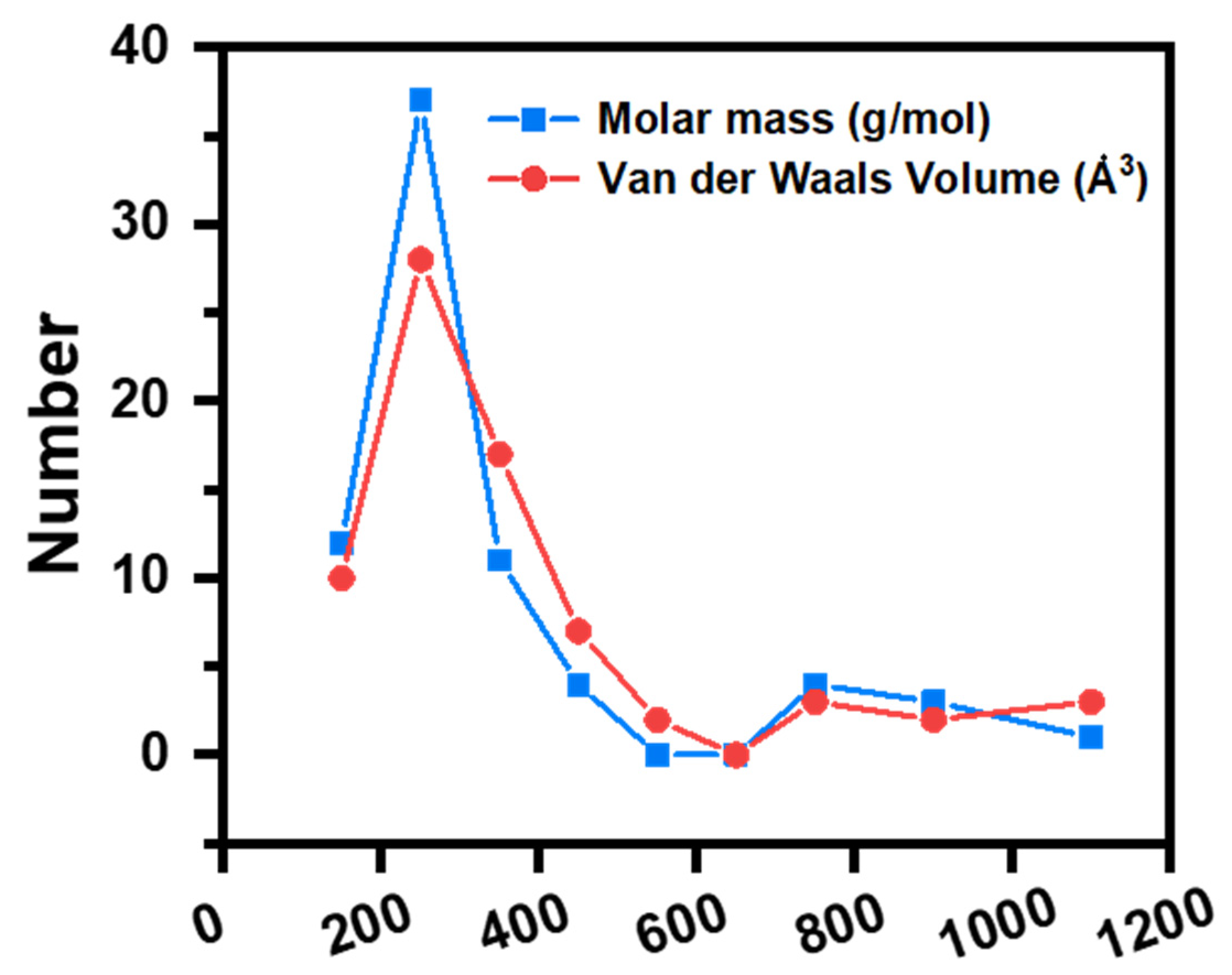
| OMP Name | Molar Mass (Da) | Formula | Charge (pH = 7) | Van der Waals Volume (Å3) |
|---|---|---|---|---|
| Methylparaben | 152.1 | C8H8O3 | Neutral | 135.76 |
| Paracetamol | 151.2 | C8H9NO2 | Neutral | 138.08 |
| Ethylparaben | 166.2 | C9H10O3 | Neutral | 152.62 |
| Ethenzamide | 165.2 | C9H11NO2 | Neutral | 155.37 |
| Caffeine | 194.2 | C8H10N4O2 | Neutral | 164.26 |
| Propylparaben | 180.2 | C10H12O3 | Neutral | 169.57 |
| Antipyrine | 188.2 | C11H12N2O | Neutral | 174.44 |
| Atrazine | 215.7 | C8H14ClN5 | Neutral | 190.9 |
| DEET | 191.3 | C12H17NO | Neutral | 198.31 |
| Primidone | 218.3 | C12H14N2O2 | Neutral | 200.65 |
| Benzylparaben | 228.2 | C14H12O3 | Neutral | 206.36 |
| Crotamiton | 203.3 | C13H17NO | Neutral | 207.44 |
| Propazine | 229.7 | C9H16ClN5 | Neutral | 207.98 |
| Carbamazepine | 236.3 | C15H12N2O | Neutral | 210.15 |
| Triclosan | 289.5 | C12H7Cl3O2 | Neutral | 212.06 |
| Bisphenol A | 228.3 | C15H16O2 | Neutral | 221.5 |
| Isopropylantipyrine | 230.3 | C14H18N2O | Neutral | 225.4 |
| Prometryn | 241.3 | C10H19N5S | Neutral | 229.85 |
| Triclocarban | 315.6 | C13H9Cl3N2O | Neutral | 236.67 |
| Bisphenol AF | 336.4 | C15H10F6O2 | Neutral | 251.77 |
| Ampicillin Sodium | 371.4 | C16H18N3NaO4S | Neutral | 269.23 |
| Norfloxacin | 319.3 | C16H18FN3O3 | Neutral | 277.46 |
| Thiamphenicol | 356.21 | C12H15Cl2NO5S | Neutral | 278.94 |
| Ciprofloxacin | 331.3 | C17H18FN3O3 | Neutral | 282.83 |
| 17α-Ethynylestradiol | 296.4 | C20H24O2 | Neutral | 291.82 |
| Omeprazole | 345.4 | C17H19N3O3S | Neutral | 301.14 |
| Levofloxacin | 361.4 | C18H20FN3O4 | Neutral | 309.96 |
| Rhodamine B | 479 | C28H31ClN2O3 | Neutral | 422.26 |
| Rifampicin | 822.9 | C43H58N4O12 | Neutral | 759.07 |
| Iodixanol | 1550.2 | C35H44I6N6O15 | Neutral | 852.98 |
| Bacitracin | 1422.7 | C66H103N17O16S | Neutral | 1314.36 |
| Metformin | 129.2 | C4H11N5 | Positive | 123.58 |
| Aspirin | 180.2 | C9H8O4 | Positive | 154.85 |
| Clofibric acid | 214.7 | C10H11ClO3 | Positive | 184.05 |
| Ibuprofen | 206.3 | C13H18O2 | Positive | 211.8 |
| Naproxen | 230.3 | C14H14O3 | Positive | 213.06 |
| Terbutaline | 225.3 | C12H19NO3 | Positive | 222.28 |
| Fenoprofen | 242.3 | C15H14O3 | Positive | 223.44 |
| Mefenamic acid | 241.3 | C15H15NO2 | Positive | 225.99 |
| Ketoprofen | 254.3 | C16H14O3 | Positive | 233.68 |
| Diclofenac | 296.1 | C14H11Cl2NO2 | Positive | 236.85 |
| Salbutamol | 239.3 | C13H21NO3 | Positive | 239.15 |
| Propranolol | 259.3 | C16H21NO2 | Positive | 257.56 |
| Atenolol | 266.3 | C14H22N2O3 | Positive | 261.34 |
| Nortriptyline | 263.4 | C19H21N | Positive | 265.01 |
| Sertraline | 306.2 | C17H17Cl2N | Positive | 266.82 |
| Fluoxetine | 309.3 | C17H18F3NO | Positive | 274.24 |
| Metoprolol | 267.4 | C15H25NO3 | Positive | 274.25 |
| Amitriptyline | 277.4 | C20H23N | Positive | 282.76 |
| Berberine chloride | 371.8 | C20H18NO4Cl | Positive | 292.73 |
| Paroxetine | 329.4 | C19H20FNO3 | Positive | 293.59 |
| Indometacin | 357.8 | C19H16ClNO4 | Positive | 300.84 |
| Sulpiride | 341.4 | C15H23N3O4S | Positive | 307.33 |
| Bezafibrate | 361.8 | C19H20ClNO4 | Positive | 319.67 |
| Diltiazem | 414.5 | C22H26N2O4S | Positive | 378.6 |
| Lincomycin | 406.5 | C18H34N2O6S | Positive | 384.58 |
| Loperamide HCl | 477 | C29H33ClN2O2 | Positive | 451.19 |
| Doxorubicin | 543.5 | C27H29NO11 | Positive | 463.44 |
| Dipyridamole | 504.6 | C24H40N8O4 | Positive | 476.19 |
| Erythromycin | 733.9 | C37H67NO13 | Positive | 727.13 |
| Clarithromycin | 747 | C38H69NO13 | Positive | 744.46 |
| Azithromycin | 749 | C38H72N2O12 | Positive | 754.48 |
| Roxithromycin | 837 | C41H76N2O15 | Positive | 824.52 |
| Tylosin | 916.2 | C46H77NO17 | Positive | 882.63 |
| Penicillin | 243 | C9H11N2O4 | Negative | 225.18 |
| Gemfibrozil | 250.3 | C15H22O3 | Negative | 255.17 |
| Cephalexin | 347.4 | C16H17N3O4S | Negative | 290.71 |
| Amoxicillin | 365.4 | C16H19N3O5S | Negative | 307.12 |
| Tetracycline | 444.4 | C22H24N2O8 | Negative | 379.67 |
| Oxytetracycline | 460.4 | C22H24N2O9 | Negative | 388.09 |
| Chlortetracycline | 478.9 | C22H23ClN2O8 | Negative | 396.69 |
| Penicillin | 243 | C9H11N2O4 | Negative | 225.18 |
| Types of Organic Framework | Nodes | Chemical Bonds | Crystallinity |
|---|---|---|---|
| MOFs | Metal ions or clusters | Coordination bond | High |
| POFs | Organic ligand | Chemical bond | Low, Moderate |
| COFs | Organic ligand | Covalent bond | Moderate or high |
| HOFs | Organic ligand | Hydrogen bond | Moderate or high |
| Membrane | Mode | Press. (bar) | Separation Performance (OMPs; Concentration, ppm; Flux, LMH/bar; Rejection, %) | Refs. |
|---|---|---|---|---|
| MIL-101(Fe)-NH2-loaded MAq | cross | 5 | Fluoxetine hydrochloride; 50; 7.76; 81.9 Sertraline hydrochloride; 50; 7.76; 75.8 Paroxetine hydrochloride; 50; 7.76; 71.5 Nortriptyline hydrochloride; 50; 7.76; 73.1 | [109] |
| PES/MOF-808@PA TFN | dead-end | 4 | Aspirin; 10; 6.04; 88.76 Naproxen; 10; 6.04; 84.51 Mefenamic acid; 10; 6.04; 70.56 | [110] |
| MOF/PA-TFC | cross | 5 | Paracetamol; 50; ~3.5; 93 Ibuprofen; 50; ~3.5; 98 Amoxicillin; 50; ~3.5; 99 | [113] |
| SurZn3/SubCo3 | cross | 4 | Doxorubicin; 25; 4.41; 89.0 | [112] |
| AMSA-MIL-101(Cr) | cross | 8 | Terbutaline; 0.2; 23; ~83 Atenolol; 0.2; 23; ~90 Fluoxetine; 0.2; 23; ~93 Ketoprofen; 0.2; 23; ~90 Diclofenac; 0.2; 23; ~90 Bezafibrate; 0.2; 23; ~90 | [111] |
| PSF-ZIF-8/PA | dead-end | 4 | Paracetamol; 100; 3.5; ~55 | [152] |
| TFN NH2-UiO-66-PL-7.6 | cross | 10 | Phenacetine; 0.5; 6.97; ~67 Nalidixic acid; 0.5; 6.97; ~85 Carbamazepine; 0.5; 6.97; ~76 Sulfamethoxazole; 0.5; 6.97; ~82 Atenolol; 0.5; 6.97; ~78 Sulpiride; 0.5; 6.97; ~92 | [153] |
| PA ZIF-93 BTFC PA HKUST-1 BTFC | dead-end | 20 | Diclofenac; 1; 33.1; 99.5 Naproxen; 1; 24.9; 99 | [154] |
| Ag@UiO-66-NH2/PA | cross | 8 | Biphenol A; 50; 8.12; 94.6 | [155] |
| TAS-Z-PiP-TFN | cross | 13.8 | Sulfamethoxazole; 10; 3.6; ~30 Amitriptyline; 10; 3.6; ~64 Omeprazole; 10; 3.6; ~60 Loperamide HCl; 10; 3.6; ~70 | [156] |
| MOF0.20-TFN | cross | 8 | Methylparaben; 0.2; 39.5; 47.4 Propylparaben; 0.2; 39.5; 45.9 Benzylparaben; 0.2; 39.5; 51.1 Bisphenol A; 0.2; 39.5; 79.8 | [157] |
| TFN-COF0.05 | cross | 4 | Bisphenol A; 2; 17.1; 98.3 Bisphenol AF; 5; 17.1; 99.1 Sodium 2-biphenylate; 5; 17.1; 99.3 | [115] |
| COF TpPa-SO3H | cross | 5 | Diclofenac; 200; 1.67; 96.4 Ketoprofen; 20; 1.67; 75.8 Naproxen; 15; 1.67; 75.2 Ibuprofen; 20; 1.67; 79.4 Sulfamethoxazole; 300; 1.67; 57.4 | [114] |
| TpPa-PEI 0.125%-10 | cross | 5 | Sulfamethazine; 0.2; 4.0; 62.1 Carbamazepine; 0.2; 4.0; 58.7 Propranolol; 0.2; 4.0; 94.1 Sulpiride; 0.2; 4.0; 97.2 Dametformin; 0.2; 4.0; 71.2 | [116] |
| TpTAPA/HPAN | cross | 3 | Ammonium glycyrrhizinate; 100; 68.1; 92 Diammonium glycyrrhizinate; 100; 68.1; 92.0 | [117] |
| COF-LZU1 | cross | 5 | Tetracycline; 0.4; 23.3; 82.5 Sulfadiazine; 0.4; 23.3; 78.0 Carbamazepine; 0.4; 23.3; 78.4 Propranolol; 0.4; 23.3; 73.6 | [118] |
| HOF-TFN-2 | cross | 1 | Congo red; 0.25 mmol/L; 546; 95.85 Coomassie brilliant blue; 0.25 mmol/L; 546; 96.47 Hodamine B; 0.25 mmol/L; 546; 97.26 Rmethyl blue; 0.25 mmol/L; 546; 83.70 Calcein; 0.25 mmol/L; 546; 92.59 | [95] |
| BILP-101x/HPAN | cross | 4 | Congo red; 0.2; 235; 99 Methyl blue; 0.2; 235; 92 Direct red 23; 0.2; 235; 99 Rhodamine B; 0.2; 235; 90 | [119] |
| GO-coated ceramic hollow fiber | cross | 5 | Rifampicin; 20; 3.5; 52 Propranolol; 0.5; 2.4; 32 | [129] |
| TFN1 | cross | 5 | Sulfamethoxazole; 100; 4.46; 96 Triclosan; 100; 4.46; 94 Diclofenac; 100; 4.46; 91 Cephalexin; 100; 4.46; 92 | [132] |
| GO NF/RGO NF | cross | 20 | Ibuprofen; 10; 94; 89 | [128] |
| TFC-GO | cross | 6.8 | Sulfamethazone; 0.8; 0.44; ~55 Ibuprofen; 0.8; 0.44; ~45 Triclosan; 0.8; 0.44; ~62 Sulfadiazine; 0.8; 0.44; ~58 Sulfamethoxazole; 0.8; 0.44; ~56 Carbamazepine; 0.8; 0.44; ~57 | [133] |
| PDA-GO/β-CD-EDA | dead-end | 5 | Carbamazepine; 0.5;6.8; ~16 Sulfadiazine; 0.5; 6.8; ~55 Propranolol; 0.5; 6.8; ~63 | [136] |
| GO membrane | cross | 5 | Secondary effluent; 175 mgO2/L; 10; 76 | [126] |
| 30 nm GO membrane | dead-end | 2 | Gemfibrozil; 10; -; 76.4 17α-ethynylestradiol; 10; -; 80.1 Diclofenac sodium salt; 10; -; 83 Iodixanol; 10; -; 95.2 | [130] |
| Ceramic GO membrane | dead-end | 3 | Ibuprofen; 10; ~5; 58 Sulfamethoxazole; 10; ~5; 48 | [127] |
| hPAN + GO | dead-end | 20.7 | Triclosan; 0.00125; ~2.5; 95 Triclocarban; 0.00125; ~2.5; 99 | [131] |
| GO/25%DA | dead-end | 5 | Propranolol; 0.2; ~4.6; 40 Carbamazepine; 0.2; ~4.6; 38 Sulfadiazine; 0.2; ~5.0; 50 | [137] |
| AIMGO-3 membrane | dead-end | 1 | Roxithromycin; 10; -; 90.2 4-dimethylaminopyridine; 1; -; 92.0 | [138] |
| GO-modified membranes | cross | 15 | Amitriptylene HCl; 10; ~2; ~90 Bisphenol-A; 10; ~2; ~100 Acetaminophen; 10; ~2; ~90 Caffeine; 10; ~2; ~80 | [158] |
| PA/GO-4 | cross | 6 | Paracetamol; 1; ~10; 4.63 Norfloxacin; 1; ~10; 53.32 Sulfamethoxazole; 1; ~10; 41.85 | [159] |
| MXMn membrane | dead-end | 7 | Caffeine; 1; 19.33; 99.99 | [140] |
| Ti3C2Tx membranes | dead-end | 1 | Bacitracin; 250; ~370; ~94 Azithromycin; 250; ~350; ~85 Erythromycin; 250; ~350; ~85 Tetracycline; 250; ~325; ~80 Penicillin; 250; ~305; ~78 | [143] |
| MP30 membrane | dead-end | 1 | Ampicillin sodium; 200; 287.5; ~90.4 Berberine chloride; 200; 291.2; ~92.9 Tetracycline; 200; 300.8; ~92.5 Erythromycin; 200; 318.8; ~94.6 | [144] |
| MNF2 | cross | 10 | Methylparaben; 0.2; -; 53.7 Ethylparaben; 0.2; -; 69.1 Propylparaben; 0.2; -; 79.1 Benzylparaben; 0.2; -; 91.3 | [160] |
| B:G (1:1) membrane | dead-end | 4 | Levofloxacin; 100; ~11; ~85 | [145] |
| TpPa-wood membrane | dead-end | 0.53 | Norfloxacin NFX; 20; 1200; ~96 Tetracycline TC; 20; 1200; ~94 | [151] |
| EDA-CQD_GOM | cross | ~ | Clofibric acid; -; -; ~90 Naproxen; -; -; ~90 Mefenamic acid; -; -; ~99 Fenoprofen; -; -; ~90 Ketoprofen; -; -; ~92 Diclofenac; -; -; ~93 Furosemide; -; -; ~88 Indometacin; -; -; ~98 Bezafibrate; -; -; ~90 Acetaminophen; -; -; ~60 Ethenzamide; -; -; ~78 Theophylline; -; -; ~62 Antipyrine; -; -; ~80 DEET; -; -; ~84 Caffeine; -; -; ~70 Crotamiton; -; -; ~80 Primidone; -; -; ~62 Isopropylantipyrine; -; -; ~78 Sulfathiazole; -; -; ~60 Cyclophosphamide; -; -; ~70 Sulfamerazine; -; -; ~69 Sulfadimidine; -; -; ~69 Sulfamonomethoxine; -; -; ~83 Thiamphenicol; -; -; ~81 Oxytetracycline; -; -; ~88 Chlortetracycline; -; -; ~99 Dipyridamole; -; -; ~96 Salbutamol; -; -; ~61 Atenolol; -; -; ~60 Trimethoprim; -; -; ~60 Sulpiride; -; -; ~60 Lincomycin; -; -; ~63 Diltiazem; -; -; ~82 Tiamulin; -; -; ~98 Clarithromycin; -; -; ~70 Roxithromycin; -; -; ~75 Tylosin; -; -; ~82 | [146] |
| UiO-66/PGP TFC | cross | 3 | Oxytetracycline HCI; 10; 17.61; 94.8 Tetracycline hydrochloride; 10; 17.61; 95.5 Ciprofloxacin; 10; 16.09; 98.6 Sulfamethoxazole; 10; 27.46; 83.05 | [147] |
| poly-Pd@RCC3/PAN | dead-end | 1 | Chlorophyll; 20; 7.5; ~97 Flavonoids; 20; 7.5; ~47 Ellagitannins; 20; 7.5; ~45 | [89] |
| Pr-MCM-41-NH2-PA/PSf | cross | 25 | Caffeine; -; ~2.24; ~97 Sulfamethoxazole; -; ~2.24; ~97 Amitriptyline; -; ~2.24; ~97 Loperamide; -; ~2.24; ~97 | [148] |
| ZnO membrane | cross | 5 | Atenolol; 0.09; 567; 96 Ibuprofen; 0.582; 561; 99 | [150] |
| MTC | dead-end | 7 | Paracetamol; 5; 19.33; 90.98 Ibuprofen; 5; 19.33; 90.98 | [149] |
| PA/TNT TFC | dead-end | 15 | Bisphenol A; 10; ~1; 89.05 Caffeine; 10; ~1; 97.89 | [161] |
| S2 | cross | 10 | Atrazine; 0.1; 2.9; ~97 Propazine; 0.1; 1.2; ~91 Prometryn; 0.1; 2.5; ~98 | [162] |
| NF90-C0.5Ag4 | cross | 8 | Ethylparaben; 200; 6.7; 67 Propylparaben; 200; 6.7; 69 Benzylparaben; 200; 6.7; 66 Bisphenol A; 200; 6.7; 99 | [163] |
| Membrane | Material | Functional Group/Active Site | Separation Mechanism | Refs. |
|---|---|---|---|---|
| MIL-101(Fe)-NH2-loaded MAq | MIL-101(Fe)-NH2 | -NH2 | Electrostatic interaction, Hydrophilicity | [109] |
| PES/MOF-808@PA TFN | MOF-808 | -COOH | Size exclusion, Electrostatic interaction | [110] |
| MOF/PA-TFC | Zn-MOF | - | Electrostatic interaction, Size exclusion | [113] |
| SurZn3/SubCo3 | ZIF-8/ZIF-67 | Metal oxide | Molecular interaction, Electrostatic interaction | [112] |
| AMSA-MIL-101(Cr) | MIL-101(Cr) | -NH2/-COOH | Electrostatic interaction | [111] |
| PSF-ZIF-8/PA | ZIF-8 | ~ | Size exclusion | [152] |
| TFN NH2-UiO-66-PL-7.6 | NH2-UiO-66 | -NH2 | Size exclusion, Electrostatic interaction | [153] |
| PA ZIF-93 BTFC PA HKUST-1 BTFC | ZIF-93/ HKUST-1 | -OH | Size exclusion, Hydrophilicity | [154] |
| Ag@UiO-66-NH2/PA | UiO66-NH2 | NH2/Ag NPs | Size exclusion, Electrostatic interaction | [155] |
| TAS-Z-PiP-TFN | ZnO | NH2 | Size exclusion, Hydrophilicity | [156] |
| MOF0.20-TFN | MIL-101(Cr) | - | Hydrophilicity, Size exclusion | [157] |
| TFN-COF0.05 | TPB-DMTP | -O-CH3 | Hydrophilicity, Molecular interaction | [115] |
| COF TpPa-SO3H | TpPa-SO3H | -SO3H | Electrostatic interaction, Size exclusion | [114] |
| TpPa-PEI 0.125%-10 | TPPA | -NH2 | Electrostatic interaction, Hydrophilicity | [116] |
| TpTAPA/HPAN | TPTAPA | -OH | Size exclusion, Electrostatic repulsion | [117] |
| COF-LZU1 | LZU-1 | -OH | Electrostatic interaction, Size exclusion | [118] |
| HOF-TFN-2 | Nano-PFC-1 | -OH | Hydrophilicity, Size exclusion | [95] |
| BILP-101x/HPAN | BILP-101x | ~NH~ | Electrostatic interaction, Size exclusion | [119] |
| GO-coated ceramic hollow fiber | GO | OH | Size exclusion | [129] |
| TFN1 | GO-NH2 | -NH2 | Size exclusion, Hydrophilicity | [132] |
| GO NF/RGO NF | GO/RGO | -OH/-COOH | Electrostatic interaction | [128] |
| TFC-GO | GO | -OH/-COOH | Electrostatic interaction | [133] |
| PDA-GO/EDA PDA-GO/β-CD-EDA | GO | -OH/-COOH | Size exclusion | [136] |
| GO membrane | GO | -OH/-COOH | Electrostatic interaction, Size exclusion | [126] |
| 30 nm GO membrane | GO | -OH/-COOH | Size exclusion | [130] |
| Ceramic GO membrane | GO | -OH/-COOH | Size exclusion, Electrostatic interaction | [127] |
| hPAN + GO | GO | -OH/-COOH/-NH2 | Size exclusion | [131] |
| GO/25%DA | GO | -OH/-COOH/-NH2 | Size exclusion | [137] |
| AIMGO-3 membrane | GO | -OH/Imidazole cationic | Size exclusion | [138] |
| GO-modified membranes | GO | -NH2 | Size exclusion, Electrostatic interaction | [158] |
| PA/GO-4 | GO | -OH/-COOH | Size exclusion, Hydrophilicity, Electrostatic interaction | [159] |
| MXMn membrane | MXene | MnO2 | Molecular interaction | [140] |
| Ti3C2Tx membranes | MXene | - | Size exclusion | [143] |
| MP30 membrane | MXene | -OH | Size exclusion, Electrostatic interaction | [144] |
| MNF2 | MoS2 | - | Size exclusion, Electrostatic interaction | [160] |
| B:G (1:1) membrane | GO + EB | OH | Size exclusion, Electrostatic interaction | [145] |
| TpPa-wood membrane | COF + wood | OH | Hydrophilicity, Size exclusion | [151] |
| EDA-CQD_GOM | GO+ CQD | OH/Pyridine | Size exclusion, Electrostatic interaction | [146] |
| UiO-66/PGP TFC | MOF + GO | -OH | Size exclusion, Electrostatic interaction | [147] |
| poly-Pd@RCC3/PAN | POC + Pd | Pd NCs | Size exclusion | [89] |
| Pr-MCM-41-NH2-PA/PSf | MCM-41 | -NH2 | Electrostatic interaction | [148] |
| ZnO membrane | ZnO | - | Hydrophilicity | [150] |
| MTC | Chitosan | MnO2 | Hydrophilicity, Molecular interaction | [149] |
| PA/TNT TFC | TiO2 | -OH | Size exclusion, Molecular interaction | [161] |
| S2 | SiO2 | Silane | Hydrophilicity, Electrostatic interaction | [162] |
| NF90-C0.5Ag4 | Ag NPs | OH-NH2 | Hydrophilicity, Size exclusion | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.; Nong, H.; Chen, L.; Zhang, S. Advanced Materials-Based Nanofiltration Membranes for Efficient Removal of Organic Micropollutants in Water and Wastewater Treatment. Membranes 2025, 15, 236. https://doi.org/10.3390/membranes15080236
Wei H, Nong H, Chen L, Zhang S. Advanced Materials-Based Nanofiltration Membranes for Efficient Removal of Organic Micropollutants in Water and Wastewater Treatment. Membranes. 2025; 15(8):236. https://doi.org/10.3390/membranes15080236
Chicago/Turabian StyleWei, Haochun, Haibiao Nong, Li Chen, and Shiyu Zhang. 2025. "Advanced Materials-Based Nanofiltration Membranes for Efficient Removal of Organic Micropollutants in Water and Wastewater Treatment" Membranes 15, no. 8: 236. https://doi.org/10.3390/membranes15080236
APA StyleWei, H., Nong, H., Chen, L., & Zhang, S. (2025). Advanced Materials-Based Nanofiltration Membranes for Efficient Removal of Organic Micropollutants in Water and Wastewater Treatment. Membranes, 15(8), 236. https://doi.org/10.3390/membranes15080236







